Abstract
BACKGROUND
Fibrinogen concentrate is increasingly considered as a hemostatic agent for trauma patients experiencing bleeding. Placing a venous access is sometimes challenging during severe hemorrhage. Intraosseous access may be considered instead. Studies of intraosseous infusion of coagulation factor concentrates are limited. We investigated in vivo recovery following intraosseous administration of fibrinogen concentrate and compared the results with intravenous administration.
METHODS
This study was performed on 12 pigs (mean [SD] body weight, 34.1 [2.8] kg). Following controlled blood loss (35 mL/kg) and fluid replacement with balanced crystalloid solution, intraosseous (n = 6) administration of fibrinogen concentrate (80 mg per kilogram of bodyweight) in the proximal tibia was compared with intravenous (n = 6) administration of the same dose (fibrinogen infusion time approximately 5 minutes in both groups). The following laboratory parameters were assessed: blood cell count, prothrombin time index, activated partial thromboplastin time, and plasma fibrinogen concentration (Clauss assay). Coagulation status was also assessed by thromboelastometry.
RESULTS
All tested laboratory parameters were comparable between the intraosseous and intravenous groups at baseline, hemodilution, and 30 minutes after fibrinogen concentrate administration. In vivo recovery of fibrinogen was also similar in the two groups (89% [23%] and 91% [22%], respectively). There were no significant between-group differences in any of the thromboelastometric parameters. Histologic examination indicated no adverse effects on the tissue surrounding the intraosseous administration site.
CONCLUSION
This study suggests that intraosseous administration of fibrinogen concentrate results in a recovery of fibrinogen similar to that of intravenous administration. The intraosseous route of fibrinogen concentrate could be a valuable alternative in situations where intravenous access is not feasible or would be time consuming.
LEVEL OF EVIDENCE
Prospective, randomized, therapeutic feasibility study in an animal model, level V.
KEY WORDS: Intraosseous access, fibrinogen concentrate, traumatic hemorrhage, hemostatic therapy, pigs
Fibrinogen concentrate is increasingly considered as a hemostatic agent for trauma patients experiencing bleeding.1–3 During ongoing bleeding, the plasma concentration of fibrinogen becomes critically low earlier than that of any other coagulation factor,4 and low plasma fibrinogen is usually the primary coagulation factor deficiency detected in trauma patients.5 An investigation of the prehospital use of fibrinogen concentrate is currently in progress, to evaluate the effects of such early intervention in trauma.6
In emergency medicine, placing a venous access is sometimes challenging, especially in hypovolemic patients during traumatic hemorrhage. In cases where intravenous access would be time consuming to establish, intraosseous access is recommended in some trauma guidelines as an alternative route for fluid and drug administration.7,8 It has been proposed that prehospital blood product administration becomes more commonplace.8 In recent years, the EZ-IO (Vidacare Corp., Shavano Park, TX) intraosseous device has been used increasingly frequently in prehospital medicine and combat care as well as in hospital emergency departments.8–13 The percentage of trauma patients in studies of EZ-IO use has varied between 5% and 100%.11
Intraosseous administration of hemostatic agents (fresh frozen plasma [FFP], activated recombinant factor VII [rFVIIa]) has been reported in single cases.9,11 However, the evidence supporting intraosseous transfusion of blood products in trauma management has recently been questioned.14 The same authors postulated that intraosseous maximum flow rates are inadequate for successful resuscitation. Intraosseous application of coagulation factor concentrates such as fibrinogen concentrate may be more feasible because of the low volumes of these treatments, but this has not yet been studied. It is unknown whether intraosseous administration of fibrinogen concentrate would restore fibrinogen levels and fibrin clot quality after severe bleeding. Furthermore, it is not known how intraosseous administration would compare with the intravenous route in terms of fibrinogen recovery or what effects intraosseous administration may have on the punctured bone.
We aimed to investigate the effects of intraosseous administration of fibrinogen concentrate on plasma fibrinogen levels and thromboelastometric parameters such as clot strength. A porcine model of severe blood loss and hemodilution was used, on the basis that pigs provide a well-established model of trauma and coagulation.15–18 We hypothesized that intraosseous administration of fibrinogen concentrate would result in the same in vivo recovery of fibrinogen as intravenous administration, without causing clinically significant alterations to the bone marrow at the puncture site.
MATERIALS AND METHODS
Animals
Twelve healthy male pigs (German domestic pigs from Münichsthal, Austria; age range, 12–16 weeks) were investigated for the study.
The experimental protocol was approved by the Animal Protocol Review Board of the City Government of Vienna, Austria, (protocol number: MA58-005750/2012/9), and our center is certified by the same review board for performing animal studies. All experiments were performed under conditions described in the Guide for the Care and Use of Laboratory Animals, as defined by the National Institutes of Health. Pairs of pigs were studied in parallel, with random allocation of one from each pair to the intraosseous (IO, n = 6) and intravenous (IV, n = 6) groups.
Anesthesia, Surgical Preparations, and Cardiorespiratory Monitoring
Anesthesia was induced intramuscularly with a combination of butorphanol (0.17 mg/kg; Alvetra and Werfft AG, Vienna, Austria), medetomidine (0.03 mg/kg; Eurovet Animal Health, Bladel, Netherlands), and midazolam (0.5 mg/kg/h; Nycomed, Linz, Austria) followed by intravenous (ear vein) ketamine (0.07 mg/kg; Pfizer, Vienna, Austria). The trachea was intubated with a 6.5-mm tracheal tube, and volume control ventilation was performed to maintain end-tidal CO2 between 4.5 and 5.5 kPa.
Anesthesia was maintained intravenously with midazolam (0.8 mg/kg/h), sufentanil (8 μg/kg/h; Janssen, Vienna, Austria), and rocuronium (5 mg/kg/h; Organon, Oss, the Netherlands). Catheters were placed by direct preparation and incision of the left external jugular vein and left carotid artery for standard fluid therapy, invasive blood pressure monitoring, and blood withdrawal (according to the standardized pig anesthesia protocol). A 14 Fr catheter was inserted suprapubically into the bladder. Mean arterial pressure, heart rate, oxygen saturation, and ventilation parameters were monitored continuously, while arterial blood gas analysis was performed and lactate was measured at blood sampling time points.
Experimental Protocol
A schematic illustration of the experimental protocol is depicted in Figure 1. After induction of anesthesia and instrumentation, hemorrhage was simulated by withdrawing 35-mL/kg blood through an arterial catheter within a period of 30 minutes (withdrawal rate approximately 30–50 mL/min). Then, for pigs randomized to the IO group, intraosseous access was established in the proximal medial tibia with a 15 gauge, 15-mm intraosseous needle set and EZ-IO drill machine (Vidacare Corp). After aspiration of blood/bone marrow for insertion control, the intraosseous access was flushed with 20 mL of saline. The immediate next step, to induce dilutional coagulopathy, was a bolus administration of 1,000 mL of a balanced crystalloid solution (ELO-MEL isoton, Fresenius Kabi, Graz, Austria) via the intraosseous access, with the flow rate adjusted for administration to be completed within 30 minutes. To achieve adequate flow rates, a standard pneumatic infusion pressure device was used as appropriate. In the IV group, dilutional coagulopathy was induced via the external jugular vein using the same solution and flow rate as in the IO group. In a third step, after hemorrhage and fluid replacement, 80-mg/kg fibrinogen concentrate was infused from 2 g/100 mL vials (Haemocomplettan P, CSL Behring, Marburg, Germany; reconstituted approximately 15 minutes before administration), via either the IO or the IV route. A 50-mL syringe was used with manual pressure to complete this step in approximately 5 minutes. After fibrinogen concentrate administration, the IO needle or IV access was flushed with an infusion of 100 mL saline over approximately 30 minutes and left in place until the end of the experiment.
Figure 1.
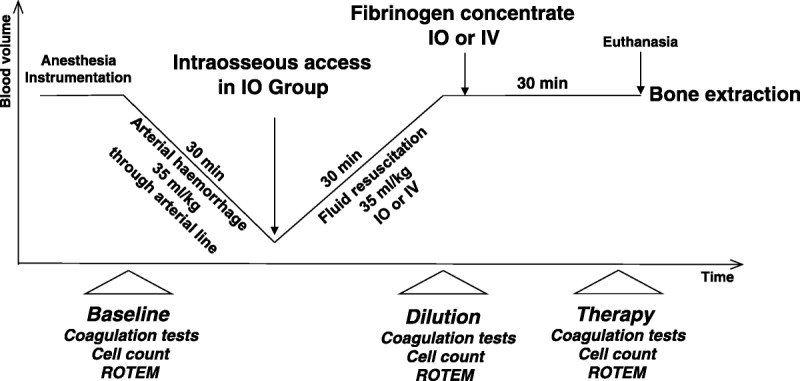
Experimental procedure and time points of laboratory investigation for comparison of IO versus IV administration of fibrinogen concentrate.
Study Measurements, Blood Sampling, and Analytic Methods
Baseline arterial blood samples were drawn at the beginning of the experimental protocol (Fig. 1) for the measurement of blood cell count, plasma fibrinogen level, prothrombin time index (PTI) and activated partial thromboplastin time (aPTT), as well as ROTEM (Tem International GmbH, Munich, Germany) analysis. The same measurements were repeated after in vivo hemodilution and 30 minutes after fibrinogen concentrate therapy.
For the coagulation analyses, blood was initially collected in 3.5-mL tubes containing 0.35-mL buffered 3.2% trisodium citrate (Vacuette, Greiner Bio-One, Linz, Austria), giving a volume ratio for citrate–whole blood of 1:9. Aliquots of citrated blood were centrifuged immediately at 2,800 G for 15 minutes to obtain plasma. Fibrinogen (Clauss method; Multifibren U, Siemens, Marburg, Germany) and aPTT (Actin FS, Siemens) were analyzed with a KC-10 steel ball coagulometer (Amelung GmbH, Lemco, Germany). PTI (Thromborel S, Siemens) was run on a Sysmex CA 1500 device (Siemens).
Measurement of hematocrit (Hct), hemoglobin concentration (Hb), white blood cell (WBC) count, and platelet (Plt) count was performed with a CELLDYN 3700 instrument (Abbott, Vienna, Austria), using appropriate animal settings.
Thromboelastometric Measurements
ROTEM analyses were then performed in two ways as follows: (1) standard EXTEM and FIBTEM assays, immediately after citrated whole blood was drawn; and (2) EXTEM assay in platelet-free plasma (PFP), obtained by centrifuging (2,800 G for 15 minutes) and filtering (0.22 µm) the citrated blood samples.
All assays were initiated by the addition of 20-μL CaCl2, 200-mmol/L and 20-μL ex-tem reagent (Tem International GmbH, Munich, Germany), providing extrinsic activation through tissue factor. In the FIBTEM assay, additional cytochalasin D inhibits platelets’ contribution to clot strength by preventing cytoskeletal reorganization. Because the commercially available FIBTEM assay does not sufficiently inhibit platelets in porcine blood samples, we also performed the EXTEM in PFP, to estimate fibrin polymerization in plasma without platelets. The following ROTEM variables were analyzed: clotting time (CT [second]; time from the start of measurement until formation of a clot of 2 mm in amplitude); clot formation time (CFT [second]; time from the end of CT until a clot firmness of 20 mm was achieved); and maximum clot firmness (MCF [mm]; maximum strength of the clot, determined by the interaction of fibrin, activated platelets and factor XIII). In FIBTEM and PFP EXTEM, only MCF was analyzed.
Fibrinogen Recovery
In vivo recovery of fibrinogen was calculated as the actual increase in fibrinogen level observed after an administration of fibrinogen concentrate, divided by the expected increase in fibrinogen, where the expected increase in fibrinogen was the dose of fibrinogen divided by the plasma volume. The plasma volume (mL) was calculated as 70 (mL/kg) × body weight (kg) × (100 − Hct) / 100.19
Histology and Other Safety Assessments
At the end of the experiment (30 minutes after fibrinogen concentrate administration), animals were euthanized. The proximal tibia (with intraosseous access) was extracted from each pig in the IO group and preserved for histologic evaluation of the bone marrow. The corresponding tibia without any puncture was extracted from IV group animals for control investigation. In three IO group animals, the tibia, which had not been used for study therapy, underwent intraosseous puncture and was flushed with 20-mL saline. These bones were investigated to provide additional histomorphologic comparison.
For each tibia, three samples (each approximately 1 cm3) from around the injection site were prepared using a clinical pneumatic bone saw and fixed in 4% neutral buffered formalin for 48 hours. Samples were rinsed with water and cut into slices 5 mm thick using a band saw. After decalcification, the slices were dehydrated in a graded series of alcohol and embedded in paraffin. Sections of 4-µm to 6-µm thickness were cut with a rotary microtome and dried overnight. After deparaffinization, sections were stained, first, with hematoxylin-eosin and, second, with Weigert’s iron hematoxylin followed by Martius/Scarlet/Blue to stain fibrin red, collagen blue, and erythrocytes yellow.
The sections were inspected for abnormalities by a senior pathology consultant.
Statistical Analysis
The number of animals included in the study was based on previous porcine studies involving trauma and coagulation. Normal distribution of data was evaluated using the Kolmogorov-Smirnov test. Normally distributed data were expressed as mean (SD), and data not following the normal distribution were expressed as median and interquartile range. A repeated-measures analysis of variance was used to detect differences between time points (baseline, dilution, therapy), and a Newman-Keuls post hoc correction for multiple comparisons was applied. The Student’s t test (normal distribution) or the Mann-Whitney U-test (nonnormal distribution) was used to assess differences between the IO and IV groups at each time point. A two-tailed p < 0.05 was considered statistically significant. All statistical calculations were performed using commercially available statistical software (GraphPad Prism 5, GraphPad Software, La Jolla, CA).
RESULTS
All animals were treated according to the experimental protocol (Fig. 1) and survived until the end of the study. The mean (SD) body weight was 34.1 (2.8) kg (range, 30.3–38.7 kg), mean (SD) blood loss was 1,195 (97) mL (not including blood sampling), and the mean dose of fibrinogen concentrate was 2.7 (0.2) g, with no significant differences between the IO and the IV group.
After hemorrhage and dilution, Hct, Hb, and Plt were lower than at baseline, while WBC count had increased (Table 1). At the same time, PTI had decreased significantly from baseline, whereas aPTT had increased. Fibrinogen administration did not influence PTI or aPTT, and all laboratory parameters were comparable between the IO and IV groups at all time points.
TABLE 1.
Blood Cell Count and Standard Coagulation Tests at Baseline, After In Vivo Hemodilution, and After IO or IV Therapy With Fibrinogen Concentrate
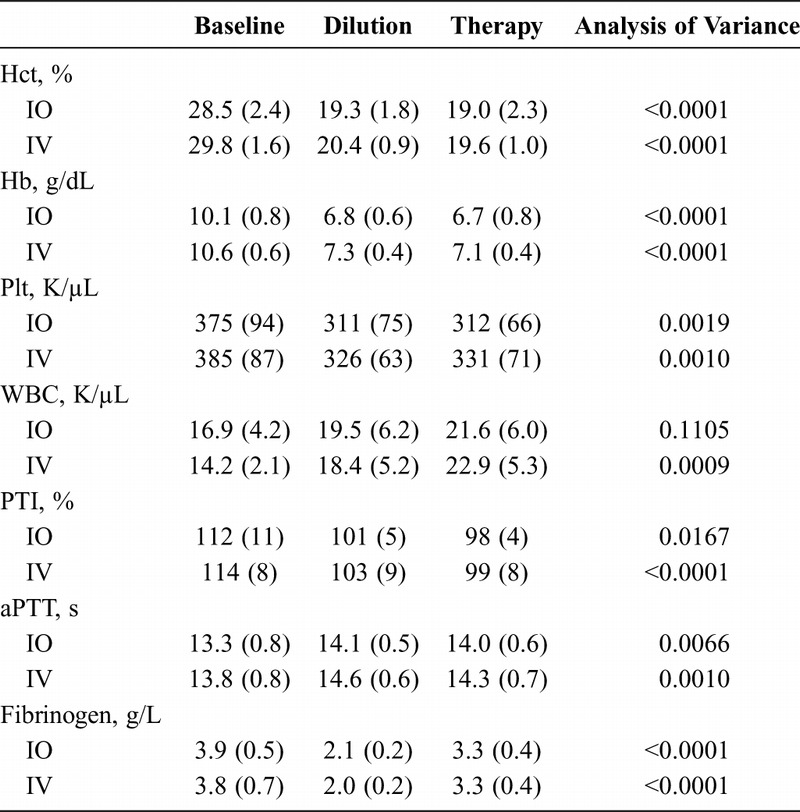
With regard to plasma fibrinogen levels and PFP MCF, the same pattern of results was observed in both study groups (Table 1, Fig. 2). No significant between-group differences were observed with either parameter at any of the study time points. Plasma fibrinogen levels decreased significantly after hemodilution and were subsequently increased by administration of fibrinogen concentrate, although baseline levels were not restored (Table 1, Fig. 2A). PFP MCF also decreased in response to hemodilution, but unlike plasma fibrinogen, levels increased significantly above baseline after the administration of fibrinogen concentrate (Fig. 2B).
Figure 2.
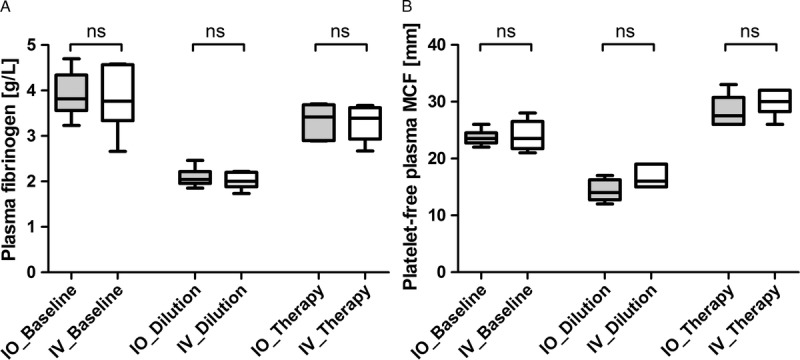
Effects of intraosseous versus intravenous fibrinogen concentrate on plasma fibrinogen level (A) and thromboelastometric MCF (B) of platelet-free plasma activated with EXTEM. Data presented are median, interquartile range, and range. Shaded boxes represent the IO group; white boxes represent the IV group. ns, not significant.
ROTEM results from the EXTEM assay (CT, CFT, MCF) and from the FIBTEM assay (MCF) are presented in Figure 3A to D. For all parameters, there were no significant differences between the IO and IV groups at baseline, hemodilution, or after the administration of fibrinogen concentrate. In both groups, hemorrhage followed by dilution resulted in a shortening of EXTEM CT, a prolongation of CFT, and a reduction of MCF. Following administration of fibrinogen concentrate, CT was shortened further, while CFT and MCF returned to baseline.
Figure 3.
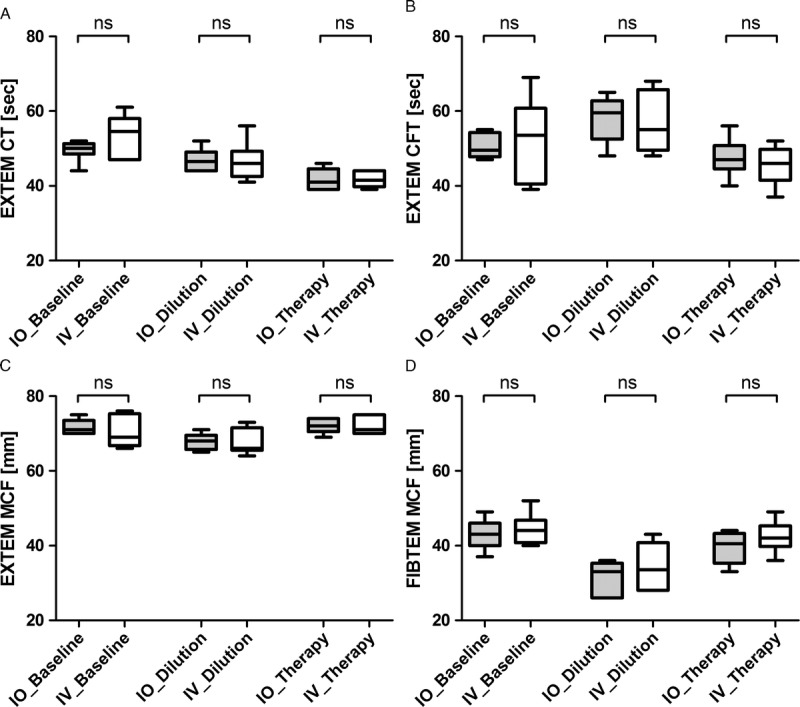
Effects of intraosseous versus intravenous fibrinogen concentrate on thromboelastometric measurement of whole blood, using EXTEM CT (A), CFT (B), and MCF (C) as well as FIBTEM MCF (D). Data presented are median, interquartile range, and range. Shaded boxes represent the IO group; white boxes represent the IV group. ns, not significant.
Fibrinogen recovery, based on Clauss assay results, was 89% (23%) in the IO group and 91% (22%) in the IV group (p = 0.85).
Microscopic investigation of the punctured bone revealed normal bone marrow and few fibrin strands around the needle channel, regardless of whether fibrinogen concentrate was administered. Fibrin strands were also found in unpunctured control bones, albeit in smaller quantities. Examples of the microscopic images are shown in Figure 4. Hemodynamic monitoring and respiratory variables showed no differences between the two study groups, with no signs of abnormalities.
Figure 4.
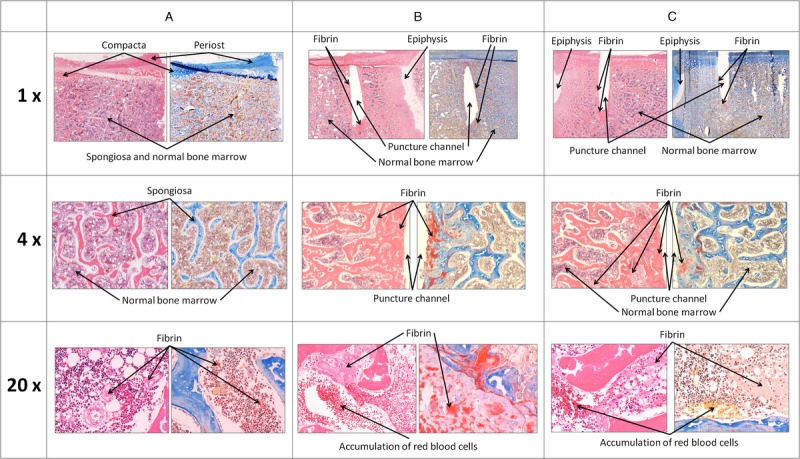
Typical examples of histologic slices (hematoxylin-eosin and Martius/Scarlet/Blue staining) from unpunctured bone marrow space (A) and from punctured bone either without (B) or with (C) fibrinogen concentrate administration. First row represents an overview of the captured slice. The puncture channel is approximately 10 mm to 15 mm in length. The second row provides 4-fold magnification, and the third row shows 20-fold enlargement of the first row slices.
DISCUSSION
In this preclinical in vivo study, we showed that intraosseous administration of fibrinogen concentrate is feasible and results in similar in vivo recovery to intravenous administration. The same pattern of results was observed in both study groups with regard to plasma fibrinogen levels and PFP MCF. Importantly, histologic examination indicated no adverse effects on bone marrow tissue surrounding the intraosseous administration site.
We are unaware of any previous studies of intraosseous fibrinogen concentrate, but a preclinical study of intraosseous rFVIIa has been published. Wright et al.20 designed a porcine study to determine whether intraosseous infusion of rFVIIa is feasible during hemorrhagic shock. They concluded that this route of administration is safe and delivers sufficient blood levels of rFVIIa to improve hemostasis during shock. Plewa et al.21 investigated the hematologic safety of intraosseous autologous whole-blood transfusion (20 mL/kg) in a porcine model of hemorrhagic shock, with follow-up to 48 hours after transfusion. All laboratory values including platelets and fibrinogen remained within normal limits at all time points, and there were no differences between animals receiving intraosseous transfusion and control animals receiving intravenous transfusion. No fat embolus was noted, and all lung and kidney specimens were free from inflammation.
With regard to intraosseous administration of hemostatic agents in humans, Cooper et al.9 reported single cases where FFP and rFVIIa were administered using the EZ-IO system in a military setting. However, they did not provide data on in vivo recovery or safety of these interventions. Burgert22 reported intraosseous infusion of red blood cells, FFP, and epinephrine via an EZ-IO device in an adult patient with hemorrhagic shock. The EZ-IO device was in place for 14 hours, and no complications were reported. However, maximum flow rates of intraosseous administration are considered inadequate for effective damage-control resuscitation with allogeneic blood products.14 Administration volumes are much reduced with coagulation factor concentrates, making them more suitable for this route of administration. Thus, in our study, a mean dose of 2.7-g fibrinogen concentrate (135 mL) was administered intraosseously with manually operated 50-mL syringes over a few minutes without using a pressure infusion device. No difficulties were encountered with this approach, and adequate recovery of plasma fibrinogen levels was observed.
In the present study, prolongation of CFT and reduction of MCF suggested hypocoagulability after hemorrhage and dilution. However, hemodilution also shortened CT. This is in line with previous in vitro observations, which showed the same effect with up to 40% hemodilution using crystalloids.23,24 Shortened CT suggests enhanced thrombin generation, and accordingly, Dunbar and Chandler25 showed increased thrombin generation following dilution of plasma proteins. This finding can be explained by the dilution of coagulation inhibitors, mainly antithrombin III.26 Administration of fibrinogen concentrate further reduced CT in our study. This effect was also observed in an in vitro study by Bolliger et al.,27 although the explanation remains to be determined. One hypothesis is that the therapeutic increase in the fibrinogen concentration in whole blood increases the availability of coagulation substrate (i.e., fibrinogen), enabling the required clot amplitude of 2 mm to be reached more quickly. In clinical practice, treatment with fibrinogen concentrate as first-line therapy may potentially correct prolonged EXTEM CT, if the prolongation is primarily caused by insufficient availability of clot substrate as opposed to deficient thrombin generation. After administration of fibrinogen concentrate, which improves substrate availability, persistence of prolonged CT could be interpreted as reduced thrombin generation, which could be increased by administering either prothrombin complex concentrate or FFP.28 This observation supports administration of fibrinogen concentrate with subsequent reevaluation by thromboelastometry before treatment with prothrombin complex concentrate or FFP in bleeding trauma patients, and this approach is proposed in coagulation management algorithms.28,29
The use of fibrinogen as first-line procoagulant therapy in bleeding trauma patients is the main reason why we chose to investigate fibrinogen concentrate. It is increasingly recognized that levels of fibrinogen are depleted in trauma to an extent greater than other coagulation factors and that fibrinogen substitution is often essential to maintain effective clotting.1,30,31 In the clinical setting, fibrinogen concentrate therapy has been shown to be effective in maintaining plasma fibrinogen concentration and fibrin-based clot strength as measured by the FIBTEM assay.32 In another study, low fibrinogen levels at admission to the emergency department were associated with poor outcomes, and mortality was reduced in trauma patients receiving early fibrinogen supplementation.33
Our examination of bone marrow following intraosseous administration of fibrinogen concentrate indicated that the physicochemical characteristics of this product (e.g., osmolality, pH) had no impact on the tissue. According to the manufacturer’s information, reconstituted fibrinogen concentrate has an osmolality of approximately 280 mosmol/L (obligatory range, 240–320 mosmol/L) and pH of 7.3 (obligatory range, 6.5–7.5) (personal communication, CSL Behring, Marburg, 2013). Histologic investigations of the punctured and infused bones also showed no signs of thrombosis or clotting in the spongiosa. Fibrin strands were seen perifocal to the intraosseous puncture site in all slices, regardless of whether fibrinogen concentrate was administered. Thus, the fibrin strands are likely to represent a physiologic response to the insertion of the intraosseous needle. It is of interest that even unpunctured bones showed small amounts of fibrin, although this is considered to be a postmortem artefact. In our study, all experiments were finished within 30 minutes of fibrinogen concentrate administration, so we cannot exclude the possibility of delayed-onset effects occurring after the observation period. However, it would have been difficult to implement a longer observation period because of endogenous fibrinogen rebound in pigs after hemorrhage and fluid resuscitation. Thus, within 24 hours, fibrinogen levels would be expected to rebound significantly above baseline values even in the absence of fibrinogen supplementation.34
Our investigation focused on the feasibility of intraosseous administration of fibrinogen concentrate and compared the effects of such treatment on laboratory parameters with those of intravenous fibrinogen concentrate. The experiments were not designed to show whether intraosseous administration alters the hemostatic capabilities of fibrinogen concentrate in acute bleeding. Previous porcine studies of intravenous fibrinogen concentrate have shown that, compared with placebo, it can reduce bleeding in models of hemorrhagic trauma.15,16 We assume that intraosseously administered fibrinogen concentrate would have similar effects, considering the similarity with intravenous administration in terms of systemic availability after administration. However, further studies would be needed to prove the hemostatic effects of intraosseously administered fibrinogen concentrate. Despite these limitations, based on the present findings, it should now be possible to investigate intraosseous administration of fibrinogen concentrate in humans with life-threatening bleeding.
CONCLUSION
Based on the results of this in vivo porcine study, intraosseous application of fibrinogen concentrate seems feasible and results in in vivo recovery of fibrinogen similar to that of intravenous application. In situations where intravenous vascular access is not possible or would be time consuming, the intraosseous route might be considered as a possible option for rapid administration of fibrinogen concentrate.
AUTHORSHIP
C.J.S., C.K., H.R., and H.S. conceived and designed the study. C.J.S., C.S., H.R., and H.S. carried out fund raising projects for this study. C.J.S., C.K., J.Z., S.N., and W.O. conducted the study. C.J.S. and H.S. managed and analyzed the data, including quality control and statistical analysis, and drafted the manuscript. All authors contributed substantially to interpretation of data and critical revision of the manuscript.
ACKNOWLEDGEMENTS
We thank the following: (a) the whole team from the animal laboratory research laboratory (Karin Brenner, Joachim Hartinger, Susanne Drechsler, Carina Penzenstadler, and Markus Kerbl) for the optimal preparation of the hemorrhagic porcine model; (b) Anna Khadem and Anton Klotz for the outstanding laboratory assistance; (c) Karl Kropik and Karl Schneider for the technical support; (d) Johanna Gaul, Renata Szölösi, Dominika Teplanova, and Patrick Heimel for the technical histology support; (e) CSL Behring, Vienna, Austria for the financial support of the study; (f) CSL Behring, Marburg, Germany for the provision of fibrinogen concentrate; and (g) Meridian HealthComms, Cheshire, UK for late-stage editorial assistance with manuscript preparation.
DISCLOSURE
C.J.S. has received speaker honoraria, travel support, and research funding from CSL Behring and research support from Tem International. C.S. is an employee of CSL Behring, has acted as a consultant for CSL Behring, has received speaker honoraria and research support from Tem International and CSL Behring, and has received travel support from Haemoscope Ltd. (former manufacturer of TEG). H.S. has received speaker honoraria and research support from CSL Behring and Tem International. Departmental funding was provided by the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, AUVA Research Centre, Vienna. Financial support was provided by CSL Behring, Vienna, Austria. Fibrinogen concentrate was provided by CSL Behring, Marburg, Germany. Late-stage editorial assistance, provided by Meridian HealthComms, was funded by CSL Behring, Marburg, Germany.
Footnotes
This study was presented at the 4th International Symposium on Critical Bleeding (ISCB 2013), September 2–3, 2013, in Copenhagen, Denmark (http://www.iscb2013.dk) at the plenary session.
CSL Behring had no involvement in the study design and in the collection, analysis, and interpretation of data.
REFERENCES
- 1. Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesth Analg. 2012; 114: 261– 274. [DOI] [PubMed] [Google Scholar]
- 2. Schochl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012; 20: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorensen B, Larsen OH, Rea CJ, Tang M, Foley JH, Fenger-Eriksen C. Fibrinogen as a hemostatic agent. Semin Thromb Hemost. 2012; 38: 268– 273. [DOI] [PubMed] [Google Scholar]
- 4. Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995; 81: 360– 365. [DOI] [PubMed] [Google Scholar]
- 5. Chambers LA, Chow SJ, Shaffer LE. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011; 136: 364– 370. [DOI] [PubMed] [Google Scholar]
- 6.Fries D. Fibrinogen Concentrate (FGTW) in Trauma Patients, Presumed to Bleed (FI in TIC) 2012. Available at: http://clinicaltrials.gov/show/NCT01475344. Accessed December 13, 2013.
- 7.European Trauma Course Organisation. The European Trauma Course Manual. 3rd ed Belgium: European Resuscitation Council; 2013. [Google Scholar]
- 8. Lyon RM, Donald M. Intraosseous access in the prehospital setting-ideal first-line option or best bailout? Resuscitation. 2013; 84: 405– 406. [DOI] [PubMed] [Google Scholar]
- 9. Cooper BR, Mahoney PF, Hodgetts TJ, Mellor A. Intra-osseous access (EZ-IO) for resuscitation: UK military combat experience. J R Army Med Corps. 2007; 153: 314– 316. [PubMed] [Google Scholar]
- 10. Davidoff J, Fowler R, Gordon D, Klein G, Kovar J, Lozano M, Potkya J, Racht E, Saussy J, Swanson E, et al. Clinical evaluation of a novel intraosseous device for adults: prospective, 250-patient, multi-center trial. JEMS. 2005; 30: suppl 20 23. [PubMed] [Google Scholar]
- 11. Santos D, Carron PN, Yersin B, Pasquier M. EZ-IO((R)) intraosseous device implementation in a pre-hospital emergency service: a prospective study and review of the literature. Resuscitation. 2013; 84: 440– 445. [DOI] [PubMed] [Google Scholar]
- 12. Schalk R, Schweigkofler U, Lotz G, Zacharowski K, Latasch L, Byhahn C. Efficacy of the EZ-IO needle driver for out-of-hospital intraosseous access—a preliminary, observational, multicenter study. Scand J Trauma Resusc Emerg Med. 2011; 19: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sunde GA, Heradstveit BE, Vikenes BH, Heltne JK. Emergency intraosseous access in a helicopter emergency medical service: a retrospective study. Scand J Trauma Resusc Emerg Med. 2010; 18: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris M, Balog R, Devries G. What is the evidence of utility for intraosseous blood transfusion in damage-control resuscitation? J Trauma Acute Care Surg. 2013; 75: 904– 906. [DOI] [PubMed] [Google Scholar]
- 15. Fries D, Krismer A, Klingler A, Streif W, Klima G, Wenzel V, Haas T, Innerhofer P. Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. Br J Anaesth. 2005; 95: 172– 177. [DOI] [PubMed] [Google Scholar]
- 16. Grottke O, Braunschweig T, Henzler D, Coburn M, Tolba R, Rossaint R. Effects of different fibrinogen concentrations on blood loss and coagulation parameters in a pig model of coagulopathy with blunt liver injury. Crit Care. 2010; 14: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grottke O, Braunschweig T, Philippen B, Gatzweiler KH, Gronloh N, Staat M, Rossaint R, Tolba R. A new model for blunt liver injuries in the swine. Eur Surg Res. 2010; 44: 65– 73. [DOI] [PubMed] [Google Scholar]
- 18. Martini J, Maisch S, Pilshofer L, Streif W, Martini W, Fries D. Fibrinogen concentrate in dilutional coagulopathy: a dose study in pigs. Transfusion. 2014. Jan;54(1):149–57. [DOI] [PubMed] [Google Scholar]
- 19. Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, Koppert W, Rahe-Meyer N. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010; 104: 555– 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright JK, Christy RJ, Tharp RV, Kalns JE. Evaluation of intraosseous delivery of factor VIIa during hemorraghic shock in the pig. Mil Med. 2009; 174: 119– 123. [DOI] [PubMed] [Google Scholar]
- 21. Plewa MC, King RW, Fenn-Buderer N, Gretzinger K, Renuart D, Cruz R. Hematologic safety of intraosseous blood transfusion in a swine model of pediatric hemorrhagic hypovolemia. Acad Emerg Med. 1995; 2: 799– 809. [DOI] [PubMed] [Google Scholar]
- 22. Burgert JM. Intraosseous infusion of blood products and epinephrine in an adult patient in hemorrhagic shock. AANA J. 2009; 77: 359– 363. [PubMed] [Google Scholar]
- 23. Egli GA, Zollinger A, Seifert B, Popovic D, Pasch T, Spahn DR. Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth. 1997; 78: 684– 689. [DOI] [PubMed] [Google Scholar]
- 24. Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schochl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, gelatine, hydroxyethyl starch or saline in vitro. Blood Transfus. 2012: 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009; 49: 2652– 2660. [DOI] [PubMed] [Google Scholar]
- 26. Ruttmann TG, Jamest MF, Lombard EH. Haemodilution-induced enhancement of coagulation is attenuated in vitro by restoring antithrombin III to pre-dilution concentrations. Anaesth Intensive Care. 2001; 29: 489– 493. [DOI] [PubMed] [Google Scholar]
- 27. Bolliger D, Szlam F, Molinaro RJ, Rahe-Meyer N, Levy JH, Tanaka KA. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: an in vitro model. Br J Anaesth. 2009; 102: 793– 799. [DOI] [PubMed] [Google Scholar]
- 28. Schochl H, Voelckel W, Grassetto A, Schlimp CJ. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J Trauma Acute Care Surg. 2013; 74: 1587– 1598. [DOI] [PubMed] [Google Scholar]
- 29. Schöchl H, Schlimp CJ. Trauma Bleeding Management: The Concept of Goal-Directed Primary Care. Anesth Analg. 2013. doi: 10.1213/ANE.0b013e318270a6f7 Anesth Analg. 2013 Jun 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Schlimp CJ, Voelckel W, Inaba K, Maegele M, Ponschab M, Schöchl H. Estimation of plasma fibrinogen levels based on hemoglobin, base excess and Injury Severity Score upon emergency room admission. Crit Care. 2013; 17: R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davenport R, Brohi K. Fibrinogen depletion in trauma: early, easy to estimate and central to trauma-induced coagulopathy. Crit Care. 2013; 17: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schlimp CJ, Voelckel W, Inaba K, Maegele M, Schochl H. Impact of fibrinogen concentrate alone or with prothrombin complex concentrate (+/− fresh frozen plasma) on plasma fibrinogen level and fibrin-based clot strength (FIBTEM) in major trauma: a retrospective study. Scand J Trauma Resusc Emerg Med. 2013; 21: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012; 10: 1342– 1351. [DOI] [PubMed] [Google Scholar]
- 34. Martini WZ. Fibrinogen availability and coagulation function after hemorrhage and resuscitation in pigs. Mol Med. 2011; 17: 757– 761. [DOI] [PMC free article] [PubMed] [Google Scholar]


