Abstract
Recently, lower thrombin generation has been associated with excess bleeding post-cardiopulmonary bypass (CPB). Therefore, treatment to correct thrombin generation is a potentially important aspect of management of bleeding in this group of patients. The objective of the present study was to investigate the effects of fresh frozen plasma (FFP), recombinant factor VIIa (rFVIIa), prothrombin complex concentrate (PCC) and tissue factor pathway inhibitor (TFPI) inhibition on thrombin generation when added ex vivo to the plasma of patients who had undergone cardiac surgery requiring CPB. Patients undergoing elective cardiac surgery were recruited. Blood samples were collected before administration of heparin and 30 min after its reversal. Thrombin generation was measured in the presence and absence of different concentrations of FFP, rFVIIa, PCC and an anti-TFPI antibody. A total of 102 patients were recruited. Thrombin generation following CPB was lower compared with pre-CPB (median endogenous thrombin potential pre-CPB 339 nmol/l per min, post-CPB 155 nmol/l per min, P < 0.0001; median peak thrombin pre-CPB 35 nmol/l, post-CPB 11 nmol/l, P < 0.0001). Coagulation factors and anticoagulants decreased, apart from total TFPI, which increased (55–111 ng/ml, P < 0.0001), and VWF (144–170 IU/dl, P < 0.0001). Thrombin generation was corrected to pre-CPB levels by the equivalent of 15 ml/kg FFP, 45 μg/kg rFVIIa and 25 U/kg of PCC. Inhibition of TFPI resulted in an enhancement of thrombin generation significantly beyond pre-CPB levels. This study shows that FFP, rFVIIa, PCC and inhibition of TFPI correct thrombin generation in the plasma of patients who have undergone surgery requiring CPB. Inhibition of TFPI may be a further potential therapeutic strategy for managing bleeding in this group of patients.
Keywords: blood coagulation factors, cardiopulmonary bypass, haemorrhage, plasma, recombinant FVIIa, thrombin
Introduction
Bleeding following cardiac surgery requiring the use of cardiopulmonary bypass (CPB) is associated with prolonged hospital admission and increased mortality [1,2]. This may be a consequence of more complex surgery or because of the increased number of red cell units transfused [3,4]. The latter has been independently associated with an increase in morbidity in a number of studies [5–7]. Although surgical causes of bleeding will always need to be addressed, bleeding caused by haemostatic failure requires the use of the best available haemostatic treatments with the objective of arresting the bleeding at an early stage, thus reducing the overall number of red cells that are transfused. Two recent studies have suggested that lower thrombin generation measured both pre-CPB and post-CPB is associated with excess postoperative bleeding [8,9]. Therefore, interventions to enhance thrombin generation may be as important as maintaining an adequate platelet count and fibrinogen concentration in controlling and preventing bleeding.
For patients who bleed because of impairment of thrombin generation, the currently available haemostatic options consist of fresh frozen plasma (FFP) and the off-label use of recombinant FVIIa (rFVIIa) and prothrombin complex concentrate (PCC). FFP is the most commonly used and readily available way to replace coagulation factors. However, the volume of FFP needed to produce a clinically relevant increase in clotting factors has been reported to be as much as 30 ml/kg [10]. In the context of surgery involving CPB, this constitutes a considerable volume load in a group of patients who may already have compromised cardiac function. In addition, the transfusion of FFP can be complicated by transfusion-related acute lung injury, and transfusion of larger volumes has been associated with an increased risk of stroke in some studies [11].
rFVIIa has been used off label and in clinical trials in the setting of massive haemorrhage, but evidence to demonstrate efficacy and safety has been limited [12–14]. Furthermore, the optimal dose is unknown and lower doses than those used to treat haemophilia-related bleeding may be preferable to minimize any risk of thrombosis, especially if these doses were shown to be effective in correcting thrombin generation. PCCs contain FII, FIX and FX or FII, FVII, FIX and FX depending on the manufacturer and the licensing jurisdiction. Successful off-label use of PCCs to manage excess bleeding following cardiac surgery has been reported in a number of studies [15–17]. However, the optimal dose is also unknown and disseminated intravascular coagulation has been reported when using high concentrations in a porcine trauma model [18], although this may relate to the type of PCC used.
Tissue factor pathway inhibitor (TFPI) is an important regulator of the initiation phase of coagulation. Heparin, the principal anticoagulant used during CPB, induces the release of TFPI from the endothelial surface [19]. In-vitro studies have shown that in the presence of TFPI, the rate of thrombin generation is reduced in a concentration-dependent manner [20]. Recently, a number of studies have reported using TFPI-inhibition to improve thrombin generation in both in-vitro and in-vivo models of haemophilia [21–25]. This raises the possibility that inhibition of TFPI may be a therapeutic target in treating bleeding in other circumstances, including cardiac surgery.
Thrombin generation assays may offer a better assessment of haemostatic function than routine coagulation tests such as the prothrombin time (PT) and activated partial thromboplastin time (APTT), and thus a better way of measuring the response to any treatment to correct haemostasis. In this study, we investigated the effects of FFP, rFVIIa, PCC and TFPI inhibition on thrombin generation when added ex vivo to the plasma of patients who had undergone cardiac surgery requiring CPB.
Methods
Patients were recruited who were undergoing heart valve surgery with or without coronary artery bypass grafting and procedures on the aorta. Informed consent was obtained and the study received approval from the South West Wales local research ethics committee (reference 11/WA/0215). The following demographic data were recorded: age, weight, sex, anticoagulant and antiplatelet medication history, type of operation, duration of aortic cross-clamping, duration of CPB, time and dose of heparin administration, time and dose of protamine given, volume and time of intravenous crystalloid, colloid and blood products administered once preoperative blood samples had been taken. The volume of cell salvage blood was also recorded. The CPB circuits and priming fluids were the same in all cases. Unfractionated heparin was used as an anticoagulant to maintain the activated clotting time more than 400 s. Protamine at a dose of 1 mg per 100 U of heparin was given after the end of the CPB prior to the removal of the arterial and venous cannulae.
Coagulation factor assays and full blood count measurement
Whole blood samples were taken into vacutainer tubes containing 3.2% trisodium citrate (Greiner Bio-One, Stonehouse, UK) and ethylenediaminetetraacetic acid (BD, Oxford, UK). Samples were taken before heparin administration and 30 min after reversal of heparin by protamine sulfate, PPP was prepared by centrifuging samples twice at 1650g before freezing in aliquots at −80°C for testing later. Full blood cell counts were performed on an ABX Pentra DX 120 automated analyser (Horiba Medical, Northampton, UK). The PT, APTT, Clauss fibrinogen and factors II, V, VII, VIII, IX, X, XI, antithrombin, protein C, free protein S and postoperative anti-Xa activity were measured on an ACL 500 Top (Instrumentation Laboratory, Cheshire, UK) automated coagulometer using standard manufacturer protocols and reagents. Factor XIII activity was measured in a flat-bottomed Immulon 2hb 96-well plate (Diagnostica-Stago, Asnières sur Seine, France) using a chromogenic assay kit from Technoclone (Vienna, Austria) and light absorbance was measured using a plate reader (BioTek, Winoosi, Vermont, USA).
Tissue factor pathway inhibitor ELISA
Quantification of full-length and total TFPI was performed using an ELISA technique as described previously [22]. Briefly, full-length and total TFPI were captured by an antic-terminus and anti-KD2 antibody respectively (both from Sanquin Blood Supply, Amsterdam, the Netherlands). A rabbit polyclonal antihuman TFPI antibody was used as the first detection antibody (American Diagnostica, Lexington, Massachusetts, USA). An antirabbit IgG peroxidase conjugate was used as the reporter antibody (Sigma-Aldrich, Bromborough, UK). An internal control and standard consisting of human full-length TFPI was provided by Baxter Innovations (Vienna, Austria). Absorbance was read at 450 nm on a plate reader (BioTek).
Von Willebrand factor ELISA
Von Willebrand factor (VWF) antigen (VWF:Ag) was measured by an ELISA technique. A 96-well DynexImmulon 4HBX plate (Fisher Scientific, Loughborough, UK) was coated with a capture antibody consisting of an antihuman VWF antibody from Dako (Ely, UK) and incubated at 4oC overnight. The plate was then blocked using a solution of 2% polyvinyl pyrrolidine. After washing the plate three times (wash buffer consisting of phosphate buffered saline and 0.05% Tween 20 v/v), 100 μl of test plasma and control plasma (CryoCheck Abnormal 1 and Abnormal 2 control plasma, Precision Biologic, Dartmouth, Nova Scotia, Canada) were added to the appropriate number of wells. A standard curve was constructed using Technoclone reference plasma from Pathway Diagnostics, UK. The plate was then covered and incubated for 2 h at room temperature. The plate was then washed three times and a rabbit anti-VWF horse-radish peroxidase immunoconjugate antibody (Dako) was added to each well. The plate was then covered and incubated for 1 h at room temperature before washing a further three times. Hundred microlitres of 3,3′,5,5′-tetramethylbenzidine liquid reagent (Skybio Ltd, Wyboston, Bedfordshire, UK) was added to each well and after 7 min 50 μl of Red-Stop reagent (Skybio Ltd) was added. Light absorbance was read at 405 nm on a plate reader (BioTek).
Thrombin generation assays
Whole blood samples were taken into vials containing 3.2% trisodium citrate with 20 μg/ml (final concentration in whole blood) corn trypsin inhibitor (CTI) at a ratio of one part anticoagulant to nine parts whole blood v/v (CTI; Cambridge Bioscience, Cambridge, UK), before heparin was given and 30 min after reversal of heparin by protamine sulphate. Platelet-poor plasma (PPP) was prepared by centrifuging samples twice at 1650 g and freezing in aliquots at −80°C for testing later. Thrombin generation was measured using the method described by Hemker et al.[6]. Experiments were performed by mixing 20 μl of commercially available trigger solution (Stago PPP Low; Diagnostica-Stago) with 80 μl of plasma in a U-bottom Immulon 2Hb 96-well plate (Diagnostica-Stago). The plate was maintained at 37°C using a plate incubator before being placed in a Fluoroscan Ascent fluorometer (Thermo Scientific, Helsinki, Finland). Thrombin generation was initiated by adding Flu-Ca (fluorogenic substrate Z-Gly-Gly-Arg; Bachem, UK, and 0.1 mol/l calcium chloride in a HEPES buffer). Fluorescence was measured using a Fluoroscan reader. Data were evaluated using Thrombinoscope software version 5.0.0.742 (Synapse BV, Maastricht, the Netherlands).
Calculation of concentrations of FFP, rFVIIa, PCC and anti-TFPI antibody to add
Thrombin generation was measured in the presence and absence of a polyclonal anti-TFPI antibody (AF2974; R&D Systems, Abingdon, UK) at a concentration of 100 nmol/l, this concentration having previously been determined to fully neutralize full-length TFPI from physiologic concentrations (∼ 0.4 nmol/l) up to 6 nmol/l (data not shown).
Fresh frozen plasma
Fresh frozen plasma was obtained from the Welsh Blood Service. The postoperative plasma volume for each patient was estimated using the formula, 0.07 × weight × (1 – haematocrit). The mean plasma volume was then calculated for the whole cohort. Mean weight was also calculated. These figures were then used to calculate the volume of FFP to add to each sample to be equivalent to 15, 20 and 50 ml/kg. This was 0.308 ml FFP, 0.411 ml FFP and 0.617 ml FFP per ml patient plasma, respectively.
rFVIIa and PCC
The mean weight of the overall cohort was used to calculate the amount of rFVIIa to add to plasma to be equivalent to doses of 45, 90 and 180 μg/kg. rFVIIa was purchased from Novonordisk (NovoSeven, Novonordisk, Denmark). The final concentrations of rFVIIa in the spiked plasma were 0.93, 1.85 and 3.7 μg/ml, respectively.
The mean weight of the overall cohort was also used to calculate the amount of PCC to add to plasma equivalent to doses of 25, 35 and 50 U/kg. PCC was a gift from CSL Behring (Beriplex, CSL; Behring UK Ltd, Haywards Heath, UK). The final concentrations of PCC in the spiked plasma were 0.51, 0.72 and 1.03 U/ml, respectively.
Statistical analysis
Data were analyzed using PASW Statistics version 18 software (SPSS Inc, released 2009; PASW Statistics for Windows, Version 18.0; Chicago, Illinois, USA). The Mann–Whitney U test was applied to examine differences between unrelated variables, whereas the Wilcoxon rank test was used to examine differences between related samples. Analysis of multiple related samples was performed using Friedman's test. Spearman's correlation coefficients were calculated to investigate the relationship between full-length TFPI and thrombin generation parameters.
Results
A total of 102 patients were recruited, median age 68 (range 28–88), male 72, female 30. Ten patients were excluded from further analysis because they had heparin anti-Xa levels greater than 0.3 anti-Xa U/ml in the post-CPB samples. This left 92 patients in the analysis. Patients who were on warfarin were included provided their international normalized ratio before surgery was less than 1.5 as per institutional protocols.
Demographic and clinical parameters are summarized in Table 1. Thrombin generation following CPB was lower compared with preoperative samples. Procoagulant factors and anticoagulants decreased significantly, apart from total TFPI, which increased significantly (median 55 ng/ml pre to 111 ng/ml post, P < 0.0001) and VWF:Ag, which increased from 144 to 170 IU/dl (P < 0.0001). The data are summarized in Table 2.
Table 1.
Demographic details and clinical parameters of study participants
| Parameter | Data |
| Age (years) | 67 |
| 28–88 (62–76) | |
| Weight (kg) | 76 |
| 48–134 (67–90) | |
| Sex (M/F) | 64/28 |
| Time on bypass (min) | 146 |
| 64–427 (117–193) | |
| Aortic cross-clamp time (min) | 115 |
| 50–34 (96–152) | |
| Intraoperative | 14 |
| volume crystalloid (ml/kg) | 0–36 (8–20) |
| Intraoperative volume of colloid | 13 |
| (ml/kg) | 0–47 (8–18) |
| Cell salvage volume (ml/kg) | 10 |
| 3–52 (7–12) |
Data shown are median, range (interquartile range).
Table 2.
Concentrations of procoagulant factors, inhibitors, platelet count and thrombin-generation parameters before and after CPB
| Parameter | Pre-CPB | Post-CPB | Comparison between pre-CPB and post-CPB (P value) |
| Platelet count (×109 cells/l) | 223 | 115 | <0.0001 |
| 124–670 (118–257) | 42–258 (98–138) | ||
| APTT (s) | 29 | 31 | <0.0001 |
| 23–38 (28–31) | 22–46 (30–35) | ||
| PT (s) | 11 | 15 | <0.0001 |
| 10–16 (10–12) | 12–22 (14–17) | ||
| vWF antigen (IU/dl) | 144 | 170 | <0.0001 |
| 61–322 (113–184) | 84-367 (131–205) | ||
| Fibrinogen (g/l) | 3.2 | 1.6 | <0.0001 |
| 1.7–7.9 (2.6–4.0) | 1.1–3.8 (1.4–1.9) | ||
| Factor II (IU/dl) | 95 | 52 | <0.0001 |
| 44–152 (85–107) | 33–83 (47–60) | ||
| Factor V (IU/dl) | 92 | 50 | <0.0001 |
| 51–135 (76–109) | 17–93 (40–58) | ||
| Factor VII (IU/dl) | 104 | 68 | <0.0001 |
| 48–192 (80–118) | 25–146 (58–80) | ||
| Factor VIII (IU/dl) | 129 | 92 | <0.0001 |
| 75–262 (106–157) | 49–216 (74–117) | ||
| Factor IX (IU/dl) | 133 | 104 | <0.0001 |
| 59–224 (117–151) | 62–157 (93–123) | ||
| Factor X (IU/dl) | 90 | 46 | <0.0001 |
| 27–148 (78–104) | 20–57 (38–54) | ||
| Factor XI (IU/dl) | 95 | 60 | <0.0001 |
| 42–183 (82–109) | 35–137 (50–72) | ||
| Factor XIII (%) | 103 | 57 | <0.0001 |
| 30–213 (89–117) | 21–107 (45–68) | ||
| Full-length TFPI (ng/ml) | 21 | 15 | <0.0001 |
| 7–39 (16–24) | 4–35 (11–22) | ||
| Total TFPI (ng/ml) | 56 | 113 | <0.0001 |
| 32–99 (48–67) | 60–165 (93–127) | ||
| Anti-thrombin (%) | 92 | 51 | <0.0001 |
| 11–144 (83–100) | 30–77 (45–58) | ||
| Free protein S (%) | 84 | 46 | <0.0001 |
| 43–125 (72–98) | 30–85 (39–55) | ||
| Protein C (%) | 106 | 61 | <0.0001 |
| 64–204 (92–119) | 39–93 (52–69) | ||
| Lag time (min) | 7 | 9 | 0.026 |
| 3–18 (6–8) | 0–36 (7–14) | ||
| Peak thrombin (nmol/l) | 35 | 11 | 0.019 |
| 1–115 (21–52) | 0–144 (2–42) | ||
| ETP (nmol/l per min) | 339 | 155 | <0.0001 |
| 10–867 (221–497) | 0–1161 (0–376) | ||
| Velocity index (nmol/l per min) | 9 | 2 | <0.0001 |
| 2–84 (5–14) | 0–53 (0–9) | ||
| Anti-Xa activity (IU/ml) | – | 0.12 | – |
| 0.00–0.30 (0.08–0.19) |
Data shown are median and range (interquartile range). APTT, activated partial thromboplastin time; CPB, cardiopulmonary bypass; ETP, endogenous thrombin potential; PT, prothrombin time; TFPI, tissue factor pathway inhibitor; vWF, von Willebrand factor.
CAT results are summarized for peak thrombin in Fig. 1, endogenous thrombin potential (ETP) in Fig. 2, lag time in Fig. 3 and velocity index in Fig. 4. There was a fall in peak thrombin, ETP and velocity index in the post-CPB samples compared with pre-CPB samples, whereas lag time increased.
Fig. 1.
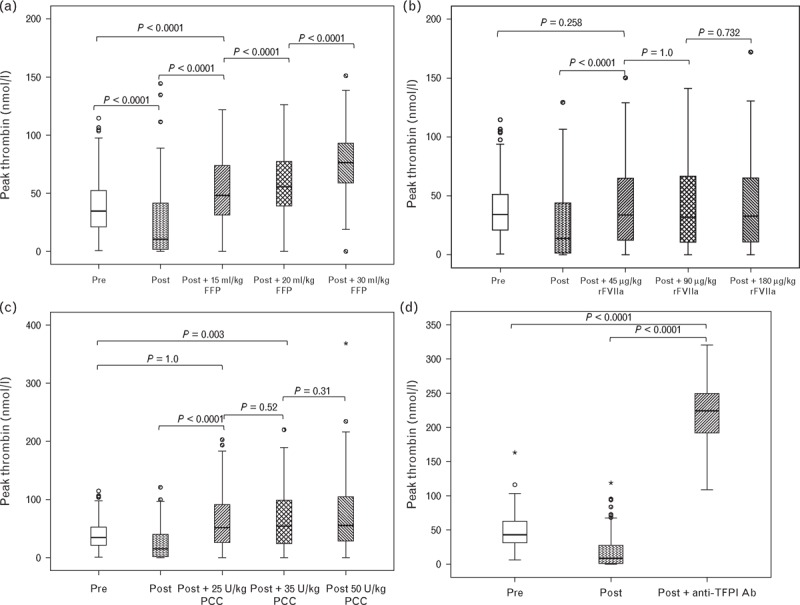
Peak thrombin in response to increasing concentrations of FFP (a), rFVIIa (b), PCC (c) and inhibition of TFPI (d). Horizontal black lines represent the median, boxes, the interquartile range, whiskers the range; asterisks and circles represent outliers. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; TFPI, tissue factor pathway inhibitor.
Fig. 2.
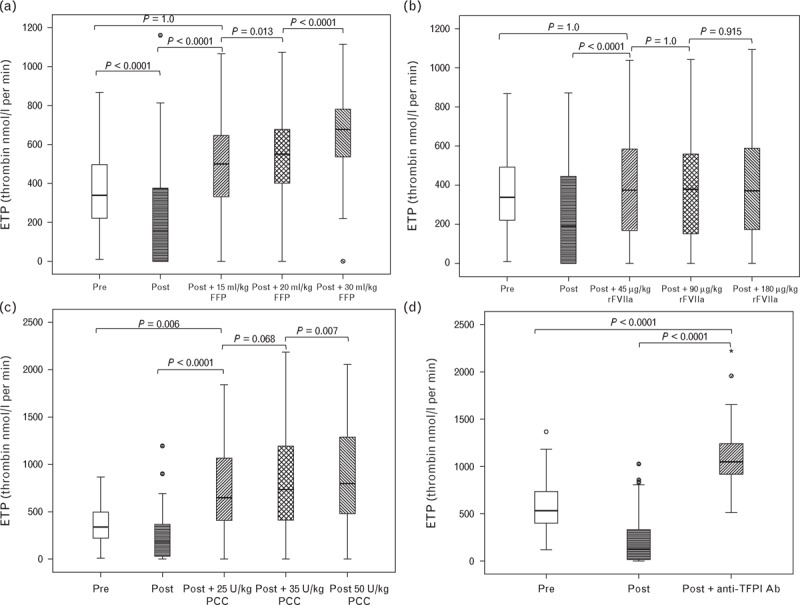
Endogenous thrombin potential (ETP) in response to increasing concentrations of FFP (a), rFVIIa (b), PCC (c) and inhibition of TFPI (d). Horizontal black lines represent the median, boxes the interquartile range, whiskers the range; asterisks and circles represent outliers. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; TFPI, tissue factor pathway inhibitor.
Fig. 3.
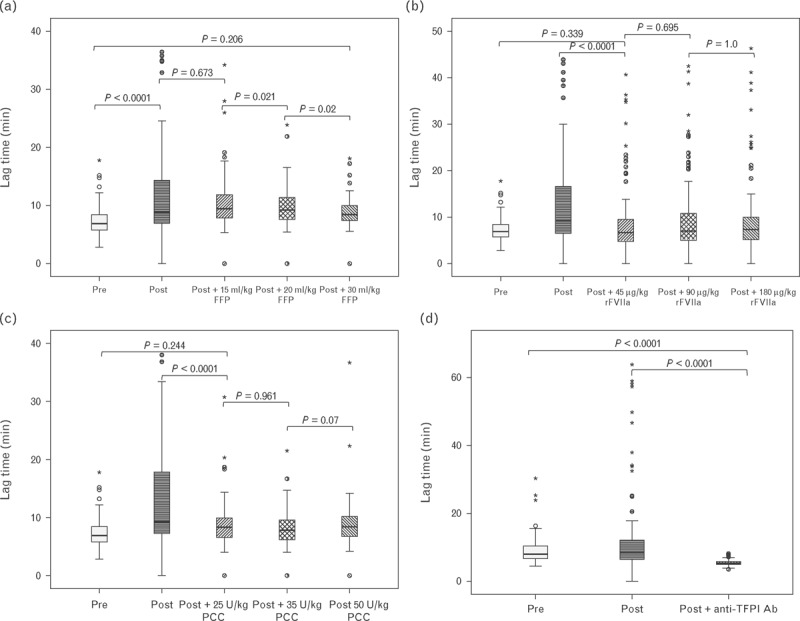
Lag time in response to increasing concentrations of FFP (a), rFVIIa (b), PCC (c) and inhibition of TFPI (d). Horizontal black lines represent the median, boxes the interquartile range, whiskers the range; asterisks and circles represent outliers. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; TFPI, tissue factor pathway inhibitor.
Fig. 4.
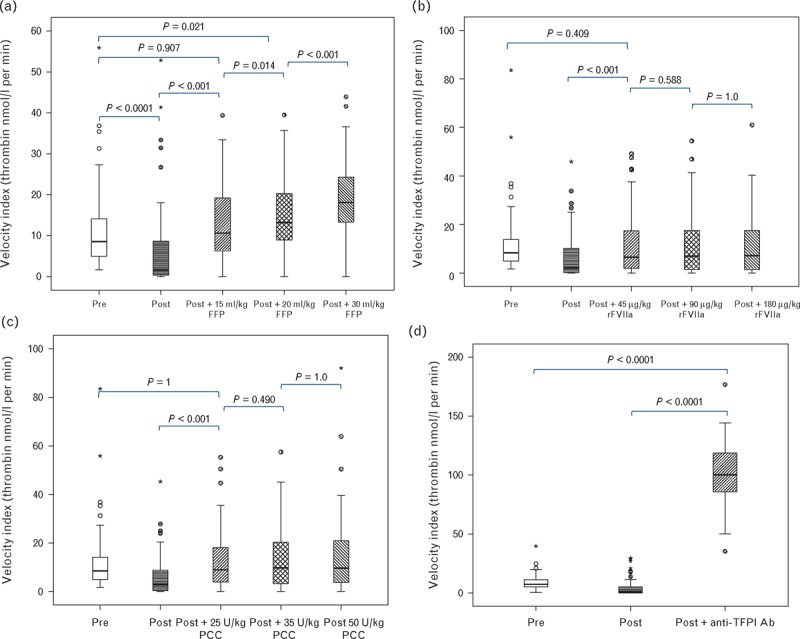
Velocity index in response to increasing concentrations of FFP (a), rFVIIa (b), PCC (c) and inhibition of TFPI (d). Horizontal black lines represent the median, boxes the interquartile range, whiskers the range; asterisks and circles represent outliers. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; TFPI, tissue factor pathway inhibitor.
The addition of FFP resulted in a progressive, concentration-dependent increase in peak thrombin, ETP and velocity index and shortening of the lag time. The largest effect was seen with a concentration equivalent to 30 ml/kg (Figs. 1–4). FFP at 15 ml/kg resulted in an increased peak thrombin to a level significantly greater than both preoperative and postoperative levels. Exceeding this volume resulted in a further dose-dependent increase. With rFVIIa, 45 μg/kg was sufficient to increase peak thrombin to preoperative levels, but not above preoperative levels, and exceeding this dose produced no further improvement. PCC at 25 U/kg resulted in peak thrombin greater than that seen preoperatively. There was no increase in peak thrombin between 25 and 35 U/kg, but there was a small but statistically significant difference comparing doses of 25 and 50 U/kg (P < 0.0001). Inhibition of TFPI resulted in a peak thrombin concentration that was much higher than the preoperative level. Results for ETP were similar to peak thrombin.
FFP at a dose of 15 ml/kg was sufficient to correct the velocity index to preoperative levels. Exceeding this dose resulted in increases statistically significantly greater than preoperative levels. For rFVIIa and PCC, 45 μg/kg and 25 U/kg, respectively, were sufficient to return the velocity index to preoperative levels. Exceeding these doses produced no further increase. Inhibition of TFPI dramatically increased the velocity index well exceeding the preoperative level. Similar results were obtained for the lag time with the exception that it required 30 ml/kg of FFP before the lag time corrected to preoperative levels.
Full-length TFPI measured post-CPB was inversely correlated with ETP, peak thrombin and velocity index in the absence and presence of all concentrations of FFP, rFVIIa and PCC, the weakest correlation being seen with FFP. Thrombin generation in the presence of the anti-TFPI antibody did not correlate the full-length TFPI concentration. Figure 5 summarizes these findings for ETP. Results for peak thrombin and velocity index were similar (data not shown).
Fig. 5.
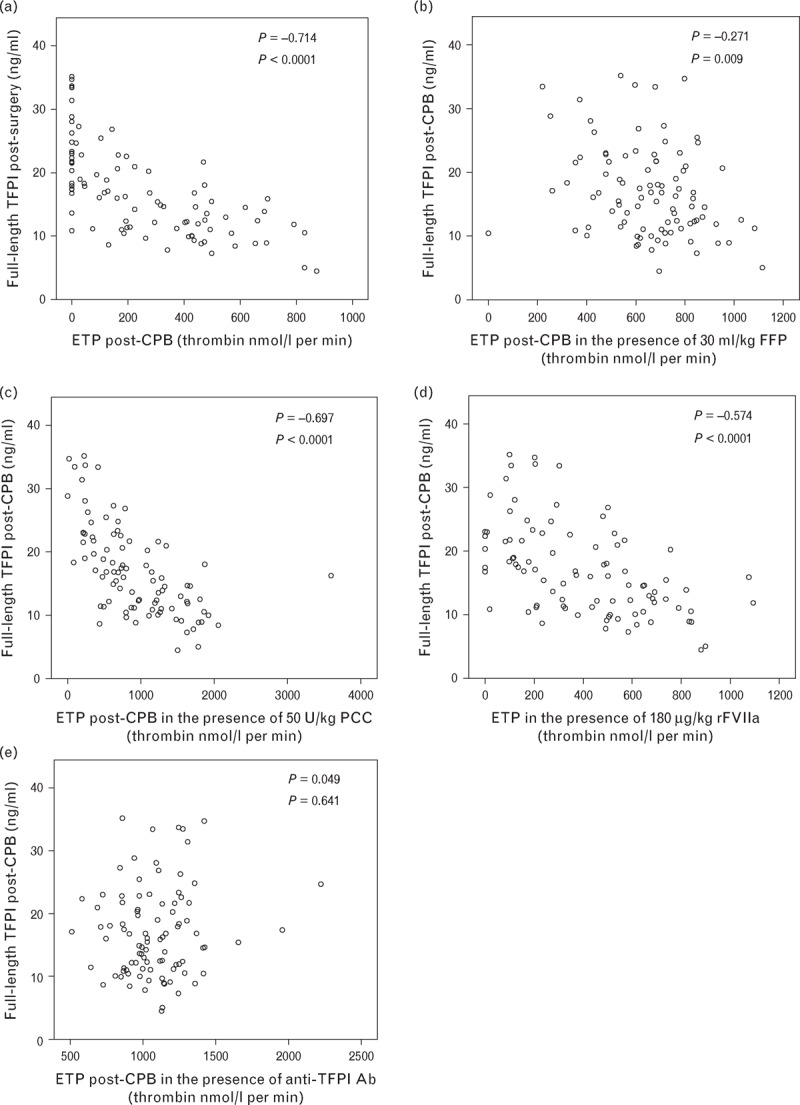
Scatter plots comparing full-length TFPI concentration post-CPB with endogenous thrombin potential (ETP) post-CPB in patient plasma alone (a), or with the addition of FFP (b), PCC (c), rFVIIa (d) and an anti-TFPI antibody (e). Spearman's correlation coefficients (ρ) are shown with P values. CPB, cardiopulmonary bypass; FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; TFPI, tissue factor pathway inhibitor.
Discussion
The results show that 15 ml/kg of FFP was sufficient to correct ETP, peak thrombin and velocity index to preoperative levels. Larger volumes of FFP improved thrombin generation above baseline. rFVIIa at a dose of 45 μg/kg and PCC at a dose of 25 U/kg were sufficient to correct all measured thrombin-generation parameters to preoperative levels. Inhibition of TFPI markedly increased the ETP, peak thrombin and velocity index beyond preoperative levels and shortened the lag time.
FFP remains the main treatment available to most clinicians for correcting any coagulopathy. The optimum dose of FFP to return the majority of thrombin-generation parameters to preoperative levels was 15 ml/kg. Some studies have reported a correlation between lag time and bleeding [26] and 30 ml/kg was required to correct this. The full-length TFPI concentration correlated with all thrombin-generation parameters postoperatively and remained so in the presence of FFP, rFVIIa and PCC. This supports the view that TFPI is an important determinant of thrombin generation and similar findings have been reported by Knappe et al.[22] and Peraramelli et al.[27]. The inverse correlation of full-length TFPI concentration with thrombin-generation parameters was weaker following the addition of FFP than after adding rFVIIa and PCC. This may be because FFP contains both procoagulant factors and inhibitors and would tend to normalize both, whereas rFVIIa and PCC only affect the procoagulant pathways. There was no correlation between full-length TFPI and thrombin generation in the presence of an anti-TFPI antibody, further supporting the importance of TFPI in thrombin generation assays activated with low concentrations of tissue factor.
The enhancing effect of FFP observed on thrombin generation may be overestimated by the in-vitro nature of the assay. Patient plasma was contact-inactivated by addition of CTI and so after addition of rFVIIa, PCC and TFPI antibody thrombin generation was activated through tissue factor. In contrast, the added FFP was not CTI-inhibited and would therefore have been prone to contact activation in addition to tissue factor activation. Contact activation has previously been shown to result in higher ETP and peak thrombin and shorter lag time when using lower concentrations of tissue factor as used in this study [28,29].
rFVIIa made no significant difference to thrombin-generation parameters beyond doses of 45 μg/kg, indicating that lower doses are as effective as higher doses in enhancing thrombin generation in vitro. This is as would be predicted given the global reduction in clotting factors observed in this patient cohort. A similar result was observed by Altman et al.[30] using a model of dilutional coagulopathy. This suggests that rFVIIa may be inappropriate to use in isolation to treat bleeding. Although there are a number of studies that have reported safety using rFVIIa in paediatric patients undergoing CPB [31], some studies have reported an increase in thrombotic events in adult patients [32]. Studies looking at adult patients from a broader population have also shown an increase in arterial events [33], something that would be particularly deleterious in the typical adult patient population undergoing cardiac surgery. Therefore, based on these in-vitro assays, if rFVIIa is used, lower doses may offer the best balance of achieving a beneficial effect while possibly reducing the chance of an adverse event.
PCC corrected thrombin-generation parameters to preoperative levels at a concentration of 25 U/kg. There was some further statistically significant improvement at concentrations of 50 U/kg; although this was so small, it is unlikely to be clinically significant. This finding is consistent with other in-vitro studies in which PCC has been shown to enhance thrombin generation [34]. In-vivo PCC has been shown to be effective in reducing blood loss in human studies [16,35]. As well as different numbers of clotting factors, different PCCs contain different amounts of heparin, with some containing more heparin than the PCC used in this study, which in turn has been shown to affect thrombin generation [36]. Although a four-factor PCC was used in this study, others have shown similar effects on thrombin generation using a three-factor PCC [34]. PCC has the advantage of additional safety over FFP for transmission of infection and smaller volumes in patients with cardiac compromise. However, large doses have been associated with disseminated intravascular coagulation in some studies [18].
Inhibition of TFPI had a marked effect on enhancing thrombin generation. TFPI in vivo is increased by administration of heparin [19]; the anticoagulant is routinely used in CPB and therefore may be a good therapeutic target in this group of patients. TFPI inhibition has previously been reported to enhance thrombin generation in plasma from people with haemophilia [21,37]. However, a number of studies have reported higher ETP and peak thrombin concentration in patients with prothrombotic tendencies [38–40]. This suggests caution may be required if TFPI inhibition was to be used as a therapeutic target especially in cardiac patients in which arterial thrombosis is often already a significant risk.
A weakness of studies investigating thrombin generation in plasma is that they take no account of other important factors that require correction in controlling bleeding following cardiac surgery: fibrinogen concentration, platelet count and function and fibrinolysis. A low fibrinogen has been associated with excessive postoperative bleeding in a number of studies [41,42]. Thrombocytopenia has also been previously described as a risk factor for bleeding following CPB [8,43]. Clot formation and durability require sufficient thrombin to cleave fibrinogen to fibrin, FXIII to cross-link fibrin monomers, platelets and reduced fibrinolysis. Therefore, correction of thrombin generation forms only one component in the management of bleeding.
In summary, this study suggests that comparatively low doses of FFP, rFVIIa and PCC may be sufficient to correct thrombin generation in patients who have undergone surgery requiring CPB. Inhibition of TFPI may offer a future therapeutic strategy for managing bleeding in this group of patients. However, these in-vitro results need to be validated in vivo.
Acknowledgements
This work was funded by the British Heart Foundation award reference FS/11/42/28753. R.H., M.D. and F.S. are employees of Baxter Innovations GmbH.
C.L.P. designed the study, recruited the patients, performed the laboratory assays, interpreted data and wrote the article. R.J. assisted in recruitment of patients, sample preparation and edited the article. R.H., M.D. and F.S. designed some of the laboratory studies, interpreted data and edited the article. S.B. and D.M. assisted in the design of the study, recruitment of patients and edited the article. J.E.H. and V.B.O. designed the study, planned experiments, interpreted data and edited the article. P.W.C. designed and supervised all aspects of the study and edited the article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Despotism G, Avian M, EBay C. Prediction and management of bleeding in cardiac surgery. J Thromb Haemost 2009; 7:111–117. [DOI] [PubMed] [Google Scholar]
- 2.Karthik S, Grayson AD, McCarron EE, Pullan DM, Desmond MJ. Reexploration for bleeding after coronary artery bypass surgery: risk factors, outcomes, and the effect of time delay. Ann Thorac Surg 2004; 78:527–534. [DOI] [PubMed] [Google Scholar]
- 3.Ranucci M, Bozzetti G, Ditta A, Cotza M, Carboni G, Ballotta A. Surgical reexploration after cardiac operations: why a worse outcome? Ann Thorac Surg 2008; 86:1557–1562. [DOI] [PubMed] [Google Scholar]
- 4.Vivacqua A, Koch CG, Yousuf AM, Nowicki ER, Houghtaling PL, Blackstone EH, et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg 2011; 91:1780–1790. [DOI] [PubMed] [Google Scholar]
- 5.Shaw RE, Johnson CK, Ferrari G, Brizzio ME, Sayles K, Rioux N, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion 2013; 54:1106–1113. [DOI] [PubMed] [Google Scholar]
- 6.van Straten AH, Bekker MW, Soliman Hamad MA, van Zundert AA, Martens EJ, Schonberger JP, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg 2010; 10:37–42. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskar B, Dulhunty J, Mullany DV, Fraser JF. Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. Ann Thorac Surg 2012; 94:460–467. [DOI] [PubMed] [Google Scholar]
- 8.Coakley M, Hall JE, Evans C, Duff E, Billing V, Yang L, et al. Assessment of thrombin generation measured before and after cardiopulmonary bypass surgery and its association with postoperative bleeding. J Thromb Haemost 2011; 9:282–292. [DOI] [PubMed] [Google Scholar]
- 9.Bosch YP, Al Dieri R, Ten Cate H, Nelemans PJ, Bloemen S, de Laat B, et al. Measurement of thrombin generation intra-operatively and its association with bleeding tendency after cardiac surgery. Thromb Res 2014; 133:488–494. [DOI] [PubMed] [Google Scholar]
- 10.Collins PW, Macchiavello LI, Lewis SJ, Macartney NJ, Saayman AG, Luddington R, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol 2006; 135:220–227. [DOI] [PubMed] [Google Scholar]
- 11.Mikkola R, Gunn J, Heikkinen J, Wistbacka JO, Teittinen K, Kuttila K, et al. Use of blood products and risk of stroke after coronary artery bypass surgery. Blood Transfus 2012; 10:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barua A, Rao VP, Ramesh B, Barua B, El-Shafei H. Salvage use of activated recombinant factor VII in the management of refractory bleeding following cardiac surgery. J Blood Med 2011; 2:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brophy GM, Candeloro CL, Robles JR, Brophy DF. Recombinant activated factor VII use in critically ill patients: clinical outcomes and thromboembolic events. Ann Pharmacother 2013; 47:447–454. [DOI] [PubMed] [Google Scholar]
- 14.Chapman AJ, Blount AL, Davis AT, Hooker RL. Recombinant factor VIIa (NovoSeven RT) use in high risk cardiac surgery. Eur J Cardiothorac Surg 2011; 40:1314–1318. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka KA, Mazzeffi MA, Grube M, Ogawa S, Chen EP. Three-factor prothrombin complex concentrate and hemostasis after high-risk cardiovascular surgery. Transfusion 2013; 53:920–921. [DOI] [PubMed] [Google Scholar]
- 16.Arnekian V, Camous J, Fattal S, Rezaiguia-Delclaux S, Nottin R, Stephan F. Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg 2012; 15:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit Care 2008; 12:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grottke O, Braunschweig T, Spronk HM, Esch S, Rieg AD, van Oerle R, et al. Increasing concentrations of prothrombin complex concentrate induce disseminated intravascular coagulation in a pig model of coagulopathy with blunt liver injury. Blood 2011; 118:1943–1951. [DOI] [PubMed] [Google Scholar]
- 19.Sandset PMA, Abildgaard U, Larsen ML. Heparin Induces release of extrinsic coagulation pathway inhibitor (EPI). Thromb Res 1988; 50:803–813. [DOI] [PubMed] [Google Scholar]
- 20.vantVeer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem 1997; 272:4367–4377. [DOI] [PubMed] [Google Scholar]
- 21.Dockal M, Hartmann R, Fries M, Thomassen MC, Heinzmann A, Ehrlich H, et al. Small peptides blocking inhibition of factor Xa and tissue factor-factor VIIa by tissue factor pathway inhibitor (TFPI). J Biol Chem 2014; 289:1732–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knappe S, Gorczyca ME, Jilma B, Derhaschnig U, Hartmann R, Palige M, et al. Plasmatic tissue factor pathway inhibitor is a major determinant of clotting in factor VIII inhibited plasma or blood. Thromb Haemost 2013; 109:450–457. [DOI] [PubMed] [Google Scholar]
- 23.Hilden I, Lauritzen B, Sorensen BB, Clausen JT, Jespersgaard C, Krogh BO, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood 2012; 119:5871–5878. [DOI] [PubMed] [Google Scholar]
- 24.Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, et al. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood 2011; 117:5514–5522. [DOI] [PubMed] [Google Scholar]
- 25.Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, et al. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood 2008; 111:672–679. [DOI] [PubMed] [Google Scholar]
- 26.Zekavat OR, Haghpanah S, Dehghani J, Afrasiabi A, Peyvandi F, Karimi M. Comparison of thrombin generation assay with conventional coagulation tests in evaluation of bleeding risk in patients with rare bleeding disorders. Clin Appl Thromb Hemost 2013; 20:637–644. [DOI] [PubMed] [Google Scholar]
- 27.Peraramelli S, Thomassen S, Heinzmann A, Rosing J, Hackeng TM, Hartmann R, et al. Direct inhibition of factor VIIa by TFPI and TFPI constructs. J Thromb Haemost 2013; 11:704–714. [DOI] [PubMed] [Google Scholar]
- 28.Spronk HMH, Dielis AWJH, Panova-Noeva M, van Oerle R, Govers-Riemslag JWP, Hamulyak K, et al. Monitoring thrombin generation: is addition of corn trypsin inhibitor needed? Thromb Haemost 2009; 101:1156–1162. [PubMed] [Google Scholar]
- 29.van Veen JJ, Gatt A, Cooper PC, Kitchen S, Bowyer AE, Makris M. Corn trypsin inhibitor in fluorogenic thrombin-generation measurements is only necessary at low tissue factor concentrations and influences the relationship between factor VIII coagulant activity and thrombogram parameters. Blood Coagul Fibrinolysis 2008; 19:183–189. [DOI] [PubMed] [Google Scholar]
- 30.Altman R, Scazziota A, de Lourdes Herrera M, Gonzalez CD. The hemostatic profile of recombinant activated factor VII. Can low concentrations stop bleeding in off-label indications? Thromb J 2010; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pychynska-Pokorska M, Pogwska-Klimek I, Krajewski W, Moll JJ. Use of recombinant activated factor VII for controlling refractory postoperative bleeding in children undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2011; 25:987–994. [DOI] [PubMed] [Google Scholar]
- 32.Hacquard M, Lecompte T, Belcour B, Geschier C, Jacquot C, Jacquot E, et al. Evaluation of the hemostatic potential including thrombin generation of three different therapeutic pathogen-reduced plasmas. Vox Sang 2012; 102:354–361. [DOI] [PubMed] [Google Scholar]
- 33.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010; 363:1791–1800. [DOI] [PubMed] [Google Scholar]
- 34.Guzzetta NA, Szlam F, Kiser AS, Fernandez JD, Szlam AD, Leong T, et al. Augmentation of thrombin generation in neonates undergoing cardiopulmonary bypass. Br J Anaesth 2014; 112:319–327. [DOI] [PubMed] [Google Scholar]
- 35.Demeyere R, Gillardin S, Arnout J, Strengers PF. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang 2010; 99:251–260. [DOI] [PubMed] [Google Scholar]
- 36.Grottke O, Rossaint R, Henskens Y, van Oerle R, Ten Cate H, Spronk HM. Thrombin generation capacity of prothrombin complex concentrate in an in vitro dilutional model. PLoS One 2013; 8:e64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gissel M, Orfeo T, Foley JH, Butenas S. Effect of BAX499 aptamer on tissue factor pathway inhibitor function and thrombin generation in models of hemophilia. Thromb Res 2012; 130:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/- thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost 2006; 96:562–567. [PubMed] [Google Scholar]
- 39.Castoldi E, Simioni P, Tormene D, Thomassen MC, Spiezia L, Gavasso S, et al. Differential effects of high prothrombin levels on thrombin generation depending on the cause of the hyperprothrombinemia. J Thromb Haemost 2007; 5:971–979. [DOI] [PubMed] [Google Scholar]
- 40.ten Cate-Hoek AJ, Dielis AW, Spronk HM, van Oerle R, Hamulyak K, Prins MH, et al. Thrombin generation in patients after acute deep-vein thrombosis. Thromb Haemost 2008; 100:240–245. [PubMed] [Google Scholar]
- 41.Yang L, Vuylsteke A, Gerrard C, Besser M, Baglin T. Postoperative fibrinogen level is associated with postoperative bleeding following cardiothoracic surgery and the effect of fibrinogen replacement therapy remains uncertain. J Thromb Haemost 2013; 11:1519–1526. [DOI] [PubMed] [Google Scholar]
- 42.Ternstrom L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A, et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res 2010; 126:E128–E133. [DOI] [PubMed] [Google Scholar]
- 43.Holloway DS, Summaria L, Sandesara J, Vagher JP, Alexander JC, Caprini JA. Decreased platelet number and function and increased fibrinolysis contribute to postoperative bleeding in cardiopulmonary bypass patients. Thromb Haemost 1988; 59:62–67. [PubMed] [Google Scholar]


