Abstract
Introduction
Electronic cigarettes (ECIGs) aerosolize a liquid that usually contains propylene glycol and/or vegetable glycerin, flavorants, and the dependence-producing drug nicotine in various concentrations. This laboratory study examined the relationship between liquid nicotine concentration on plasma nicotine concentration and puffing behavior in experienced ECIG users.
Methods
Sixteen ECIG-experienced participants used a 3.3-Volt ECIG battery attached to a 1.5-Ohm dual-coil “cartomizer” loaded with 1 ml of a flavored propylene glycol/vegetable glycerin liquid to complete four sessions, at least 2 days apart, that differed by nicotine concentration (0, 8, 18, or 36 mg/ml). In each session, participants completed two 10-puff ECIG use bouts (30-sec puff interval) separated by 60 minutes. Venous blood was sampled to determine plasma nicotine concentration. Puff duration, volume, and average flow rate were measured.
Results
Immediately after bout 1, mean plasma nicotine concentration was 5.5 ng/ml (SD=7.7) for 0 mg/ml liquid, with significantly (p<0.05) higher mean concentrations observed for the 8 (mean=17.8 ng/ml, SD=14.6), 18 (mean=25.9 ng/ml, SD=17.5), and 36 mg/ml (mean=30.2 ng/ml; SD=20.0) concentrations; a similar pattern was observed for bout 2. For bout 1, at 36 mg/ml, the mean post- minus pre-bout difference was 24.1 ng/ml (SD=18.3). Puff topography data were consistent with previous results and revealed few reliable differences across conditions.
Discussion
This study demonstrates a relationship between ECIG liquid nicotine concentration and user plasma nicotine concentration in experienced ECIG users. Nicotine delivery from some ECIGs may exceed that of a combustible cigarette. The rationale for this higher level of nicotine delivery is uncertain.
Keywords: Nicotine, Electronic nicotine delivery devices, Addiction
INTRODUCTION
Electronic cigarettes (ECIGs) are an evolving class of products that use an electric heating element to aerosolize a liquid that often contains propylene glycol, vegetable glycerin, flavorants, and the dependence-producing drug nicotine. ECIG use is growing in the US and elsewhere,[1–5] including among tobacco cigarette smokers using them as smoking cessation aids.[2,6] Effective ECIG-mediated smoking cessation may depend on a given product’s ability to deliver nicotine to the user, and ECIG nicotine delivery can be variable.[7–9] Interestingly, experience with a product may give users the opportunity to learn how to extract nicotine efficiently from it.10 Experienced users puff differently than ECIG-naïve combustible cigarette smokers,[10–12] and this modified puffing behavior likely influences ECIG nicotine yield.[13] Other factors also can influence nicotine yield, including device features and liquid nicotine concentration. The combination of user behavior, device, and liquid nicotine concentration can lead to dramatic differences in yield,[13] suggesting that some experienced ECIG users might self-administer more nicotine as ECIG users than they did as tobacco cigarette smokers. To date there has been little systematic exploration of how these factors might influence nicotine delivery in experienced ECIG users. The primary purpose of this clinical laboratory study was to examine the extent to which liquid nicotine concentration influences the plasma nicotine concentration of experienced ECIG users. A secondary purpose was to examine how puff topography was influenced by liquid nicotine concentration.
METHODS
Participants
Sixteen experienced ECIG users completed this Institutional Review Board-approved study. Participants were eligible if they were healthy, aged 18–55, used ≥1 ml ECIG liquid/day for ≥3 months at a liquid nicotine concentration of ≥12mg/ml. Individuals were ineligible if they reported a history of chronic disease or psychiatric condition, regular use of a prescription medication (except vitamins and/or birth control), marijuana use >10 days and/or alcohol use >25 days in the past 30, and use of other illicit drugs in the past 30 days. Women were excluded if they tested positive for pregnancy by urinalysis at screening.
Procedure
Similar to another report,14 all participants completed four independent, double-blind laboratory sessions preceded by instructions to abstain for >12 hours of tobacco/nicotine, separated by >48 hours, and randomized. In each session, participants were provided with an “eGo” 3.3-Volt, 1000 mAh battery with a 1.5-Ohm, dual-coil, 510-style cartomizer pre-loaded (by staff with no participant contact) with 1-ml of a flavored (tobacco or menthol), 70% propylene glycol/30% vegetable glycerin liquid. Sessions differed by liquid nicotine concentration: 0, 8, 18, or 36 mg/ml. All ECIG liquids were tested to verify nicotine concentration throughout the study, and results indicated that, on average, the actual nicotine content was +/− 1 mg of the labeled nicotine content. In each session, participants completed two, 10-puff ECIG use bouts (separated by 60 minutes as in previous work)15 with a 30-second inter-puff interval. Venous blood was sampled ten times in each session (10 minutes prior to bout 1, and 5, 15, 30, 45, and 55 minutes after bout 1 and 5, 15, 30, and 45 minutes after bout 2) for later analysis of plasma nicotine concentration.[16] Puff duration, volume, and peak flow were measured using ECIG-specific equipment.[10] Other outcomes (e.g., heart rate, subjective effects) were assessed but are not the focus of this preliminary report and are not discussed further.
Statistical Analyses
For plasma nicotine data, in order to maintain statistical power in this preliminary report while limiting Type I error, we conducted a set of a priori comparisons using dependent samples t-tests in which, at each measurement time point, the mean plasma nicotine concentration for the 0 mg/ml condition was compared to the corresponding mean of the 8, 18, and 36 mg/ml condition. Because these comparisons were non-orthogonal at each time point, a Bonferroni correction was applied.[17] For topography data, we used the same analytic strategy within each bout (i.e., for each measure, comparing 0 to 8, 18, and 36 mg/ml, with a Bonferroni correction) but compared across bouts within each dose using uncorrected dependent samples t-tests for these orthogonal comparisons.
RESULTS
Participant Characteristics
Fifteen men and 1 woman participated in this study, of which 11 self-identified as White, 3 African American, 1 native Hawaiian/Pacific Islander, and 1 other. Mean (SD) age was 29.6 (5.8) years. On average, participants had been using ECIGs for 1.4 (0.9) years and consumed 2.0 (1.4) ml nicotine liquid daily at 19.8 (5.9) mg/ml nicotine concentration. Thirteen did not smoke any cigarettes and three smoked an average of 2.3 (0.9) cigarettes/day for 1.7 (2.3) years. At screening, average expired air carbon monoxide (CO) concentration was 3.5 (2.4) parts/million.
Plasma Nicotine
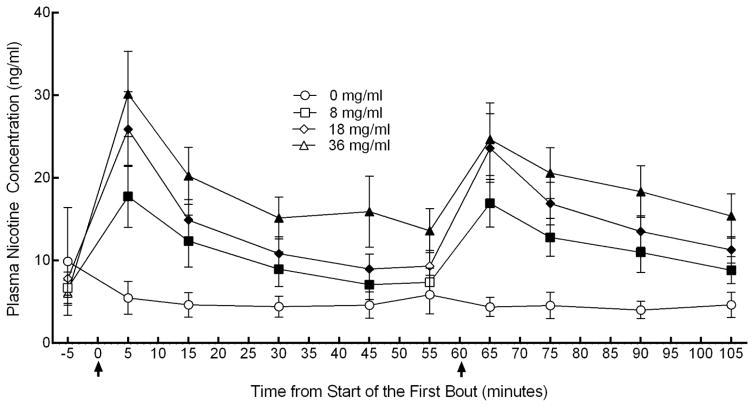
Figure 1 depicts mean plasma nicotine concentration over time by liquid nicotine concentration. Significant (p<0.05) differences were observed between 0 and 8 mg/ml immediately after the first bout (timepoint 5 minutes) through 45 minutes and then also immediately after the second bout (timepoint 65) through 105 minutes [ts(15) < −3.2], between 0 and 18 mg/ml immediately after the first bout through 45 minutes and immediately after the second bout through 105 minutes [ts(15) < −3.2], and between 0 and 36 mg/ml immediately after the first bout and then throughout the rest of the session [ts(15) < −3.0]. Immediately following the first bout, mean (SD) plasma nicotine concentration for the 0 mg/ml liquid nicotine concentration was 5.4 (7.7) ng/ml, for 8 mg/ml was 17.8 (14.6) ng/ml, for 18 mg/ml was 25.9 (17.5) ng/ml, and for 36 mg/ml was 30.2 (20.0) ng/ml. In terms of difference from baseline, results indicated a mean difference following the first bout of −4.4 (17.9) ng/ml for 0 mg/ml, 11.1 ng/ml (9.4) for 8 mg/ml, 18.1 ng/ml (15.5) for 18 mg/ml, and 24.1 (18.2) ng/ml for 36 mg/ml. Immediately following the second bout, mean (SD) plasma nicotine concentration for the 0 mg/ml liquid nicotine concentration was 4.4 (4.6) ng/ml, for 8 mg/ml 16.9 (11.2) ng/ml, for 18 mg/ml 23.6 (16.1) ng/ml, and 24.7 (17.0) ng/ml for 36 mg/ml.
Figure 1. Plasma nicotine concentration versus time as a function of ECIG nicotine concentration.
Mean (± SEM) plasma nicotine values for 16 experienced ECIG users using ECIGS that varied by liquid nicotine concentration. Bouts consisting of ECIG use for 10 puffs with a 30-sec IPI are denoted by arrows. Filled symbols indicate a significant (p<0.05) difference from 0 mg/ml ECIG liquid at that time point.
Puff Topography
For bout 1, mean (SD) volume was 154.5 ml (155.5) for 0 mg/ml, 176.0 ml (131.6) for 8 mg/ml, 114.7 ml (61.9) for 18 mg/ml, and 78.5 ml (39.5) ml for 36 mg/ml. The difference between 0 mg/ml and 36 mg/ml was not significant with the Bonferroni correction though it would have achieved conventional levels of significance were the correction not applied. Also for bout 1, mean puff duration was 5.5 s (2.04) for 0 mg/ml, 5.5 s (2.11) for 8 mg/ml, 4.97 (1.69) for 18 mg/ml, 3.98 s (1.54) for 36 mg/ml. The difference between 0 mg/ml and 36 mg/ml was significant with the Bonferroni correction on this measure [t (15)=4.7]. Mean flow rate was 33.8 ml/s (33.1) for 0 mg/ml, 30.8 ml/s (20.7) for 8 mg/ml, 23.3 ml/s (10.8) for 18 ml/s, and 19.7 ml/s (6.43) for 36 mg/ml; no significant differences from 0 mg/ml were observed. For bout 2, mean (SD) volume was 210.8 ml (261.13) for 0 mg/ml, 208.4 ml (170.2) for 8 mg/ml, 124.2 ml (70.7) for 18 mg/ml, and 84.3 ml (44.25) ml for 36 mg/ml, mean puff duration was 5.8 s (2.07) for 0 mg/ml, 6.1 s (2.21) for 8 mg/ml, 5.35 (1.98) for 18 mg/ml, 4.17 s (1.55) for 36 mg/ml, and mean flow rate was 33.0 ml/s (31.5) for 0 mg/ml, 31.5 ml/s (21.7) for 8 mg/ml, 22.8 ml/s (10.1) for 18 ml/s, and 20.4 ml/s (8.7) for 36 mg/ml. For bout 2, a significant (p<0.05) difference in puff duration was observed for 36 mg/ml compared to 0 mg/ml in bout 2 [t (15)=4.1].
DISCUSSION
These results demonstrate that, in experienced ECIG users, mean plasma nicotine concentration after 10 puffs from a 3.3-Volt ECIG with a 1.5-Ohm dual-coil cartomizer is related directly to liquid nicotine concentration. At the highest concentration tested (36 mg/ml), we observed a difference (post-bout minus pre-bout) in plasma nicotine concentration of 24.1 ng/ml (18.3). This “nicotine boost” appears greater than typically is observed in combustible cigarette smokers under similar puffing conditions (i.e., ~15 ng/ml).[15,18] Thus, some ECIGs are so efficient at delivering nicotine that they appear capable of exceeding the nicotine delivery profile of a combustible tobacco cigarette. We speculate that this excessive nicotine delivery may be harmful if it leads to a greater level of nicotine dependence, which could make ECIG cessation difficult if users eventually choose to try to quit their ECIG use. Alternatively, uses of higher nicotine liquid concentration may control nicotine intake by altering their puffing behavior. Nonetheless, there is no clear rationale for a product that delivers nicotine more efficiently than a tobacco cigarette, thus public health policymakers worldwide may want to consider limiting access to ECIG device/liquid combinations that demonstrate this nicotine delivery profile.
The puff topography results reported in this study were consistent with some previous reports.[10,11] but not one other [19] in which lower puff volume and shorter puffs were observed. This difference also may reflect different product used (“cigalikes”) and/or sensitivity differences in topography measurement systems that were designed for combustible tobacco cigarettes as compared to those that were designed an calibrated for ECIGs.[10] Interestingly, there was a significant (p<0.05) difference in puff duration in the 36 mg/ml condition relative to the 0 mg/ml condition in bout 1 (and a non-significant trend for lower puff volume in this bout) and these results suggest that participant may have altered their puff topography in order to titrate nicotine delivery in the 36 mg/ml condition by taking shorter (and possibly smaller) puffs. If so, the plasma nicotine results in the 36 mg/ml condition are all the more striking, as they indicated that even when attempting to titrate nicotine delivery, users may still receive more nicotine from some ECIGs than from a combustible cigarette over a 10-puff use bout.
This study has important limitations. As a preliminary report the study lacked sensitivity for many comparisons that may be of interest and a larger sample size would allow for these analyses using statistical techniques that take into account the overall experiment-wise error rate.[17] Also, there is no clear understanding in this study of the nicotine delivery profile of users’ usual liquid/device combination because no naturalistic observations were made of topography and exposure while using own ECIGs at home, work, and play. Another limitation, not unique to this study, is that assessing ECIG abstinence prior to a study session is problematic, as there are no rapid measures that can be used for this purpose (as expired air CO is used to assess abstinence from combustible tobacco). The baseline plasma nicotine concentrations are higher than is reported in combustible tobacco users following 12-hour abstinence, which may indicate that at least some participants did not comply fully with the pre-session abstinence requirement. Finally, the results reported here were obtained from a homogenous sample that was primarily male and white. In future work we hope to be able to explore the extent to which gender and ethnicity play a role in determining ECIG effects.
What this paper adds.
Previous studies examining the ability of electronic cigarettes to delivery nicotine used a variety of devices and liquid nicotine concentrations and demonstrated wide variability with some device/liquid combinations delivering no nicotine and others approximating the nicotine delivery profile of a combustible cigarette. This is the first study to hold all device and liquid characteristics constant while varying liquid nicotine concentration and it demonstrates that user plasma nicotine concentration increases with the nicotine concentration of the liquid and, at the highest liquid nicotine concentration tested, the nicotine delivery profile of the electronic cigarette exceeded that of a tobacco cigarette tested under similar use conditions. There is no clear rationale for an electric cigarette that exceeds the nicotine delivery of a combustible tobacco cigarette, and policymakers may want to limit access to such liquids/devices.
Acknowledgments
Funding
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Footnotes
Contributors
The authors thank CSTP staff Barbara Kilgalen, Kathleen Osei, and Kendall Pettaway for their contribution to this study.
Competing interests
The authors have no conflict of interest to report.
Ethics approval
The Virginia Commonwealth Institutional Review Board.
References
- 1.Bunnell R, Agaku I, Arrazola R, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res. 2015;17(2):228–35. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Newcombe R, Walton D. The prevalence, correlates and reasons for using electronic cigarettes among New Zealand adults. Addict Behav. 2015;45:245–51. doi: 10.1016/j.addbeh.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 3.McMillen R, Gottlieb M, Shaefer R, et al. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu213. pii:ntu213 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.King B, Alam S, Promoff G, et al. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res. 2013;15(9):1623–7. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan A, Promoff G, Dube S, et al. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 6.Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014;174(5):812–3. doi: 10.1001/jamainternmed.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 8.Farsalinos K, Spyrou A, Tsimopoulou K, et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vansickel A, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–70. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spindle T, Breland A, Karaoghlanian N, et al. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22(2):103–6. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 12.Farsalinos K, Romagna G, Tsiapras D, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–14. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17(2):150–7. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez A, Hiler M, Soule E, et al. Effects of Electronic Cigarette Liquid Nicotine Concentration on Plasma Nicotine and Puff Topography in Tobacco Cigarette Smokers: A Preliminary Report. doi: 10.1093/ntr/ntv182. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vansickel A, Cobb C, Weaver M, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breland A, Kleykamp B, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob Res. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- 17.Keppel G. Design and analysis, a researcher’s handbook. Englewood Cliffs, New Jersey: Prentice Hall; 1991. [Google Scholar]
- 18.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Behar R, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]