Abstract
Recent studies have demonstrated that repeated short‐term nutrient withdrawal (i.e. fasting) has pleiotropic actions to promote organismal health and longevity. Despite this, the molecular physiological mechanisms by which fasting is protective against metabolic disease are largely unknown. Here, we show that, metabolic control, particularly systemic and liver lipid metabolism, is aberrantly regulated in the fasted state in mouse models of metabolic dysfunction. Liver transcript assays between lean/healthy and obese/diabetic mice in fasted and fed states uncovered “growth arrest and DNA damage‐inducible” GADD45β as a dysregulated gene transcript during fasting in several models of metabolic dysfunction including ageing, obesity/pre‐diabetes and type 2 diabetes, in both mice and humans. Using whole‐body knockout mice as well as liver/hepatocyte‐specific gain‐ and loss‐of‐function strategies, we revealed a role for liver GADD45β in the coordination of liver fatty acid uptake, through cytoplasmic retention of FABP1, ultimately impacting obesity‐driven hyperglycaemia. In summary, fasting stress‐induced GADD45β represents a liver‐specific molecular event promoting adaptive metabolic function.
Keywords: FABP1, hormesis, lipid, metabolism, stress
Subject Categories: Metabolism
Introduction
The incidence of obesity is at an epidemic level worldwide and is a strong risk factor for a number of ageing‐related diseases including type 2 diabetes (T2D), cardiovascular disease and the metabolic syndrome, and thus poses a tremendous burden on quality of life and health care systems worldwide (Popkin et al, 2012). Thus, there is a desperate need for more effective strategies to curtail this trend, whether through prescription of behavioural or pharmacological treatments.
A hallmark of obesity‐driven T2D is insulin resistance (Bjorntorp, 1997), and thus, much effort is placed into treatments that promote “insulin sensitisation” (Connor et al, 2015). While there is no doubt that insulin is an important mediator of metabolic control in the prandial state (Boucher et al, 2014), insulin resistance likely represents a physiological feedback mechanism to actually retard the development of obesity‐driven complications (Hoehn et al, 2009), prompting speculation that “insulin sensitisation” may be a flawed strategy (Connor et al, 2015). This is exemplified by studies demonstrating that tissue‐restricted loss of function of key insulin signalling nodes actually extends health span of mice (Bluher et al, 2003; Taguchi et al, 2007) and that insulin per se can promote the progression of obesity‐related metabolic dysfunction (Mehran et al 2012). Thus, alternative strategies are warranted, such as mild and intermittent activation of stress‐responsive pathways that are pro‐adaptive (Ristow & Zarse, 2010; Kolb & Eizirik, 2012).
One such strategy could be intermittent nutrient withdrawal. Nutrient withdrawal (i.e. fasting) is protective against modern chronic diseases such as cancer, neurodegeneration, cardiovascular disease and diabetes (Longo & Mattson, 2014). In particular, a recent study demonstrated that across multiple species including humans, a periodic diet that mimics fasting had a positive effect on lifespan and biomarkers of cognitive, immune and cardiometabolic function (Brandhorst et al, 2015). Concerning metabolic function, although it is known that there are several systemic adaptive changes in metabolism with nutrient withdrawal (Cahill, 2006), little is known of the tissue‐specific molecular mechanisms by which fasting can improve organismal metabolic control and retard the development of metabolic disease (Longo & Mattson, 2014).
Although it is well accepted that the liver is a pivotal organ contributing to metabolic control (van den Berghe, 1991), whether the liver actually contributes in many aspects of metabolic control during fasting, and molecular mechanisms therein, remains largely unknown. It is known that the liver is the primary site of glucose production and can provide alternate substrates such as ketone bodies under fasting conditions (van den Berghe, 1991). Interestingly, the liver transcriptome starvation response correlates with lifespan prolonging processes (Bauer et al, 2004) and it is hypothesised that resistance to stress is an important determinant of survival and longevity (Calabrese et al, 2011). Hence, one strategy to uncover the molecular mechanisms contributing to metabolic dysfunction could be to dissect the liver transcriptome to uncover select genes which are aberrantly regulated during fasting. To this end, our studies here identify a member of the “growth arrest and DNA damage‐inducible” (GADD45) gene family, namely GADD45β, as one such gene. Importantly, there are no studies that have systematically examined the role of GADD45β in metabolic function in vivo, and here, we demonstrate that liver GADD45β acutely regulates fatty acid handling under fasting stress, ultimately coordinating proper metabolic function under conditions of chronic nutrient oversupply.
Results
An altered lipid profile in mouse models of metabolic dysfunction is most pronounced in the fasted state
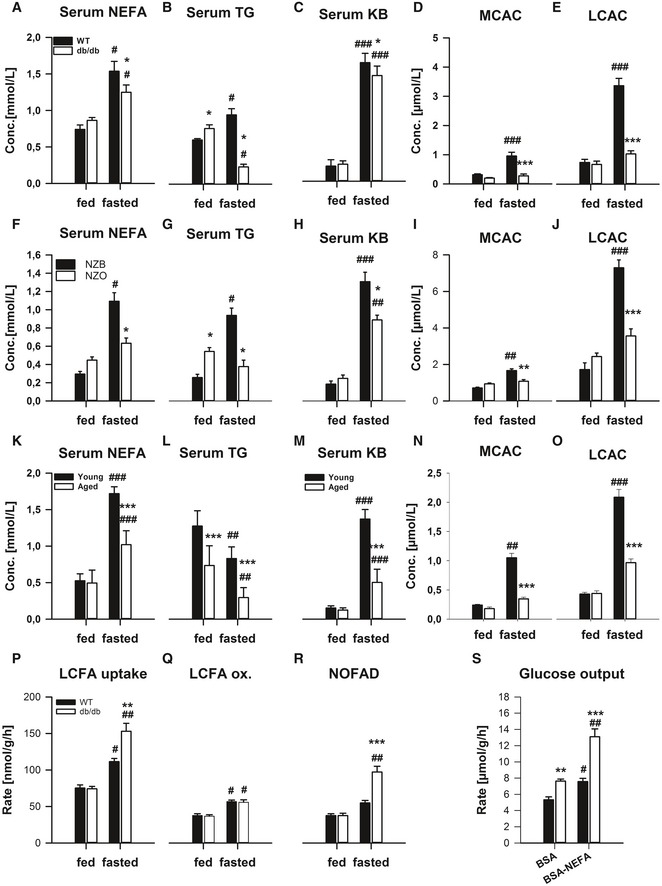
To examine the molecular bases of metabolic (dys)regulation during fasted and fed states, we required suitable mouse models. To this end, we examined mouse models of obesity (Kanasaki & Koya, 2011) including the obese/diabetic db/db mouse (Figs 1A–E and EV1A–D), pre‐diabetic young New Zealand Obese mice (Figs 1F–N and EV1E–H) and aged mice (Figs 1K–O and EV1I–L). In particular, we subjected these mouse models to a fasting‐refeeding regimen and could demonstrate heightened blood glucose (BG; Fig EV1A, E and I) most prominent in the fed and but also in the fasted state. In contrast, although there was evidence of higher serum non‐esterified fatty acids (NEFA; Fig 1A, F and K) in the fed state, surprisingly these levels were lower in models of metabolic dysfunction in the fasted state. This pattern was also reflected in the level of triglycerides (TG; Fig 1B, G and L), ketone bodies (KB; Fig 1G, H and M) as well as medium‐chain (MCAC; Fig 1D, I and N) and long‐chain (LCAC; Fig 1E, J and O) acylcarnitines, with only mild differences in free‐ (Fig EV1B, F and J), acyl‐ (Fig EV1C, G and K) and short‐chain (SCAC; Fig EV1D, H and L) acylcarnitines.
Figure 1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state.
-
A–OMale 12‐weeks‐old wild‐type (WT; C57Bl/6J) or obese/diabetic monogenic (db/db; BKS.Cg‐m+/+ Lepr DB/J; n = 4/group, A–E), New Zealand Black (NZB) and polygenic obese/pre‐diabetic New Zealand Obese (NZO; n = 4/group; F–J), as well as young (i.e. 3 months) and aged (i.e. 22 months; n = 5/group; K–O), mice were fed ad libitum (fed) or fasted for 24 h (fasted). Serum non‐esterified fatty acids (A, F, K), triglycerides (B, G, L) and ketone bodies (C, H, M) were measured. In addition, serum acylcarnitine profiling was conducted and medium‐chain (D, I, N) and long‐chain (E, J, O) acylcarnitine concentrations are shown.
-
P–SIn another cohort of mice, ex vivo long‐chain fatty acid (LCFA) metabolism, including uptake (P), oxidation (Q) and non‐oxidative LCFA disposal (NOFAD; R), in precision‐cut liver slices from fed and fasted WT and db/db mice (n = 3/group; 4 slices per mouse), were determined. In addition, in slices from fasted mice, glucose output was determined in the presence of incubation with NEFA (BSA‐NEFA) or vehicle (BSA) (S).
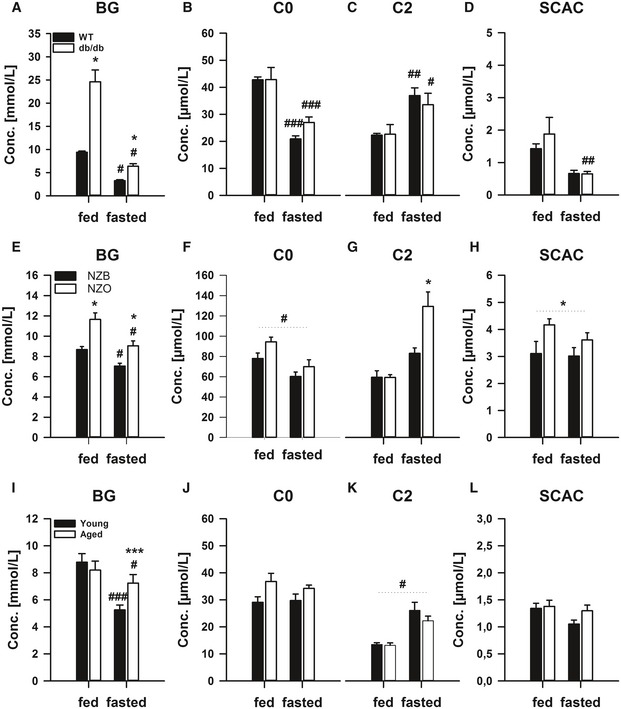
Figure EV1. The dysregulated lipid metabolic phenotype of mouse models of metabolic dysfunction is most pronounced in the fasted state.
-
A–LMale 12‐weeks‐old wild‐type (WT; C57Bl/6J) or obese/diabetic monogenic (db/db; BKS.Cg‐m+/+ Lepr DB/J; n = 4/group, A–D), New Zealand Black (NZB) and polygenic obese/pre‐diabetic New Zealand Obese (NZO; n = 4/group; E–H), as well as young (i.e. 3 months) and aged (i.e.22 months; n = 5/group; I–L), mice were fed ad libitum (fed) or fasted for 24 h (fasted). Blood glucose (BG, A, E, I) and serum acylcarnitine species including free carnitine (C0; B, F, J), acylcarnitine (C2; C, G, K) and short‐chain acylcarnitines (SCAC; D, H, L) were measured. Data are mean ± SEM. n = 4/group. Effect of genotype, *P < 0.05, **P < 0.01, ***P < 0.001. Effect of nutritional state: # P < 0.05, ## P < 0.01, ### P < 0.001. The statistical test used and respective P‐value outputs can be found in Appendix Table S1.
We then examined liver‐specific metabolic control in a separate cohort using ex vivo liver metabolic tracing. In particular, long‐chain fatty acid (LCFA) uptake was higher in the fasted state, and this was exacerbated in db/db mice (Fig 1P). The higher fasting LCFA uptake in WT mice was largely explained by higher LCFA oxidation (Fig 1Q). However, in the db/db mice, the heightened LCFA oxidation could not account for the higher uptake rate, meaning that non‐oxidative LCFA disposal was enhanced. We examined incorporation into lipids but this was unchanged (data not shown). As fatty acids can indirectly affect hepatic glucose production (Ross et al, 1967), we examined liver glucose output, and indeed, it was higher in db/db mice, particularly in the presence of exogenous NEFA (Appendix Fig S1).
Taken together, our preliminary studies exemplify that systemic lipid metabolism is particularly disturbed in the fasted state in mice with metabolic dysfunction, with either enhanced clearance or reduced production of systemic NEFA and TG, the former of which might be explained by a liver‐specific mechanism. Thus, our subsequent studies were focussed on the discovery of a molecular control point regulating (mal)adaptive lipid metabolism during fasting.
Liver GADD45β is an inflexibly regulated gene transcript upon fasting stress in multiple mouse models of metabolic dysfunction
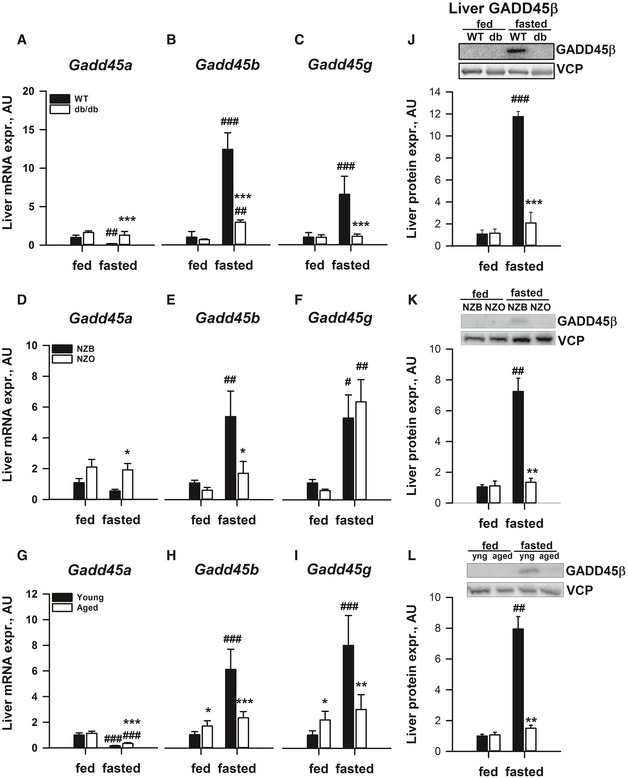
Previous studies of metabolic tissue transcriptomes in rodents (Bauer et al, 2004; Sokolovic et al, 2008; Hakvoort et al, 2011; Zhang et al, 2011; Schupp et al, 2013) identified members of the Gadd45 gene family potently affected by fasting stress. Given that there are three members of the GADD45 family, we then decided to take a targeted approach to the discovery of novel molecular regulatory mechanisms, and examine the expression of all members in multiple models of metabolic dysfunction. To this end, we examined mRNA expression in the liver from the db/db mice (Fig 2A–C) and observed an upregulation of Gadd45b (~12‐fold; Fig 2B) and Gadd45g (~sevenfold; Fig 2C), which was largely blunted in the liver of db/db mice; with only mild regulation of Gadd45a (Fig 2A). Furthermore, we employed less severe models of metabolic dysfunction such as a monogenic model of obesity‐driven pre‐diabetes (Kanasaki & Koya, 2011), the young ob/ob mouse (data not shown), a polygenic model of obesity‐driven pre‐diabetes, the young New Zealand Obese mouse (NZO; Fig 2D–F) and aged mice (Fig 2G–I). In these models, we could observe a consistent pattern of regulation of Gadd45b (Fig 2A, E and H), but not Gadd45g (Fig 2C, F and I), with again only mild regulation of Gadd45a (Fig 2A, D and G). Importantly, similar to the Gadd45b mRNA, which we could show is dynamically regulated upon fasting and refeeding (Appendix Fig S1A), we could observe a similar pattern of upregulation of GADD45β protein in the liver of fasted mice, which was absent in the liver of models of metabolic dysfunction (Fig 2J–L). In addition, although we could show mild differential regulation in perigonadal white adipose tissue (pgWAT; Appendix Fig S1B–D), brown adipose tissue (BAT; Appendix Fig S1E–G) and gastrocnemius complex skeletal muscle (GCM; Appendix Fig S1H–J) of Gadd45a (Appendix Fig S1B, E and H), Gadd45b (Appendix Fig S1C, F and I) and Gadd45g (Appendix Fig S1D, G and J) between fed and fasted states comparing lean and obese/T2D mice, none of these regulations were as striking as observed with the liver Gadd45b (Fig 2).
Figure 2. Liver/hepatocyte Gadd45b expression is consistently dysregulated upon fasting in several mouse models of metabolic dysfunction.
-
A–ILiver mRNA expression of growth arrest and DNA damage‐inducible 45 alpha (Gadd45a; A, D, G), beta (Gadd45b; B, E, H) and gamma (Gadd45g; C, F, I) was measured in fed and fasted obese/diabetic monogenic (db/db (n = 4/group); A–C), obese/pre‐diabetic polygenic New Zealand Obese (NZO (n = 4/group; D–F) and aged C57Bl/6J (22 months; n = 5/group; G–I) as well as corresponding lean, young wild‐type (WT) mice (n = 4/group). Matched controls for the NZO and aged mice were New Zealand Black (NZB) and 12‐weeks‐old C57Bl/6J mice, respectively.
-
J–LProtein expression of liver GADD45β, as well as the housekeeping protein valosin‐containing protein (VCP), was measured from liver samples. Number of replicates for each sample set are outlined above.
GADD45β affects metabolic regulation under conditions of heightened lipid metabolism
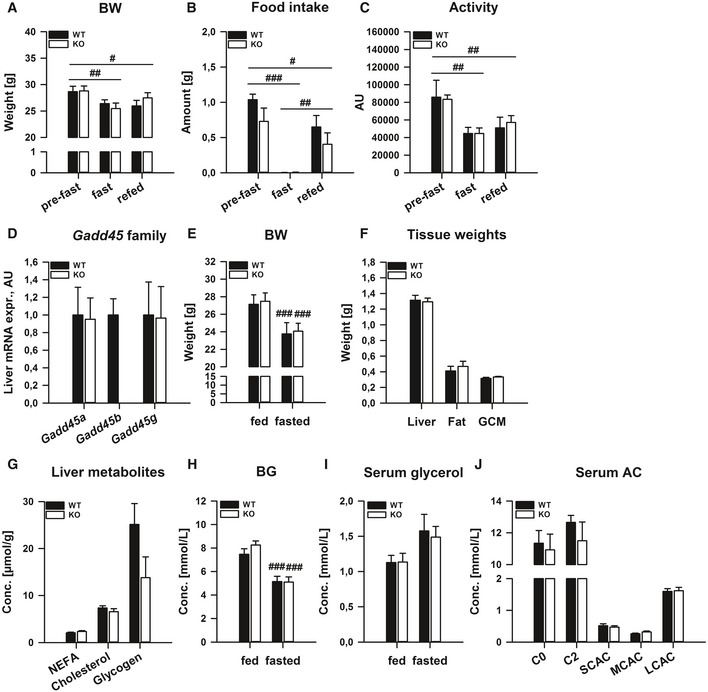
Given that we could observe a stark regulation of liver GADD45β under fasting stress, we next tested whether GADD45β expression/activity affects the ability of the organism to handle such nutritional stress by subjecting whole‐body GADD45β knockout mice to starvation and refeeding. In particular, absolute levels as well as changes in body mass (Fig EV2A), food intake (Fig EV2B) and physical activity (Fig EV2C) were unaffected in GADD45β KO mice. Furthermore, systemic oxidative metabolism, as reflected at the rate of O2 consumption (Fig 3A), CO2 production (Fig 3B) and respiratory exchange ratio (Fig 3C), was also unaffected. In a separate study, we could demonstrate that there was no compensatory upregulation of the other GADD45 family members with loss of GADD45β (Fig EV2D) and could confirm the lack of effect of GADD45β loss on body mass regulation upon starvation (Fig EV2E). When we examined the masses of selected metabolic organs/tissues, there were also no differences (Fig EV2F). On the other hand, while we could not observe any differences in selected liver metabolites (Fig EV3G) or blood serum levels of glucose (Fig EV2H), TG (Fig 3D), ketone bodies (Fig 3E), glycerol (Fig EV2I) and acylcarnitines (Fig EV2J), we could observe a significantly lower increase in serum NEFA (Fig 3F) with a much higher level of accumulation of TG (~twofold; Fig 3G) in the liver of GADD45β KO mice, pointing to a role for GADD45β in coordinating an aspect of systemic lipid metabolism under starvation stress. Importantly, even though we could observe changes in Gadd45γ upon starvation (Fig 2), there was no discernible differential metabolic phenotype in starved GADD45γ KO mice (Appendix Fig S2).
Figure EV2. Systemic GADD45β deletion affects metabolic regulation under conditions of heightened lipid metabolism.
-
A–CMale GADD45β+/+ (WT) or GADD45β−/− (KO) were fed ad libitum (fed) or fasted for 24 h (fasted), and subsequently refed for 24 h (n = 4–6/group). Body weight (A), food intake (B) and physical activity (C) were measured.
-
D–JIn a distinct cohort, male GADD45β+/+ (WT) or GADD45β−/− (KO) mice were fasted for 24 h (fasted) (n = 5–8/group) with blood samples taken before and during fasting. Liver mRNA expression of Gadd45 family members (D). Body weight (E) before and after fasting and fasted tissue weights (F). Selected liver metabolites (G) as well as blood glucose (H) and serum glycerol (I) and acylcarnitines (J).
Figure 3. Systemic GADD45β deletion affects metabolic regulation under conditions of heightened lipid metabolism.
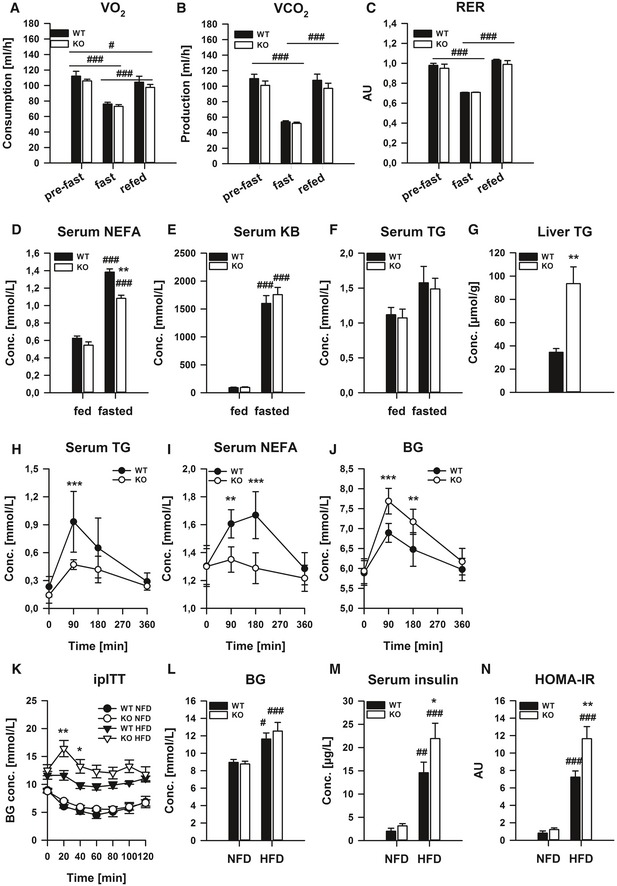
-
A–GMale GADD45β+/+ (WT; n = 6) or GADD45β−/− (KO; n = 4) mice were fed ad libitum (fed) or fasted for 24 h (fasted) and subsequently refed for 24 h. O2 consumption rate (A), CO2 production rate (B) and respiratory exchange ratio (C) were measured by indirect calorimetry. In a distinct cohort, male GADD45β+/+ (WT; n = 5) or GADD45β−/− (KO; n = 8) were fed ad libitum (fed) or fasted for 24 h (fasted). Serum non‐esterified fatty acids (D) and ketone bodies (E) as well as serum (F) and liver (G) triglycerides (TG) were measured.
-
H–JSerum TG (H), NEFA (I) and blood glucose (BG, J) concentrations during an oral lipid tolerance test in overnight fasted GADD45β+/+ (WT; n = 5) or GADD45β−/− (KO; n = 5) mice.
-
K–NBlood glucose excursion during and intraperitoneal insulin tolerance test (K) as well as fasting blood glucose (L), serum insulin (M) and HOMA‐IR (N) in GADD45β+/+ (WT; n = 6) or GADD45β−/− (KO, n = 9) chronically fed a normal‐ (NFD) or high (HFD)‐fat diet.
Figure EV3. Liver‐restricted GADD45β manipulation affects systemic metabolic homoeostasis.
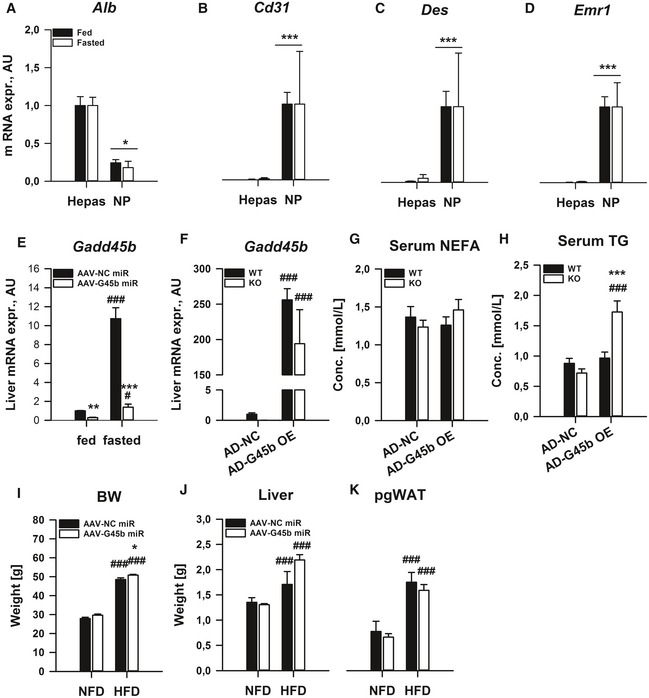
-
A–DAlbumin (A), Cluster of differentiation 31 (B), desmin (C) and EGF‐like module‐containing mucin‐like hormone receptor‐like 1 (D) mRNA expression were measured from fractionated parenchymal hepatocytes (Hepas) as well as non‐parenchymal cells (NP) from C57Bl/6J mice fed ad libitum (fed) or fasted for 24 h (fasted) (n = 3/group).
-
EMale C57Bl/6J mice with (AAV‐G45b miR) or without (AAV‐NC miR) liver/hepatocyte‐restricted GADD45β silencing were fed or fasted for 24 h (n = 6/group). Liver Gadd45b mRNA expression was measured.
-
F–HMale GADD45β+/+ (WT) or GADD45β−/− (KO) mice fasted for 24 h (fasted) with (AD‐G45b OE) or without (AD‐NC) liver‐restricted Gadd45b over‐expression (n = 7–8/group). Liver mRNA expression of Gadd45b (F). Serum non‐esterified fatty acids (G) and triglycerides (H) were measured.
-
I–KMale C57Bl/6J mice with (AAV‐G45b miR) or without (AAV‐NC miR) liver/hepatocyte‐restricted GADD45β silencing were chronically fed a normal‐fat diet (NFD) or high ‐fat diet (HFD) and were sacrificed in the ad libitum fed state (n = 6–8/group). Body (I), liver (J) and perigonadal white adipose tissue (K) masses.
Given that we could detect changes in lipid metabolism during starvation, we next directly tested lipid homoeostasis by conducting an oral lipid tolerance test. Strikingly, systemic lipid clearance was accelerated in fasted GADD45β KO mice, with substantially lower serum TG (Fig 3H) and NEFA (Fig 3I). Perhaps surprisingly, blood glucose levels rose to a greater extent in the GADD45β KO mice following oral lipid provision (Fig 3J), indicating that GADD45β might be a molecular regulator of lipid–glucose metabolic crosstalk. To test this further, we conducted a chronic high‐fat diet (HFD) study, which should then reveal whether GADD45β is operative in affecting metabolic control during more mild but chronic fasting‐feeding rhythms, and while there were no substantial effects of GADD45β loss on glucose homoeostasis on the normal‐fat diet (NFD), there was impaired glucose homoeostasis upon high‐fat diet treatment as demonstrated by intraperitoneal insulin tolerance testing (ipITT; Fig 3K) as well as fasting glucose (BG; Fig 3J), insulin (Fig 3M) and a calculated insulin resistance index (HOMA‐IR; Fig 3N), which were independent of compensatory GADD45 expression, body and liver mass in the ad libitum fed state (Appendix Fig S3).
Liver GADD45β modulates metabolic regulation under conditions of heightened lipid metabolism
We next wanted to test whether liver‐specific GADD45β affects systemic metabolism. Indeed, we could demonstrate that similar to the lipid tolerance test results (Fig 3C and D), ex vivo liver lipid handling is affected by GADD45β loss (Fig 4A–D), selectively in the fasted state when Gadd45b is upregulated (Fig 2). In particular, long‐chain fatty acid uptake was enhanced (LCFA; Fig 4A), without effects on LCFA oxidation (Fig 4B), and thus largely driven by an enhanced non‐oxidative metabolism (NOFAD; Fig 4C). Similar to the exaggerated glycemic response to oral lipid (Fig 3E), the glucose production rate from liver ex vivo was exacerbated by GADD45β loss (Fig 4D), highlighting a potential role of GADD45β in coordinating proper liver fatty acid–glucose metabolism crosstalk.
Figure 4. Liver GADD45β modulates metabolic regulation under conditions of heightened lipid metabolism.
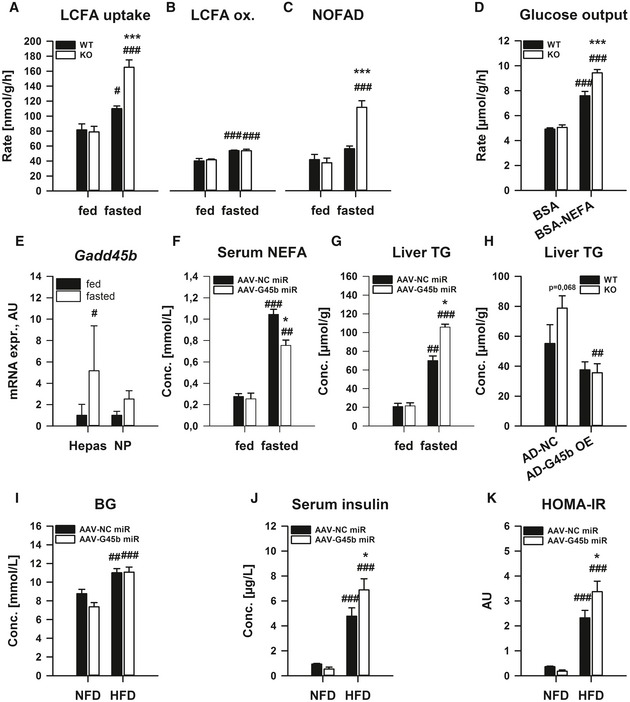
-
A–DMale, GADD45β+/+ (WT) or GADD45β−/− (KO) mice were fed ad libitum (fed) or fasted for 24 h (fasted), and ex vivo liver slice long‐chain fatty acid (LCFA) metabolism was measured including LCFA uptake (A), oxidation (B) and non‐oxidative LCFA disposal (NOFAD) was calculated (C). In addition, glucose production was measured (D) in the presence (BSA‐NEFA) or absence (BSA) of extracellular fatty acids (A‐D: n = 4/group with four liver slices per mouse liver).
-
EGadd45b mRNA expression was measured from fractionated parenchymal hepatocytes (Hepas) as well as non‐parenchymal cells (NP) from C57Bl/6J mice fed ad libitum (fed) or fasted for 24 h (fasted); n = 3/group.
-
F, GMale C57Bl/6J mice with (AAV‐G45b miR) or without (AAV‐NC miR) liver/hepatocyte‐restricted GADD45β silencing were fed or fasted for 24 h (n = 6/group), and serum levels of non‐esterified fatty acids (NEFA; F) as well as liver triglyceride (TG) concentration (G) were measured.
-
HMale GADD45β+/+ (WT; n = 16) or GADD45β−/− (KO; n = 15) mice fasted for 24 h (fasted) with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression (n = 7–8/group). Liver TG concentration was measured.
-
I–KMale C57Bl/6J mice with (AAV‐G45b miR; n = 15) or without (AAV‐NC miR, n = 13) liver/hepatocyte‐restricted GADD45β silencing were chronically fed a normal‐fat diet (NFD) or high ‐fat diet (HFD) (n = 6–8/group). Fasting blood glucose (I) and serum insulin (J) were measured from which HOMA‐IR was calculated (K).
To examine whether the upregulation of Gadd45b mRNA during fasting was occurring within the parenchymal cell of the liver, namely the hepatocyte, we performed a liver cell‐type fractionation experiment from livers of fasted and fed mice. While the fractionation of hepatocytes leaked into the non‐parenchymal fraction to a mild extent (Alb; Fig EV3A), the hepatocyte fraction was devoid of non‐parenchymal markers of endothelial (Cd31; Fig EV3B), stellate (Des; Fig EV3C) and Kupffer (Emr1; Fig EV3D) cells, and there was only an upregulation of Gadd45b in the hepatocyte fraction (Fig 4E), indicating that the increase of Gadd45b expression within the liver upon fasting occurs within the hepatocyte.
Given the above findings, we then wanted to assess whether GADD45β affects metabolic control in a liver‐specific manner in vivo. As such, we conducted a study whereby we silenced Gadd45b in a liver/hepatocyte selective manner (Graham et al, 2008; Rose et al, 2011) via AAV‐mediated delivery of a Gadd45b‐specific miRNA. Using this strategy, we were able to substantially blunt the expression of Gadd45b, particularly during fasting (Fig EV3E). Similar to the results from the germline whole‐body KO (Fig 3), there was a blunting of the higher serum NEFA (Fig 4F) and exacerbation of the higher liver TG (Fig 4G) in the fasted liver‐specific Gadd45b silenced mice. To confirm that hepatic GADD45β affects lipid metabolism, we conducted a study whereby we overexpressed GADD45β in the liver in GADD45β KO mice (Fig EV3F). Indeed, GADD45β re‐introduction into the liver of whole‐body GADD45β KO, despite not affecting serum NEFA (Fig EV3G), affected serum TG (Fig EV3H) and alleviated the heightened accumulation of TG in the liver of GADD45β KO mice upon fasting (Fig 4H). To test this further, we conducted a chronic high‐fat diet (HFD) study, which should then reveal whether GADD45β is operative in affecting metabolic control during more mild but chronic fasting‐feeding rhythms. While there were no substantial effects of GADD45β loss on biometric parameters (Fig EV3I–K), similar to the whole‐body KO study (Fig 3), hepatocyte‐specific GADD45β silencing worsened the progression of insulin resistance upon high‐fat diet‐induced obesity (Fig 4I–K).
Impaired liver GADD45β expression correlates with metabolic dysfunction in obesity‐driven type 2 diabetes in mouse and man
Prompted by our results that GADD45β loss can affect glucose homoeostasis in obesity (Figs 3 and 4), we tested whether restoration of liver GADD45β can improve metabolic homoeostasis in type 2 diabetes. To this end, we employed our prior strategy and overexpressed GADD45β in the liver of obese/diabetic db/db mice (Fig EV4A). While biometrics (Fig EV4B–D) were not drastically affected, fasting blood glucose (Fig 5A) and insulin (Fig 5B) were lower in diabetic mice, which led to an overall reduction in systemic insulin resistance (HOMA‐IR; Fig 5C). Since GADD45β expression in liver affects lipid and glucose homoeostasis in mice, we wondered if the same were true in humans. Indeed, during fasting, GADD45B expression was significantly lower in livers of type‐2 diabetic patients (T2D) when compared with aged‐matched individuals with normal glucose tolerance (NGT; Fig 5D). Furthermore, although there was a non‐significant negative correlation with HOMA‐IR (Fig EV4E), liver GADD45B expression negatively correlated with fasting TG (Fig 5E) and glucose (FPG; Fig 5F) levels. Hence, similar to mice, effective GADD45B expression during fasting may also confer proper metabolic control in humans.
Figure EV4. Liver GADD45β expression modulates metabolic control in type 2 diabetes.
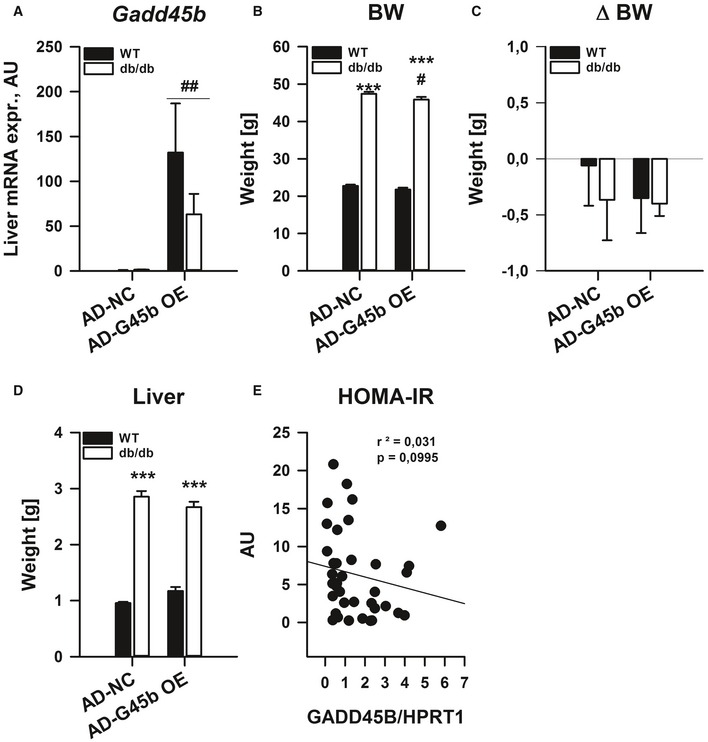
-
A–DMale 12‐weeks‐old wild‐type (WT; C57Bl/6J) or obese/diabetic (db/db; BKS.Cg‐m+/+ Lepr DB/J) mice with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression were fasted for 24 h (n = 4–6/group). Liver mRNA expression of Gadd45b (A). Body mass (B), the change in body mass during the experiment (C) and liver mass (D). Data are mean ± SEM. Effect of genotype, *P < 0.05, **P < 0.01, ***P < 0.001. Effect of viral manipulation state: # P < 0.05, ## P < 0.01, ### P < 0.001.
-
EFasting HOMA‐IR in correlation with liver GADD45B mRNA expression in men as in Fig 5 (n = 37). Inserts show r 2 values and P‐values from Spearman's correlation test.
Figure 5. Liver GADD45β expression modulates glucose homoeostatic control in type 2 diabetes.
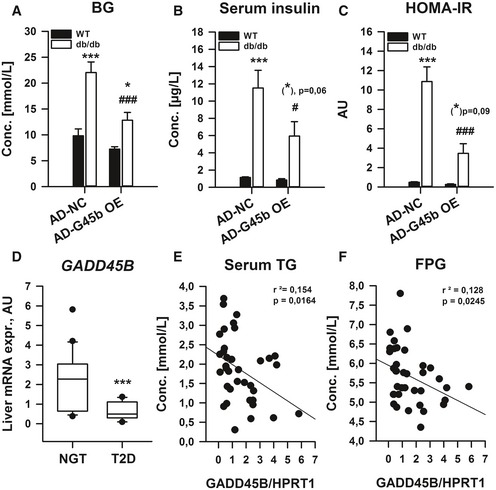
-
A–CMale 12‐weeks‐old wild‐type (WT; C57Bl/6J) or obese/diabetic (db/db; BKS.Cg‐m+/+ Lepr DB/J) mice with (AD‐G45b OE) or without (AD‐NC) prior liver‐restricted GADD45β over‐expression were fasted, and blood glucose (A) and serum insulin (B) were measured from which HOMA‐IR was calculated (C) (n = 4–6/group). Data are mean ± SEM. Effect of genotype/viral manipulation, *P < 0.05, **P < 0.01, ***P < 0.001. Effect of nutritional state: # P < 0.05, ## P < 0.01, ### P < 0.001.
-
D–FLiver GADD45B mRNA expression from men with (T2D) or without (NGT) type 2 diabetes (n = 14–23/group). ***P < 0.001. Scatter plots fasting plasma triglycerides (E) and glucose (F) in correlation with liver GADD45B mRNA expression (n = 37). Inserts show r 2 values and P‐values from Spearman's correlation test.
Liver GADD45β controls liver fatty acid handling by cytosolic FABP1 retention
Given the effects of GADD45β on metabolism, we then searched for a mechanism by which it might exert such control. Given that prior studies have demonstrated a role for GADD45β to control various aspects of cellular functions though transcriptional or signalling mechanisms we first focussed on this. In particular, overexpression of GADD45β in the livers of diabetic mice did not enhance key insulin signalling nodes (Fig EV5A and B). Furthermore, although previously implicated in other studies (Keil et al, 2013; Tian & Locker, 2013), GADD45β expression did not affect autophagy, mTORC1, MAPK or ER stress signalling pathways (Fig EV5C and D). Nor did GADD45β (Fig 6A) affect expression of key genes involved in liver fatty acid transport/metabolism at the mRNA (Fig 6B) or protein (Fig EV5C and D) level. Consistent with a role for localisation of key FA transport/handling proteins in metabolic control (Glatz et al, 2002; Kazantzis & Stahl, 2012), we could, however, observe a role for GADD45β in regulating FABP1, but not FATP2 nor CD36, localisation (Fig 6C). In particular, FABP1 localisation was redistributed from the cytoplasm to the low‐density microsomal (i.e. plasma membrane and microsomes) membranes with a lack of GADD45β (Figs 6C and D, and EV5E), which could be reversed by re‐expression of GADD45β in a liver‐specific manner (Fig 6E). Furthermore, similar to the liver of GADD45β knockout mice, obese/diabetic db/db mice had a redistribution of FABP1 towards microsomes and away from the cytosol, which could be reversed by GADD45B overexpression (Fig 6F).
Figure EV5. Liver GADD45β controls liver fatty acid handling by cytosolic FABP1 retention.
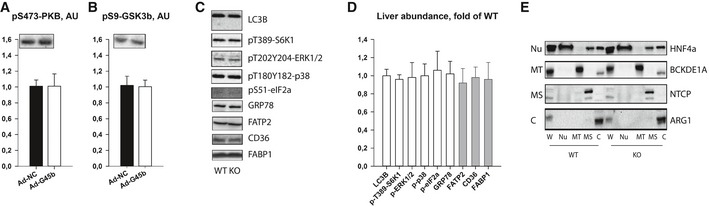
-
A, BMale 12‐weeks‐old wild‐type (WT; C57Bl/6J) or obese/diabetic (db/db; BKS.Cg‐m+/+ Lepr DB/J) mice with (Ad‐G45b OE) or without (Ad‐NC) prior liver‐restricted GADD45β over‐expression were fasted and insulin was injected with livers harvested shortly thereafter and subsequently liver proteins were subjected to immunoblotting for insulin signalling proteins including phosphoprotein kinase B (PKB/Akt; A) and glycogen synthase kinase beta (GSK3b; B). Inserts show representative blots (n = 6/group).
-
C, DRepresentative immunoblots (C) and relative abundance quantifications (D; n = 6) of proteins and phosphoproteins including light chain 3 isoform B (LC3B), S6 kinase 1 (S6K1), p42/44 mitogen activated protein kinase (ERK1/2), eukaryotic initiation factor 2 alpha (eIF2a), glucose regulated protein 78 (GRP78/HSPA5), fatty acid transport protein 2 (FATP2/SLC27A2), cluster determinant 36 (CD36/FAT) and fatty acid binding protein 1 (FABP1) from fasted GADD45β+/+ (WT) or GADD45β−/− (KO) mice.
-
ERepresentative immunoblots of HNF4a (nuclear marker), BCKDE1A (mitochondrial marker), NTCP (microsomal marker) and ARG1 (cytosolic marker) from liver whole tissue lysate (W) as well as fractionated organelles/intraceuular structures including nuclei (N), mitochondria (MT), microsomes (MS) and cytoplasm (C), from GADD45β+/+ (WT) and GADD45β−/− (KO) mice.
Figure 6. Liver GADD45β controls liver fatty acid handling by cytosolic FABP1 retention.
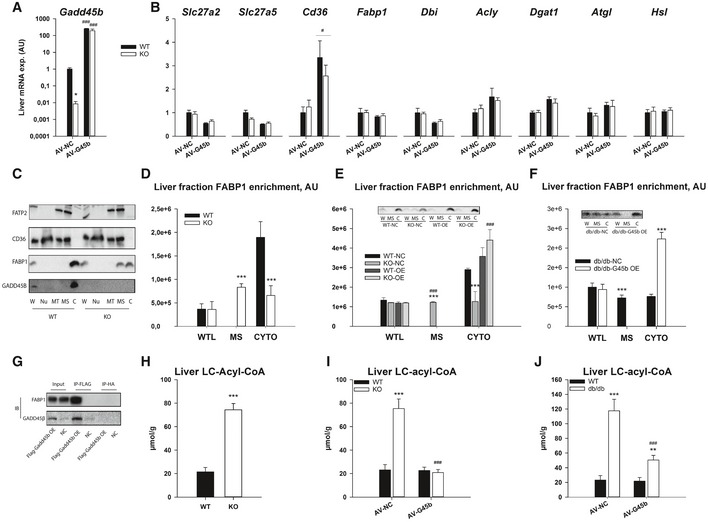
-
A, BMale GADD45β+/+ (WT; n = 16) or GADD45β−/− (KO; n = 15) mice fasted for 24 h (fasted) with (AV‐G45b OE) or without (AV‐NC) liver‐restricted GADD45β over‐expression (n = 7–8/group). Liver mRNA expression of Gadd45b (A) as well as fatty acid metabolic genes (B) encompassing transport (Slc28a2, Slc27a5, Cd36), intracellular binding (Fabp1, Dbi) and metabolism (Acly, Dgat1, Atgl, Hsl).
-
CRepresentative immunoblots of FATP2, CD36, FABP1 and GADD45β from liver whole tissue lysate (W) as well as fractionated organelles/intracellular structures including nuclei (N), mitochondria (MT), microsomes (MS) and cytoplasm (C), from GADD45β+/+ (WT) and GADD45β−/− (KO) mice.
-
DQuantified band densities of FABP1 enrichment from fractions in C (n = 4/group).
-
ELiver fraction enrichment of FABP1 from male GADD45β+/+ (WT) or GADD45β−/− (KO) mice fasted for 24 h with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression (n = 4/group). Insert shows a representative FABP1 immunoblot.
-
FLiver fraction enrichment of FABP1 from obese/diabetic male db/db mice fasted for 24 h with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression (n = 4/group). Insert shows a representative FABP1 immunoblot.
-
GFABP1 and GADD45B immunoblots from Flag immunoprecipitations (IP‐FLAG) or mock IP (IP‐HA) from liver input samples from mice with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression. Shown is a representative immunoblot from 3 separate experiments using 3 different input samples per condition.
-
H–JLiver tissue long‐chain acyl‐CoA (LC‐acyl‐CoA) concentrations were determined in GADD45β+/+ (WT) or GADD45β−/− (KO) mice (H; n = 6/group) with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression (I; n = 5/group). Liver LC‐acyl‐CoA concentrations were determined in wild‐type (WT; C57Bl/6J) or obese/diabetic (db/db; BKS.Cg‐m+/+ Lepr DB/J) mice with (AD‐G45b OE) or without (AD‐NC) liver‐restricted GADD45β over‐expression (J; n = 4/group).
Given that immunoprecipitation experiments demonstrated that GADD45β can physically bind in a complex with FABP1 (Fig 6G) and that GADD45β is exclusively expressed in the cytoplasm (Fig 6C), GADD45β might operate as a cytosolic adapter molecule for FABP1. Lastly, expression of GADD45β and associated altered localisation of FABP1 consistently negatively correlated with liver levels of activated long‐chain fatty acids in multiple experiments (Fig 6H–J), consistent with a direct role for GADD45β to modulate hepatocellular FA metabolism.
Discussion
Here, we demonstrate that liver GADD45β expression is dynamically regulated upon fasting stress, perhaps by increased oxidative stress (Zhang et al, 2013; Kim et al, 2014), where it aids in the coordination of liver lipid metabolism, by limiting hepatocellular fatty acid (FA) uptake via cytosolic FABP1 retention. Furthermore, in mouse and human metabolic dysfunction such as type 2 diabetes, obesity/pre‐diabetes and the aged, liver GADD45β expression is dysregulated thereby contributing to aberrant lipid/glucose homoeostasis.
The GADD45 gene family composes of three structurally and functionally related genes named as GADD45α, GADD45β and GADD45γ, encoding small, highly acidic, nuclear proteins (Liebermann & Hoffman, 2002). Depending on the cellular stress and the physical interactions with other proteins, GADD45 proteins serve as stress sensors, which participate in cell cycle arrest, apoptosis and cell survival (Liebermann & Hoffman, 2008). Despite their structural and physical similarities, their response to stress conditions is cell type and stimulus specific (Yang et al, 2009). Indeed, similar to prior studies (Bauer et al, 2004; Zhang et al, 2011), here, we could show that fasting preferentially promotes GADD45β expression in the liver, whereas others have demonstrated that physical exertion (Hoene & Weigert, 2010), cold stress (Gantner et al, 2014) and atrophy (Ebert et al, 2012) activated GADD45 isoform expression in other diverse metabolic tissues, with functional metabolic effects. Furthermore, there are several lines of evidence that GADD45β is involved in hyperplasia of hepatocytes in liver regeneration (Columbano et al, 2005; Papa et al, 2008; Tian et al, 2011), and although hepatic TG accumulation occurs during liver regeneration, the effects GADD45β on lipid metabolism identified here are unlikely to be of relevance in this setting (Newberry et al, 2008). Even though GADD45β has been linked to epigenetic control (Ma et al 2009), it is unlikely that the altered metabolism observed within GADD45β knockout mice results from developmental “programming” as we could show congruent phenotypes when GADD45β was manipulated in adult mice.
Through liver‐specific GADD45β manipulation in vivo, we demonstrated that the altered liver fatty acid metabolism in obesity/diabetes and ageing might result from altered liver GADD45β expression, particularly during the fasted state. In particular, fasted obese/T2D mice had enhanced liver LCFA uptake, with a similar phenotype observed in fasted GADD45β KO mice, suggesting that the blunted regulation of liver GADD45β and metabolic derangements in mouse models of metabolic dysfunction may be causally linked. We speculate that the persistent fast‐feeding cycles intermittently activate liver GADD45β expression, leading to proper coordination of liver lipid metabolism during fasting, ultimately impacting the maintenance of metabolic health, particularly when dietary lipid/nutrient intake is high. Indeed, liver lipid metabolism is intimately linked to systemic glucose control (Perry et al, 2015).
While it is conceivable that GADD45β could achieve its effects via both cell‐autonomous and non‐autonomous mechanisms, we could demonstrate that GADD45β modulates LCFA metabolism ex vivo, indicating that the former is likely to be the case, and that factors within hepatocytes per se are likely to be affected by GADD45β. In particular, unlike other studies that have shown a role for the activation of transcriptional co‐activator PGC1α to retard metabolic dysfunction by promoting fatty acid oxidation (Morris et al, 2012), we could demonstrate that fasting liver LCFA oxidation was not affected, but rather non‐oxidative FA metabolism independent of storage was involved in GADD45β‐mediated effects on hepatic lipid metabolism. This is reminiscent of studies of skeletal muscle, where an unknown FA metabolic fate correlates with obesity‐related metabolic dysfunction (Koves et al, 2008), and probably relates to a relative mitochondrial substrate “overload” (Muoio & Neufer, 2012), which seems to be also present in the liver (Satapati et al, 2012). Furthermore, chronically enhanced liver FA supply/uptake can negatively affect systemic metabolic function (Mittelman et al, 1997; Koonen et al, 2007; Doege et al, 2008; Falcon et al, 2010; Perry et al, 2015) the mechanisms by which are not fully resolved. On this, we could demonstrate that induced GADD45β restrained liver fatty acid uptake during fasting ultimately preventing an accumulation of long‐chain (LC) acyl‐CoAs in the liver. Given that liver LC‐acyl‐CoAs levels positively correlate with insulin resistance (present work and (Chen et al, 1992; Kamath et al, 2011), they may be directly or indirectly related to aberrant glucose metabolism under conditions of enhanced fatty acid supply such as obesity (Li et al, 2010), the mechanisms by which are likely to be diverse (Faergeman & Knudsen, 1997; Cooney et al, 2002; Li et al, 2010). Indeed, here we observed that restoration of low liver GADD45β expression in obese/diabetic mice reduced the higher liver LC‐acyl‐CoA amount in liver, which correlated with improved glucose homoeostasis.
Here, we demonstrate that GADD45β binds to and restrains FABP1 to the hepatocyte cytoplasmic compartment, ultimately preventing an accumulation of activated long‐chain fatty acids. Importantly, although normally heavily abundant in the cytoplasm, redistribution of FABP1 has been described previously in other contexts (Wolfrum et al, 2001; Antonenkov et al, 2006). Consistent with a key role for FABP1 localisation in explaining the altered FA metabolism modulated by GADD45β expression, FABP1 is a highly abundant protein in the liver which has a role in cellular uptake of FAs and affects the development of insulin resistance in obesity (Atshaves et al, 2010). In particular, in vitro gain‐ and loss‐of‐function studies (Wolfrum et al, 2001; Linden et al, 2002; Newberry et al, 2003) have implicated FABP1 in hepatocellular FA uptake, and studies of germline FABP1 knockout mice demonstrate reduced hepatic FA uptake particularly during conditions of heightened FA supply such as fasting (Martin et al, 2003; Newberry et al, 2003), which is consistent with our studies here. Thus, we hypothesise that aberrant localisation of FABP1 towards microsomal membranes, including the surface membranes and microsomes, is a molecular event linking excess hepatic fatty acid uptake and low GADD45β expression during fasting.
Of note, the aberrant lipid metabolic phenotype, including serum NEFA, triglycerides, ketone bodies, acylcarnitines and liver triglycerides, of obese/aged mice was particularly observed in the fasted state, which corresponds to studies in humans demonstrating that differential metabolic phenotypes are best revealed by challenges (Krug et al, 2012). In flies, D‐GADD45 overexpression results in increased longevity, and a hallmark of long‐lived animals is resistance to stressors (Calabrese et al, 2011). Indeed, we (data not shown) and others (Kim et al, 2015) have demonstrated that GADD45β protects against liver damage upon toxicity stress and fasting activates pleiotropic adaptive processes that are beneficial to organismal health (Longo & Mattson, 2014). Given that liver toxicity response is exacerbated in obese/diabetic mice (Aubert et al, 2012), of which have dysregulated GADD45β expression, the coordination of metabolism by GADD45β may represent of molecular metabolic link in adaptive stress biology. Taken together, we hypothesise that GADD45β might represent a “vitagene” conferring hormetic metabolic adaptive processes (Calabrese et al, 2011), and that the concept of “metabolic inflexibility” (Storlien et al, 2004) may simply reflect a lack of response of such hormetic mechanisms (Kolb & Eizirik, 2012), of which liver GADD45β induction may be one component.
In summary, here, we identify a role of a liver transcript, GADD45β, in modulating systemic and liver‐specific adaptive metabolism under nutrient‐starvation stress, which ultimately aids in the coordination of metabolic homoeostasis upon chronic nutrient overload.
Materials and Methods
Mouse experiments
Mouse strains used included male wild‐type C57Bl/6J (000664; Charles River Laboratories, DEU), db/db (12 weeks; 000642, BKS.Cg‐m+/+ Lepr DB/J, Charles River Laboratories, DEU), ob/ob (7 weeks; 000632, B6.Cg‐Lep ob/J, Jackson Laboratories, USA), New Zealand Black (7 weeks; NZB/BlNJ, 000993, Jackson Laboratories, USA) and New Zealand Obese (7 weeks; NZO/HlLtJ, 002105, Jackson Laboratories, USA). Furthermore, germline male GADD45β (B6.CgGADD45βtm1Daa; Gupta et al, 2005) and female (XX) GADD45γ (B6.CgGADD45γtm1Mhol; Cai et al, 2006) knockout mice from −/+ × −/+ crossings were used. Importantly, SNP marker testing demonstrated that the GADD45β mouse line was of 99.1% pure C57Bl/6 background strain (Charles River Genetic Testing Services; data not shown). The animals were housed according to international standard conditions with a 12‐h dark–light cycle and regular unrestricted diet with free access to water if not stated otherwise. For studies with overexpression of GADD45β in the murine liver, 1 × 109 infectious units per recombinant adenovirus (AD) were administered via tail vein injection into either male db/db and C57Bl/6J control mice or GADD45β WT and KO littermates 1 week after acclimation on control diet, all at the age of 12 weeks. Another week later, mice were fasted for 24 h before sacrifice. In other studies, hepatocyte‐specific GADD45β knockdown was accompanied with fasting or HFD treatments. After 1 week of acclimation on control diet, at the age of 9 weeks, 2 × 1011 virus particles per adeno‐associated virus (AAV) were administered via tail vein injection into male C57Bl/6J mice. The fasting study was carried out, half the mice from each virus group either fed ad libitum or were subjected for fasting for 24 h. For the HFD study, 1 week after virus administration, half of the animals switched to HFD and studied for further 16 weeks. Inclusion criteria were mice of a certain age (i.e. 9–12 weeks at the beginning of the experiment. Criteria for exclusion of mice from study groups were obvious infections/wounds which would impact on feeding behaviour as well as metabolic profile. These criteria were pre‐established. Animal experiments were conducted according to local, national and EU ethical guidelines and approved by local regulatory authorities (Regierungspräsidium Karlsruhe, DEU) and conformed to ARRIVE guidelines.
Metabolic phenotyping
For starvation experiments, mice were placed in fresh cages and food was withdrawn while maintain access to drinking water for 24 h from ZT1. For all GADD45 experiments, a control diet (Research diets D12450B, New Brunswick, USA) was used, and experiments were conducted after at least 1‐week adaptation to the diet. To study diet‐induced obesity, a high‐fat diet was used (Research diets D12492, New Brunswick, USA). For comprehensive metabolic/behavioural phenotyping, the TSE Phenomaster system was used that permits automated and simultaneous monitoring of indirect calorimetry, body mass, food and water intake and 3D activity of individually housed mice (Tschop et al, 2012). Mice were acclimated to the system 4 days prior to the initiation of the experiments. For standard experiments, blood was taken after cervical dislocation and organs including liver, adipose tissue depots and gastrocnemius muscles were collected, weighed, snap‐frozen in liquid nitrogen and stored at −80°C until further analysis.
To study the dynamics of systemic metabolism, we performed tolerance tests according to established guidelines (Ayala et al, 2010). In particular, we performed an intraperitoneal insulin (1 IU/kg; Huminsulin Normal, DEU) tolerance test in the 5–6 h fasted state between ZT5‐9. An oral lipid (100 μl/mouse olive oil delivered by oral gavage; O1514, Sigma‐Aldrich, DEU) tolerance test was conducted in the overnight (i.e. 14–16 h) fasted state between ZT1‐4. Furthermore, blood samples were collected from the tail vein in the 5–6 h fasted state (ZT6‐7) for assessment of blood glucose and serum insulin levels for the calculation of HOMA‐IR ((glucose (mM) × insulin (pM))/3857), which is a good surrogate measure of whole‐body insulin action in mice (Lee et al, 2008). To assess tissue‐specific insulin signalling, mice were fasted for 6 h, then insulin (Huminsulin, Lilly) was injected (10 mU/g body weight) and 15 min later, liver tissue was rapidly harvested and frozen in LN2 following euthanisation by cervical dislocation (Agouni et al, 2010).
Human subjects
Liver tissue samples were obtained from 37 Caucasian lean and obese men, 14 with and 23 without type 2 diabetes, who underwent open abdominal surgery for Roux‐en‐Y bypass, sleeve gastrectomy or elective cholecystectomy. Liver biopsy was taken during the surgery, immediately frozen in liquid nitrogen and stored at −80°C until further use. The phenotypic characterisation of the cohort has been performed as described previously (Kloting et al, 2010). Serum samples and liver biopsies were taken between 8 am and 10 am after an overnight fast. The study was approved by the local ethics committee of the University of Leipzig, Germany (363‐10‐13122010 and 017‐12‐230112). All patients gave preoperative written informed consent for the use of their samples.
Blood metabolites & hormones
Blood glucose levels were determined using an automatic glucose monitor (One Touch, LifeScan). In addition, commercially available kits were used to measure serum non‐esterified fatty acids (NEFA; NEFA‐HR, Wako), glycerol/triglyceride (TG; TR‐0100; Sigma‐Aldrich), ketone bodies (KB; Autokit 3‐HB, Wako), cholesterol (CH200, Randox) and insulin (80‐INSMS‐E01, Alpco) essentially according to manufacturer's instructions. All samples were loaded in order to fit within the assay range of the reagents supplied. Acylcarnitines were determined in serum by electrospray ionisation tandem mass spectrometry (ESI‐MS/MS) according to a modified method as previously described (Sauer et al, 2006), using a Quattro Ultima triple quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ion source and a Micromass MassLynx data system.
Tissue metabolite extraction and assay
For tissue lipid determinations, frozen tissue samples were pulverised, weighed and extracted. Lipid analyses were conducted according to established guidelines (Argmann et al, 2006) using glycerol/triglyceride (TR‐0100; Sigma‐Aldrich), NEFA (NEFA‐HR, Wako) and cholesterol (CH200, Randox) assay kits. For liver glycogen determination, ~50 mg of liver powder was carefully weighed and extracted in 30% KOH followed by 70% ethanol precipitation. Resuspended glycogen was digested with amyloglucosidase (A7095, Sigma‐Aldrich, DEU) and glycogen digests were then assayed using a glucose assay kit (GAHK20, Sigma‐Aldrich, DEU). Liver tissue long‐chain acyl‐CoA species were extracted (Golovko & Murphy, 2004) and measured using an enzymatic assay (Barber & Lands, 1971). Values were calculated as molar concentration per gram wet tissue.
Ex vivo long‐chain fatty acid metabolism
These experiments were carried out on precision‐cut liver slices (de Graaf et al, 2010) according to established guidelines for long‐chain fatty acid (LCFA) metabolism experiments (Watt et al, 2012). In particular, liver slices were taken and prepared (in absence of Insulin and Dexamethasone) from fed or fasted mice and pre‐incubated in Williams media E containing 50 μg/ml gentamycin, 5% dialysed FBS, 5 mM d‐glucose, 0.3 mM pyruvate, 0.1 μM methyl‐linoleate and an amino acid mixture resembling the hepatic portal vein concentrations (Patti et al, 1998). After 1 h, slices were then incubated with the same media containing 0.5 mM carnitine and BSA‐conjugated palmitic acid (50 μM) plus BSA‐conjugated oleic and linoleic acid mix (150 μM; L9655, Sigma‐Aldrich), representing the major (i.e. 67%) LCFA species in blood serum (Masood et al, 2005) together with trace amounts of [9,10‐3H(N)]‐palmitic acid (ART0129; American Radiolabeled Chemicals, USA), to trace LCFA metabolism, and [1‐14C]‐R‐2‐bromopalmitic acid (ARC3623; American Radiolabeled Chemicals, USA), a non‐metabolisable palmitate analogue (Oakes et al, 1999) to trace LCFA uptake. Incubations were conducted for 3 h after which media was collected and slices were washed in ice‐cold PBS, spot‐dried and snap‐frozen in LN2. Liver slice samples were homogenised in Solvable™ (PerkinElmer, DEU) and media were extracted (twice) according to Folch method (Folch et al, 1957) in order to count only the aqueous 3H2O reflecting FA oxidation. Samples were then analysed by dual‐dpm counting (Packard 2200CA Tri‐Carb Liquid Scintillation analyzer; Packard Instruments, USA) using multi‐purpose scintillant (Rotiszint® eco plus, Carl‐Roth, DEU), and LCFA metabolism was calculated based upon the media tracer:tracee ratio (dpm/mol). Non‐oxidative LCFA disposal (NOFAD) rate was calculated as the difference between LCFA uptake and oxidation rates. In addition, liver slices from the same mice were allowed to incubate in media described above without glucose but with (BSA‐NEFA) or without (fatty acid‐free BSA vehicle) for 18 h and glucose concentration of the media was measured (GAHK20, Sigma‐Aldrich, DEU) and subsequently glucose production rate was calculated.
Plasmids, RNA interference and recombinant viruses
AAV encoding control or specific miRNAs under the control of a hepatocyte‐specific promoter were established, purified and tittered as described previously (Graham et al, 2008; Rose et al, 2011). For miRNA experiments, oligonucleotides targeting mouse GADD45β (5′‐ GGCGGCCAAACTGATGAATGT ‐3′) and non‐specific oligonucleotides (5′‐AAATGTACTGCGCGTGGAGAC‐3′) were cloned into pcDNA6.2‐GW/EmGFP‐miR [“BLOCK‐iT™ PolII miR RNAi Expression Vector Kit” (Invitrogen, Darmstadt, DEU)]. For the overexpression of GADD45β in the murine liver AD virus were produced. Therefore, mGadd45b cDNA (GenBank: BC023815.1; Source Bioscience, UK) was subcloned into pENTR‐FLAG vector and subsequently recombined with the pAD/BLOCK‐IT™ DEST vector (Invitrogen, DEU). Linearised plasmid was subsequently transfected into HEK293A cells to amplify viruses, and these were subsequently purified were purified by the caesium chloride method and dialysed against phosphate‐buffered saline buffer containing 10% glycerol prior to animal injection.
Tissue RNA/protein extraction and analysis
Liver cell fractionation was performed using a simplified method adapted from Jiang and colleagues (Liu et al, 2011). Liver organelle fractionation was conducted via density‐based separation (Cox & Emili, 2006). RNA was extracted from tissues using Qiazol and cDNA synthesised using the First Strand cDNA synthesis kit (Fermentas, DEU). Quantitative PCR was conducted using Taqman master mix and Taqman primer‐probe assays (Life Technologies, DEU). Tissue protein extraction and immunoblotting was performed using standard methods using GADD45β (sc‐8776), FATP2 (sc‐161311), FABP1 (sc‐50380), HNF4a (sc‐6556), BCKDE1A (sc‐67200), NTCP (sc‐98485) and ARG1 (sc‐21050) (Santa‐Cruz Biotechnology, DEU); LC3B (2275), pT39‐S6K1 (9205), p‐ERK (9101), p‐p38 (9211), p‐eIF2a (9721) and GRP78 (3183) (Cell Signaling Technologies, USA); CD36 (AF2519, RnD Systems, USA); and the housekeeping protein VCP (ab11433, Abcam, UK) antibodies. Immunoprecipitation was conducted using anti‐FLAG (A2220, Sigma‐Aldrich, DEU) and anti‐HA (A2095, Sigma‐Aldrich, DEU) agarose from tissue lysates using standard protocols.
Study design criteria and statistical analyses
Based upon preliminary data showing the expected effect size of major outcome variables, a power analysis was conducted in order to determine the minimal number of animals to be used for each experiment. For genotype difference studies, offspring mice from Het × Het breedings were initially randomised to each experiment group. Afterwards, counterbalancing was done in order to realise equal sample sizes per experimental group. When conducting studies, the investigators were aware of which mouse was in which experimental group due to prior genotyping and allocation. However, the technical assistants involved in the studies were blinded.
Statistical analyses were performed using t‐tests (two‐sided), or 2‐way analysis of variance (ANOVA) with or without repeated measures, where appropriate, with Holm–Sidak‐adjusted post‐tests. Nonparametric tests (e.g. Mann–Whitney–Wilcoxon) were conducted when data were not normally distributed. Correlation was determined using Spearman's correlation coefficient. All analyses were carried out with SigmaPlot v.12 software (Systat Software GmbH, DEU).
Author contributions
JF, AZ, TPS, OS, SC, KS, NV, RMdG, KN, MBD, AM and AJR performed the experiments and analysed the samples. JGO coordinated the blood serum acylcarnitine profiling. MB conducted the human studies and provided data thereof. JF, TPS and AJR analysed the data. SH & AJR co‐directed the research project. AJR and JF wrote the manuscript. Dr. Adam J. Rose is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
The paper explained.
Problem
A hallmark of obesity‐driven T2D is insulin resistance, and thus “insulin sensitisation” has been an attractive strategy for treatment. However, insulin resistance likely represents a physiological feedback mechanism to actually retard the development of obesity‐driven complications. Thus, alternative strategies are warranted, such as mild and intermittent activation of stress‐responsive pathways that are pro‐adaptive.
Results
Here, we show that “growth arrest and DNA damage‐inducible” GADD45β as a dysregulated gene transcript during fasting in several models of metabolic dysfunction including ageing, obesity/pre‐diabetes and type 2 diabetes, in both mice and humans. Using whole‐body knockout mice as well as liver/hepatocyte‐specific gain‐ and loss‐of‐function strategies, we revealed a role for liver GADD45β in the coordination of liver fatty acid uptake, through cytoplasmic retention of FABP1, ultimately impacting obesity‐driven hyperglycaemia.
Impact
We identified liver GADD45β as a novel regulator of systemic and hepatic lipid metabolism. Our findings demonstrate the importance of fasting hepatic lipid metabolism in systemic metabolic control and provide insight into the development of new therapies for metabolic dysfunction.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank Prof. Christof Niehrs (IMB, Mainz, DEU) and his colleagues Dr. Matthias Gierl and Dr. Andrea Schäfer for providing the Gadd45 mouse breeding pairs as well as experimental support and advice. Furthermore, we appreciate the experimental support of Emil Karaulanov (IMB, Mainz), Prachiti Narvekar, Astrid Wendler, Katharina Sowodniok, Yvonne Feuchter (A170, DKFZ) and Marcus Jabs (A270, DKFZ) for helpful advice regarding the LC‐Acyl‐CoA assay. This work was supported by grants from the Helmholtz Association (ICEMED, Cross‐Program Topic Metabolic Dysfunction) and the Deutsche Forschungsgemeinschaft (He3260/4‐2) to S.H.
EMBO Mol Med (2016) 8: 654–669
References
- Agouni A, Owen C, Czopek A, Mody N, Delibegovic M (2010) In vivo differential effects of fasting, re‐feeding, insulin and insulin stimulation time course on insulin signaling pathway components in peripheral tissues. Biochem Biophys Res Commun 401: 104–111 [DOI] [PubMed] [Google Scholar]
- Antonenkov VD, Sormunen RT, Ohlmeier S, Amery L, Fransen M, Mannaerts GP, Hiltunen JK (2006) Localization of a portion of the liver isoform of fatty‐acid‐binding protein (L‐FABP) to peroxisomes. Biochem J 394: 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argmann CA, Houten SM, Champy MF, Auwerx J (2006) Lipid and bile acid analysis. Curr Protoc Mol Biol Chapter 29: Unit 29B 22 [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F (2010) Liver fatty acid‐binding protein and obesity. J Nutr Biochem 21: 1015–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Delannoy M, Morel I, Pajaud J, Ribault C, Lepage S, McGill MR, Lucas‐Clerc C, Turlin B et al (2012) Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db mice. J Pharmacol Exp Ther 342: 676–687 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP (2010) Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3: 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber ED, Lands WE (1971) Determination of acyl‐CoA concentrations using pancreatic lipase. Biochim Biophys Acta 250: 361–366 [DOI] [PubMed] [Google Scholar]
- Bauer M, Hamm AC, Bonaus M, Jacob A, Jaekel J, Schorle H, Pankratz MJ, Katzenberger JD (2004) Starvation response in mouse liver shows strong correlation with life‐span‐prolonging processes. Physiol Genomics 17: 230–244 [DOI] [PubMed] [Google Scholar]
- van den Berghe G (1991) The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metab Dis 14: 407–420 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P (1997) Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 13: 795–803 [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574 [DOI] [PubMed] [Google Scholar]
- Boucher J, Kleinridders A, Kahn CR (2014) Insulin receptor signaling in normal and insulin‐resistant states. Cold Spring Harb Perspect Biol 6: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M et al (2015) A periodic diet that mimics fasting promotes multi‐system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22: 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GF Jr (2006) Fuel metabolism in starvation. Annu Rev Nutr 26: 1–22 [DOI] [PubMed] [Google Scholar]
- Cai Q, Dmitrieva NI, Ferraris JD, Michea LF, Salvador JM, Hollander MC, Fornace AJ Jr, Fenton RA, Burg MB (2006) Effects of expression of p53 and Gadd45 on osmotic tolerance of renal inner medullary cells. Am J Physiol Renal Physiol 291: F341–F349 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ (2011) Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med 32: 279–304 [DOI] [PubMed] [Google Scholar]
- Chen MT, Kaufman LN, Spennetta T, Shrago E (1992) Effects of high fat‐feeding to rats on the interrelationship of body weight, plasma insulin, and fatty acyl‐coenzyme A esters in liver and skeletal muscle. Metabolism 41: 564–569 [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda‐Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, Tian J, Locker J (2005) Gadd45beta is induced through a CAR‐dependent, TNF‐independent pathway in murine liver hyperplasia. Hepatology 42: 1118–1126 [DOI] [PubMed] [Google Scholar]
- Connor T, Martin SD, Howlett KF, McGee SL (2015) Metabolic remodelling in obesity and type 2 diabetes: pathological or protective mechanisms in response to nutrient excess? Clin Exp Pharmacol Physiol 42: 109–115 [DOI] [PubMed] [Google Scholar]
- Cooney GJ, Thompson AL, Furler SM, Ye J, Kraegen EW (2002) Muscle long‐chain acyl CoA esters and insulin resistance. Ann N Y Acad Sci 967: 196–207 [DOI] [PubMed] [Google Scholar]
- Cox B, Emili A (2006) Tissue subcellular fractionation and protein extraction for use in mass‐spectrometry‐based proteomics. Nat Protoc 1: 1872–1878 [DOI] [PubMed] [Google Scholar]
- Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A (2008) Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet‐induced non‐alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem 283: 22186–22192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, Dierdorff JM, Foster ED, Adams CM (2012) Stress‐induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem 287: 27290–27301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J (1997) Role of long‐chain fatty acyl‐CoA esters in the regulation of metabolism and in cell signalling. Biochem J 323(Pt 1): 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A (2010) FATP2 is a hepatic fatty acid transporter and peroxisomal very long‐chain acyl‐CoA synthetase. Am J Physiol Endocrinol Metab 299: E384–E393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509 [PubMed] [Google Scholar]
- Gantner ML, Hazen BC, Conkright J, Kralli A (2014) GADD45gamma regulates the thermogenic capacity of brown adipose tissue. Proc Natl Acad Sci USA 111: 11870–11875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz JF, Luiken JJ, van Bilsen M, van der Vusse GJ (2002) Cellular lipid binding proteins as facilitators and regulators of lipid metabolism. Mol Cell Biochem 239: 3–7 [PubMed] [Google Scholar]
- Golovko MY, Murphy EJ (2004) An improved method for tissue long‐chain acyl‐CoA extraction and analysis. J Lipid Res 45: 1777–1782 [DOI] [PubMed] [Google Scholar]
- de Graaf IA, Olinga P, de Jager MH, Merema MT, de Kanter R, van de Kerkhof EG, Groothuis GM (2010) Preparation and incubation of precision‐cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc 5: 1540–1551 [DOI] [PubMed] [Google Scholar]
- Graham T, McIntosh J, Work LM, Nathwani A, Baker AH (2008) Performance of AAV8 vectors expressing human factor IX from a hepatic‐selective promoter following intravenous injection into rats. Genet Vaccines Ther 6: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Gupta SK, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA (2005) Hematopoietic cells from Gadd45a‐ and Gadd45b‐deficient mice are sensitized to genotoxic‐stress‐induced apoptosis. Oncogene 24: 7170–7179 [DOI] [PubMed] [Google Scholar]
- Hakvoort TB, Moerland PD, Frijters R, Sokolovic A, Labruyere WT, Vermeulen JL, Ver Loren van Themaat E, Breit TM, Wittink FR, van Kampen AH et al (2011) Interorgan coordination of the murine adaptive response to fasting. J Biol Chem 286: 16332–16343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn KL, Salmon AB, Hohnen‐Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ et al (2009) Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 106: 17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoene M, Weigert C (2010) The stress response of the liver to physical exercise. Exerc Immunol Rev 16: 163–183 [PubMed] [Google Scholar]
- Kamath S, Chavez AO, Gastaldelli A, Casiraghi F, Halff GA, Abrahamian GA, Davalli AM, Bastarrachea RA, Comuzzie AG, Guardado‐Mendoza R et al (2011) Coordinated defects in hepatic long chain fatty acid metabolism and triglyceride accumulation contribute to insulin resistance in non‐human primates. PLoS ONE 6: e27617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Koya D (2011) Biology of obesity: lessons from animal models of obesity. J Biomed Biotechnol 2011: 197636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantzis M, Stahl A (2012) Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 1821: 852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil E, Hocker R, Schuster M, Essmann F, Ueffing N, Hoffman B, Liebermann DA, Pfeffer K, Schulze‐Osthoff K, Schmitz I (2013) Phosphorylation of Atg5 by the Gadd45beta‐MEKK4‐p38 pathway inhibits autophagy. Cell Death Differ 20: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Qu A, Reddy JK, Gao B, Gonzalez FJ (2014) Hepatic oxidative stress activates the Gadd45b gene by way of degradation of the transcriptional repressor STAT3. Hepatology 59: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Hwang JH, Kim KS, Noh JR, Choi DH, Kim DK, Tadi S, Yim YH, Choi HS, Lee CH (2015) Metformin ameliorates acetaminophen hepatotoxicity via Gadd45beta‐dependent regulation of JNK signaling in mice. J Hepatol 63: 75–82 [DOI] [PubMed] [Google Scholar]
- Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M (2010) Insulin‐sensitive obesity. Am J Physiol Endocrinol Metab 299: E506–E515 [DOI] [PubMed] [Google Scholar]
- Kolb H, Eizirik DL (2012) Resistance to type 2 diabetes mellitus: a matter of hormesis? Nat Rev Endocrinol 8: 183–192 [DOI] [PubMed] [Google Scholar]
- Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR (2007) Increased hepatic CD36 expression contributes to dyslipidemia associated with diet‐induced obesity. Diabetes 56: 2863–2871 [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56 [DOI] [PubMed] [Google Scholar]
- Krug S, Kastenmuller G, Stuckler F, Rist MJ, Skurk T, Sailer M, Raffler J, Romisch‐Margl W, Adamski J, Prehn C et al (2012) The dynamic range of the human metabolome revealed by challenges. FASEB J 26: 2607–2619 [DOI] [PubMed] [Google Scholar]
- Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ (2008) Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 294: E261–E270 [DOI] [PubMed] [Google Scholar]
- Li LO, Klett EL, Coleman RA (2010) Acyl‐CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta 1801: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B (2002) Myeloid differentiation (MyD)/growth arrest DNA damage (GADD) genes in tumor suppression, immunity and inflammation. Leukemia 16: 527–541 [DOI] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B (2008) Gadd45 in stress signaling. J Mol Signal 3: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D, Lindberg K, Oscarsson J, Claesson C, Asp L, Li L, Gustafsson M, Boren J, Olofsson SO (2002) Influence of peroxisome proliferator‐activated receptor alpha agonists on the intracellular turnover and secretion of apolipoprotein (Apo) B‐100 and ApoB‐48. J Biol Chem 277: 23044–23053 [DOI] [PubMed] [Google Scholar]
- Liu W, Hou Y, Chen H, Wei H, Lin W, Li J, Zhang M, He F, Jiang Y (2011) Sample preparation method for isolation of single‐cell types from mouse liver for proteomic studies. Proteomics 11: 3556–3564 [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Guo JU, Ming GL, Song H (2009) DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 8: 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B (2003) Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid‐binding protein gene. J Biol Chem 278: 21429–21438 [DOI] [PubMed] [Google Scholar]
- Masood A, Stark KD, Salem N Jr (2005) A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res 46: 2299–2305 [DOI] [PubMed] [Google Scholar]
- Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ et al (2012) Hyperinsulinemia drives diet‐induced obesity independently of brain insulin production. Cell Metab 16: 723–737 [DOI] [PubMed] [Google Scholar]
- Mittelman SD, Fu YY, Rebrin K, Steil G, Bergman RN (1997) Indirect effect of insulin to suppress endogenous glucose production is dominant, even with hyperglucagonemia. J Clin Invest 100: 3121–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA (2012) PGC‐1alpha overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD (2012) Lipid‐induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO (2003) Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid‐binding protein gene. J Biol Chem 278: 51664–51672 [DOI] [PubMed] [Google Scholar]
- Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, Graham MJ, Davidson NO (2008) Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology 48: 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes ND, Kjellstedt A, Forsberg GB, Clementz T, Camejo G, Furler SM, Kraegen EW, Olwegard‐Halvarsson M, Jenkins AB, Ljung B (1999) Development and initial evaluation of a novel method for assessing tissue‐specific plasma free fatty acid utilization in vivo using (R)‐2‐bromopalmitate tracer. J Lipid Res 40: 1155–1169 [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K, Christiansen PA, Anders RA, Franzoso G (2008) Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest 118: 1911–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR (1998) Bidirectional modulation of insulin action by amino acids. J Clin Invest 101: 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM et al (2015) Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160: 745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70: 3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45: 410–418 [DOI] [PubMed] [Google Scholar]
- Rose AJ, Diaz MB, Reimann A, Klement J, Walcher T, Krones‐Herzig A, Strobel O, Werner J, Peters A, Kleyman A et al (2011) Molecular control of systemic bile acid homeostasis by the liver glucocorticoid receptor. Cell Metab 14: 123–130 [DOI] [PubMed] [Google Scholar]
- Ross BD, Hems R, Freedland RA, Krebs HA (1967) Carbohydrate metabolism of the perfused rat liver. Biochem J 105: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez‐Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC (2012) Elevated TCA cycle function in the pathology of diet‐induced hepatic insulin resistance and fatty liver. J Lipid Res 53: 1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer SW, Okun JG, Fricker G, Mahringer A, Muller I, Crnic LR, Muhlhausen C, Hoffmann GF, Horster F, Goodman SI et al (2006) Intracerebral accumulation of glutaric and 3‐hydroxyglutaric acids secondary to limited flux across the blood‐brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl‐CoA dehydrogenase deficiency. J Neurochem 97: 899–910 [DOI] [PubMed] [Google Scholar]
- Schupp M, Chen F, Briggs ER, Rao S, Pelzmann HJ, Pessentheiner AR, Bogner‐Strauss JG, Lazar MA, Baldwin D, Prokesch A (2013) Metabolite and transcriptome analysis during fasting suggest a role for the p53‐Ddit4 axis in major metabolic tissues. BMC Genom 14: 758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovic M, Sokolovic A, Wehkamp D, Loren V, van Themaat E, de Waart DR, Gilhuijs‐Pederson LA, Nikolsky Y, van Kampen AH, Hakvoort TB et al (2008) The transcriptomic signature of fasting murine liver. BMC Genom 9: 528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien L, Oakes ND, Kelley DE (2004) Metabolic flexibility. Proc Nutr Soc 63: 363–368 [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF (2007) Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317: 369–372 [DOI] [PubMed] [Google Scholar]
- Tian J, Huang H, Hoffman B, Liebermann DA, Ledda‐Columbano GM, Columbano A, Locker J (2011) Gadd45beta is an inducible coactivator of transcription that facilitates rapid liver growth in mice. J Clin Invest 121: 4491–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Locker J (2013) Gadd45 in the liver: signal transduction and transcriptional mechanisms. Adv Exp Med Biol 793: 69–80 [DOI] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C et al (2012) A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Hoy AJ, Muoio DM, Coleman RA (2012) Distinct roles of specific fatty acids in cellular processes: implications for interpreting and reporting experiments. Am J Physiol Endocrinol Metab 302: E1–E3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Borrmann CM, Borchers T, Spener F (2001) Fatty acids and hypolipidemic drugs regulate peroxisome proliferator‐activated receptors alpha – and gamma‐mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci USA 98: 2323–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Song L, Huang C (2009) Gadd45 proteins as critical signal transducers linking NF‐kappaB to MAPK cascades. Curr Cancer Drug Targets 9: 915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu X, Zhou B, He Z, Zhai Q (2011) Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding. PLoS ONE 6: e27553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Wu KC, Klaassen CD (2013) Genetic activation of Nrf2 protects against fasting‐induced oxidative stress in livers of mice. PLoS ONE 8: e59122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


