Summary
Many bacterial pathogens can cause acute infections that are cleared with onset of adaptive immunity, however a subset of these pathogens can establish persistent, and sometimes lifelong infections. While bacteria causing chronic infections are phylogenetically diverse, they share common features in their interactions with the host that enable a protracted period of colonization. This chapter will compare the persistence strategies of two chronic pathogens from the Proteobacteria, Brucella abortus, and Salmonella enterica serovar Typhi (S. Typhi) to consider how these two pathogens, which are very different at the genomic level, can utilize common strategies to evade immune clearance to cause chronic intracellular infections of the mononuclear phagocyte system.
INTRODUCTION
Persistent bacterial infections such as Brucellosis and Typhoid Fever are characterized by a long incubation period to leads to chronic, sometimes lifelong, debilitating disease with serious clinical manifestations (1). Therefore, chronic bacterial diseases have a significant impact on public health, due to the utilization of resources for long-term treatment of patients (2). Additionally, chronic infections affect the ability of the ill to provide for their families, resulting in a significant socioeconomic burden in affected countries (3).
Brucellosis, caused by intracellular Gram-negative coccobacilli of the Brucella spp., is considered one of the most relevant bacterial zoonoses worldwide, with more than 500,000 new human cases reported each year (4). The disease targets organs of the mononuclear phagocyte system, resulting in a chronic debilitating infection with serious clinical manifestations such as fever, arthritis, hepatomegaly, and splenomegaly (3, 5).
Typhoid fever, caused by the human-adapted Salmonella enterica serovar Typhi (S. Typhi) affects between 10 and 20 million people each year (6, 7), causing an estimated 190,000 deaths (8). Similarly to Brucella, S. Typhi causes a systemic infection, which targets the mononuclear phagocyte system, and has the ability to persist inside host tissues for long periods, causing a chronic debilitating disease (9, 10). Interestingly, one study of brucellosis patients noted that over half had initially been misdiagnosed as having typhoid fever, which highlights the similar clinical presentation of these very different infections (11).
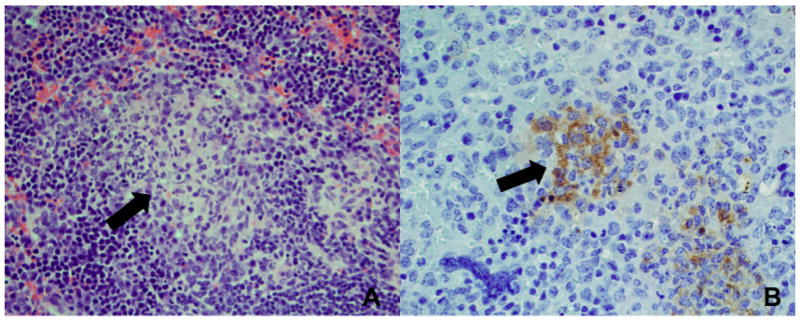
In the host, one of the preferential target cells for both Salmonella and Brucella spp. are macrophages (3, 12), in which the bacterium can persist and replicate (3, 12, 13). A hallmark of these chronic bacterial infections is the formation of granulomas, which contain epithelioid macrophages and are known to be a site of bacterial persistence during infection (Figure 1) (3). The granulomatous response is viewed as an attempt by the host to isolate bacteria that have been taken up but not killed by macrophages (14) and is the result of an inefficient and/or insufficient immune response to these pathogens.
Figure 1. Microgranuloma formation in spleen of Brucella infected mice.
(A) Fully developed microgranuloma (black arrow) at 30 days postinfection. Granuloma is composed of epithelioid macrophages surrounded by lymphocytes. Hematoxylin and eosin stain, 400x magnification. (B) Immunolabeling of B. abortus within microgranulomas in spleen at 30 days postinfection. Note the presence of bacteria inside macrophages (black arrow). Immunohistochemistry, 400x magnification.
Intracellular Brucella survival involves a temporary fusion of the Brucella-containing vacuole (BCV) with the lysosome, and subsequent exclusion of the lysosomal proteins (15). Interestingly enough, after this process, the BCV becomes associated with the rough endoplasmic reticulum, creating the compartment in which intracellular replication of Brucella occurs (16–18). Once inside the ER-associated compartment, Brucella spp. becomes practically invisible to the immune system (13), as demonstrated by a low production of cytokines and antibodies during the chronic phase of infection (19, 20). Therefore, the initial immune response becomes key factor for the control of Brucella spp. infection.
Salmonella enters the host through the gastrointestinal tract, mainly through epithelial barrier translocation via microfold (M) cell invasion or via phagocytoisis by CD-18+ antigen presenting cells (21). Like Brucella, after bypassing the intestinal barrier, Salmonella is able to survive within macrophages residing in systemic tissues (12). Intracellularly, Salmonella is able to avoid complete fusion with the lysosome through translocation of effector proteins that direct maturation of a Salmonella-containing vacuole (SCV) (reviewed by (22)). Once inside the SCV, Salmonella is able to manipulate host cell functions, leading to replication and persistence.
Although B. abortus, and S. Typhi have the common goal to avoid elimination by the host, these pathogens use different strategies to persist. In this chapter we will discuss the different mechanisms used by these chronic bacterial pathogens to evade the initial host immune defense and colonize the host. Moreover, we will discuss the recent concept that bacterial pathogens have evolved to take advantage of the host cell metabolism and nutrient availability to survive and replicate inside target cells.
TRICKING THE HOST IMMUNE SYSTEM
Entry into the host: the role of secretion systems
In spite of its well established immunoevasive character, Brucella spp. do rely on an important virulence factor for intracellular survival, the type IV secretion system (T4SS) encoded by the genes virB1-virB12 (13, 23–25). The critical role of Brucella T4SS is demonstrated by the inability of T4SS deficient mutants to persist in vivo, as demonstrated in the murine (25–27) and the caprine infection models (28). This phenotype could be attributed to the essential role of the T4SS in establishing the ER-associated niche for Brucella replication (18), since virB mutants remain inside macrophage lysosomes and are degraded (3).
Interestingly, it has been demonstrated that the T4SS is required not only for establishment of long-term infection, but also for the induction of Th1 immune response in infected mice (29). This function was confirmed by the fact that a functional T4SS is necessary for B cell maturation, activation of CD4+ T cells and for initial secretion of IL-12 and IFN-γ (30, 31). Moreover, B. abortus detection by Nod-like receptors (NLRs), leading to apoptotic specklike protein with a caspase recruitment domain (ASC)-inflammasome mediated production of IL-1β and IL-18, was also shown to be dependent on the type IV secretion system (32).
S. Typhi enters the host through the gastrointestinal tract and uses different strategies to reach systemic sites where it can persist for long periods (33). Salmonella serovars encode two different type III secretion systems, T3SS-1 and T3SS-2. Studies in the murine typhoid model using S. Typhimurium have demonstrated that the T3SS-1 is essential for the initial contact of the pathogen with intestinal epithelial cells and invasion of the ileal and colonic mucosa (34). Subsequently, the T3SS-2 is activated to mediate Salmonella survival inside macrophages and persistence in systemic sites (35). While few studies have addressed directly whether findings from the mouse typhoid model hold true during human typhoid, a screen for S. Typhi genes involved in infection of humanized mice identified mutants in either structural genes or regulators of both T3SS-1 and T3SS-2, suggesting that both of these virulence factors are involved in the bacteremic phase of typhoid (36).
Innate immune system evasion
The innate immune system is considered the first line of host-defense against invading pathogens. Therefore, the host has evolved mechanisms to detect the presence of bacteria in tissue through an innate immune surveillance system, which is able to recognize conserved pathogen-associated molecular patterns (PAMPs). These pathogen recognition receptors (PRRs), present in cell membranes (Toll-like receptors; TLRs) or in the cytosol (NOD-like receptors; NLRs) are able to detect products considered unique to bacteria, such as lipopolysaccharides (LPS), lipoteichoic acids, lipoproteins, and flagellin (37), leading to induction of the initial pro-inflammatory response. However, chronic pathogens have evolved passive and active mechanisms to evade detection by both TLRs and NLRs of the innate immune system (Table 1).
Table 1.
Strategies for Persistence
| Bacterial Pathogen | TLR evasion | Complement evasion | Secretion Systems |
|---|---|---|---|
| Brucella spp. |
|
|
|
| S. typhi |
|
|
|
The stealthy nature of Brucella species
The LPS of Brucella spp. has several features that contribute to its near invisibility to the innate immune system. Brucella spp. can avoid the detection by TLR4 via modification of the lipid A moiety of its LPS. While most bacterial pathogens such as Enterobacteriaceae have a lipid A moiety containing short fatty acid residues (C12–C16), Brucella lipid A contains a much longer one (C28), resulting in its greatly reduced TLR4 agonist and endotoxic properties (38). TLR4 agonist activity is further reduced by glycosylation of the LPS core, which reduces its affinity for the TLR4 co-receptor MD-2 (39). Another LPS component, the O-antigen moiety, is recognized by complement (40). Therefore, an additional anti-inflammatory feature of Brucella LPS is its resistance to deposition of complement component C3 (41, 42), avoiding the generation of the anaphylatoxins C3a and C5a, which synergize with TLRs in the induction of proinflammatory cytokines (43, 44).
Recently, an additional role for B. abortus LPS in evasion of innate immunity has been described, namely inhibition of neutrophil function. Once B. abortus has been phagocytosed by neutrophils, release of LPS within the pathogen vacuole appears to trigger a novel form of non-inflammatory cell death, thereby preventing killing of the engulfed bacteria (45). It is not yet known whether B. abortus LPS can be recognized by caspase-11 (or its human orthologs caspase-4 and caspase-5), the lack of pyroptotic cell death observed during infection of macrophages suggests that either LPS is not released to the cytosol where it can be accessed by these sensor caspases, or that it does not activate them in the same manner as described for other bacterial pathogens (46).
While Brucella spp. are non-motile, their genomes encode the structural components of an unconventional flagellum of unknown function, which is sheathed by LPS (47, 48). Interestingly, Brucella flagellin is able to avoid TLR5 detection, as it lacks a domain that is essential for its recognition by this receptor (49). However, recent work has demonstrated that the cytosolic receptor NLCR4 is able to detect Brucella flagellin, and is important for the pathogen control in the mouse model of infection (50).
In addition to TLR4, TLR2 and TLR9 have also been implicated in sensing Brucella infection (2, 51, 52). Therefore, as another strategy to avoid immune recognition, the Brucella genome encodes a protein that contains Toll-interleukin-1 receptor (TIR) domain, named Btp1/BtpA in B. abortus and TcpB in B. melitensis (53, 54). Btp1/TcpB acts by degrading the MyD88 adaptor-like (MAL), which is required for both TLR2 and TLR4 signaling, but not for TLR9 (53, 55). In consequence, Btp1/TcpB is able to inhibit dendritic cell maturation and production of pro-inflammatory cytokines, contributing to long-term Brucella persistence. Recently, a second Brucella TIR-containing effector protein has been described, named BtpB (56). BtpB is also believed to interfere with TLR signaling in a MyD88-dependent manner, although its role in modulating Brucella-induced inflammatory responses and bacterial persistence remains to be determined.
The viaB locus: the “cloaking device” of S. Typhi
Differently from Brucella, it has been demonstrated that the lipid A moiety of purified S. typhi LPS is a potent TLR4 agonist (57), that S. typhi flagellin is recognized by TLR5 (58), and the O-antigen of purified S. typhi LPS activates complement (59). Therefore, in order to persist, S. typhi has evolved different strategies to avoid recognition by PRRs.
Whole-genome sequencing revealed the presence of a S. Typhi specific pathogenicity island, named Salmonella pathogenicity island 7 (SPI-7) (60) that contains the viaB locus that encodes for the production and export of the Vi capsular polysaccharide antigen, also known as Vi-antigen (61). Initial studies demonstrated that the expression of Vi-antigen was linked to reduced flagellin secretion and lower Salmonella invasiveness. Moreover, the expression of the Vi capsule was tightly regulated by osmolarity conditions, since high-osmolarity conditions suppressed Vi-antigen expression and led to increased Salmonella invasiveness and flagellin secretion (62, 63). Further studies demonstrated that the first gene in the viaB locus, named tviA, encoded the regulatory protein TviA, which is responsible for these osmolarity-dependent phenotypic changes (64).
Interestingly, regulation of tviA expression is directly linked to conditions encountered by S. Typhi in the intestinal lumen (65), and is key for this pathogen’s ability to bypass the intestinal barrier. It turns out that TviA is not only responsible for regulation of the Vi-antigen, but also for the suppression of flagella production and regulation of the T3SS-1 gene expression (64, 66). Therefore, the high-osmolarity environment encountered in the lumen leads to inhibition of tviA expression, which allows S. Typhi to be motile and invasive as it approaches the mucosal epithelium (66). In contrast, once S. Typhi reaches the intestinal lamina propria, it encounters an environment characterized by low osmolarity, which leads to rapid tviA expression (65). As a result, several S. Typhi PAMPs and pathogen-induced processes can no longer be detected by the host’s immune system.
As described above, TviA-mediated repression of flagellin expression prevents detection of S. Typhi by host TLR5 and the consequent induction of the TLR5-dependent production of the pro-inflammatory cytokine IL-8 by colonocytes (67). Additionally, it has been demonstrated that S. Typhi is able to evade TLR4 recognition, in a Vi-antigen dependent manner (reviewed in (66). Recent studies suggest that this TLR4 evasion could result indirectly from the ability of the Vi antigen to prevent complement activation (43), and consequent generation of the anaphylatoxins C3a and C5a, which are two known enhancers of the TLR4-mediated induction of pro-inflammatory cytokines in response to lipid A recognition (44). The Vi-dependent inhibition of complement activation also prevents deposition of C3b in the bacterial surface, which in turn inhibits phagocytosis of S. Typhi by neutrophils, a cell type crucial for avoiding Salmonella dissemination (43, 66). Moreover, the S. Typhi Vi-capsule is also able to inhibit bacteria-guided neutrophil chemotaxis in a C5a-dependent manner (68). Taken together, these mechanisms help explain the lack of neutrophils in intestinal infiltrates from S. Typhi infected individuals (69, 70), which greatly contribute to this pathogens ability to evade host immune defenses and leads to an invasive persistent infection.
Both Brucella and S. Typhi are able to conceal two crucial molecular signatures that would otherwise allow the hosts immune system to identify them as Gram-negative bacteria. The host’s inability to detect these molecular patterns through TLR receptors as well as the complement system prevents the induction of an appropriate initial antibacterial host response. As a consequence, the pathogen clearance and infection control is significantly impaired (3, 71).
Induction of anti-inflammatory cytokines by Brucella
Interleukin 10 (IL-10) is considered an immunoregulatory cytokine that can be produced by different cell types, including B cells, T cells, macrophages and keratinocytes (72). The main cell type responsible for IL-10 production in defined situations is dependent on the kind of stimulus, type of affected tissue, and time point in an immune process (73). Therefore, IL-10 is able to function at different stages of an immune response, affirming its crucial role as a regulator of both Th1 and Th2 cell responses (72, 74).
Therefore, a plausible strategy for persistent pathogen would be the induction of a cytokine that is able to modulate the host pro-inflammatory response. Indeed, in addition to an early pro-inflammatory Th1 response, B. abortus also induces the anti-inflammatory cytokine IL-10 (1, 2, 75). Interestingly, anti-Brucella effector functions of IFNγ activated macrophages such as bactericidal capacity and production of pro-inflammatory cytokines were dampened by IL-10 during in vitro infection (75, 76). In vivo experiments demonstrated that production of IL-10 by CD4+CD25+ T cells was key for modulation of macrophage function during early Brucella infection, since mice lacking IL-10 production by T cells or lacking the presence of the IL-10R in macrophages presented decreased bacterial survival in spleen and liver, as well as increased production of pro-inflammatory cytokines and pathology in affected organs (1). Moreover, a B. abortus proline racemase PrpA was shown to both stimulate a mitogenic activity on B cells and induce IL-10 secretion by splenocytes, suggesting that it may be one of the factors involved in induction of IL-10 during infection (77). Taken together, these data suggest an important role of IL-10 in modulating the initial immune response to Brucella infection through regulation of macrophage function and resulting in increased pathogen survival and long-term persistence.
TAKING ADVANTAGE OF HOST CELL METABOLISM
The interactions of persistent bacterial pathogens with the host immune system have been extensively studied and contribute greatly to the ability of such pathogens to cause chronic infection. However, evasion of the immune response is not the sole mechanisms for pathogen persistence, since studies have shown that factors required for establishment of chronic disease in vivo may not be necessarily dependent on the induction of an immune response. Interestingly, a variety of genes required for Brucella persistence for example, are related to changes in bacterial metabolism and to the ability of the pathogen to use a specific nutrient (26). This fact gives rise to the possibility that chronic bacterial pathogens may have adapted not only to the different immune environment present during persistent infection, but also to differences in nutrient availability in target cells during this period.
Macrophages subsets and their different metabolism
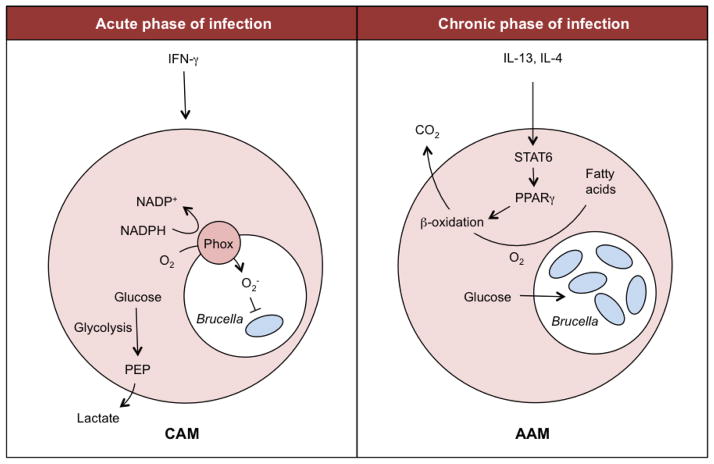
Macrophage activation by IFNγ and TLRs leads to upregulation of the inducible form of nitric oxide synthase (iNOS) (78) and production of reactive oxygen species (ROS) (79). Therefore, ROS and nitric oxide (NO) production are key functional features of the inflammatory and bactericidal classically activated macrophage (CAM; Figure 2), and the metabolic alterations that occur are integral to this process (80). Interestingly, NO competes with oxygen to inhibit cytochrome c oxidase, the terminal electron acceptor of the respiratory chain. This fact prevents the reoxidation of NADH, which in turn limits flux through the tricarboxylic acid (TCA) cycle. Moreover, increased generation of ROS by mitochondria also contributes to reduced macrophage reliance on the TCA cycle and the respiratory chain for energy and ATP production. However, CAM macrophages still need to maintain ATP levels for biosynthesis, as well as to maintain mitochondrial membrane potential and to prevent apoptosis (80). Therefore, decreased TCA flux in CAM leads to ATP production through anaerobic glycolysis and lactate production. Consequently, these cells show elevated expression of the glucose transporter GLUT1 as well as marked switch from expression of the liver isoform of the enzyme 6-phosphofructo-2-kinase (encoded by PFKFB1) to the PFKFB3 isoform (81). This leads to increased glucose uptake and consumption, as well as to accumulation of fructose-2,6-bisphosphate which, in turn, increases glycolytic flux (80).
Figure 2. Macrophage metabolism during Brucella infection.
During the acute phase of B. abortus infection (left), IFN-γ is transiently produced, resulting in a predominance of classically activated macrophages (CAM). In these cells, oxygen is consumed by NADPH oxidase (Phox) to generate superoxide radicals, and energy is produced by anaerobic glycolysis. Since anaerobic glycolysis yields only 2ATP, the cell has to consume more glucose to meet its energy needs. In contrast, during the chronic infection phase (right), IFN-γ is absent, but IL-4 and IL-13 signal via STAT6 to induce the alternatively activated macrophage (AAM) phenotype. Activation of STAT6 increases the expression and activation of PPARγ, which in turn upregulates genes controlling β-oxidation, thereby shifting cellular physiology toward oxidative pathways. As a result, less glucose is consumed for cellular metabolism, and the intracellular glucose concentration increases. This glucose can be utilized by B. abortus for growth within infected macrophages.
The opposite is true when macrophages are activated by IL-4 and IL-13, which promote development of alternatively activated macrophages (AAM; Figure 2). This macrophage subpopulation exhibits a profound increase in the entire program of fatty-acid metabolism, including uptake and oxidation of fatty acids and mitochondrial biogenesis, as well as much lower rates of glycolysis (81, 82). Consequently, while CAM preferentially utilize glucose, the alternative program of macrophage activation switches over to fatty acid oxidation for energy homeostasis (82). Since AAM are involved in chronic processes and tissue repair, it is possible that the more energy efficient oxidative metabolism is better suited to long-term roles of this subpopulation (80).
Interestingly, the control of the genetic program for long-term activation is dependent on STAT6 phosphorylation (80, 83). As consequence, phosphorylated STAT6 dimerizes and translocates to the nucleus where it induces expression of its target genes, including markers (Arg1, Ym1, Fizz1, Cd301) and regulators of macrophage metabolism and alternative activation (i.e.: Pparγ, Pparδ and PGC-1β) (84).
Macrophage metabolism and Brucella persistence
It is well established that macrophages represent the main target cell for Brucella persistence in many tissue types (13, 85–89). Therefore, interactions between Brucella and the different macrophages subpopulations are key for understanding the bacterial survival and disease progression. Interestingly, during infection of C57BL/6 mice the macrophage subpopulations differ significantly between acute and chronic stages of Brucella infection. During the acute and more pro-inflammatory stage of infection, there is a significant increase in the numbers of bactericidal CAM, and this fact correlates well with higher IFNγ levels as well as the decrease in B. abortus survival in spleen of infected mice (88). Conversely, during chronic infection, there is a shift in the macrophage subpopulation with predominance of the wound-healing AAM subtypes leading to a persistent Brucella survival over time. Indeed, AAM were shown to be more permissive for B. abortus survival and replication in vitro. Furthermore, during chronic infection of mice, two lines of evidence show persistence of B. abortus in AAM, firstly, viable B. abortus was cultured primarily from the CD11b+ fraction, which consists predominantly of AAM during chronic infection, and secondly, bacteria were localized by flow cytometry to splenic cells expressing markers of the AAM phenotype, CD301+CD11b+. The presence of B. abortus-infected AAM was shown to be dependent on the activation of the intracellular receptor Peroxisome proliferator-activated receptor gamma; PPARγ.
PPARγ, is a nuclear receptor activated by fatty acids, that has recently been linked to the polarization of macrophage phenotype (90). Therefore, even though PPARγ is best known for its influence in adipocyte development and insulin-resistance (84), it can also have an widespread influence on macrophage biology (91, 92). Interestingly, studies using PPARγ –deficient cells have demonstrated that, in the absence of PPARγ signaling, macrophages neither appropriately suppress inflammatory cytokine production nor acquire an oxidative metabolic program that is associated with the AAM phenotype (84, 90).
As previously discussed, one consequence of macrophage polarization is the shift in their cellular metabolism, which means that CAM and AAM utilize different sources of carbon and energy (93). This fact raises the possibility that different nutrients are available intracellularly in different macrophage subpopulations. Indeed, CAM rely on glycolysis for energy production and, therefore, consume most intracellular glucose. Conversely, AAM obtain their ATP via degradation of fatty-acids via the β-oxidation pathway in a PPAR-dependent manner. In consequence, there is an accumulation of glucose inside the cell, shown by higher intracellular glucose levels in AAM when compared to CAM (88, 94). Interestingly, Brucella makes use of this nutrient availability for long-term persistence, since a gluP mutant, which lacks the ability to take up intracellular glucose, is no longer able to persist inside AAM in the mouse model. Moreover, this phenotype was dependent on the PPARγ expression by macrophages (26, 88).
Macrophage metabolism and Salmonella persistence
While S. Typhi is a strictly human-adapted pathogen, work done modelling S. Typhi infection by studying chronic infection of Salmonella enterica serotype Typhmiurium in mice has provided significant insights into mechanisms underlying persistence at systemic sites.
Recent work in the mouse has shown that there is a shift in the immune environment during Salmonella infection, characterized by predominance of a pro-inflammatory Th1 response during acute infection and the presence of Th2 cytokines like interleukin-4 (IL-4) during chronic infection. As a result, there is an increase in the percentage of AAM during the persistence phase of the disease, and this cell-type was shown to harbor the majority of Salmonella population in infected organs (94). Interestingly, the increased susceptibility of this particular cell-type was dependent on its metabolic program, rather than on its immunological status.
Interestingly, survival of Salmonella was dependent on the activation of one of the PPAR receptors, named PPARδ. As previously described for PPARγ, PPARδ functions to regulate the host-cell energy metabolism, mainly fatty acid β-oxidation (95). Indeed, Salmonella infection actively upregulated the expression of Ppard, which in turn led to shift in the metabolism of infected cells to the oxidation of fatty acids. Consequently, infected AAM presented increased intracellular levels of glucose, the carbon source used by macrophages when β-oxidation is downregulated (81, 94). This fact raised the possibility that Salmonella was taking advantage of this new available energy source to persist and proliferate inside AAM. The inability of glucose uptake-deficient Salmonella mutants to survive inside AAM, confirmed that intracellular glucose utilization was key for Salmonella long-term persistence in AAM, and consequent establishment of chronic infection.
Although AAM-polarized cells of the human-derived monocytic cell line THP1 were shown to support higher levels of intracellular S. Typhi replication, it will be interesting to see if S. Typhi uses increased glucose availability to persist in the host, as was shown for S. Typhimurium, and whether PPARδ expression is linked with intracellular persistence of S. Typhi in human macrophages (94).
CONCLUSION
Recent work in both Brucella and Salmonella fields of research has revealed shared strategies utilized by chronic bacterial pathogens to persist in the host. It is becoming more evident that both immune evasion and interactions with the host-cell metabolism play key roles during establishment of chronic infection. Therefore, the picture emerging from these studies is that persistence is determined not only by the pathogen’s ability to evade the host immune response, but also by its ability to develop mechanisms to exploit the nutrients available during the chronic stages of infection. Since both preventive and therapeutic interventions remain difficult and costly, a better understanding of the new mechanisms responsible for bacterial pathogen persistence will be crucial for a proper control and treatment of such infections.
References
- 1.Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TM, Baumler AJ, Muller W, Santos RL, Tsolis RM. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 2013;9:e1003454. doi: 10.1371/journal.ppat.1003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svetic A, Jian YC, Lu P, Finkelman FD, Gause WC. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-γ in CD4+ T cells. Int Immunol. 1993;5:877–883. doi: 10.1093/intimm/5.8.877. [DOI] [PubMed] [Google Scholar]
- 3.Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis ReM. Interactions of the Human Pathogenic Brucella Species with Their Hosts. Annual Review of Microbiology. 2011;65:523–541. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- 4.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 5.Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 7.Keestra-Gounder AM, Tsolis RM, Baumler AJ. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol. 2015;13:206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Global Burden of Disease. 2013. [Google Scholar]
- 9.House D, Bishop A, Parry C, Dougan G, Wain J. Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis. 2001;14:573–578. doi: 10.1097/00001432-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 10.DelVecchio VG, Kapatral V, Elzer P, Patra G, Mujer CV. The genome of Brucella melitensis. Vet Microbiol. 2002;90:587–592. doi: 10.1016/s0378-1135(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 11.Jennings GJ, Hajjeh RA, Girgis FY, Fadeel MA, Maksoud MA, Wasfy MO, El-Sayed N, Srikantiah P, Luby SP, Earhart K, Mahoney FJ. Brucellosis as a cause of acute febrile illness in Egypt. Trans R Soc Trop Med Hyg. 2007;101:707–713. doi: 10.1016/j.trstmh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNγ neutralization. J Exp Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xavier MN, Paxão TA, den Hartigh AB, Tsolis RM, Santos RL. Pathogenesis of Brucella spp. The Open Veterinary Science Journal. 2010;4:109–118. [Google Scholar]
- 14.Adams DO. The granulomatous inflammatory response. A review. Am J Pathol. 1976;84:164–192. [PMC free article] [PubMed] [Google Scholar]
- 15.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson TD, Cheville NF. Ultrastructural morphometric analysis of Brucella abortus-infected trophoblasts in experimental placentitis. Bacterial replication occurs in rough endoplasmic reticulum. Am J Pathol. 1986;124:226–237. [PMC free article] [PubMed] [Google Scholar]
- 17.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Zapata M, Matias MJ, Prieto A, Jonde MA, Monserrat J, Sanchez L, Reyes E, De la Hera A, Alvarez-Mon M. Human brucellosis is characterized by an intense Th1 profile associated with a defective monocyte function. Infect Immun. 2010;78:3272–3279. doi: 10.1128/IAI.01385-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martirosyan A, Moreno E, Gorvel J-P. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunological Reviews. 2011;240:211–234. doi: 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 21.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 22.Bakowski MA, Braun V, Brumell JH. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9:2022–2031. doi: 10.1111/j.1600-0854.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 24.Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, De Bolle X, Tibor A, Gorvel JP, Letesson JJ. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 25.den Hartigh AB, Rolan HG, de Jong MF, Tsolis RM. VirB3-VirB6 and VirB8-VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system. J Bacteriol. 2008 doi: 10.1128/JB.00406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong PC, Tsolis RM, Ficht TA. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect Immun. 2000;68:4102–4107. doi: 10.1128/iai.68.7.4102-4107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Hartigh AB, Sun YH, Sondervan D, Heuvelmans N, Reinders MO, Ficht TA, Tsolis RM. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect Immun. 2004;72:5143–5149. doi: 10.1128/IAI.72.9.5143-5149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zygmunt MS, Hagius SD, Walker JV, Elzer PH. Identification of Brucella melitensis 16M genes required for bacterial survival in the caprine host. Microbes Infect. 2006;8:2849–2854. doi: 10.1016/j.micinf.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Roux CM, Rolan HG, Santos RL, Beremand PD, Thomas TL, Adams LG, Tsolis RM. Brucella requires a functional Type IV secretion system to elicit innate immune responses in mice. Cell Microbiol. 2007;9:1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 30.Rolan HG, Tsolis RM. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect Immun. 2007;75:2965–2973. doi: 10.1128/IAI.01896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolán HG, Tsolis RM. Inactivation of the Type IV Secretion System Reduces the Th1 Polarization of the Immune Response to Brucella abortus Infection. Infection and Immunity. 2008;76:3207–3213. doi: 10.1128/IAI.00203-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol. 2013;190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- 33.Monack DM. Salmonella persistence and transmission strategies. Curr Opin Microbiol. 2012;15:100–107. doi: 10.1016/j.mib.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Galan JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoramian-Falsafi T, Harayama S, Kutsukake K, Pechere JC. Effect of motility and chemotaxis on the invasion of Salmonella typhimurium into HeLa cells. Microb Pathog. 1990;9:47–53. doi: 10.1016/0882-4010(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 36.Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, Cookson BT, Karlinsey JE, Kinkel TL, Porwollik S, Canals R, Cummings LA, Fang FC. Humanized nonobese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A. 2010;107:15589–15594. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 38.Lapaque N, Takeuchi O, Corrales F, Akira S, Moriyon I, Howard JC, Gorvel JP. Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell Microbiol. 2006;8:401–413. doi: 10.1111/j.1462-5822.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 39.Conde-Alvarez R, Arce-Gorvel V, Iriarte M, Mancek-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacon-Diaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, Grillo MJ, Jerala R, Brandenburg K, Llobet E, Bengoechea JA, Moreno E, Moriyon I, Gorvel JP. The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog. 2012;8:e1002675. doi: 10.1371/journal.ppat.1002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joiner KA, Puentes SM, Warren KA, Scales RA, Judd RC. Complement binding on serum-sensitive and serum-resistant transformants of Neisseria gonorrhoeae: effect of presensitization with a non-bactericidal monoclonal antibody. Microb Pathog. 1989;6:343–350. doi: 10.1016/0882-4010(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 41.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzman-Verri C, Chacon-Diaz C, Rucavado A, Moriyon I, Moreno E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE. 2007;2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann EM, Houle JJ. Failure of Brucella abortus lipopolysaccharide (LPS) to activate the alternative pathway of complement. Vet Immunol Immunopathol. 1983;5:65–76. doi: 10.1016/0165-2427(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 43.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tukel C, Baumler AJ. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun. 2011;79:830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barquero-Calvo E, Mora-Cartin R, Arce-Gorvel V, de Diego JL, Chacon-Diaz C, Chaves-Olarte E, Guzman-Verri C, Buret AG, Gorvel JP, Moreno E. Brucella abortus Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide. PLoS Pathog. 2015;11:e1004853. doi: 10.1371/journal.ppat.1004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Ferooz J, Letesson JJ. Morphological analysis of the sheathed flagellum of Brucella melitensis. BMC Res Notes. 2010;3:333. doi: 10.1186/1756-0500-3-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fretin D, Fauconnier A, Kohler S, Halling S, Leonard S, Nijskens C, Ferooz J, Lestrate P, Delrue RM, Danese I, Vandenhaute J, Tibor A, DeBolle X, Letesson JJ. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol. 2005;7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 49.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terwagne M, Ferooz J, Rolan HG, Sun YH, Atluri V, Xavier MN, Franchi L, Nunez G, Legrand T, Flavell RA, De Bolle X, Letesson JJ, Tsolis RM. Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell Microbiol. 2013;15:942–960. doi: 10.1111/cmi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–1087. doi: 10.4049/jimmunol.180.2.1080. [DOI] [PubMed] [Google Scholar]
- 52.Copin R, De Baetselier P, Carlier Y, Letesson JJ, Muraille E. MyD88-Dependent Activation of B220-CD11b+LY-6C+ Dendritic Cells during Brucella melitensis Infection. J Immunol. 2007;178:5182–5191. doi: 10.4049/jimmunol.178.8.5182. [DOI] [PubMed] [Google Scholar]
- 53.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel JP. Brucella Control of Dendritic Cell Maturation Is Dependent on the TIR-Containing Protein Btp1. PLoS Pathog. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008 doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 55.Sengupta D, Koblansky A, Gaines J, Brown T, West AP, Zhang D, Nishikawa T, Park SG, Roop RM, 2nd, Ghosh S. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J Immunol. 2010;184:956–964. doi: 10.4049/jimmunol.0902008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salcedo SP, Marchesini MI, Degos C, Terwagne M, Von Bargen K, Lepidi H, Herrmann CK, Santos Lacerda TL, Imbert PR, Pierre P, Alexopoulou L, Letesson JJ, Comerci DJ, Gorvel JP. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front Cell Infect Microbiol. 2013;3:28. doi: 10.3389/fcimb.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bignold LP, Rogers SD, Siaw TM, Bahnisch J. Inhibition of chemotaxis of neutrophil leukocytes to interleukin-8 by endotoxins of various bacteria. Infect Immun. 1991;59:4255–4258. doi: 10.1128/iai.59.11.4255-4258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyant TL, Tanner MK, Sztein MB. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gewurz H, Mergenhagen SE, Nowotny A, Phillips JK. Interactions of the complement system with native and chemically modified endotoxins. J Bacteriol. 1968;95:397–405. doi: 10.1128/jb.95.2.397-405.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker S, Dougan G. The genome of Salmonella enterica serovar Typhi. Clin Infect Dis. 2007;45(Suppl 1):S29–33. doi: 10.1086/518143. [DOI] [PubMed] [Google Scholar]
- 61.Tischler AD, McKinney JD. Contrasting persistence strategies in Salmonella and Mycobacterium. Curr Opin Microbiol. 2010;13:93–99. doi: 10.1016/j.mib.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey PS, Popoff MY. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhao L, Ezak T, Li ZY, Kawamura Y, Hirose K, Watanabe H. Vi-Suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer’s patches. Microbiol Immunol. 2001;45:149–158. doi: 10.1111/j.1348-0421.2001.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 64.Winter SE, Winter MG, Thiennimitr P, Gerriets VA, Nuccio SP, Russmann H, Baumler AJ. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol. 2009;74:175–193. doi: 10.1111/j.1365-2958.2009.06859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winter SE, Winter MG, Godinez I, Yang HJ, Russmann H, Andrews-Polymenis HL, Baumler AJ. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 2010;6:e1001060. doi: 10.1371/journal.ppat.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wangdi T, Winter SE, Baumler AJ. Typhoid fever: “you can’t hit what you can’t see”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winter SE, Raffatellu M, Wilson RP, Russmann H, Baumler AJ. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 68.Wangdi T, Lee CY, Spees AM, Yu C, Kingsbury DD, Winter SE, Hastey CJ, Wilson RP, Heinrich V, Baumler AJ. The Vi capsular polysaccharide enables Salmonella enterica serovar Typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014;10:e1004306. doi: 10.1371/journal.ppat.1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kraus MD, Amatya B, Kimula Y. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod Pathol. 1999;12:949–955. [PubMed] [Google Scholar]
- 70.Mukawi TJ. Histopathological study of typhoid perforation of the small intestines. Southeast Asian J Trop Med Public Health. 1978;9:252–255. [PubMed] [Google Scholar]
- 71.Tsolis RM, Young GM, Solnick JV, Baumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- 72.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 73.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 74.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 75.Fernandes DM, Baldwin CL. Interleukin-10 downregulates protective immunity to Brucella abortus. Infection and Immunity. 1995;63:1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandes DM, Jiang X, Jung JH, Baldwin CL. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunology & Medical Microbiology. 1996;16:193–203. doi: 10.1111/j.1574-695X.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 77.Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc Natl Acad Sci U S A. 2006;103:16514–16519. doi: 10.1073/pnas.0603362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Neill LAJ, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 82.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 84.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 85.Ilhan F, Yener Z. Immunohistochemical detection of Brucella melitensis antigens in cases of naturally occurring abortions in sheep. J Vet Diagn Invest. 2008;20:803–806. doi: 10.1177/104063870802000616. [DOI] [PubMed] [Google Scholar]
- 86.Magnani DM, Lyons ET, Forde TS, Shekhani MT, Adarichev VA, Splitter GA. Osteoarticular tissue infection and development of skeletal pathology in murine brucellosis. Dis Model Mech. 2013;6:811–818. doi: 10.1242/dmm.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xavier MN, Paixao TA, Poester FP, Lage AP, Santos RL. Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J Comp Pathol. 2009;140:149–157. doi: 10.1016/j.jcpa.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Xavier MN, Winter MG, Spees AM, den Hartigh AB, Nguyen K, Roux CM, Silva TM, Atluri VL, Kerrinnes T, Keestra AM, Monack DM, Luciw PA, Eigenheer RA, Baumler AJ, Santos RL, Tsolis RM. PPARγ-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages. Cell Host Microbe. 2013;14:159–170. doi: 10.1016/j.chom.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meador VP, Deyoe BL, Cheville NF. Pathogenesis of Brucella abortus infection of the mammary gland and supramammary lymph node of the goat. Vet Pathol. 1989;26:357–368. doi: 10.1177/030098588902600501. [DOI] [PubMed] [Google Scholar]
- 90.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L, Chawla A. Role of PPARγ in macrophage biology and atherosclerosis. Trends Endocrinol Metab. 2004;15:500–505. doi: 10.1016/j.tem.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Roop RM, 2nd, Caswell CC. Bacterial persistence: finding the “sweet spot”. Cell Host Microbe. 2013;14:119–120. doi: 10.1016/j.chom.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. Salmonella require the fatty acid regulator PPARδ for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14:171–182. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]