Abstract
Following stroke-like lesions to the sensorimotor cortex in rats, experience with the ipsi-to-lesion (ipsilesional, “nonparetic”) forelimb worsens deficits in the contralesional (“paretic”) forelimb. We tested whether the maladaptive effects of experience with the nonparetic limb are mediated through callosal connections and the contralesional sensorimotor cortex. Adult male rats with proficiency in skilled reaching with their dominant (for reaching) forelimb received ischemic bilateral sensorimotor cortex lesions, or unilateral lesions with or without callosal transections. After assessing dominant forelimb function (the paretic forelimb in rats with unilateral lesions), animals were trained with their non-dominant/nonparetic forelimb or underwent control procedures for 15 days. Animals were then tested with their dominant/paretic forelimb. In animals with unilateral lesions only, nonparetic forelimb training worsened subsequent performance with the paretic forelimb, as found previously. This effect was not found in animals with both callosal transections and unilateral lesions. After bilateral lesions, training the non-dominant limb did not worsen function of the dominant limb compared with controls. Thus, the maladaptive effects of training the nonparetic limb on paretic forelimb function depend upon the contralesional cortex and transcallosal projections. This suggests that this experience-dependent disruption of functional recovery is mediated through interhemispheric connections of the sensorimotor cortex.
Keywords: corpus callosum, motor skill, unilateral, nonparetic, recovery
Stroke affects approximately 795,000 Americans annually and accounts for one of every 18 deaths in the US (Lloyd-Jones et al., 2009). A prevalent problem after stroke is loss of function in the hand and arm contralateral to the side of injury (the “paretic” side). As a result, stroke survivors begin to rely on the ipsilesional side, despite the presence of mild impairment in this side. A now established treatment approach for upper arm impairments is constraint induced movement therapy (CIMT; (Mark, Taub, & Morris, 2006; Taub, Uswatte, Mark, & Morris, 2003), where use of the paretic arm is encouraged through restraint of the nonparetic hand for most waking hours. This is intended to counteract the effects of learned nonuse of the paretic arm, which is thought to result from repeated experience with its incompetence. Data from clinical trials indicate that this therapy can significantly improve upper arm deficits (Wolf et al., 2006; Park, Wolf, Blanton, Winstein, Nichols-Larsen, 2008). Frequently, however, stroke survivors learn to use their nonparetic side in order to carry out daily tasks (e.g., Dobkin, 2006). Though learning how to compensate with this body side may convey immediate functional benefits, its long-term neural and behavioral consequences are not well understood.
Unilateral sensorimotor cortex (SMC) damage in the caudal forelimb representation area in rats results in sensory and motor impairments in the contralesional forelimb and a compensatory reliance on the ipsilesional forelimb, mimicking some aspects of upper extremity impairment and learned non-use in human stroke (e.g., Allred & Jones, 2004; Bury & Jones, 2002; Hsu & Jones, 2005; Luke, Allred, & Jones, 2004). We use the terms “nonparetic” and “paretic” to refer to the two limbs in this animal model to be consistent with the clinical terminology used in reference to the weakness and partial loss of motor function found after unilateral cerebral stroke.
Recently, we found that rats trained with their nonparetic forelimb early after unilateral SMC damage have worsened motor function, decreased responsiveness to rehabilitative training of the paretic forelimb and a reduced peri-lesion neuronal activation of FosB/ΔFosB compared to rats without nonparetic forelimb training (Allred & Jones, 2008; Allred, Maldonado, Hsu, & Jones, 2005). This may indicate plasticity-inhibiting effects of the nonparetic forelimb on the remaining cortex of the injured hemisphere, and that learning to compensate with the nonparetic body side is limiting neural recovery mechanisms of the paretic limb. However, the mechanisms underlying this effect are entirely unknown.
Following unilateral brain injury there are abnormalities in interhemispheric activity that are associated with reduced functional outcome. For example, after visual cortex lesions in cats, there is increased activity in contralesional regions. Visual neglect to stimuli presented in the contralesional field can be reduced with transient lesions of the contralateral cortex (Rushmore, Valero-Cabre, Lonber, Hilgetag, Payne, 2006; Ward & Cohen, 2004). Interhemispheric inhibition (as measured using a paired-pulse transcranial magnetic stimulation protocol) from the contralesional to the lesion hemisphere is increased following stroke in humans (Duque et al., 2005; Murase, Duque, Mazzocchio, & Cohen, 2004; see also Perez & Cohen, 2009), and this is correlated with deficits in motor performance (Murase et al., 2004). In functional magnetic resonance imaging (fMRI) studies, better functional outcome tends to correspond with more normal lateralized cortical activity during hand movements (reviewed in Cramer, 2008). It seems logical to think that experience with the ipsilesional body side may also further disrupt interhemispheric activity and contribute to worsened recovery.
The present studies were designed to test whether the maladaptive effects of nonparetic forelimb experience are mediated by the contralesional SMC and intercortical connections. If so, then loss of these intercortical connections, which can be induced with partial transections of the corpus callosum (Bury et al., 2000), should mitigate the maladaptive effect of nonparetic forelimb experience on functional recovery of the paretic forelimb. Furthermore, the effect should not be found in animals with bilateral SMC lesions.
Method
Subjects
Forty-four, 6 to 7 month old adult male Long-Evans rats were housed in pairs on a 12:12 light/dark cycle in standard laboratory cages. Animals were provided with standardized housing supplementation (a 10.5 cm diameter PVC pipe, small wooden objects, and cardboard paper rolls). At the beginning of each experiment, animals were fed a restricted diet of 16-19g/day/rat standard rat chow so that they were motivated to perform the skilled reaching task. Animal protocols were approved by the University of Texas Institutional Animal Care and Use Committee.
Experimental Designs
Experiment 1
This study was designed to test whether the nonparetic forelimb training effects on paretic forelimb function depend upon callosal projections of the sensorimotor cortex (SMC). If so, then the impairing effects of nonparetic forelimb training on skilled motor behavior in the paretic limb should be present in animals with an intact corpus callosum, but absent or reduced if callosal connections of the SMC are partially severed by transection (CCX). Rats were divided into 4 groups based on post-operative contralesional forelimb reaching deficits: two groups received control procedures (Cont, n = 7; CCX_Cont, n = 10) and two groups received training of the nonparetic, ipsilesional limb (NonParT, n = 7; CCX_NonParT, n = 9).
Experiment 2
This study was designed to test whether the worsening of paretic limb function by training the other limb depends upon the contralesional cortex. If so, then the effect should not be found in animals with bilateral sensorimotor cortex (SMC) damage. It was hypothesized that animals with bilateral SMC damage trained with their non-dominant (for the task) forelimb (Bilat_ND, n = 6) would perform at a similar level with their dominant (for the task) forelimb compared to animals receiving control procedures (Bilat_Cont, n = 5). Figure 1 outlines the experimental designs.
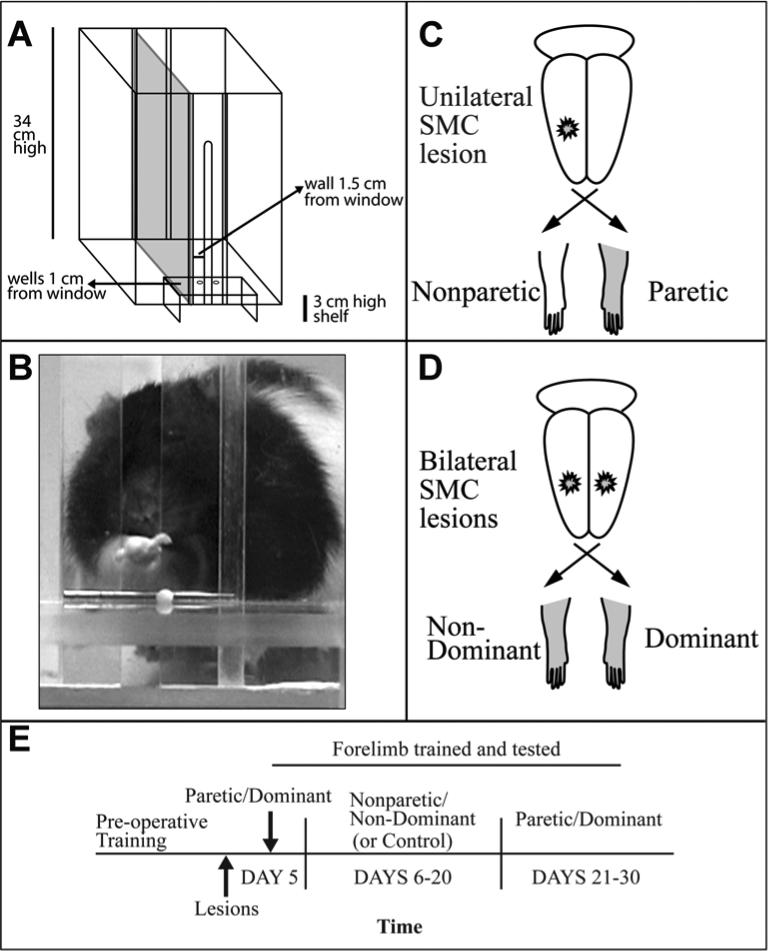
Figure 1. Experimental Design.
A. Schematic of the reaching chamber. The inner chamber wall and pellet placement are adjusted to train the left versus right limb. This chamber is configured for reaching with the right forelimb. B. A rat aiming and reaching for a banana flavored food pellet. C. Unilateral SMC lesion and corresponding non-paretic (ipsilesional) and paretic (contralesional) forelimbs. Transections of the corpus callosum are not depicted. D. Bilateral SMC lesions and corresponding non-dominant- and dominant-for-reaching forelimbs. E. Time line of experimental procedures. Transections, in Experiment 1, were given at the same time as ischemic lesions.
Ischemic Sensorimotor Cortex (SMC) Lesions
All animals were given unilateral SMC lesions opposite their dominant-for-reaching forelimb (Experiment 1) or in both hemispheres (Experiment 2) using the endothelin-1 (ET-1) method, which results in localized transient ischemia (Fuxe et al., 1997; Adkins, Voorhies, & Jones, 2004). Animals were anesthetized with i.p. injections of ketamine (10mg/kg) and xylazine (120mg/kg) and maintained under a surgical plane of anesthesia throughout the procedure, with ketamine boosters when necessary. A craniectomy was made by connecting four drill holes (A/P: −1.0, +2.0, M/L: 2.0, 4.5) and dura was removed just prior to topical application of 3.0 μl (Experiment 1) or 4.0 μl (Experiment 2) of ET-1 (80 pmol, American Peptide, Inc.). (Animals in Experiment 2 received a greater amount of ET-1 to ensure that the lesions were large enough to adequately test the importance of the non-dominant SMC in dominant forelimb recovery.) Animals were then left undisturbed for 10 minutes before suturing. Buprenorphine (10mg/kg), an analgesic, was administered subcutaneously post-surgery when the animal began to arise from anesthesia. Behavioral assessment of reaching ability began 5 days after surgeries. For animals with corpus callosum transections (see below), ET-1 was applied to the cortical surface directly following the completion of the transection. Animals in Experiment 2 received bilateral craniectomies, and ET-1 was applied to one cortex and then immediately to the other cortex, with the first side chosen randomly.
Corpus Callosum Transections
Transections were made using methods that focus callosal lesions in the region of the interhemispheric projections of the SMC (Bury et al., 2000; Bury & Jones, 2002). As a control, midline skull between A/P −2.0 to +1.5 mm relative to Bregma was thinned and removed in all animals in Experiment 1. To prevent mechanical damage, the electrode was not lowered into the brain of Cont or NonParT animals. For those animals receiving a transection (CCX_Cont, CCX_NonParT), the dura and sagittal sinus were pushed to the side and a size 00 ethyl cyanocrylate coated insect pin with an exposed tip was lowered 4.7 mm into the brain at −1.5 mm posterior to Bregma. The side of approach was opposite the SMC lesion. After lowering, 0.7 mV of anodal current was passed through the electrode while it was moved rostrally to Bregma over 9 seconds. The electrode was then raised 0.7 mm and current was again passed while the electrode was moved rostrally 1 mm anterior to Bregma over 6 seconds.
Single Pellet Retrieval Task
Reach training was carried out as previously described (Allred & Jones, 2008; Hsu & Jones, 2005; Maldonado, Allred, Felthauser, & Jones, 2005) adapted from Whishaw and others (Miklyaeva & Whishaw, 1996; Whishaw, 1992; Whishaw, Pellis, & Gorny, 1992). Briefly, for shaping, animals were placed in a Plexiglas reaching chamber with their cage mate for 10 minutes. Forty-five mg banana flavored pellets (Bioserve, Inc.) were dropped into the chamber and placed on a 3 cm high shelf located outside of the reaching chamber (see Figure 1). On each subsequent day animals were placed into the reaching chamber alone for 10 minutes and permitted to reach for pellets on the shelf. Once a limb of preference was established (15 of 20 reach attempts made with same forelimb), this was considered the dominant-for-reaching limb and, the next day the task was configured so that they could only successfully reach pellets with the dominant forelimb. A Plexiglas wall was placed ipsilaterally to the reaching limb and pellets were placed in a shallow well 1 cm from the center window for 30 trials or 10 minutes, whichever came first. To discourage tongue use, a small 2 mm diameter drill bit was adhered to the platform where it made contact with the reaching chamber. Pre-operatively, animals were trained to a proficient level (≥ 50% success/reach attempt).
On each trial, animals were given up to 5 reach attempts to obtain a single pellet. Trials concluded when the pellet was knocked from its well or greater than 5 reach attempts took place (failures), the pellet was retrieved but dropped inside the chamber before consumption (drop), or the pellet was retrieved and taken directly to the mouth (success). Post-operative non-dominant (nonparetic in Exp. 1) limb training was for 60 trials/day for 15 days. Post-operative performance was calculated based on % successful retrievals (success + drops/total reach attempts). Reach training focused on the paretic/dominant forelimb was used to assay the initial effects of the lesions and the effects of experience with the other limb (see Figure 1). Pre-operatively and during the post-operative paretic/dominant forelimb assessment and training periods, animals received up to 30 trials per day for either 9 days (Experiment 1) or 13 days (Experiment 2).
Reach training focused on the nonparetic/non-dominant forelimb was used as an experimental manipulation. Post-operative reach training of the non-dominant (nonparetic in Experiment 1) side was for 60 trials/day for 15 days. (The larger number of trials in this phase was intended to ensure its robustness as an experimental manipulation, which may vary with training intensity (Allred et al., 2008). Animals in control conditions were placed in a reaching chamber, without an inner wall, and given banana flavored food pellets on the cage floor at approximately the same rate as trained animals.
Schallert Cylinder Test
To test forelimb use asymmetries, the Schallert cylinder test (Schallert, sKozlowski, Humm, & Cocke, 1997) was used pre-operatively and post-operatively. Animals were placed into a 19 cm diameter Plexiglas cylinder for approximately 2 minutes to encourage upright postural support behaviors. Use of the forelimbs (ipsilateral, contralateral, or bilateral) on the cylinder walls was recorded from slow motion playbacks of videotape. Percent use of the non-dominant forelimb was calculated based on: no. of non-dominant touches/sum of all touches.
Histology and Lesion Evaluation
At the conclusion of each experiment, animals were overdosed with sodium pentobarbital (100mg/kg) and perfused transcardially with .1M phosphate buffer and 4% paraformaldehyde in the same buffer. Brains were removed and sliced coronally with a vibratome into 50μm thick sections collected in six alternating sets. Sliced brains were stored in cryoprotectant at 4 C°. One set of sections was immediately mounted onto gelatin-coated slides and stained with toluidine blue, a Nissl stain.
The volume of remaining cortex in the SMC region was measured by tracing seven 50μm (between 2.2 mm anterior to and 0.80 mm posterior to Bregma) coronal Nissl stained sections using Neurolucida (Microbrightfield Inc.) perimeter tracing software at 17X magnification. Moving caudally, the first section containing the head of the caudate was chosen and subsequent sections were 600 μm apart. Volume was obtained by applying the formula: ΣA*section thickness where ΣA is the total area summed across all sections (Gundersen et al., 1988).
Statistical Analyses
All statistical analyses were carried out using SPSS statistical software (SPSS, Inc) with a priori planned comparisons. We chose to perform planned comparisons rather than an omnibus ANOVA comparing all 4 groups as literature has shown that a priori designs are a more powerful approach to test specific planned (prior to the experiment) comparisons (e.g., Kuehne, 1993; Benton, 1989; DuRapau, 1988). The comparisons were designed to test whether reaching performance in the dominant/paretic forelimb testing period was: 1) affected by prior nonparetic limb training after unilateral lesions alone (Cont vs. NonParT) and 2) affected by prior nonparetic limb training after unilateral lesions with callosal transections (CCX_Cont vs. CCX_NonParT) and 3) affected by prior non-dominant limb training after bilateral lesions (Bilat_Cont vs. Bilat_ND). This analysis plan tests the effects of the primary behavioral manipulation (training the non-dominant/nonparetic limb) by only comparing groups with similar injuries to one another, which avoids potential complications in the interpretations related to differences in injury extent.
Performance during the nonparetic forelimb training period was also compared between NonParT and CCX_NonParT. Behavioral analyses were performed with repeated-measures ANOVAs and student's t tests. Volume analyses were performed with one-way ANOVAs or paired sample t-tests. All data are expressed as means ± SEM. Effects were considered significant at p < .05. Three rats in the CCX_Cont group (Experiment 1) had particularly large damage resulting from the callosal transection procedure. Excluding these animals did not change statistical outcome on behavioral measures and therefore they remained in the study, however they are considered separately, as described below.
Results
Corpus Callosum Transections Mitigate the Negative Impact of Nonparetic Forelimb Experience
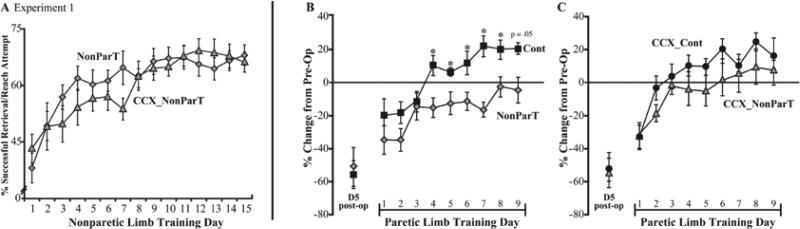
After unilateral SMC lesions (Experiment 1), there was no difference in acquisition of the skilled reaching task with the nonparetic forelimb in rats with or without callosal transections (F(1, 15) < 1.0, p > .05, Fig. 2A). Consistent with previous findings, this nonparetic forelimb training led rats with SMC lesions to perform significantly worse than Cont rats when later trained with their paretic forelimb (F(1,12) = 5.82, p < .05, Figure 2B). A significant group by day interaction effect was also found (F(8,96) = 2.13, p < .05) and subsequent post-hoc analyses for day revealed significant differences on days 4 through 8 (F's > 5.0, p's < .05) and day 9 (F = 4.74, p = .05). However, in rats with both unilateral lesions and transections of the corpus callosum, there was no significant effect of the nonparetic/ipsilesional forelimb training on the paretic forelimb (F(1,17) = 1.44, p > .05); CCX_Cont vs. CCX_NonParT, Figure 2C) though there was a tendency for CCX_Cont rats to perform better than CCX_NonParT rats. These results cannot be explained by differences in reaching activity of the paretic forelimb. There was no significant group or group by day interaction for the number of reach attempts made with the paretic limb over the days of training this limb.
Figure 2. Nonparetic forelimb training worsens performance of the paretic forelimb in rats without corpus callosum transections.
A. Performance during the period of training of the nonparetic, ipsilesional, limb (NonParT) after unilateral SMC lesions in rats with or without callosal transections (CCX). There was no significant difference in acquisition of the skilled reaching task with the nonparetic forelimb between these two groups. The first day of nonparetic limb training was 6 days after lesions. B. After training the nonparetic limb, NonParT rats had major deficits in the paretic, contralesional forelimb compared to control animals. C. In contrast, in rats with unilateral SMC lesions and callosum transections performance with the paretic forelimb was not significantly affected by prior nonparetic forelimb training. Day 1 of the paretic limb training period in B and C corresponds to 22 days after the lesions. Data in panels B and C were calculated as %[(pre-operative- postoperative)/preoperative] successful retrievals per reach attempt. Data in all figures are means ± SEM. * p < .05.
Transections did not result in significant deficits in reaching behavior in the paretic forelimb. As measured on day 5 post-lesion, rats without transections had a 57.1 ± 7.83% reduction from pre-operative performance levels whereas rats with both unilateral lesions and callosal transections had a 53.3 ± 7.21% drop from pre-operative baseline (Figures 2B-C). CCX_NonParT rats tended to perform better than NonParT rats with their paretic forelimb, however this effect failed to reach significance (F(1,14) = 1.25, p > .05).
Lack of Non-dominant Forelimb Training Effects after Bilateral SMC Lesions
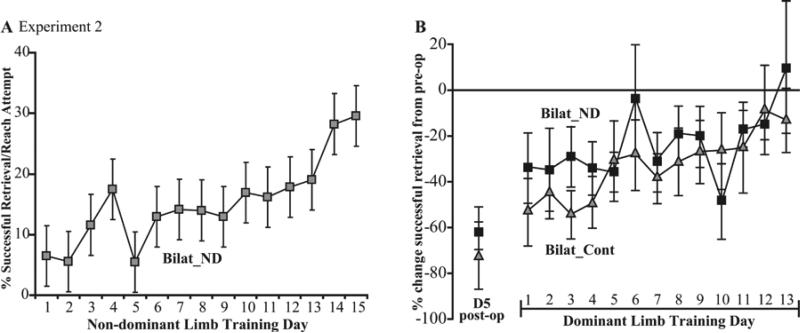
Non-dominant forelimb training after bilateral lesions did not worsen subsequent performance with the dominant forelimb compared to control rats (F(1,9) < 1.0, p > .05, Figure 3). Animals with bilateral lesions also did not differ in the number of reach attempts made with the dominant limb during this training period (Bilat_ND = 37.27 ± 0.85; Bilat_Cont = 35.67 ± 1.36, means ± SEM).
Figure 3. Non-dominant forelimb training in rats with bilateral SMC lesions does not worsen performance of the dominant forelimb.
A. Non-dominant limb learning curve of rats with bilateral lesions (Experiment 2). Rats that had learned the task with the dominant limb were learning it for the first time with the non-dominant limb after the lesion. The first training day was 6 days post-lesion. B. Rats with bilateral lesions had a similar rate of re-acquisition of the skilled reaching task with their dominant forelimb regardless of whether they received earlier post-lesion training with the non-dominant forelimb (Bilat_ND) or earlier control procedures (Bilat_Cont). Panel B shows %[(pre-operative-postoperative)/preoperative] successful retrievals per reach attempt. The first dominant limb training day in panel B was 22 days after lesions. Note the differences in scales in comparison to Figure 2.
In addition to the lesion-induced deficits in the dominant limb, rats with bilateral lesions tended to perform poorly during the non-dominant forelimb training period (Fig. 3A) compared to animals in Experiment 1 (as expected because, unlike Experiment 1, this limb was contralateral to a SMC lesion).
Training the Non-dominant/Nonparetic Forelimb Led to its Perseverative Use
Rats with nonparetic forelimb training attempted to use this limb more than controls during the subsequent paretic limb training period (even though the apparatus was configured to only permit successful retrievals with the paretic limb). However, perseverative reaching with the nonparetic forelimb cannot explain the worsening of function of the paretic forelimb in NonParT compared to controls, because this perseverance effect was also found in rats with callosal transections and bilateral SMC lesions.
In Experiment 1, all animals with nonparetic forelimb training made futile reaches with this forelimb (mean = 20.10 ± 2.87 reaches) on the first day of the paretic forelimb training period. This compares with only one reach attempt made by only one animal in the Cont group. This effect did not vary significantly as a result of corpus callosum transections. All animals in the CCX_NonParT group made reach attempts with the nonparetic limb on the first day of the switch in sides (23.67 ± 4.65 reaches). In contrast, 4 of 10 rats in the CCX_Cont group made nonparetic reach attempts (1.9 ± 0.95 attempts on day 1 averaged over all animals in this group). The number of reaches with the nonparetic forelimb in these groups significantly declined over days of paretic forelimb training and, by day 4, the NonParT groups were no longer significantly different from Cont in this measure.
Consistent with data from Experiment 1, animals trained with their non-dominant forelimb after bilateral lesions in Experiment 2 also made significantly more reaches with this limb (mean = 22.0 ± 4.51 reaches) on the first day of dominant limb training compared to Bilat_Cont animals (mean = 6.6 ± 1.78 reaches, p < .05). Reach attempt number with this limb significantly declined over days of training, though in contrast to Experiment 1, animals in both groups (n = 6, Bilat_ND; n =3, Bilat_Cont) were still making reach attempts with this limb on day 13 of dominant limb training (Bilat_ND = 7.2 ± 2.03; Bilat_Cont = 2 ± 0.95).
This perseverance effect was not linked with success levels on the skilled reaching task during the earlier nonparetic/non-dominant forelimb training period. There were no significant correlations in either experiment between nonparetic/non-dominant forelimb success levels and the number of reach attempts made with this forelimb during the subsequent paretic/dominant training period (r's < .5, p's > .05). Furthermore, there was no relationship between the severity of the perseverance and paretic/dominant forelimb performance in any group (NonParT, CCX_NonParT, or Bilat_ND, r's < .6, p's > .05).
Unilateral, but not Bilateral, Lesions Resulted in Postural Support Asymmetries
Consistent with previous findings (Allred & Jones, 2004; Allred et al., 2008; Barth, Jones, & Schallert, 1990), unilateral, but not bilateral, SMC lesions increased reliance on one forelimb (i.e., the nonparetic forelimb in Experiment 1) as measured on the Schallert cylinder test. Before the unilateral lesions, rats used the to-be-nonparetic forelimb solely for 36.47 ± 2.12% of wall touches and after the lesions they used it for 57.92 ± 2.68% (calculated as %ipsilesional/(ipsi+contra+bilateral)). There were no significant differences in the initial post-lesion effects in rats with transections versus no transections (57.54 ± 3.81% and 58.44 ± 3.94%, respectively). Also consistent with previous findings (Allred et al., 2005), training the nonparetic forelimb tended to increase reliance on this forelimb compared with controls (61.43 ± 3.16 vs. 55.12 ± 3.67%), however, this failed to reach significance (F(1,12) = 3.61, p = .11). This same tendency was not found in animals with transections (CCX_NonParT = 53.73 ± 5.69%; CCX_Cont = 54.23 ± 6.39%) and it was also not found in animals with bilateral lesions (Bilat_ND = 41.38 ± 5.03%; Bilat_Cont = 40.0 ± 2.36%).
Differences in Injuries Do Not Explain Differential Effects of Nonparetic/Non-dominant Limb Training
Sensorimotor cortex lesions
SMC lesions produced damage to the forelimb representation area in the region between approximately 2.7 mm rostral and 0.8 mm caudal to bregma (Figure 4). Lesions also frequently resulted in some superficial white matter damage directly below the lesion (72% of animals in Exp. 1; 55% of animals in Exp. 2). The striatum was considered damaged if the lesions penetrated the white matter under the lesions. With this criterion, striatal damage was incurred in approximately one third of the rats (38% of animals in Exp. 1; 36% of animals in Exp. 2). However, more than superficial striatal damage was not found in any animal.
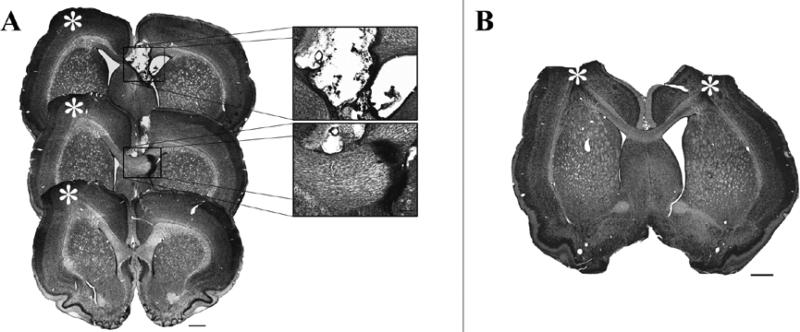
Figure 4. Representative lesions and callosal transections.
A. Experiment 1, representative lesion and corpus callosum transection. B. Representative lesion from Experiment 2. Scale bars for low magnification images in panels A and B are 1 mm. Scale bar in inset is 250μm. * indicates SMC damage.
Placement and extent of SMC lesions were similar between groups within experiments. All animals with unilateral lesions had a significantly smaller dominant hemisphere (due to the lesion) compared to the non-dominant hemisphere (NonParT, t(6) = −4.15, p < .01; Cont, t(6) = −4.74, p < .01; CCX_NonParT, t(8) = −5.03, p < .01; CCX_Cont, t(9) = −4.93, p < .01). There was no difference between groups in either experiment in volume of remaining SMC of the dominant hemisphere (Table 1) though animals with bilateral lesions in Experiment 2 tended to have larger lesions than animals with the unilateral lesions in Experiment 1 (as was intended due to the larger amount of endothelin-1 used in Experiment 2).
Table 1.
Sensorimotor Cortex Volume (mm3).
| Experiment 1 | Lesion/Dominant Hemisphere | No-Lesion/Non-Dominant Hemisphere |
|---|---|---|
| Cont | 84.51(2.68) | 95.43(1.61) |
| NonParT | 82.11(3.28) | 95.61(1.11) |
| CCX_Cont | 82.53(1.22) | 88.63(1.79)* |
| CCX_NonParT | 83.52(2.07) | 94.38(1.36) |
| Experiment 2 | Lesion/Dominant Hemisphere | Lesion/Non-Dominant Hemisphere |
|---|---|---|
| Bilat_Cont | 80.85(2.63) | 81.96(5.25) |
| Bilat_ND | 76.61(1.29) | 81.30(5.94) |
Note. Data are means with SE in ().
p < .05, significantly different from CCX_NonParT.
Callosal transections
All transections resulted in some damage to the corpus callosum between A/P +1.2 and −0.3 relative to Bregma. Most transections also produced damage to the septal nucleus (n = 8, CCX_NonParT; n = 9, CCX_Cont; See Fig. 4). In four brains, complete dorsal to ventral transections were not found in any single coronal plane, but major superficial damage was evident in several coronal planes between A/P 0.7 and −0.3 mm relative to Bregma. These variations in callosal injury characteristics were not clearly linked to differences in reaching performance with either limb.
Callosal transections were made using a side of approach opposite the SMC lesions and, as intended, there was no damage from the electrode track evident in the cortex of the dominant hemisphere (the side of the SMC lesions) in any animal. However, despite being matched for initial impairment levels and despite similarity in white matter damage, in histological analysis, the CCX_Cont group was found to have significantly more cortical tissue loss in the side of the transection procedure compared to the CCX_NonParT group (F(1,18) = 6.31, p < .05). This is unlikely to contribute to the results because the matched groups had similar deficits in the paretic forelimb as measured 5 days post-operatively (CCX_NonParT = 54.26 ± 9.54% drop from pre-operative levels; CCX_Cont = 52.20 ± 10.79% drop from pre-operative levels) as a result of matching groups for initial impairment levels. Furthermore, the transected groups were not different on the first day of paretic forelimb training (see Figure 2C). Finally, in secondary analyses of the behavioral results, excluding animals with larger cortical tissue loss on the side of the transection approach did not change statistical outcome on behavioral measures. For example, when 3 animals with the largest cortical damage in the CCX_Cont group were excluded such that, in the remaining animals, the cortical volume (91.78 ± 1.15 mm3) was similar to that of CCX_NonParT (Table 1), this resulted in little effect on mean values of reaching success during the paretic limb training period. In another example, the subgroup of CCX_Cont (excluding the three animals with the largest cortical damage) performed at 17.43 ± 6.38% of preoperative levels on paretic limb training days 7-9, which was similar to the inclusive group and to CCX_NonParT (Figure 2C). The subgroup of CCX_Cont continued to be non-significant compared with CCX_NonParT (F(1,14) < 1.0, p = .85). Furthermore, there was no correlation between transection-associated cortical injury and paretic limb performance in either group (r's < .5, p's > .05). Thus, there is no clear relationship between this secondary cortical damage and the attenuation of the nonparetic (NonParT) limb training effects by CCX.
Discussion
The present results add more support to the finding that learning a skilled motor task with the nonparetic forelimb worsens performance and re-learning with the paretic forelimb (Allred et al., 2005; Allred & Jones, 2008). This maladaptive effect was absent in animals with transections of the corpus callosum. Furthermore, the effect was not reproduced in rats with bilateral lesions of the sensorimotor cortex that underwent equivalent sequential training of the two limbs. These data suggest an involvement of both the SMC of the contralesional hemisphere and of transcallosal projections in the maladaptive effects of nonparetic forelimb training on the function of the paretic forelimb.
This present study identifies a circuit that is required for the maladaptive effects of training the nonparetic limb, but the mechanisms remain to be uncovered. Reorganization in peri-lesion cortex is thought to be important for recovery of the paretic side after unilateral damage in both humans and animal models (e.g., Kleim, Barbay, & Nudo, 1998; Cramer, 2008). Previously, we found that nonparetic forelimb training disrupts the perilesion neuronal expression of FosB/ΔFosB resulting from paretic limb training (Allred & Jones, 2008). ΔFosB is a cumulatively expressed transcription factor involved in instigating structural plasticity (McClung et al., 2004) that is likely to be sensitive to the repetitive practice involved in re-acquisition of the skilled reaching task with the paretic limb. Thus, it is possible that activity-dependent plasticity in perilesion cortex is disrupted by experience with the nonparetic forelimb.
Several other lines of evidence suggest that one hemisphere and body side can constrain activity and plasticity in the other, even in intact animals. Unilateral deprivation of sensory input in one arm (Floel et al., 2004), forelimb (O'Bryant, Bernier, & Jones, 2007), eye (Iny, Heynen, Sklar and Bear, 2006) or whisker pad (Li et al., 2005) enhances somotosensory and motor abilities and experience-dependent plasticity (Glazewski et al., 2007) of the non-deprived side. Lidocaine inactivation of primary motor cortex in rats results in an expansion of the motor map in the contralateral hemisphere (Maggiolini, Viaro, & Franchi, 2008). It may be that this “normal” constraint is exaggerated after unilateral lesions and further exaggerated by experience with the nonparetic limb, and that these effects can be attenuated when transcallosal connections are severed. Unilateral lesions or transient inactivation of cortex are known to alter activity in the other hemisphere (Li, Rema & Ebner, 2005; Clarey, Tweedale & Calford, 1996). For example, there is increased excitability in the homotopic contralesional cortex (e.g., Que, Schiene, Witte, & Zilles,1999; Witte, Bidmon, Schiene, Redecker, & Hagemann, 2000; Witte & Stoll, 1997). Furthermore, in humans, an abnormal inhibitory drive from the contralesional motor cortex to the damaged hemisphere is found in stroke patients preceding voluntary paretic hand movement (Murase et al., 2004).
Individuals with severe hemiplegic cerebral palsy develop an increase in ipsilateral corticospinal projections from the “intact” hemisphere, which Eyre and colleagues (2007) conclude may be competitively displacing contralateral projections from the infarcted cortex thereby making impairments worse. This raises the possibility that the experience with the nonparetic limb confiscates circuits that might otherwise mediate recovery of the paretic limb. If so, the role of callosal fibers in such a confiscation, and the timing of their involvement remains to be established. Training one forelimb in rats results in increases in synapses (Luke et al., 2004) and dendrites in the hemisphere opposite the trained limb (Greenough et al., 1985; Bury and Jones, 2002; Allred and Jones, 2004) and involves mechanisms similar to long-term potentiation (LTP; Rioult-Pedotti, Friedman, & Donoghue, 2000; Monfils & Teskey, 2004). The LTP-like changes have been found to be specific to the contra-to-training hemisphere (Rioult-Petdotti et al., 2000; Monfils & Teskey, 2004). Furthermore, after unilateral SMC lesions, the training-related neuroplastic effects are enhanced in the cortex opposite the lesions (Bury & Jones, 2002; Jones, 1999; Jones, Chu, Grande, & Gregory, 1999; Luke et al., 2004) and this is linked with an increased capacity to learn a motor skills task with the nonparetic forelimb (Allred & Jones, 2004; Bury & Jones, 2002; Hsu & Jones, 2006). However, some bilateral dendritic growth of layer II/III pyramidal neurons has been observed after unilateral training in intact animals (Greenough, Withers, & Larson, 1989). If such ipsi-to-training neural plasticity occurs in perilesion cortex, this might reduce the ability to create further changes by training the paretic forelimb.
Rats trained with the nonparetic limb also perseverated in the attempt to use this forelimb after switching to the paretic forelimb, despite the task configuration making reaches with this limb futile. However, this effect cannot be responsible for exacerbating deficits in the paretic limb as it was seen in all animals with nonparetic/non-dominant training, including those with corpus callosum transections and bilateral lesions. Furthermore in correlation analyses, it was not associated with deficits in paretic forelimb skilled reaching performance. This suggests that use of the nonparetic forelimb while the paretic forelimb is being used is not necessarily maladaptive. This adds to earlier findings that training rats to reach with both forelimbs after unilateral lesions does not worsen paretic forelimb function (Allred & Jones, 2008). Furthermore, the task learned with the nonparetic forelimb does not have to be one that was originally performed with the paretic side. In rats naïve to the reaching task prior to the lesions, post-lesion training of the nonparetic forelimb results in a pronounced deficit in learning this task later with the paretic forelimb compared with controls (Allred et al., 2005). This may indicate that establishment of pre-injury dominance for the task is not a critical factor in this effect.
While all rats did show some motor recovery with training of the paretic forelimb, the rate of relearning was slowed greatly after nonparetic limb training compared to control rats. It is possible that longer training of the paretic forelimb could overcome the maladaptive effects of prior nonparetic forelimb experience and learned disuse of the paretic forelimb. Furthermore, though the effect was not significant, there was a tendency for rats to perform worse with the paretic limb after nonparetic limb training even in callosally transected rats. This could be because the transection approach reduces, rather than eliminates, callosal fibers, but it might also indicate that there are at least some non-callosally mediated effects of this behavioral manipulation. Additionally, CCX_NonParT had a tendency to perform better with their paretic forelimb compared to NonParT rats. This effect may have failed to attain significance because the transections used in this study were partial and most likely did not completely obliterate interhemipsheric communication.
Following unilateral SMC lesions, even in the absence of any training, animals begin to rely more on their nonparetic forelimb. Compensatory reliance on this side for postural support is evident in home cage observations (Jones & Schallert, 1992) and it may be that these self-taught behaviors are also limiting recovery of the paretic forelimb. By training them in a skilled motor task that is not performed in the home cage environment, we may be exaggerating these effects. Nevertheless, the nonparetic limb training effects have been found to generalize to non-reaching behaviors, including coordinated forelimb placement in a grid walking task and postural support in upright exploratory movements (Allred & Jones, 2008). Motor skill training with the nonparetic limb in animals with unilateral lesions may therefore induce a greater use of this limb, at a cost of disuse of the paretic forelimb. However, in the present study, this postural support effect failed to reach significance, in contrast with previous findings. A more sensitive measurement of forelimb use for postural support may reveal greater experience-dependent effects in asymmetrical forelimb use for postural support behaviors.
The present results provide further support that learning new ways of using the nonparetic limb to compensate for impairments can be detrimental to recovery of function with the paretic forelimb (Allred & Jones, 2008; Allred et al., 2005), and probably exacerbate learned nonuse or learned bad use (Taub et al., 2003; Alaverdashvili et al., 2008). The exact mechanism(s) mediating this effect are still unknown; however, data from this study point to interhemispheric involvement and disruptive behavioral experience on recovery. The maladaptive effects of experience with the nonparetic body side may need to be overcome with treatment approaches, such as CIMT (Taub et al., 2003) and facilitating stimulation of the perilesion cortex (Adkins-Muir & Jones, 2003; Plow, Carey, Nudo & Pascual-Leone, 2009). However, the optimal application of these strategies might be improved with a better understanding of the exact neural mechanisms of the present phenomenon, including the time periods in which intercortical interference is high. Though the present results indicate that the effect is mediated by intercortical connections, further investigation is needed to isolate the time period of their involvement as well as to understand exactly how they are disrupting the function of the paretic forelimb. A better understanding of the phenomenon seems likely to illuminate processes involved in neglect and learned nonuse.
Acknowledgments
We thank Dr. Jui-En Hsu for help with perfusions, Truc Garcia, Georgina Singh and Angelica McPartlin for cylinder tape rating and Dr. Lawrence Cormack for statistical advice. Supported by MS 64586 and AAUW Educational Foundation.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurology Research. 2003;25:780–787. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Experimental Neurology. 2004;190:433–445. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Experimental Neurology. 2008;210(1):172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, Maldonado MA, Hsu JE, Jones TA. Training the “less- affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurology and Neuroscience. 2005;23(5-6):297–302. [PubMed] [Google Scholar]
- Alaverdashvili M, Foroud A, Lim DH, Whishaw IQ. “Learned baduse” limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behavioral Brain Research. 2008;188(2):281–290. doi: 10.1016/j.bbr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat sensorimotor cortex. Behavioural Brain Research. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Benton R. Paper presented at annual meeting of Mid-South Educational Research Association. Little Rock; 1989. Planned comparisons as better alternatives to ANOVA omnibus test. [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. Journal of Neuroscience. 2009;29(19):6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SD, Adkins DL, Ishida JT, Kotzer CM, Eichhorn AC, Jones TA. Denervation facilitates neuronal growth in the motor cortex of rats in the presence of behavioral demand. Neuroscience Letters. 2000;287(2):85–88. doi: 10.1016/s0304-3940(00)01138-1. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. Journal of Neuroscience. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Callosal terminals in the rat prefrontal cortex: synaptic targets and association with GABA-immunoreactive structures. Synapse. 1998;29(3):193–205. doi: 10.1002/(SICI)1098-2396(199807)29:3<193::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cisse Y, Grenier F, Timofeev I, Steriade M. Electrophysiological properties and input-ouput organization of callosal neurons in cat association cortex. Journal of Neurophysiology. 2003;89(3):1402–1413. doi: 10.1152/jn.0871.2002. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Tweedale R, Calford MB. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somattosensory cortex. Cerebral Cortex. 1996;6(2):196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Annals of Neurology. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Dobkin DH. Rehabilitation after Stroke. New England Journal of Medicine. 2006;352:1677–84. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel M, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- DuRapau TM. Paper presented at the annual meeting of the Mid- South Educational Research Association. Louisville; 1988. Benefits of using planned comparisons rather than post hoc tests: A brief review with examples. [Google Scholar]
- Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Annals of Neurology. 2007;62(5):493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Floel A, Nagorsen U, Werhahn KJ, Ravindran S, Birbaumer N, Knecht S, Cohen LG. Influence of somatosensory input on motor function in patients with chronic stroke. Annals of Neurology. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8(11):2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behavavioral Neural Biology. 1985;44(2):301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1988;96:379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less affected forelimb after unilateral sensorimotor cortex lesions in rats. European Journal of Neuroscience. 2005;22:2069–2080. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Experimental Neurology. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Clarke S, Kraftsik R. Interchange of callosal and association projections in the developing visual cortex. Journal of Neuroscience. 1986;6(5):1384–1409. doi: 10.1523/JNEUROSCI.06-05-01384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iny K, Heynen AJ, Sklar E, Bear MF. Bidirectional modifications of visual acuity induced by monocular deprivation in juvenile and adult rats. Journal of Neuroscience. 2006;26(28):7368–7374. doi: 10.1523/JNEUROSCI.0124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. Journal of Comparative Neurology. 1999;414:57–66. [PubMed] [Google Scholar]
- Jones TA, Allred RP, Adkins DL, Hsu JE, O'Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40(Suppl 3):S136–S138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. Journal of Neuroscience. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Research. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Karayannis T, Huerta-Ocampo I, Copogna M. GABAergic and pyramidal neurons of deep cortical layers directly receive and differently integrate callosal input. Cerebral Cortex. 2007;17(5):1213–1226. doi: 10.1093/cercor/bhl035. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Receptor subtypes involved in callosally-induced postsynaptic potential in rat frontal agranular cortex in vitro. Experimental Brain Research. 1992;88(1):33–40. doi: 10.1007/BF02259126. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. Journal of Neurophysiology. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kuehne CC. Paper presented at the annual meeting of the Mid-South Educational Research Association. New Orleans: 1993. The advantages of using planned comparisons over post hoc tests. [Google Scholar]
- Li L, Rema V, Ebner FF. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. Journal of Neurophsyiology. 2005;94(5):3342–3356. doi: 10.1152/jn.00357.2005. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;199(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Maggiolini E, Viaro R, Franchi G. Suppression of activity in the forelimb motor cortex temporarily enlarges forelimb representation in the homotopic cortex in adult rats. European Journal of Neuroscience. 2008;27:2733–2746. doi: 10.1111/j.1460-9568.2008.06248.x. [DOI] [PubMed] [Google Scholar]
- Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary running exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabilitation and Neural Repair. 2005;22(3):250–61. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark VW, Taub E, Morris DM. Neuroplasticity and constraint-induced movement therapy. Europa Medicophysica. 2006;42(3):569–284. [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Molecular Brain Research. 2004;132(2):146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Miklyaeva EI, Whishaw IQ. Hemiparkinson analogue rats display active support in good limbs versus passive support in bad limbs on a skilled reaching task of variable height. Behavioral Neuroscience. 1996;110:117–125. doi: 10.1037//0735-7044.110.1.117. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- O'Bryant A, Bernier B, Jones TA. Abnormalities in skilled reaching movements are improved by peripheral anesthetization of the nonparetic forelimb after sensorimotor cortical infarcts in rats. Behavioral Brain Research. 2007;177(2):298–307. doi: 10.1016/j.bbr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabilitation and Neural Repair. 2008;22(5):486–493. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? Journal of Physiology. 2009;587(Pt4):725–726. doi: 10.1113/jphysiol.2008.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: A critical appraisal. Stroke. 2009;40(5):1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que M, Schiene K, Witte OW, Zilles K. Widespread up-regulation of N-methyl-D-aspartate receptors after focal photothrombotic lesion in rat brain. Neuroscience Letters. 1999;273:77–80. doi: 10.1016/s0304-3940(99)00598-4. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Advanced Neurology. 1997;73:229–238. [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cerebral Cortex. 2009:19435709. doi: 10.1093/cercor/bhp075. Epub ahead of print. PMID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Europa Medicophysica. 2003;42(3):241–256. [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Archives of Neurology. 2004;61:1844–1888. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. Lateralization and reaching skill related: results and implications from a large sample of Long-Evans rats. Behavioral Brain Research. 1992;52:45–48. doi: 10.1016/s0166-4328(05)80323-7. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP. Medial frontal cortex lesions impair the aiming component of rat reaching. Behavioral Brain Research. 1992;50(1-2):93–104. doi: 10.1016/s0166-4328(05)80291-8. [DOI] [PubMed] [Google Scholar]
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27(1):61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. Journal of Cerebral Blood Flow Metabolism. 2000;20:1149–1165. doi: 10.1097/00004647-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Witte OW, Stoll G. Delayed and remote effects of focal cortical infarctions: secondary damage and reactive plasticity. Advanced Neurology. 1997;73:207–227. [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Journal of American Medical Association. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]