Abstract
Purpose of review
This article reviews recently published evidence for common pathways explaining bone and muscle wasting in normal ageing and pathological conditions.
Recent findings
Numerous studies support the concept of a bone–muscle unit, where constant cross-talking between the two tissues takes place, involving molecules released by the skeletal muscle secretome, which affects bone, and osteokines secreted by the osteoblasts and osteocytes, which, in turn, impact muscle cells.
Summary
New chemical entities aiming at concomitantly treating osteoporosis and sarcopenia could be developed by targeting pathways that centrally regulate bone and muscle or emerging pathways that facilitate the communication between the two tissues.
Keywords: bone, muscle, myostatin, osteoporosis, sarcopenia
INTRODUCTION
Loss of bone and muscle with advancing age represents a huge threat to loss of independence in later life [1]. Osteoporosis is defined as a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture. Osteoporotic fractures, a major cause of morbidity in the population, are associated with increased mortality and generate direct costs in excess of 35 billion euros, in 2010, in the 27 EU countries [2]. Sarcopenia corresponds to a progressive and generalized loss of muscle mass with either a loss of muscle strength or a loss of physical performance. However, a single consensual operational definition of sarcopenia is lacking and none of the definitions, proposed so far, unequivocally emerge as providing benefits over previous ones [3], leading to inconsistent reports across cohorts on its prevalence [3,4]. Nevertheless, there is a wide consensus to consider that consequences of sarcopenia, including physical disability, nursing home admissions, depression, hospitalizations and mortality are linked to direct healthcare costs estimated in 2000, in the USA, to raise up to 18.5 billion USD [5]. During the last decade, bone and muscle were increasingly recognized as interacting tissues, not only because of their adjacent surfaces or as a result of the mechanical effects of muscle loading on bone function [6▪]. In this perspective, the ‘bone–muscle’ unit would be the site of privileged exchanges in which the two tissues communicate via paracrine and endocrine signals to coordinate their development and adapt their response to loading and injury from embryologic stages to involution [6▪,7▪▪]. Growing evidence shows that sarcopenia and osteoporosis share many common pathways including the sensitivity to reduced anabolic hormone secretion, increased inflammatory cytokine activity, anabolic or catabolic molecules released by the skeletal muscle or by the bone cells (i.e. myokines and osteokines) and eventually, reduced physical activity [7▪▪,8]. With adipose tissue and cartilage being also involved in their complex interactions [7▪▪] came the suggestion that obesity, sarcopenia and osteoporosis could be concomitantly found in a subset of the population, presenting with an entity called osteosarcopenic obesity (OSO) with health outcomes likely to be worse compared with individuals with only one of these disorders [9,10]. This manuscript will review publications issued over the last 18 months which help to better understand the complex relationship between osteoporosis and sarcopenia, hopefully paving the way for the development of chemical entities that are able to target both diseases.
Box 1.
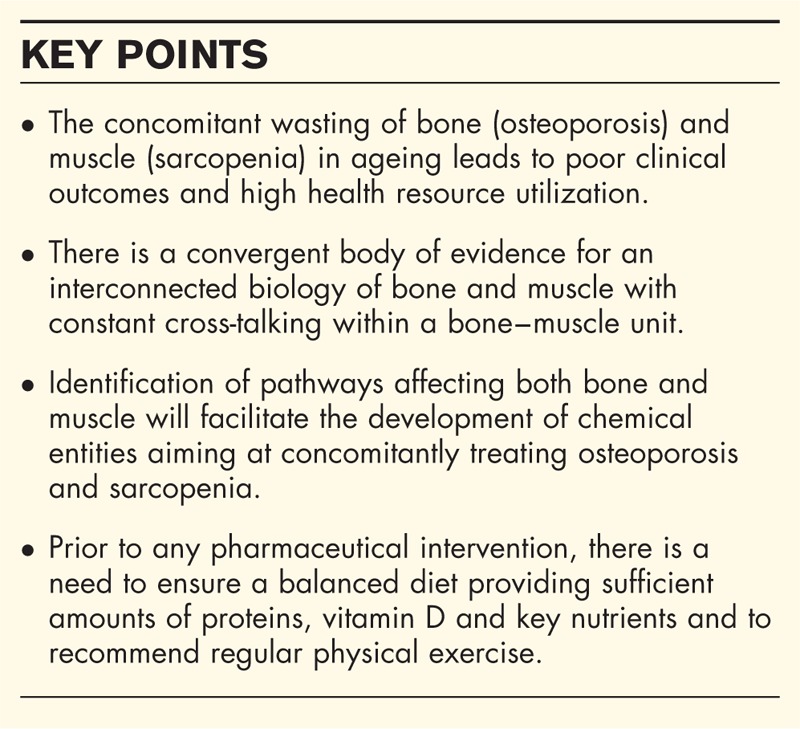
no caption available
INTERACTIONS BETWEEN BONE AND MUSCLE: REVIEW ARTICLES
Several reviews are published with the aim to summarize the current knowledge on the connections between bone, muscle and cartilage, and in some cases fat tissue, or to better understand the common pathogenic pathways between osteoporosis and sarcopenia. We shall discuss here the major features of what we consider as the prominent and most relevant of these review articles.
Isaacson and Brotto [11] elegantly discuss the emerging research on the putative ‘bone–muscle cross-talk’ departing from the traditional view of prominent mechanical interactions, suggesting a biochemical channel of communication between bones and muscles. Their review focuses on the paracrine and endocrine communication between these tissues. After reviewing the respective roles of bones and muscles as biochemical communicators, the authors discuss the physiological relevance of the endocrine properties observed in these tissues. They suggest that these interactions serve to sense and transduce biochemical signals such as unloading, loading, inactivity or exercise and maybe as a translation of systemic hormonal simulation into effective biochemical signals. They take osteocalcin (OCN), an osteoblast-derived protein, as an example of the endocrine function of bone cells, proposing that OCN may play a role in the regulation of muscle mass and be a target for prevention or treatment of sarcopenia. The paracrine nature of the bone–muscle cross-talk is suggested to take place at the muscle fibre insertion sites along the periosteal interfaces. Inflammatory molecules from adjacent muscle fibres may penetrate the underlying bone and promote fracture healing. They also quote studies from their own group suggesting that prostaglandin E2 is secreted 1000 times more by osteocytes than from muscle cells interplay with injured muscles, and aid in regeneration and repair. The authors eventually suggest the development of highly specific bone–muscle models to allow manipulation of skeletal genes and factors and observe their impact on the bone–muscle units [11].
Tagliaferri et al.[7▪▪] provide an in-depth analysis of the studies considering bone as a target of skeletal muscle secretory pattern or describing the potential effects of bone on muscle metabolism. They appropriately include in their analysis the potential role of cartilage, tendon and adipose tissue in the musculoskeletal control loop. They first review the concept of the ‘bone–muscle unit’ which is evidenced phenotypically by the observation of a linear relationship at various ages between bone mineral content or density (BMD) and lean body mass. The muscle–bone cross-talk is also supported by preclinical data, showing its presence even before birth in mammals. Emphasizing that bone remodelling appears to be sensitive to both external loads arising from gravitational loading as well as to internal loads generated by muscular activity, they describe the cellular and molecular mechanisms responsible for the adaptations made in response to mechanosensation. The authors adequately point out that the muscle secretome consists of several hundred secreted peptides, providing a whole new paradigm for understanding how muscles communicate with other organs including bones. They extensively review the evidence that several molecules released by the muscle affect bone, including insulin-like growth factor-1, fibroblast-growth factor-2, IL-6, IL-15, myostatin, osteoglycin (OGN), family with sequence similarity 5, member C (FAM5C), transmembrane protein 119 (Tmem119) and osteoactivin. However, much less studies were dedicated to studying the reverse channel (i.e. from bone to muscle); both osteoblasts and osteocytes were shown to secrete osteokines. The effects on muscle of prostaglandin E2 and wingless-type MMTV integration site family, member 3A (Wnt3a), which are secreted by osteocytes, OCN and insulin-like growth factor-1 produced by osteoblasts and sclerostin (SOST) which is secreted by both cell types are convincingly described and discussed. The authors also further approach the role of adipose tissue on both skeletal muscle and bone, including the production of leptin, adiponectin and IL-6 by adipocytes. A major feature of this article is the inclusion of the chondrocytes, the cartilage cells, in a more global system of paracrine communication. Indeed, cartilage shares the mesenchymal stem cells origin with bone and muscle and is located in close proximity. Chondrocytes release Dickkopf-1, a regulatory molecule of the Wnt-pathway and Indian hedgedog, both substances that significantly influence the bone resorption/formation balance. As in the previously reported study [11], Tagliaferri et al.[7▪▪] suggest to avoid neglecting the components (i.e. ligament and tendon) of the zone of interaction between bone and muscle. They conclude their article by re-emphasizing the beneficial impact of physical exercise and/or a balanced diet, including key ingredients like proteins, calcium, vitamin D and various micronutrients or specific fatty acids, a comment in accordance with recent recommendations for bone and muscle health issued by international scientific societies [12▪].
Kaji [13] also describes the interaction between muscle and bone and the genetic, endocrine and mechanical factors affecting both muscle and bone simultaneously. The author describes fibrodysplasia ossificans progressiva as an important pathologic situation linking bone to muscle. Within the local factors affecting muscle ossification, he suggests the role of Tmem119, a parathyroid hormone-responsive osteoblast differentiation factor, also mentioned in [7▪▪], to be a putative local inducer of muscle ossification. He also discusses the potential role of OGN, a small leucine-rich proteoglycan as a muscle-derived bone anabolic factor and the interest of myostatin, a member of the transforming growth factor-beta family, which apart from its well-known inhibition of muscle growth, might have a potential role for preventing osteocytes apoptosis trough activation of beta-catenin. The properties of these molecules were also discussed in the Tagliaferri et al. review [7▪▪].
Compared with the previous review articles, Ormsbee et al. [9] focus their manuscript on the population presenting with an interconnection of osteopenia/osteoporosis, sarcopenia and obesity, and suggest to use the term ‘osteosarcopenic obesity’ to describe the appearance of obesity in patients with low bone and muscle mass. They compare the tools used for the assessment of abnormal body composition phenotypes, also extensively reviewed by Cooper et al. [14], in the historical perspective of the OSO concept and the challenges related to its operationalization and applicability, mainly linked to the debate existing on its diagnosis. They review the hypothetical mechanisms underlying the condition. They suggest that an increase in total and/or abdominal adipose tissue causes an increase in pro-inflammatory cytokines, as well as some hormonal disturbances leading to losses of both muscle and bone. The decrease in muscle and bone is associated with a decrease in physical activity leading to a vicious cycle of progressive loss of muscle and bone and a gain in fat. They describe the potential clinical implications. OSO generates direct but even higher indirect costs (linked to absenteeism, disability and premature mortality), and it is likely that these individuals will present with poorer clinical outcomes caused by the cascade of metabolic abnormalities associated with the changes in their body composition. They conclude by recommending a multifactorial nonpharmacological approach including long-term resistance training, and an adjustment of the proteins/carbohydrate ratio in the diet for reducing adiposity and maintaining muscle and bone mass. This article should be linked to Ilich et al. who offer a similar description of the OSO syndrome [15].
Cederholm et al. [8] concentrate their review article on the tight relationship existing between sarcopenia and the occurrence of osteoporotic fractures. They re-emphasize the critical role played by mechanical forces created by muscle contractions on bone density, strength and architecture. They elegantly discuss the common pathogenic pathways for osteoporosis and sarcopenia including the sensitivity to reduced anabolic hormone secretion, increased inflammatory cytokine activity and reduced physical activity. The importance of sufficient vitamin D levels for bone and muscle health is reported, as in [12▪], with the appropriate suggestion to concentrate on sun exposure in younger individuals but to rely more on dairy or pharmacological supplementation in elderly patients. As suggested in [9], regular and long-term resistance training, together with adequate access to energy and protein, is considered as the basis of treatment to improve muscle health and reduce the risk of fractures.
INTERACTION BETWEEN BONE AND MUSCLE: RESEARCH ARTICLES
The molecular factors, through which bone and muscle communicate, via biochemical messengers, are not yet fully identified. A genome-wide association study identified methyl-transferase-like 21C, a member of the seven-beta-strand methyl-transferase superfamily, recently also identified as a member of a new group of distantly related lysine methyl-transferase, as a potential pleiotropic gene for both bone and muscle. Huang et al. [16▪] provide evidence that methyl-transferase-like 21C exerts its pleiotropic function through the regulation of the nuclear factor kappa B (NF-kB) signalling pathway. NF-kB is one of the most critical signalling pathways in muscles with its activation leading to muscle loss. In bone, NF-kB signalling is involved in corticosteroid-induced osteocyte apoptosis but also mediates receptor activator of NF-kB ligand-induced osteoclastogenesis. These results are the first in-vitro studies validating potential bone–muscle pleiotropic genes.
In the previously discussed review articles [9,15], the authors foresee poorer clinical outcomes for individuals presenting the triad constitutive of OSO compared with patients suffering from sarcopenia, osteoporosis or obesity alone. A small sample of patients with OSO, with sarcopenia (defined only on morphological parameters) and obesity or with osteoporosis/osteopenia (defined by a BMD T score ≤ −1) and obesity, were compared for handgrip strength, normal/brisk walking speed and leg stance with a group of obese-only women. Women with OSO presented with the lowest handgrip scores, slowest normal and brisk walking speed and shortest time for each leg stance. These results support the assumption that OSO patients have a poorer functionality than women with isolated disorders, hence being more prone to falls, osteoporotic fractures, hypodynamism and combined decline in muscle and bone mass [17].
The presence of sarcopenia (European Working Group on Sarcopenia in Older People definition) in patients who recently experienced hip fracture is identified in 58% of the cases and is associated with lower ability to function in activities of daily living, compared with presarcopenic women. This may reflect an increased risk of posthip fracture complications, additional health resource utilization and higher incidence of recurrent contralateral hip fracture [18]. In a population of young patients (20–69 years) with a femoral neck fracture, those with low-energy trauma have significantly lower femoral neck BMD and fat-free mass index than patients with other trauma mechanisms. These results re-emphasize the association between low bone and muscle mass in patients experiencing hip fractures and the need for a comprehensive management of such patients [19]. Two reports from a large sample of Chinese community-dwelling men [20] or men and women [21] aged 65 and older demonstrate that sarcopenia is a predictor of fracture risk independent of BMD and other clinical risk factors [20], and that the diagnosis of sarcopenia adds incremental value to the fracture risk assessment tool algorithm [2] in predicting incident fracture in men but not in women [21]. Similarly, in an elderly Korean population including volunteers from both sexes, individuals with sarcopenia identified by dual energy X-ray absorptiometry (DXA) have a significantly higher risk of osteoporosis (low BMD). This relationship appears in men but not in women, in accordance to what is seen in the Chinese population [22]. A few differences are observed in a comprehensive list of parameters reflecting nutritional status in Australian volunteers (mean age 79 years) from both sexes, at increased risk for falls and presenting with osteopenia/osteoporosis, sarcopenia, the combination of both diseases (SOP) or none of them (normal). Compared with normal patients, SOP are more likely to have a lower multinutritional assessment, lower BMI and lower serum haemoglobin. Lower albuminaemia is associated with osteoporosis and sarcopenia alone compared with normal. No differences are seen in vitamin D, glomerular filtration rates, calcium, phosphate, red blood cells folate or vitamin B12 levels between the groups [23]. These results slightly differ from those observed in Italian hip-fractured patients in which patients with slow muscle mass, assessed by bioelectrical impedance (comparison of bioimpedance and DXA is discussed in [24]), have a lower intake of calories, proteins and leucine [25]. The latter study shows the importance of detecting nutritional deficits predisposing to muscle atrophy in patients with osteoporotic fractures.
INTERVENTIONS AND TREATMENTS
At this stage, no randomized controlled trial assessing the concomitant effects of a new chemical entity (NCE) on bone and muscle is published. Gingis et al. [6▪] suggest that better understanding the interconnected biology of bone and muscle may shift our treatment paradigm for musculoskeletal disease to, as mentioned in the title of their article, ‘Kill two birds with one stone’ (i.e. treat frail or sarcopenic patients and prevent fractures). As potential strategies, they recommend either to target pathways that centrally regulate both bone and muscle (e.g. growth hormone and growth hormone secretagogues, androgens, selective androgen receptor modulators, vitamin D, etc.) or investigate newly emerging pathways that might facilitate the communication between the two tissues [e.g. activin signalling inhibitors, including myostatin-neutralizing antibodies-propeptides (also discussed in [26]), recombinant follistatin, follistatin derivatives and soluble activin receptors or myokines (extensively discussed in [7▪▪])]. These authors, as it is also the case in [7▪▪], [8], [9], [12▪] and [27], acknowledge the critical importance of regular exercise and adequate nutrition to optimize peak bone mass and maintain bone and muscle health throughout life. In this perspective, two intervention trials are worth a comment. Bauer et al.[28] report, in a well designed 13-week randomized controlled trial, the beneficial effects of a vitamin D and leucine-enriched whey protein oral nutraceutical supplement on muscle mass and lower-extremity function among sarcopenic older adults [28]. Knowing the above-mentioned importance of vitamin D and proteins for bone health [12▪], this nutritional supplement could also take place in the armamentarium against osteoporosis. Chahal et al. [29] investigate the impact of various intensities and frequencies of loading doses of physical activity on knee extension torque and broadband ultrasound attenuation at the heel in middle-aged women. They conclude that physical activity, especially at high intensity level and high frequency range may have beneficial effects on muscle strength and bone density in this particular population. A lot of discussions take place on the usefulness of active vitamin D metabolites in the management of osteoporosis [30]. However, Tanaka et al. [31] investigate the effects of 1,25-dihydroxyvitamin D3 on myoblastic and osteoblastic differentiation of precursor cells through OGN expression. They conclude that active vitamin D treatment may rescue the advanced glycation end products-induced sarcopenia as well as advanced glycation end products-suppressed osteoblastic differentiation via OGN expression in myoblasts.
Whole body vibration (WBV) is a popular exercise where individuals stand on an oscillating plate and the motor transmits vertical acceleration to muscles and bones [32]. This intervention was previously shown to increase the anabolic effect of bone tissues as well as to increase bone volume and area. WBV is also suggested to improve muscle performance [33]. During the period covered by the present review, three studies assessing the effects of WBV on bone and muscle were published. In osteopenic postmenopausal women, 12 months of WBV do not positively impact and even decrease calcaneal broadband ultrasound attenuation at the heel [34]. In another study, 6 months of high-frequency and high-magnitude WBV yield significant increase in spinal BMD in postmenopausal women [32]. In elderly institutionalized volunteers from both sexes, 6 months of low doses of WBV fail to influence functional and motor abilities, measured after 1 year [35]. Apparently, these discrepant results may be linked to a lack of consensus on the optimal WBV equipment, dose, energy, frequency or duration.
CONCLUSION
Osteoporosis and sarcopenia are two disorders, predominantly affecting elderly patients and responsible for a major clinical and financial burden. Increase in life expectancy in most countries and in both sexes makes their diagnosis, prevention and treatment a major social and ethical, yet unmet, medical need. Genetic, developmental, paracrine, endocrine and lifestyle factors have dual effects on bone muscle and bone mass and function. The evidence of biochemical and molecular interactions between the two tissues need to be further explored for the development of NCE against these twin conditions of ageing, osteoporosis and sarcopenia. Targeting pathways that centrally regulate bone and muscle or newly pathways that facilitate communication between the two tissues are the privileged directions for the identification of NCE, which could simultaneously prevent, reduce or restore bone and muscle age-related wasting. It seems wise for companies developing such agents for the management of osteoporosis or sarcopenia to include, within the secondary endpoint of their trials, outcomes parameters (e.g. DXA, biochemical markers, imaging, etc) reflecting the effect of these drugs on bone if developed against sarcopenia or on muscle if developed against osteoporosis. However, the importance of physical exercise (i.e. long-term resistance training) and the need for a balanced diet providing sufficient amounts of proteins, calcium, vitamin D and various micronutrients should not be underestimated.
Acknowledgements
The authors would like to thank Francine Bonvalet and Fabienne Damblon for their secretarial assistance in the preparation of the manuscript.
Financial support and sponsorship
None.
Conflicts of interest
J.-Y.R. has received research grant and/or consulting fees from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed-Takeda, NPS, IBSA-Genevrier, Theramex, UCB, Asahi Kasei, Endocyte, Merck Sharp and Dohme, Rottapharm, Teijin, Teva, Analis, NovoNordisk, Ebewee Pharma, Zodiac, Will Pharma, Meda, Bristol Myers Squibb. C.B. and F.B. have no conflicts of interest. O.B. has received research grant and/or consulting fees from IBSA, Merck Sharp & Dohme, Novartis, Nutraveris, Pfizer, Rottapharm, Servier, SMB, Theramex, Bayer, and Genevrier.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol 2015; 230:2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013; 24:23–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuy C, Lauwers-Cances V, Guyonnet S, et al. Searching for a relevant definition of sarcopenia: results from the cross-sectional EPIDOS study. J Cachexia Sarcopenia Muscle 2015; 6:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol 2015; 61:31–37. [DOI] [PubMed] [Google Scholar]
- 5.Beaudart C, Rizzoli R, Bruyere O, et al. Sarcopenia: burden and challenges for public health. Arch Public Health 2014; 72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Girgis CM, Mokbel N, Digirolamo DJ. Therapies for musculoskeletal disease: can we treat two birds with one stone? Curr Osteoporos Rep 2014; 12:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]; An interesting review summarizing the various pathways likely to provide insight for the development of concomitant treatments for osteoporosis and sarcopenia.
- 7▪▪.Tagliaferri C, Wittrant Y, Davicco MJ, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015; 21:55–70. [DOI] [PubMed] [Google Scholar]; An exhaustive, clear, and robust overview of the current knowledge in the field of bone–muscle cross-talking.
- 8.Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 2013; 49:111–117. [PubMed] [Google Scholar]
- 9.Ormsbee MJ, Prado CM, Ilich JZ, et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 2014; 5:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidya R. Obesity, sarcopenia and postmenopausal osteoporosis: an interlinked triad!. J Midlife Health 2014; 5:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacson J, Brotto M. Physiology of mechanotransduction: how do muscle and bone ‘talk’ to one another? Clin Rev Bone Miner Metab 2014; 12:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Rizzoli R, Stevenson JC, Bauer JM, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014; 79:122–132. [DOI] [PubMed] [Google Scholar]; An expert consensus offering clear recommendations for dietary protein and vitamin D intake in postmenopausal women.
- 13.Kaji H. Interaction between muscle and bone. J Bone Metab 2014; 21:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 2013; 93:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilich JZ, Kelly OJ, Inglis JE, et al. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev 2014; 15:51–60. [DOI] [PubMed] [Google Scholar]
- 16▪.Huang J, Hsu YH, Mo C, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J Bone Miner Res 2014; 29:1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]; A genetic study providing rationale for cellular and muscular validation of genome-wide association studies in the musculoskeletal field.
- 17.Ilich JZ, Inglis JE, Kelly OJ, McGee DL. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos Int 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Di Monaco M, Castiglioni C, De Toma E, et al. Presarcopenia and sarcopenia in hip-fracture women: prevalence and association with ability to function in activities of daily living. Aging Clin Exp Res 2015; 27:465–472. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ani AN, Cederholm T, Saaf M, et al. Low bone mineral density and fat-free mass in younger patients with a femoral neck fracture. Eur J Clin Invest 2015; 45:800–806. [DOI] [PubMed] [Google Scholar]
- 20.Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc 2014; 15:551–558. [DOI] [PubMed] [Google Scholar]
- 21.Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc 2014; 15:918–923. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Won CW, Kim BS, et al. The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci 2014; 29:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo YR, Suriyaarachchi P, Gomez F, et al. Comprehensive nutritional status in sarco-osteoporotic older fallers. J Nutr Health Aging 2015; 19:474–480. [DOI] [PubMed] [Google Scholar]
- 24.Buckinx F, Reginster JY, Dardenne N, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord 2015; 16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvani R, Martone AM, Marzetti E, et al. Prehospital dietary intake correlates with muscle mass at the time of fracture in older hip-fractured patients. Front Aging Neurosci 2014; 6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buehring B, Binkley N. Myostatin—the holy grail for muscle, bone, and fat? Curr Osteoporos Rep 2013; 11:407–414. [DOI] [PubMed] [Google Scholar]
- 27.Daly RM, Duckham RL, Gianoudis J. Evidence for an interaction between exercise and nutrition for improving bone and muscle health. Curr Osteoporos Rep 2014; 12:219–226. [DOI] [PubMed] [Google Scholar]
- 28.Bauer JM, Verlaan S, Bautmans I, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE Study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015; 16:740–747. [DOI] [PubMed] [Google Scholar]
- 29.Chahal J, Lee R, Luo J. Loading dose of physical activity is related to muscle strength and bone density in middle-aged women. Bone 2014; 67:41–45. [DOI] [PubMed] [Google Scholar]
- 30.Cianferotti L, Cricelli C, Kanis JA, et al. The clinical use of vitamin D metabolites and their potential developments: a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine 2015; 50:12–26. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Kanazawa I, Yamaguchi T, et al. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochemical and biophysical research communications 2014; 450:482–487. [DOI] [PubMed] [Google Scholar]
- 32.Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging 2013; 8:1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards JH, Reilly GC. Vibration stimuli and the differentiation of musculoskeletal progenitor cells: review of results in vitro and in vivo. World J Stem Cells 2015; 7:568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slatkovska L, Beyene J, Alibhai SM, et al. Effect of whole-body vibration on calcaneal quantitative ultrasound measurements in postmenopausal women: a randomized controlled trial. Calcif Tissue Int 2014; 95:547–556. [DOI] [PubMed] [Google Scholar]
- 35.Buckinx F, Beaudart C, Maquet D, et al. Evaluation of the impact of 6-month training by whole body vibration on the risk of falls among nursing home residents, observed over a 12-month period: a single blind, randomized controlled trial. Aging Clin Exp Res 2014; 26:369–376. [DOI] [PubMed] [Google Scholar]


