Abstract
Positioning releasable vesicles near voltage-gated calcium channels may ensure transmitter release upon calcium influx. Disruption of vesicle positioning may underlie short-term synaptic depression. However, how this positioning is achieved is unclear. In this issue of Neuron, Young and Neher find that synaptotagmin 2 helps to align readily releasable vesicles near calcium channels at nerve terminals.
The communication between neurons in the nervous system relies on synaptic transmission. When an action potential invades the nerve terminal, voltage-sensitive calcium channels open, and a flood of calcium pours in, resulting in a transient and local calcium microdomain, which triggers fusion of vesicles that are ready or primed for release at the active zone. The distance between calcium channels and releasable or primed vesicles is likely within tens of nanometers (Meinrenken et al., 2002). Conceivably, primed vesicles distant from calcium channels would experience lower calcium concentrations and thus have a lower release probability. Proper vesicle positioning is therefore required to ensure transmitter release upon calcium influx.
Regulation of vesicle positioning was proposed as a mechanism underlying the decrease of release probability, which contributes to the generation of short-term synaptic depression (Wu and Borst, 1999). This hypothesis has recently received supporting evidence from an elegant study showing that, during short-term depression, release evoked by calcium influx via calcium channels decreases more than that evoked by experimentally induced homogeneous calcium elevation across the whole nerve terminal (Wadel et al., 2007). Thus, the decrease in release evoked by calcium influx is not caused by a decreased sensitivity of the calcium sensor, but likely by an increased distance between primed vesicles and calcium channels (Wadel et al., 2007).
Although proper vesicle positioning is crucial for synaptic function, how it is achieved and regulated remained unclear. In this issue of Neuron, Young and Neher (2009) found that vesicle positioning is regulated, surprisingly, by synaptotagmin 2 (syt2) (Figure 1), the calcium sensor that mediates synchronous release at the calyx of Held nerve terminal (Sun et al., 2007).
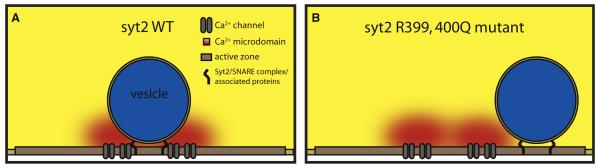
Figure 1. Overexpression of syt2 R399, 400Q Dissociates Ca2+ Channels and Synaptic Vesicles.
(A) In wild-type terminals, vesicles are positioned at the active zone close to calcium channels. This allows the vesicle to be exposed to the high calcium concentrations at the local calcium microdomain during calcium channel opening and ensures vesicle fusion.
(B) In terminals overexpressing the syt2 R399, 400Q mutant, vesicles show positioning defects, resulting in exposure of the vesicle to lower concentrations of calcium during calcium channel opening and impaired fusion. In this cartoon, the vesicle is misaligned with calcium channels at the active zone. Other interpretations are possible; for example, calcium channels may be misaligned at the active zone.
Synaptotagmins (syts) are a family of multifunctional proteins containing two C2 domains. Among them, syt1, syt2, and syt9 can act as calcium sensors mediating synchronized calcium-triggered release (Xu et al., 2007). Syt1 and syt2 are expressed in different areas of the brain but are highly homologous and are thought to act in much the same way (Xu et al., 2007). Knocking out syt1 or syt2 abolishes calcium-triggered synchronous release (Geppert et al., 1994; Sun et al., 2007), and, although asynchronous release continues, its calcium sensitivity is significantly reduced (Sun et al., 2007). Recently, it was found that a mutation in syt1, changing arginines at positions 398 and 399 to glutamines, abolishes synchronous release in autaptic cultured hippocampal neurons, while presumably leaving calcium binding unaffected (Xue et al., 2008). This study implicates these residues in the transduction of the calcium signal, though it is possible that they have another function unrelated to calcium sensing.
Young and Neher (2009) studied whether the reciprocal mutation in syt2 (R399, R400Q) affects vesicle positioning at the calyx of Held, a giant nerve terminal that arises from globular bushy cells in the ventral cochlear nucleus (VCN) and makes an axosomatic synapse in the medial nucleus of the trapezoid body. Both the calyx and the postsynaptic neuron can be patch-clamped, allowing for recordings of presynaptic ionic currents and EPSCs from the same synapse. Caged calcium compounds and calcium indicators can be dialyzed into the calyx, allowing for an experimentally induced global increase in calcium concentration upon calcium uncaging (Bollmann et al., 2000; Schneggenburger and Neher, 2000). Furthermore, vesicle fusion pore opening and fission pore closure can be studied by recording membrane capacitance changes at the calyx (He et al., 2006; Sun and Wu, 2001).
Application of these powerful biophysical techniques to the calyx has significantly advanced our understanding of biophysical mechanisms regulating exocytosis and endocytosis. However, the molecular nature of these biophysical mechanisms is poorly understood, partly because the calyx has not been successfully cultured, making most molecular biology tools available for cultured neurons inapplicable to the calyx. One of the most impressive components of Young and Neher’s study is their solution to this problem, to deliver the syt2 R399, R400Q mutant to the calyx in vivo via adenovirus. Viral delivery of proteins to the calyx has already been demonstrated (Wimmer et al., 2004). However, the size of the calyx may require extraordinary measures to achieve high expression levels to compete with the number of syt2 molecules in the calyx. Young and Neher (2009) developed a highly expressing adenoviral vector dubbed pUNISHER to do the job and devised a stereotaxic injection procedure in p1 rats to deliver the virus to the VCN, giving the viral machinery 7–9 days to build up the levels needed before optimal recording conditions at p8–p10.
Using pUNISHER to express the R399, R400Q mutant in the calyces, Young and Neher (2009) found that the EPSC induced by an action-potential (AP)-like stimulus was significantly reduced. Although quite a large variation was seen in the EPSC, the results are clearly specific to the construct and not the technique, as over-expression of syt2 actually enhanced the EPSC. A longer delay between the stimulus and response was also observed, consistent with the lack of synchronous fusion seen in syt2 knockouts (Sun et al., 2007).
The decrease of the EPSC by syt2 R399, R400Q may arise from a smaller release probability or number of vesicles in the readily releasable pool (RRP). To distinguish them, Young and Neher (2009) analyzed the EPSCs evoked by a pair of pulses. A smaller second EPSC often is interpreted as indicating that the first pulse depleted much of the RRP and that the initial release probability is high. Conversely, a higher second/first EPSC ratio may reflect a lower release probability. The second/first EPSC ratio in R399, R400Q mutant synapses was much higher than in wild-type and syt2-overexpressing pairs, suggesting a drop in the release probability (Young and Neher, 2009). Release probability can also be calculated more directly by evoking release with an AP and then dividing by the RRP size, which is measured as release induced by a prolonged depolarization at the calyx. Although this calculation was not provided, the R399, R400Q mutant was found to reduce the RRP size, but to a level insufficient to account for the decreased EPSC. Thus, the EPSC reduction is mostly caused by the decreased release probability. Interestingly, the syt2 mutant did not increase the mEPSC frequency, contrary to findings in syt2 knockout (Sun et al., 2007), suggesting that the mutant preserves at least some regular function.
The R399, R400Q mutant-induced decrease of the release probability could plausibly be due to disruption of the calcium sensor or to uncoupling of vesicles from calcium channels. Young and Neher (2009) discerned between these possibilities by uncaging various concentrations of calcium homogeneously in the calyx, and measuring release from EPSC recordings at the same synapse. They found that the calcium sensitivity was similar to controls, leading to the provocative hypothesis that syt2 mutation uncouples vesicles from calcium channels, dramatically reducing the release probability during calcium influx through calcium channels (Figure 1).
The findings by Young and Neher (2009) may have a long-lasting impact. On the technical side, this study introduces a technique that, at last, brings the powerful molecular biology tools to the calyx. For instance, syt mutants can be introduced in a syt2 null background to clarify the role of each domain in the absence of wild-type syt2. Also, the prospect of adenovirus-delivered RNAi is an enticing alternative to the still-elusive targeted-knockout approach in the VCN. Refinement of the technique to reduce variability in expression will permit further study of more subtle effects, such as a potential small change in the calcium sensitivity of release in the mutant. From a conceptual standpoint, this study posits a new function for syt2 as a protein that contributes to the structure of the active zone. The authors suggest that, in addition to its role as calcium sensor, syt2 may bring vesicles closer to calcium channels and facilitate release (Figure 1). Perhaps this will prompt a re-evaluation of the syt2 knockout—might a positioning defect account for some of the altered properties of release? And what effects would a positioning defect have on endocytosis, since endocytosis requires the same calcium microdomain that triggers exocytosis (Hosoi et al., 2009; Wu et al., 2009)?
The paper of Young and Neher raises many interesting questions. During short-term depression, the decrease in the release probability (Wu and Borst, 1999) is caused by uncoupling between calcium channels and primed vesicles at the calyx (Wadel et al., 2007). Is this uncoupling due to an impaired ability of syt2 to colocalize vesicles and calcium channels? Furthermore, how syt2 could carry out a role in vesicle positioning remains to be elucidated. This is made more difficult by the confusion over what the R399, R400Q mutant actually does to the properties of syt2. As the authors note, various biochemical studies on the reciprocal mutations in syt1 have yielded conflicting results, and while the authors favor that the mutations block association of syt2 with SNARE proteins that mediate vesicle fusion, the complete effect is simply not known. One clue referenced by the authors as to this new role is that neurotoxin-effected cleavage of VAMP-2, the synaptic vesicle SNARE, also reduces evoked release without lowering the calcium sensitivity of the release apparatus. Does this mean that syt2 and VAMP-2 combine to localize vesicles to calcium channels? Do they associate independently with calcium channels, or must they bind to the other SNAREs first? When syt2 or VAMP-2 is compromised, is the mispositioning due to loosened tethering of vesicles to active zones or to calcium channels drifting away from active zones? Is there an increase in the physical distance between calcium channels and primed vesicles or perhaps an increase in the endogenous calcium buffer concentration, which would limit long-range calcium diffusion? It would be of great interest to see how these questions are addressed in the future.
REFERENCES
- Bollmann JH, Sakmann B, Borst JG. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. J. Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Nevian T, Kuner T. Pflugers Arch. 2004;449:319–333. doi: 10.1007/s00424-004-1327-9. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JGG. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Nat. Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. Nat. Struct. Mol. Biol. 2008;15:1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Neher E. Neuron. 2009;63(this issue):482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]