Abstract
Although the hippocampus is thought to play a central role in the regulation of the cortisol awakening response (CAR), results from past studies examining the relationship between the CAR and hippocampally-mediated memory and cognition have been mixed. Inconsistent findings may be due to the use of cortisol samples collected on only 1 to 2 days since reduced sampling can permit unstable situational factors to bias results. We used cortisol assessments from 10 consecutive days to test the relationship of the CAR to episodic memory, working memory, and processing speed in a sample of healthy young, middle-aged, and older adults (age range: 23 – 79 years; N = 56). We tested if the relationship between the CAR and cognition would depend upon age and also tested if other cortisol measures, specifically waking cortisol, diurnal cortisol output (i.e., area under the curve) and diurnal cortisol slope (linear and quadratic), would be related to cognition. We found that a more positive CAR slope was related to better episodic memory and that this relationship did not depend upon age. The CAR was not significantly related to working memory. The relationship of the CAR to processing speed was not significant when using a CAR measure that corrected for non-compliant cortisol sampling. We also found that higher waking cortisol was significantly related to better working memory, but not episodic memory or processing speed. Neither diurnal cortisol output nor diurnal linear cortisol slope was significantly related to cognitive functioning. Future work should investigate the mechanisms underpinning the relationship of the cortisol awakening process to cognitive functioning.
Keywords: Cortisol awakening response, Waking cortisol, Hippocampus, Episodic Memory, Working Memory
1. Introduction
The cortisol awakening response (CAR) is the rapid rise in cortisol that lasts 30 – 45 min immediately following morning awakening (Pruessner et al., 1997; Wilhelm, Born, Kudielka, Schlotz, & Wϋst, 2007). Some correlational studies indicate that the hippocampus plays a central role in its regulation (Bruehl, Wolf, & Convit, 2009; Buchanan, Kern, Allen, Tranel, & Kirschbaum, 2004; Pruessner, Pruessner, Hellhammer, Pike, & Lupien, 2007; Wolf, Fujiwara, Luwinski, Kirschbaum, & Markowitsch, 2005). For example, the magnitude of the CAR is positively related to hippocampal volume (Bruehl et al., 2009; Pruessner et al., 2007). In people with unilateral and bilateral hippocampal damage and severe global amnesia, the CAR is not observed even though cortisol levels decrease normally across the rest of the day (Buchanan et al., 2004; Wolf et al., 2005). Adequate hippocampal function may therefore be necessary for the CAR to occur. Additionally, the amount of cortisol produced during the CAR may influence hippocampal functioning. Pharmacologic suppression of the morning rise in cortisol has been found to inhibit free recall of previously learned content in texts and pictures (Rimmele, Meier, Lange, & Born, 2010). In contrast, recognition of individual items, a cognitive process less dependent upon the hippocampus than free recall (Holdstock, Mayes, Gong, Roberts, & Kapur, 2005; Mayes, Holdstock, Isaac, Hunkin, & Roberts, 2002), was not impaired. Since the CAR and hippocampal function appear to be closely linked, one would expect to find a relationship between the CAR and hippocampal-dependent memory, such as episodic memory, a cognitive domain known to be supported by the hippocampus (e.g., Cohen, Ryan, Hunt, Romine, Wszalek, & Nash, 1999; Davachi, Mitchell, & Wagner, 2003; Henke, Weber, Kneifel, Wieser, & Buck, 1999; Scoville & Milner, 1957).
There have been few studies examining the relationship between CAR magnitudes and cognition. Most have focused upon middle aged and older adults (Almela, van der Meij, Hidalgo, Villada, & Salvador, 2012; Evans et al., 2011; Evans, Hucklebridge, Loveday, & Clow, 2012; Franz et al., 2011; Rickenbach, Almeida, Seeman, & Lachman, 2014) because higher cortisol, specifically higher diurnal cortisol output, has been linked to poorer cognition in this age group (Lupien et al., 2005). Long-term increases in diurnal cortisol output have been related to worse declarative memory (Li et al., 2006; Lupien et al., 1994, 1998; Seeman, McEwen, Singer, Albert, & Rowe, 1997) and executive functioning (Li et al., 2006), cognitive domains typically found to decline with age (Park et al., 2002; Rönnlund, Nyberg, Bäckman, & Nilsson, 2005; Schaie & Willis, 1996). In those studies specifically investigating the relationship between the CAR and cognition, episodic memory has been assessed through word list learning, paragraph recall, and visual-spatial memory (Almela et al., 2012; Evans et al., 2012; Franz et al., 2011; Singh-Manoux et al., 2014). The dynamic of the CAR has been measured as: 1) the amount of increase in cortisol from awakening to 30 min post awakening (Franz et al., 2011; Rickenbach et al., 2014; Singh-Manoux et al., 2014), 2) the mean increase (MnInc, see Wϋst, Federenko, Hellhammer, & Kirschbaum, 2000) (Evans et al., 2012), and 3) area under the curve with respect to increase (AUCi) (Almela et al., 2012). In one study, higher CAR values were related to lower visual-spatial memory (i.e., Franz et al., 2011); however, this association was not maintained when controlling for diurnal cortisol output (i.e., cortisol area under the curve). CAR magnitudes have not been found to be associated with list learning (Evans et al., 2012; Franz et al., 2011; Singh-Manoux et al., 2014) and its association with paragraph recall has been inconsistent: one study reported a significant negative relationship (Almela et al., 2012) while another found no association (Franz et al., 2011). The CAR was found to moderate the relationship between cognitive decline and everyday memory problems (Rickenbach et al., 2014). For adults with less cognitive decline, a higher CAR was significantly related to more reports of memory problems; however, for adults with greater cognitive decline, a higher CAR was marginally related to fewer reports of memory problems.
Results from the few studies in middle-aged and older adults examining the relationship between the CAR and working memory/executive functioning have also been inconsistent. An elevated CAR was related to better working memory (Almela et al., 2012) and executive functioning (Evans et al., 2012) in two reports; however, in another study, positive associations between the CAR and working memory/executive functioning were not found (Franz et al., 2011). Because the production of the CAR is associated temporally with the reactivation of the prefrontal cortex (Balkin et al., 2002), further investigations examining the relationship between the CAR and cognitive tests of prefrontal function seem warranted.
The inconsistent findings across studies could be due to several factors including the use of different methods to assess the CAR (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010a) and participant non-adherence to the cortisol collection protocol (Clow, Hucklebridge, & Thorn, 2010b). The use of cortisol samples collected on only 1 to 2 days may also produce inconsistent results since reduced frequency of sampling allows unstable situational factors to have a stronger influence on cortisol outcomes (Hellhammer et al., 2007). To the extent that the CAR is a stable characteristic of individuals, the reliability of estimates of individual differences in CAR responses – and hence estimated correlations of the CAR with cognition -- will benefit from aggregation over multiple samples (Rushton, Brainerd, & Pressley, 1983). In the present study we used cortisol samples collected over 10 consecutive mornings to investigate the association between the CAR and both episodic memory and working memory in a sample of healthy adults ranging in age from 23 to 79 years. To our knowledge, this is the only study that has assessed the relationship between the CAR and memory aggregating CAR values over an extended sampling period.
We also tested if the CAR-cognition relationship is related to processing speed, a construct known to decline with age (Schaie, 1989; Verhaeghen & Salthouse, 1997) but which is typically not linked to hippocampal or prefrontal function. This approach allowed us to evaluate whether any association of CAR to cognition in adulthood is more specific to memory. To test for general HPA axis activity influences upon cognition, we examined the relationship of cognitive performance to both diurnal cortisol output (i.e., area under the curve) and diurnal cortisol slope. We also tested the relationship between waking cortisol and cognition because waking cortisol is thought to reflect pre-awakening mechanisms that influence the magnitude of the CAR (Clow et al., 2010a). We hypothesized a significant association between the CAR and hippocampal-dependent memory (i.e. episodic memory); however, we did not hypothesize the direction of that relationship because results from studies investigating the relationship of the CAR to episodic memory have been mixed.
2. Material and Methods
2.1 Participants
Participants were community-dwelling volunteers from the Atlanta, GA metropolitan area who were initially recruited for a study examining everyday problem solving and emotion regulation. People were excluded if they were pregnant, indicated use of recreational drugs, or reported a diagnosis of post-traumatic stress disorder, bipolar disorder, psychosis, eating disorder, dementia, or the endocrine conditions of Cushing's or Addison's disease (see Nater, Hoppmann, & Scott, 2013 for additional information). Individuals who endorsed at least 3 out of 5 questions on an alcohol abuse questionnaire (e.g., “You drank alcohol even though a doctor suggested that you stop drinking because of a problem with your health”) were determined to have a history of alcohol abuse and were excluded. Participants were also excluded if they experienced a major life event (e.g. death in the family or having surgery) or if they had a schedule that would interfere with data collection (e.g., shift work). Sixty-four out of 185 participants from the initial study agreed to participate in the present study, which occurred 8 to 38 months later. One participant who had insufficient cortisol data (i.e., 3 out of 70 assessments) was excluded. Six participants who did not have complete cognitive data were excluded and one young adult whose 3-back score was greater than 3 standard deviations lower than the sample mean was also excluded. The final sample (N = 56) ranged in age from 23 to 79 years (M = 53.04, SD = 16.94; female = 29) and contained comparable numbers of young (N = 17), middle-aged (N = 21), and older adults (N = 18). We examined if the participants in the final sample differed in age and CAR from the remaining participants in the initially recruited sample. The participants in the final sample did not significantly vary in age, t(183) = -1.21, p = .23, or CAR, t(182) = -.29, p = .77, from the remaining participants in the initially recruited sample.
2.2 Cortisol (nmol/L) measures
On 10 consecutive days, participants used Starstedt Salivettes to collect 7 saliva samples: upon waking (M = 7:00 AM, SD = 53.4 min), 30 min later (M = 7:30, SD = 54.6 min), and then approximately every three hours until 9:17 PM on average. Cortisol was analyzed from saliva using a commercial chemiluminescence immunoassay (IBL, Hamburg, Germany) (see Nater et al., 2013 for collection details). Cortisol values for each collection time were compared to the respective 10-day within-person mean; values deviating 3 standard deviations from that mean were removed prior to calculation of cortisol measures. Following removal of outliers, a total of 508 out of a maximum of 560 (56 participants × 10 days of data) awakening and post 30 min awakening cortisol samples were available for the calculation of the CAR.
Because we had 10 consecutive days of data, we calculated within-person means for each cortisol measure. To calculate the within-person mean, we averaged cortisol across days for each person. All cortisol measures (i.e., the CAR, waking cortisol, cortisol output, and cortisol slope) represented the mean of all the within-person means in the sample. Nine days of data on average were used to calculate the CAR. Ninety-one percent of participants had 8 to 10 days of CAR data. No participant had less than 5 days of CAR data.
2.2.1 Cortisol awakening response (CAR)
The CAR was a slope measure representing the nmol/L change in morning cortisol per hour. It was calculated by taking the difference between the 30 min post awakening and awakening samples and dividing by the duration of time between the collection of the two samples: (30 min post awakening cortisol – waking cortisol)/(30 min post awakening time – waking time) (Almeida, Piazza, & Stawski, 2009). Accurate CAR values rely upon compliance with the collection protocol. In a healthy sample, negative CAR values most likely represent non-compliance with the study protocol (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Chida & Steptoe, 2009; Kupper et al., 2005) and indicate that the waking sample was most likely not collected upon awakening (Thorn, Hucklebridge, Evans, & Clow, 2006; Franz et al., 2010). Non-compliance can also be detected if the difference in collection times between the first morning and 30 min post awakening samples deviates from 30 min. To account for potential non-compliance, we calculated a corrected CAR. We first calculated a CAR by excluding samples where the time from waking to 30 min post awakening was less than 15 min or greater than 45 min and then excluded CAR values less than 0. This procedure is very similar to one employed by Franz et al. (2010). Because we had multiple days of data, calculating a corrected CAR only resulted in the exclusion of 1 young adult male, whose CAR values ranged from -3.05 to -38.61 (nmol/L)/hr.
2.2.2 Waking cortisol
Waking cortisol represented the first sample collected upon waking in the morning. Participants were instructed to collect this sample after waking-up while still lying in bed.
2.2.3 Diurnal cortisol output
Area under the curve with respect to ground (AUC) was calculated using the trapezoid formula (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) to measure diurnal cortisol output. AUC was not calculated for days when the awakening specimen and/or greater than 3 specimens were missing.
2.2.4 Diurnal cortisol slope
Linear and quadratic diurnal cortisol slopes were estimated using a linear mixed model. To prevent the CAR from obscuring the slope calculation, the 30 min post awakening cortisol sample was not included (Kumari et al., 2009). Linear and quadratic slopes were calculated using the awakening sample, sample 3 (collected on average at 9:23 AM), and the remaining 4 cortisol samples collected every three hours until 9:17 PM on average. Linear and quadratic terms for measurement occasion were group-mean centered and entered as Level 1 predictors. Slopes were estimated for each day of data. Convergence criteria were met for 7 out of the 10 days of data. More negative linear slope values represented a more rapid decline in diurnal cortisol. More positive quadratic slope values indicated a more rapid decline in cortisol across the morning and a slightly greater increase in cortisol in the evening.
2.3 Cognitive domains
Participants were assessed on the following cognitive domains: episodic memory, working memory, and processing speed.
2.3.1 Episodic memory
Episodic memory was assessed using a yes-no associative recognition task (e.g., Hines, Hertzog, & Touron, 2009). Participants were presented with sixty semantically unrelated noun-noun paired associates in random order on a computer screen and were allowed 5 s to study each pair. In the recognition test that followed immediately after study of all items was completed, half of the words in the noun-noun pairs were rearranged and the other half were left intact. Each pair was presented for 10 s and participants pressed the Y key to indicate “yes” if they remembered the pair or the N key to indicate “no” if they did not. Memory accuracy was determined by subtracting the proportion of false alarms from the proportion of hits.
2.3.2 Working memory
The 3-back and Reading Span were used to assess working memory. In the 3-back participants were presented with a series of single digit numbers and were asked on each trial to recall the number that was 3-back (Mackworth, 1959). 3-back accuracy was measured by the percentage of correct responses. In the Reading Span task, participants were asked to judge whether a series of sentences made sense while remembering a set of letters presented after each sentence (Kane et al., 2004). Sentences were presented in blocks of 3, 4, 5, 6, or 7. Participants were asked to recall the letters at the end of each block in the order that they had appeared. The Reading Span and 3-back were moderately correlated, r(54) = .52, p < .001. A composite measure of working memory capacity was computed by summing standardized scores for the 3-back and Reading Span accuracy scores (Schmiedek, Lövdén, & Lindenberger, 2014).
2.3.3 Processing speed
Letter Comparison and Pattern Comparison were used to measure processing speed. Participants viewed pairs of letters in Letter Comparison and pairs of line-segment patterns in Pattern Comparison and classified the pairs in each task as “same” or “different” as rapidly as possible (Salthouse & Babcock, 1991). One-half of the pairs were the same and the other half were different. Each test is scored as the number of correct responses in a fixed 2-minute time limit. The two measures were moderately correlated, r(54) = .64, p < .001. A composite perceptual speed score was computed by summing standardized scores for the Letter Comparison and Pattern Comparison tests.
2.4 Procedure
For 10 consecutive days, participants collected 7 saliva samples: upon awakening, 30 min later, and every 3 hours until 9:17 PM on average. A Tungsten T handheld computer (Palm, Inc.) reminded participants with a beep to collect a specimen and was used by participants to document collection times. Participants returned 8 to 38 months (M = 28.75; SD = 7.32) following cortisol collection to take cognitive tests.
2.5 Statistical approach
2.5.1 Covariates
Because of the well-recognized relationship between age and cognition, we controlled for age in all linear regression analyses. The following measures, previously shown to be related to cortisol levels or the CAR were considered as potential covariates: sex (female vs. male) (Kirschbaum et al., 1999; Wϋst et al., 2009), education (years) (Cohen et al., 2006), body mass index1 (BMI) (Stalder et al., 2013), race (White vs. not White) (Cohen et al., 2006), smoking status1 (no cigarettes per day vs. > 0 cigarettes per day) (Dmitrieva, Almeida, Dmitrieva, Loken, & Pieper, 2013), and waking time (Clow, Thorn, Evans, & Hucklebridge, 2004). Because of our small sample size, including all covariates would have resulted in model overfitting; thus, we selected variables as covariates if their zero-order relationship with a cognitive measure had a p value < .20. Only education, race, sex, and waking time fit this criterion. There were non-significant relationships of education and race to episodic memory; education: r(54) = .25, p = .07; race: rpb(54) = -.19, p = .15. A correlation was also found between sex and working memory, rpb(54) = .23, p = .08. Waking time was significantly related to processing speed, r(54) = .32, p = .02. These variables were entered into regression models predicting the particular cognitive measure to which a relationship had been detected. Thus, in addition to age, which was included in all models, education and race were included for models predicting episodic memory, sex was entered for models predicting working memory, and waking time was included for models predicting processing speed.
2.5.2 Analyses
We first computed zero-order correlations between selected covariates, cortisol, and cognition. We tested the relationship between each cortisol index and cognition using hierarchical regression. The three cognitive domains (i.e., episodic memory, working memory, and processing speed) were the dependent variables. Covariates were entered into a first step (Model 1) and cortisol was entered in the second step (Model 2). This approach allowed us to see whether the variance in cognition explained by cortisol was significantly greater than the overall variance explained by age and additional covariates. For CAR only, we tested if its relationship to cognition was dependent upon age in a third step (Model 3). CAR and age were mean-centered to reduce multicollinearity effects (Aiken & West, 1991). We also examined if the CAR explained additional variance in cognition beyond that contributed by diurnal cortisol measures (i.e., diurnal cortisol output and diurnal cortisol slope). We did this by entering covariates in Model 1, diurnal cortisol output and diurnal cortisol slope (linear and quadratic) in Model 2, and the CAR in Model 3. These analyses were designed to examine if CAR-specific mechanisms or general HPA axis function accounted for the association between the CAR and cognitive performance. A similar approach has been suggested by Clow et al. (2010b) to disentangle mechanisms responsible for an abnormal CAR. If waking cortisol was significantly related to cognition, we entered the CAR as a covariate in Model 1 and waking cortisol in Model 2 in order to isolate whether pre-awakening mechanisms underpinning the CAR remain related to cognitive performance after controlling for the CAR.
3. Results
3.1 Participant characteristics
Descriptive statistics of the study sample can be found in Table 1.
Table 1.
Descriptive statistics of study variables (N = 56, except for BMI and corrected CAR, see below).
| N (%) | Mean (SD) | Range | |
|---|---|---|---|
| Age (years) | 53.04 (16.94) | 23 - 79 | |
| Sex (female) | 29 (51.8) | ||
| Race (white) | 44 (78.6) | ||
| Education (years) | 15.91 (1.77) | 12 - 20 | |
| Smoker (yes) | 6 (10.7%) | ||
| BMI (N = 52) | 25.57 (4.68) | 14.20 – 36.74 | |
| Waking time (hours from 12 AM) | 7.00 (.89) | 4.62 – 8.88 | |
| CAR ([nmol/L]/hour) | 11.43 (12.17) | -11.00 – 43.71 | |
| Corrected CAR ([nmol/L]/hour) (N = 55) | 17.66 (8.60) | 4.12 – 40.30 | |
| Waking cortisol (nmol/L) | 15.57 (4.57) | 4.92 – 25.07 | |
| 30 min post waking cortisol (nmol/L) | 20.61 (6.57) | 7.99 – 40.07 | |
| Diurnal cortisol output (AUC) | 101.43 (28.75) | 37.53 – 177.23 | |
| Diurnal cortisol slope (linear) | -.91 (.24) | -1.55 - -.46 | |
| Diurnal cortisol slope (quadratic) | .06 (.03) | .00 - .12 | |
| Associative recognition (hits – false alarms) | 0.74 (.14) | 0.53 – 1.00 | |
| Reading span accuracy | 29.30 (21.66) | 0 - 75 | |
| 3-Back accuracy | .76 (.13) | 0.28 – 0.96 | |
| Pattern Comparison | 36.61 (7.92) | 20 - 59 | |
| Letter Comparison | 20.38 (4.50) | 12 - 34 |
3.2 Zero-order correlations
The correlations are reported in Table 2. CAR and corrected CAR were not significantly related to age or any of the covariates (i.e., sex, race, education, and waking time). Higher CAR and corrected CAR slopes were significantly associated with better episodic memory, but both CAR values were not related to working memory or processing speed. The CAR and corrected CAR were significantly correlated to diurnal cortisol output and the linear diurnal cortisol slope, but neither were related to the quadratic diurnal cortisol slope or waking cortisol. Higher waking cortisol was related to better episodic memory and working memory, but waking cortisol was not significantly related to processing speed. A more negative linear diurnal slope was related to better episodic memory and working memory, but the linear slope was not significantly related to processing speed. The quadratic diurnal slope was positively related to episodic memory, but it was not significantly related to working memory or processing speed. Diurnal cortisol output was not significantly related to any cognitive measure.
Table 2.
Zero-order correlation coefficients between age, covariates, cortisol, and cognition.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | - | |||||||||||||
| 2. Sex | .08 | - | ||||||||||||
| 3. Race | -.24 | .11 | - | |||||||||||
| 4. Education | -.09 | .03 | .03 | - | ||||||||||
| 5. Waking time | -.23 | .03 | .02 | -.06 | - | |||||||||
| 6. CAR | .11 | -.07 | -.05 | -.07 | .01 | - | ||||||||
| 7. Corrected CAR | .16 | -.16 | -.11 | -.02 | -.02 | .72*** | - | |||||||
| 8. Waking cortisol | .003 | -.04 | -.19 | .29* | .10 | .05 | .17 | - | ||||||
| 9. Diurnal output | .07 | .07 | -.08 | .26* | -.24 | .48*** | .60*** | .39** | - | |||||
| 10. Linear slope | .12 | .02 | .16 | -.28* | -.44** | -.33* | -.44** | -.69*** | -.60*** | - | ||||
| 11. Quadratic slope | .01 | -.11 | -.20 | .31* | .21 | -.16 | -.03 | .70*** | -.07 | -.39** | - | |||
| 12. Episodic memory | -.23 | -.04 | -.19 | .25 | .06 | .35** | .37** | .32* | .20 | -.33* | .31* | - | ||
| 13. Working memory | -.50*** | .23 | -.002 | .10 | .10 | .14 | .08 | .29* | .10 | -.31* | .14 | .34** | - | |
| 14. Processing speed | -.68*** | -.11 | .06 | .09 | .32* | .02 | -.02 | .20 | -.18 | -.19 | .21 | .37** | .59*** | - |
Note:
p <.05,
p <.01,
p <.001.
3.3 Episodic memory
3.3.1 Variance explained by cortisol after accounting for covariates
Model 1, containing age, race, and education, was significantly related to episodic memory and explained 17% of its variance (see Table 3). Participants who were younger and White (vs. not White) had better memory. Of the cortisol indices assessed, only the CAR and corrected CAR were significant predictors in Model 2, increasing the overall explained variance in episodic memory by 15% and 16%, respectively (see Table 3). Participants with larger positive CAR slopes had higher memory scores (see Figure 1). The Age X CAR interaction term was not significant in Model 3 indicating that the relationship between the CAR and episodic memory was not moderated by age (see Table 3).
Table 3.
Summary of hierarchical regression analyses for each cortisol predictor where episodic memory is the dependent variable.
| Cortisol predictor → | CAR | Waking cortisol | Diurnal cortisol output (AUC) | Diurnal cortisol slope | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | t | p | r | B | t | p | r | B | t | p | r | B | t | p | r | ||
| Model 1 | |||||||||||||||||
| Age | -.002 | -2.12 | .04 | -.28 | -.002 | -2.12 | .04 | -.28 | -.002 | -2.12 | .04 | -.28 | -.002 | -2.12 | .04 | -.28 | |
| Race | -.09 | -2.05 | .046 | -.27 | -.09 | -2.05 | .046 | -.27 | -.09 | -2.05 | .046 | -.27 | -.09 | -2.05 | .046 | -.27 | |
| Education | .02 | 1.80 | .08 | .24 | .02 | 1.80 | .08 | .24 | .02 | 1.80 | .08 | .24 | .02 | 1.80 | .08 | .24 | |
| Model 2 | |||||||||||||||||
| Age | -.003 | -2.62 | .01 | -.35 | -.002 | -2.12 | .04 | -.29 | -.002 | -2.22 | .03 | -.30 | -.002 | -1.93 | .06 | -.26 | |
| Race | -.09 | -2.17 | .04 | -.29 | -.08 | -1.70 | .10 | -.23 | -.09 | -1.98 | .054 | -.27 | -.07 | -1.48 | .14 | -.21 | |
| Education | .02 | 2.15 | .04 | .29 | .01 | 1.25 | .22 | .17 | .02 | 1.42 | .16 | .20 | .01 | .97 | .34 | .14 | |
| Cortisol | .005 | 3.30 | .002 | .42 | .007 | 1.72 | .09 | .23 | .001 | 1.17 | .25 | .16 | Linear | -.10 | -1.20 | .24 | -.17 |
| Quadratic | .83 | 1.20 | .24 | .17 | |||||||||||||
| Model 3 | |||||||||||||||||
| Age | -.003 | -2.64 | .01 | -.35 | |||||||||||||
| Race | -.09 | -2.21 | .03 | -.30 | |||||||||||||
| Education | .02 | 2.17 | .04 | .29 | |||||||||||||
| Cortisol | .005 | 3.37 | .001 | .43 | |||||||||||||
| Age X Cortisol | .000 | .81 | .42 | .11 | |||||||||||||
| Model 1 | |||||||||||||||||
| R2 | .17 | .17 | .17 | .17 | |||||||||||||
| F(3,52) | 3.60 | 3.60 | 3.60 | 3.60 | |||||||||||||
| p | .02 | .02 | .02 | .02 | |||||||||||||
| Model 2 | |||||||||||||||||
| ΔR2 | .15 | .05 | .02 | .06 | |||||||||||||
| F(change) | 10.91 | 2.96 | 1.38 | 2.04 | |||||||||||||
| p | .002 | .09 | .25 | .14 | |||||||||||||
| Model 3 | |||||||||||||||||
| ΔR2 | .01 | ||||||||||||||||
| F(change)(1,50) | .65 | ||||||||||||||||
| p | .42 | ||||||||||||||||
Note: r = partial correlation coefficient
Figure 1.
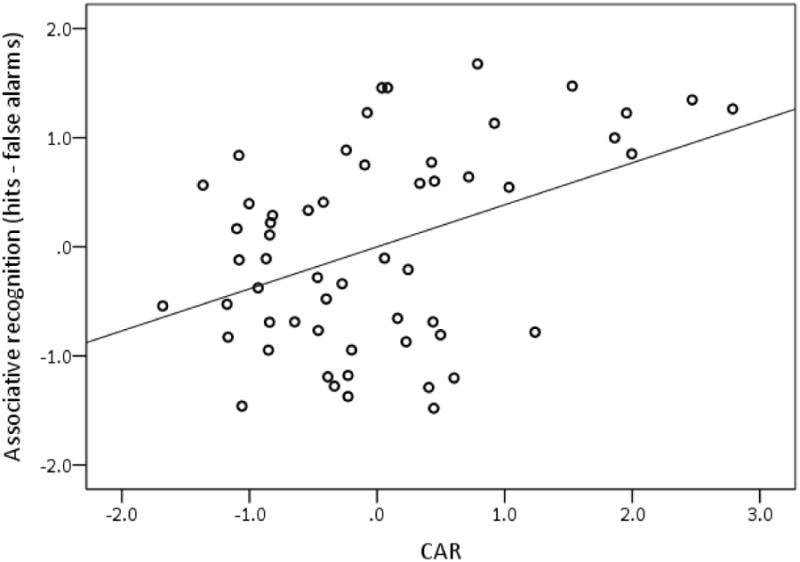
Partial regression plot of the relationship between the CAR and associative recognition (i.e., episodic memory), controlling for age, race, and education. Standardized values are represented on each axis.
3.3.2 Variance explained by CAR after accounting for covariates and diurnal cortisol measures
The addition of diurnal cortisol output (i.e., AUC) and diurnal cortisol slope (linear and quadratic) in Model 2 did not significantly increase the variance explained in episodic memory by Model 1 (see Table 4). Controlling for age, covariates, and the diurnal measures, the CAR was a significant predictor in Model 3 and increased the overall explained variance in episodic memory by 14%. Participants with larger positive CAR slopes had higher memory scores. Results were the same for the corrected CAR, B = .008, t(47) = 3.30, p = .002, r = .43. When controlling for the CAR in Model 3, the quadratic slope became a significant predictor of episodic memory. More positive quadratic slope values were related to better memory.
Table 4.
Summary of hierarchical regression analyses where the CAR and diurnal cortisol measures are predictors of cognitive outcomes.
| Episodic memory | Working memory | Processing Speed | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | t | p | r | B | t | p | r | B | t | p | r | |||
| Model 1 | Model 1 | Model 1 | ||||||||||||
| Age | -.002 | -2.12 | .04 | -.28 | Age | -.05 | -4.55 | <.001 | -.53 | Age | -.07 | -6.31 | <.001 | -.66 |
| Race | -.09 | -2.05 | .046 | -.27 | Sex | .95 | 2.42 | .02 | .32 | Waking time | .34 | 1.64 | .11 | .22 |
| Education | .02 | 1.80 | .08 | .24 | ||||||||||
| Model 2 | Model 2 | Model 2 | ||||||||||||
| Age | -.002 | -2.10 | .04 | -.29 | Age | -.05 | -4.26 | <.001 | -.52 | Age | -.07 | -6.36 | <.001 | -.67 |
| Race | -.07 | -1.48 | .15 | -.21 | Sex | 1.00 | 2.58 | .01 | .34 | Waking time | -.04 | -.13 | .90 | -.02 |
| Education | .008 | .68 | .50 | .10 | ||||||||||
| AUC | .001 | .97 | .34 | .14 | AUC | -.001 | -.10 | .92 | -.01 | AUC | -.02 | -1.35 | .18 | -.19 |
| Linear slope | -.02 | -.22 | .83 | -.03 | Linear slope | -1.61 | -1.35 | .18 | -.19 | Linear slope | -1.70 | -1.03 | .31 | -.14 |
| Quadratic slope | 1.18 | 1.51 | .14 | .21 | Quadratic slope | 5.38 | .68 | .50 | .10 | Quadratic slope | 6.99 | . 9 3 | .36 | .13 |
| Model 3 | Model 3 | Model 3 | ||||||||||||
| Age | -.003 | -2.69 | .01 | -.36 | Age | -.05 | -4.54 | <.001 | -.54 | Age | -.07 | -6.78 | <.001 | -.70 |
| Race | -.07 | -1.69 | .10 | -.24 | Sex | 1.08 | 2.84 | .007 | .38 | Waking time | -.11 | -.34 | .74 | -.05 |
| Education | .01 | 1.32 | .20 | .19 | ||||||||||
| AUC | .00007 | .09 | .93 | .01 | AUC | -.005 | -.52 | .60 | -.07 | AUC | -.02 | -1.84 | .07 | -.25 |
| Linear slope | .04 | .41 | .67 | .06 | Linear slope | -1.21 | -1.01 | .32 | -.14 | Linear slope | -1.52 | -.94 | .35 | -.13 |
| Quadratic slope | 1.58 | 2.19 | .03 | .30 | Quadratic slope | 8.68 | 1.09 | .28 | .15 | Quadratic slope | 9.83 | 1.32 | .19 | .19 |
| CAR | .005 | 3.35 | .002 | .44 | CAR | .03 | 1.70 | .10 | .24 | CAR | .03 | 2.02 | .049 | .28 |
| Model 1 | ||||||||||||||
| R2 | .17 | .32 | .49 | |||||||||||
| F | 3.60 | 12.50 | 25.05 | |||||||||||
| p | .02 | <.001 | <.001 | |||||||||||
| Model 2 | ||||||||||||||
| ΔR2 | .08 | .07 | .05 | |||||||||||
| F(change) | 1.67 | 1.93 | 1.85 | |||||||||||
| p | .19 | .14 | .15 | |||||||||||
| Model 3 | ||||||||||||||
| ΔR2 | .14 | .03 | .04 | |||||||||||
| F(change) | 11.23 | 2.87 | 4.09 | |||||||||||
| p | .002 | .10 | .049 | |||||||||||
Note: r = partial correlation coefficient
3.4 Working memory
3.4.1 Variance explained by cortisol after accounting for covariates
Model 1, containing age and sex, was significantly related to working memory, explaining 32% of its variance (see Table 5). Participants who were younger and male had better working memory. Of the cortisol indices measured, only waking cortisol was a significant predictor in Model 2, increasing the overall explained variance in working memory by 9%. Participants with higher waking cortisol had better working memory (see Figure 2). The relationship between the CAR and working memory was not moderated by age (see Table 5). Because there was a trend for a relationship between the CAR and working memory (p = .06), we tested the association between waking cortisol and working memory again by adding the CAR into Model 1 and waking cortisol in Model 2. The addition of the CAR in Model 1 did not change the variance in working memory explained by waking cortisol in Model 2, ΔR2 = .09, F(change) (1, 51) = 8.18, p = .006.
Table 5.
Summary of hierarchical regression analyses for each cortisol predictor where working memory is the dependent variable.
| Cortisol predictor → | CAR | Waking cortisol | Diurnal cortisol output (AUC) | Diurnal cortisol slope | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | t | p | r | B | t | p | r | B | t | p | r | B | t | p | r | ||
| Model 1 | |||||||||||||||||
| Age | -.05 | -4.55 | <.001 | -.53 | -.05 | -4.55 | <.001 | -.53 | -.05 | -4.55 | <.001 | -.53 | -.05 | -4.55 | <.001 | -.53 | |
| Sex | .95 | 2.42 | .02 | .32 | .95 | 2.42 | .02 | .32 | .95 | 2.42 | .02 | .32 | .95 | 2.42 | .02 | .32 | |
| Model 2 | |||||||||||||||||
| Age | -.06 | -4.85 | <.001 | -.56 | -.05 | -4.87 | <.001 | -.56 | -.05 | -4.62 | <.001 | -.54 | -.05 | -4.44 | <.001 | -.53 | |
| Sex | 1.00 | 2.62 | .01 | .34 | .99 | 2.69 | .01 | .35 | .93 | 2.36 | .02 | .31 | .99 | 2.60 | .01 | .34 | |
| Cortisol | .03 | 1.91 | .06 | .26 | .12 | 2.87 | .006 | .37 | .007 | 1.04 | .31 | .14 | Linear | -1.53 | -1.80 | .08 | -.24 |
| Quadratic | 5.72 | .80 | .43 | .11 | |||||||||||||
| Model 3 | |||||||||||||||||
| Age | -.06 | -4.81 | <.001 | -.56 | |||||||||||||
| Sex | 1.00 | 2.59 | .01 | .34 | |||||||||||||
| Cortisol | .03 | 1.85 | .07 | .25 | |||||||||||||
| Age X Cortisol | .000 | -.18 | .86 | -.03 | |||||||||||||
| Model 1 | |||||||||||||||||
| R2 | .32 | .32 | .32 | .32 | |||||||||||||
| F(2,53) | 12.50 | 12.50 | 12.50 | 12.50 | |||||||||||||
| p | <.001 | <.001 | <.001 | <.001 | |||||||||||||
| Model 2 | |||||||||||||||||
| ΔR2 | .05 | .09 | .01 | .07 | |||||||||||||
| F(change) | 3.66 | 8.25 | 1.07 | 2.95 | |||||||||||||
| p | .06 | .006 | .31 | .06 | |||||||||||||
| Model 3 | |||||||||||||||||
| ΔR2 | .00 | ||||||||||||||||
| F(change)(1,51) | .03 | ||||||||||||||||
| p | .86 | ||||||||||||||||
Note: r = partial correlation coefficient
Figure 2.
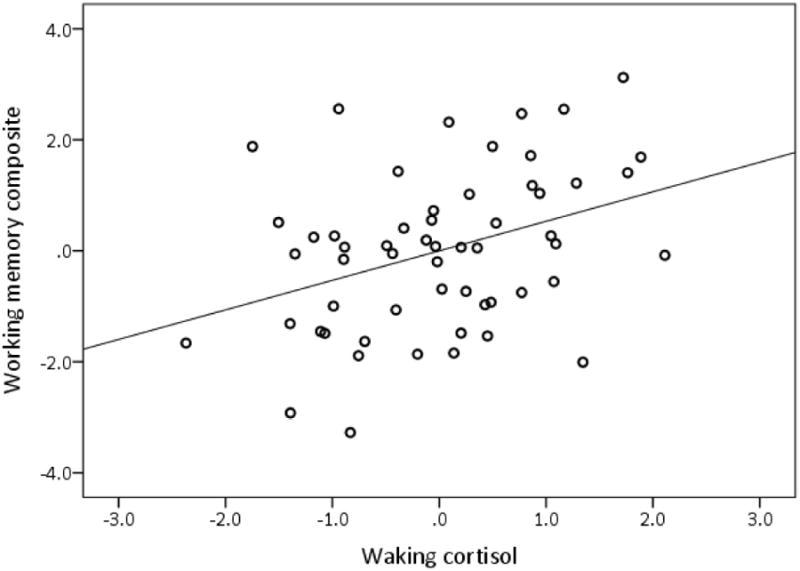
Partial regression plot of relationship between waking cortisol and working memory controlling for age and sex. Standardized values are represented on each axis.
3.4.2 Variance explained by CAR after accounting for covariates and diurnal cortisol measures
The addition of diurnal cortisol output (i.e., AUC) and diurnal cortisol slope (linear and quadratic) in Model 2 and the CAR in Model 3 did not significantly increase the variance already explained in working memory by age and sex in Model 1 (see Table 4).
3.5 Processing speed
3.5.1 Variance explained by cortisol after accounting for covariates
Model 1, containing age and waking time, was significantly related to processing speed, explaining 49% of its variance. Younger age was related to better processing speed (see Table 6). None of the cortisol measures were significant predictors of processing speed in Model 2 and the relationship between the CAR and processing speed was not moderated by age (see Table 6).
Table 6.
Summary of hierarchical regression analyses for each cortisol predictor where processing speed is the dependent variable.
| Cortisol predictor → | CAR | Waking cortisol | Diurnal cortisol output (AUC) | Diurnal cortisol slope | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | t | p | r | B | t | p | r | B | t | p | r | B | t | p | r | ||
| Model 1 | |||||||||||||||||
| Age | -.07 | -6.31 | <.001 | -.66 | -.07 | -6.31 | <.001 | -.66 | -.07 | -6.31 | <.001 | -.66 | -.07 | -6.31 | <.001 | -.66 | |
| Waking time | .34 | 1.64 | .11 | .22 | .34 | 1.64 | .11 | .22 | .34 | 1.64 | .11 | .22 | .34 | 1.64 | .11 | .22 | |
| Model 2 | |||||||||||||||||
| Age | -.07 | -6.36 | <.001 | -.66 | -.07 | -6.51 | <.001 | -.67 | -.07 | -6.28 | <.001 | -.66 | -.07 | -6.52 | <.001 | -.67 | |
| Waking time | .33 | 1.60 | .12 | .22 | .30 | 1.47 | .15 | .20 | .29 | 1.38 | .17 | .19 | .28 | 1.23 | .23 | .17 | |
| Cortisol | .01 | .91 | .37 | .13 | .07 | 1.91 | .06 | .26 | -.006 | -.93 | .36 | -.13 | Linear | .23 | .27 | .79 | .04 |
| Quadratic | 12.25 | 1.88 | .07 | .25 | |||||||||||||
| Model 3 | |||||||||||||||||
| Age | -.07 | -6.31 | <.001 | -.66 | |||||||||||||
| Waking time | .35 | 1.66 | .10 | .23 | |||||||||||||
| Cortisol | .01 | .78 | .44 | .11 | |||||||||||||
| Age X Cortisol | -.001 | -.79 | .43 | -.11 | |||||||||||||
| Model 1 | |||||||||||||||||
| R2 | .49 | .49 | .49 | .49 | |||||||||||||
| F(2,53) | 25.05 | 25.05 | 25.05 | 25.05 | |||||||||||||
| p | <.001 | <.001 | <.001 | <.001 | |||||||||||||
| Model 2 | |||||||||||||||||
| ΔR2 | .008 | .03 | .008 | .03 | |||||||||||||
| F(change) | .82 | 3.65 | .87 | 1.83 | |||||||||||||
| p | .37 | .06 | .36 | .17 | |||||||||||||
| Model 3 | |||||||||||||||||
| ΔR2 | .006 | ||||||||||||||||
| F(change)(1,51) | .63 | ||||||||||||||||
| p | .43 | ||||||||||||||||
Note: r = partial correlation coefficient
3.5.2 Variance explained by CAR after accounting for covariates and diurnal cortisol measures
Diurnal cortisol output and diurnal cortisol slope were not significant predictors of processing speed in Model 2 (see Table 4). The CAR reached the threshold for statistical significance in Model 3 when controlling for the diurnal cortisol measures. More positive CAR slopes were related to better processing speed. The corrected CAR, however, was not a significant predictor in this model, B = .05, t(48) = 1.94, p = .06, r = .27.
4. Discussion
When controlling for the covariates age, race, and education, but not diurnal cortisol measures, we found that only the CAR, out of all the cortisol measures assessed, was significantly related to episodic memory, predicting 15% of its variance. Individuals with more positive CAR slopes had better episodic memory than those with less positive slopes. The CAR was not significantly related to working memory. The CAR was positively related to processing speed when controlling for diurnal cortisol measures in addition to the covariates of age and waking time; however, this relationship was not significant when the corrected CAR, which adjusts for non-compliant sampling, was used as a predictor.
The significant relationship of the CAR to episodic memory is sensible given the linkage of hippocampal structure and function to associative memory. It is also congruent with research demonstrating elimination of the CAR in people with unilateral and bilateral hippocampal damage and severe global amnesia (Bruehl et al., 2009; Buchanan et al., 2004; Wolf et al., 2005). We found that people with larger positive CAR slopes had better episodic memory than people with smaller positive slopes, suggesting that a dynamic increase in cortisol following morning awakening is a marker of healthy hippocampal function. The production of a robust CAR may also influence hippocampal function, consequently benefiting episodic memory.
An inverted U-shaped relationship has been reported between the CAR and episodic memory (Almela et al., 2012) and spatial working memory (Moriarty et al., 2014), suggesting that intermediate CAR responses are better for memory than low or high responses, a notion consistent with findings of an inverted U-shaped relationship between corticosteroid doses and memory (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson, 2003; Lupien, Buss, Schramek, Maheu, & Pruessner, 2005; Schilling et al., 2013). In a supplementary analysis, we tested for but did not find a quadratic relationship between the CAR and episodic memory, B = .02, p = .12. It is possible that none of the participants in our sample had a CAR large enough to negatively influence memory.
Because we assessed the CAR using 10 consecutive morning cortisol samples and measured episodic memory 8 to 38 months following cortisol collection, the significant relationship between the CAR and episodic memory was probably due to more stable (trait-like or long-term) conditions influencing cortisol production rather than labile (state-like or situational) factors. Indeed, one of the strengths of this study is that aggregating cortisol data over an extended time period undoubtedly increased the reliability of all of the diurnal cortisol indicators for capturing stable individual differences (Doane, Chen, Sladek, Van Lenten, & Granger, 2015). Stable chronic conditions, such as chronic physical and mental health disorders, have been related to a reduced CAR (for review, see Fries, Dettenborn, & Kirschbaum, 2009) and could possibly explain the relationship between a reduced CAR and poorer episodic memory. However, since our sample on average reported to be physically and mentally healthy and we excluded people with posttraumatic stress disorder and other serious psychiatric conditions from study recruitment, we believe this explanation is unlikely for our findings. The CAR appears to be significantly influenced by genetic traits more so than cortisol measures across the rest of the day (Franz et al., 2010; Kupper et al., 2005; Wϋst, Federenko, Hellhammer, & Kirschbaum, 2000). Medium sized heritability estimates (e.g., h2 = .48 for area under the cortisol awakening response curve in Wϋst et al., 2000) have been reported for the CAR in contrast to statistically nonsignificant heritability estimates for cortisol collected later in the morning and throughout the day until the evening (Wϋst et al., 2000). Genetic determinants of the CAR could be related to hippocampal function as manifested in episodic memory performance.
In addition to genetic factors, chronic stress has been related to both an increased (Schulz, Kirschbaum, Pruessner, & Hellhammer, 1998; Wϋst et al., 2000) and decreased CAR (Buchanan et al., 2004; Juster et al., 2011; Marchand, Juster, Durand, & Lupien, 2014), with prolonged exposure to stress hypothesized to result in a hypoactive HPA axis (Fries et al., 2005) and a reduced CAR (Fries et al., 2009). Consistent with this hypothesis, a reduced CAR has been found in people who have experienced situations of chronic stress and burden, such as childhood maltreatment (Li, Chassan, Bruer, Gower, & Shelton, 2015) social strain (Friedman, Karlamangla, Almeida, & Seeman, 2012), minority status (Karlamangla, Friedman, Seeman, Stawski, & Almeida, 2013), caregiving burden (Buchanan et al., 2004), and burnout (Juster et al., 2011). The biological cost of prolonged chronic stress is allostatic load, a process which can lead to loss of neural resilience and aging of brain structure and function, especially in the prefrontal cortex, amygdala, and hippocampus (McEwen et al., 2015). Our results suggest that a reduced CAR is not beneficial to hippocampally-mediated memory function. Future work should investigate whether allostatic load related reductions in the CAR are related to hippocampal dysfunction and episodic memory impairment.
Recently, studies have linked within-person fluctuation in cortisol and daily stress with fluctuations in cognitive function, indicating that stress-related psychological states, including anxiety and rumination, influence constructs like working memory (Sliwinski, Smyth, Hofer, & Stawski, 2006; Sliwinski, Smyth, Stawski, & Wasylyshyn, 2005). We view the present results as complementary to this line of work. Our results indicate that stable individual differences in CAR are related to individual differences in episodic memory, even when controlling on level of cortisol production. Within individuals, however, memory may covary with daily fluctuations in stress-related experiences. An interesting question for future research would be whether the type of between-person difference seen here moderates within-person variability in stress reactivity and its effects on memory performance, which might be expected given the Rickenbach et al. (2014) finding that within-person covariation of stress (including CAR) moderates the relationship of long-term cognitive decline and self-reported memory problems.
The significant relationship between the CAR and episodic memory was maintained when the corrected CAR was used as a predictor. Because the corrected CAR was calculated after removing CAR values indicative of sampling non-compliance (e.g., negative CAR values), non-compliance with the cortisol collection protocol had minimal influence upon the results. This was probably due to the use of aggregated data to calculate the CAR, which likely reduced the influence of measurement error.
Measures of global HPA axis function, specifically diurnal cortisol output (AUC) and the diurnal linear cortisol slope, and waking cortisol, a measure of pre-awakening cortisol secretion, were not related to episodic memory. The quadratic slope was significantly related to episodic memory, but only when controlling for diurnal cortisol measures and the CAR. Interpretation of this relationship is difficult considering the lack of a significant relationship with episodic memory when only age, race, and education were controlled. When accounting for the diurnal cortisol slope (linear and quadratic) and diurnal cortisol output, the CAR remained a significant predictor of episodic memory and explained 14% of its variance. Similar results were obtained with the corrected CAR. Although speculative, it is possible that post-awakening mechanisms specific to CAR regulation and independent of global HPA axis functioning, may account to some extent for the observed relationship between the CAR and episodic memory. Past studies relating the CAR to cognition have interpreted the CAR as a marker of hypothalamic pituitary adrenal (HPA) axis function (e.g., Almela et al., 2012). While the HPA axis influences the CAR, it may not be the CAR's sole regulator (Clow et al., 2010a; Fries et al., 2009). HPA-independent mechanisms involving the suprachiasmatic nucleus (SCN) and the hippocampus have also been proposed to regulate the CAR (Clow et al., 2010a). The SCN not only controls the HPA axis through direct innervations to the paraventricular nucleus of the hypothalamus but also influences adrenal sensitivity to adrenocorticotropin (ACTH) through an extra-pituitary pathway (Buijs, van Eden, Goncharuk, & Kalsbeek, 2003). As mentioned previously, an intact hippocampus has been consistently shown to be necessary for the occurrence of the CAR but not for the production of cortisol across the rest of the day. Some postulate that one way the hippocampus assists in regulating the CAR is by modulating the extra-pituitary pathway between the SCN and adrenal cortex (Clow et al., 2010a,b). The HPA regulation of the CAR also appears to be somewhat different from HPA regulation of cortisol across the remainder of the diurnal cycle (Clow et al., 2010b; Edwards, Clow, Evans, & Hucklebridge, 2001). For example, the post-awakening peak in cortisol has been negatively related to increases in cortisol at night (Wilhelm et al., 2007). Thus, the CAR is not only a marker of general HPA axis activity but also of mechanisms specific for CAR regulation. The current results suggest that post-awakening mechanisms specific to CAR regulation may account partly for the relationship between the CAR and episodic memory.
The interaction between age and the CAR was not a significant predictor of any cognitive measure indicating that the relationship between the CAR and cognition was independent of age. Several longitudinal studies have found that higher cortisol levels predict worse cognition in older adults (e.g., Li et al., 2006; Lupien et al., 1994, 1998; Seeman et al., 1997); however, these studies measured cortisol as an average over the diurnal cycle and did not assess the CAR. Further, they did not include younger adults in their sample; thus, it is unknown if the relationship between long-term increases in cortisol and worse cognition is a sole phenomenon of older and not younger adulthood. Results from studies investigating the relationship of the CAR to cognition have not explored age as a moderator.
Neither diurnal cortisol slope (linear and quadratic) nor total cortisol output (AUC) was significantly related to working memory or processing speed. Waking cortisol, however, was significantly related to working memory, explaining 9% of its variance after controlling for age and sex. Individuals with higher waking cortisol had better working memory. Waking cortisol, however, was not related to processing speed when controlling for age and waking time. There are few studies that have examined the relationship of waking cortisol to cognition. O'Hara et al. (2007) found that higher waking cortisol was related to worse delayed recall in older adults. However, this effect was found among individuals who had at least one ‘s’ allele of the serotonin transporter gene polymorphism. Working memory was not tested in that study and cortisol was collected only on two occasions. Somewhat supportive of our findings, Stawski et al. (2011) found that better cognitive function (a composite of episodic memory and executive functioning) was related to higher waking cortisol (collected over 4 days) in a sample of young, middle-aged, and older adults. Executive functioning, like working memory, is supported in part by the prefrontal cortex (Collette, Hogge, Salmon, & Van der Linden, 2006; Owen, McMillan, Laird, & Bullmore, 2005). It is difficult to explain the relationship between higher waking cortisol and better prefrontal function. One possible mechanism might be cortisol-associated increases in arousal that facilitates executive/working memory processing. In support of this, higher waking cortisol has been related to lower levels of fatigue over a three day period (Adam et al., 2006).
There are several limitations to our study. Participants did not collect cortisol at the same time and there was a delay of 8 to 38 months between the time of cortisol collection and cognitive testing. Although there was an extended time period between cortisol collection and cognitive testing, we argue that an aggregated CAR, calculated using up to 10 days of cortisol data, represents a stable trait CAR (see Hellhammer et al., 2007) that can be used to predict cognitive functioning months following cortisol collection. Further, measuring cognition months to years following cortisol assessment is not uncommon. Cortisol has been used as a predictor of cognition measured at a time point 1 to 7 years after cortisol collection in numerous studies (Comijs et al., 2010; Csernansky et al., 2006; Gerritsen, Comijs, Deeg, Penninx, & Geerlings, 2011; Greendale, Kritz-Silverstein, Seeman, & Barrett-Connor, 2000; Kuningas et al., 2006; Li et al., 2006; Singh-Manoux et al., 2014). Greendale et al. (2000) assessed cognition an average of 4.9 years (SD = 1.1 years) after the collection of morning cortisol. Controlling for multiple variables (e.g. age, education, and BMI), they found that higher morning cortisol predicted worse category fluency. Any potential confounding variable due to the delay in time between cortisol collection and cognitive testing should serve to weaken relationships between cortisol and cognition, not strengthen them. We measured potential confounds of the relationship of cortisol to cognition (e.g., age and education) at the time of cognitive testing and controlled for these when appropriate. Age at time of testing was controlled in all of our analyses.
Though we had 10 consecutive days of cortisol data, we had only two cortisol assessments, at waking and 30 min post waking, for the calculation of the CAR slope. Additional cortisol measures throughout the awakening period may have better captured the dynamics of the CAR (Clow et al., 2004; Hellhammer et al., 2007). For example, we were unable to calculate total cortisol secretory activity during morning awakening (i.e., area under the curve relative to ground), which requires at least three cortisol measures, and differentiate whether the association to cognition was due to total cortisol output during awakening or the dynamic of the cortisol response (Clow et al., 2004).
Our study was correlational, so we cannot claim that higher CAR values improve episodic memory. We can only speculate as to the possible mechanisms that drive this association. Future studies should investigate if interindividual differences (e.g., age, chronic stress, genetic traits) explain some of the variability in the relationship between the CAR and episodic memory measured over time.
Finally, we found that the relationship of the CAR to cognition did not depend upon age. Our limited sample size may have precluded identification of small to medium sized effects due to being underpowered; having said that, the partial r squared associated with the moderated regression coefficient was essentially zero (see Table 3), indicating no evidence of age moderation in this sample.
4.1 Summary
We found that a more positive CAR slope was related to better episodic memory in an adult sample and that this relationship did not depend upon age. The CAR was not significantly related to working memory and the corrected CAR was not related to processing speed. We also found that higher waking cortisol was significantly related to better working memory, but not episodic memory or processing speed. Future work should investigate the mechanisms underpinning the relationship of the cortisol awakening process to cognitive functioning.
Cortisol measures were aggregated over 10 days (7 samples per day).
More positive CAR slopes were significantly related to better episodic memory.
Higher waking cortisol was significantly related to better working memory.
Diurnal cortisol output and linear slope were not related to cognition.
The relationship between the CAR and cognition did not depend upon age.
Acknowledgments
We gratefully acknowledge the assistance of Katie Umberson and Jared Holder in collection of cognitive data at the Center for Advanced Brain Imaging for this project, and Jared Holder for data management support.
Financial Support: This research was supported by a grant from the National Institute on Aging, one of the National Institutes of Health (R01 AG015019; Christopher Hertzog, Principal Investigator), and a Ruth L. Kirschstein PHS training grant (T32 AG000175).
Footnotes
Smoking status and BMI were obtained during cortisol collection.
Conflict of Interest: The authors report no conflict of interest and are alone responsible for the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gilda E. Ennis, Email: gilda.ennis@psych.gatech.edu.
Scott D. Moffat, Email: scott.moffat@psych.gatech.edu.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117(3):505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychol Aging. 2009;24(4):819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almela M, van der Meij L, Hidalgo V, Villada C, Salvador A. The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology. 2012;37(12):1929–1940. doi: 10.1016/j.psyneuen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, et al. Herscovitch P. The process of awakening: a PET study of regional brain activity patterns mediating the reestablishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34(6):815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry. 2004;56(9):651–656. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Buijs R, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. Journal of Endocrinology. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010a;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Thorn L. The cortisol awakening response in context. International Review of Neurobiology. 2010b;93:153–175. doi: 10.1016/S0074-7742(10)93007-9. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139(1):209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Gerritsen L, Penninx BWJH, Bremmer MA, Deeg DJH, Geerlings MI. The association between serum cortisol and cognitive decline in older persons. The American Journal of Geriatric Psychiatry. 2010;18(1):42–50. doi: 10.1097/JGP.0b013e3181b970ae. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. American Journal of Psychiatry. 2006;163(12):2164–2169. doi: 10.1176/ajp.2006.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva NO, Almeida DM, Dmitrieva J, Loken E, Pieper CF. A day-centered approach to modeling cortisol: diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology. 2013;38(10):2354–2365. doi: 10.1016/j.psyneuen.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA. Latent trait cortisol (LTC) levels: Reliability, validity, and stability. Psychoneuroendocrinology. 2015;55:21–35. doi: 10.1016/j.psyneuen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal secretory activity. Life Sciences. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Evans P, Hucklebridge F, Loveday C, Clow A. The cortisol awakening response is related to executive function in older age. Int J Psychophysiol. 2012;84(2):201–204. doi: 10.1016/j.ijpsycho.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Evans PD, Fredhoi C, Loveday C, Hucklebridge F, Aitchison E, Forte D, Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. Int J Psychophysiol. 2011;79(3):371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Franz CE, O'Brien RC, Hauger RL, Mendoza SP, Panizzon MS, Prom-Wormley E, et al. Kremen WS. Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: the Vietnam Era twin study of aging. Psychoneuroendocrinology. 2011;36(7):1040–1052. doi: 10.1016/j.psyneuen.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, et al. Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behav Genet. 2010;40(4):467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Karlamangla AS, Almeida DM, Seeman TE. Social strain and cortisol regulation in midlife in the US. Soc Sci Med. 2012;74(4):607–615. doi: 10.1016/j.socscimed.2011.11.003. doi:0.1016/j.socscimed.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, Deeg DJH, Penninx BWJH, Geerlings MI. Salivary cortisol, APOE-ε4 allele and cognitive decline in a prospective study of older persons. Neurobiology of Aging. 2011;32(9):1615–1625. doi: 10.1016/j.neurobiolaging.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Kritz-Silverstein D, Seeman T, Barrett-Connor E. Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo Study. Journal of the American Geriatrics Society. 2000;48(12):1655–1658. doi: 10.1111/j.1532-5415.2000.tb03878.x. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser H, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci U S A. 1999;96:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JC, Touron DR, Hertzog C. Metacognitive influences on study time allocation in an associative recognition task: An analysis of adult age differences. Psychol Aging. 2009;24(2):462–475. doi: 10.1037/a0014417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15(2):203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, Lupien SJ. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 2011;36(6):797–805. doi: 10.1016/j.psyneuen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen. 2004;133(2):189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawski RS, Almeida DM. Daytime trajectories of cortisol: Demographic and socioeconomic differendes. Findings from The National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38(11):2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, et al. Kivimaki M. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology. 2009;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kuningas M, de Rijk RH, Westendorp RGJ, Jolles J, Eline Slagboom P, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2006;32(6):1295–1301. doi: 10.1038/sj.npp.1301260. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus EJ, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30(9):857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, et al. Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiol Aging. 2006;27(11):1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Li L, Chassan RA, Bruer EH, Gower BA, Shelton RC. Childhood maltreatment increases the risk for visceral obesity. Obesity. 2015;23:1625–1632. doi: 10.1002/oby.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S, Buss C, Schramek TE, Maheu F, Pruessner JC. Hormetic influence of glucocorticoids on human memory. Nonlinearity in Biology, Toxicology, and Medicine. 2005;3:23–56. doi: 10.2201/nonlin.003.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney M. Basal cortsol levels and cognitive deficits in human aging. The Journal of Neuroscience. 1994;14(5):2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, De Leon M, De Santi S, Convit A, Tarshish C, Nair NP, et al. Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Mackworth JF. Paced memorizing in a continuous task. J Exp Psychol. 1959;58:206–211. doi: 10.1037/h0049090. [DOI] [PubMed] [Google Scholar]
- Marchand A, Juster RP, Durand P, Lupien SJ. Burnout symptom sub-types and cortisol profiles: what's burning most? Psychoneuroendocrinology. 2014;40:27–36. doi: 10.1016/j.psyneuen.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12(3):325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Nature Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty AS, Bradley AJ, Anderson KN, Watson S, Gallagher P, McAllister-Williams RH. Cortisol awakening response and spatial working memory in man: a U-shaped relationship. Hum Psychopharmacol. 2014;29(3):295–298. doi: 10.1002/hup.2399. [DOI] [PubMed] [Google Scholar]
- Nater UM, Hoppmann CA, Scott SB. Diurnal profiles of salivary cortisol and alpha-amylase change across the adult lifespan: evidence from repeated daily life assessments. Psychoneuroendocrinology. 2013;38(12):3167–3171. doi: 10.1016/j.psyneuen.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, et al. Hallmayer JF. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12(6):544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. doi: 10.1037//0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, Lupien SJ. The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Res. 2007;155(1):1–10. doi: 10.1016/j.pscychresns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rickenbach EH, Almeida DM, Seeman TE, Lachman ME. Stress magnifies the association between cogntive decline and everyday memory problems: An integration of longitudinal and diary methods. Psychol Aging. 2014;29(1):852–862. doi: 10.1037/a0038072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Meier F, Lange T, Born J. Suppressing the morning rise in cortisol impairs free recall. Learn Mem. 2010;17(4):186–190. doi: 10.1101/lm.1728510. [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging. 2005;20(1):3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Rushton JP, Brainerd CJ, Pressley MJ. Behavioral development and construct validity: The principle of aggregation. Psychological Bulletin. 1983;94:18–38. [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27(5):763–776. [Google Scholar]
- Schaie KW. Perceptual speed in adulthood: Cross-sectional and longitudinal studies. Psychol Aging. 1989;4(4):443–453. doi: 10.1037//0882-7974.4.4.443. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Willis SL. Psychometric intelligence and aging. In: Blanchard-Fields F, Hess TM, editors. Perspectives on cognitive change in adulthood and aging. New York: McGraw-Hill; 1996. pp. 293–322. [Google Scholar]
- Schilling TM, Kolsch M, Larra MF, Zech CM, Blumenthal TD, Frings C, Schachinger H. For whom the bell (curve) tolls: cortisol rapidly affects memory retrieval by an inverted U-shaped dose-response relationship. Psychoneuroendocrinology. 2013;38(9):1565–1572. doi: 10.1016/j.psyneuen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. A task is a task is a task: putting complex span, n-back, and other working memory indicators in psychometric context. Front Psychol. 2014;5:1475. doi: 10.3389/fpsyg.2014.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Prϋbner J, Hellhammer DH. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Medicine. 1998;14:91–97. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiat. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur Studies of Successful Aging. Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Elbaz A, Shipley M, Kivimaki M, Kumari M. No evidence of a longitudinal association between diurnal cortisol patterns and cognition. Neurobiol Aging. 2014;35(10):2239–2245. doi: 10.1016/j.neurobiolaging.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Smyth JM, Hofer SM, Stawski RS. Intraindividual coupling of daily stress and cognition. Psychol Aging. 2006;21(3):545–557. doi: 10.1037/0882-7974.21.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Smyth JM, Stawski RS, Wasylyshyn C. Stress and working memory: Between-person and within-person relationships. In: Engle R, Sedek G, von Hecker U, McIntosh D, editors. Cognitive limitations in aging and psychopathology: Attention, working memory, and executive functions. Cambridge: Cambridge University Press; 2005. pp. 73–96. [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, et al. Fischer JE. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573–2580. doi: 10.1210/jc.2013-1056. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol B Psychol Sci Soc Sci. 2011;66 Suppl 1:i71–81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31(8):1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122(3):231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wϋst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Fujiwara E, Luwinski G, Kirschbaum C, Markowitsch HJ. No morning cortisol response in patients with severe global amnesia. Psychoneuroendocrinology. 2005;30(1):101–105. doi: 10.1016/j.psyneuen.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wϋst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wϋst S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34(7):972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]


