Abstract
Ibogaine, an alkaloid derived from the African shrub Tabernanthe iboga, has shown promising anti-addictive properties in animals. Anecdotal evidence suggests that ibogaine is also anti-addictive in humans. Thus, it alleviates drug craving and impedes relapse of drug use. Although not licensed as therapeutic drug, and despite evidence that ibogaine may disturb the rhythm of the heart, this alkaloid is currently used as an anti-addiction drug in alternative medicine. Here we report that therapeutic concentrations of ibogaine reduce currents through human ERG potassium channels. Thereby, we provide a mechanism by which ibogaine may generate life-threatening cardiac arrhythmias.
Keywords: Anti-addiction drug, cardiac arrhythmias, hERG potassium channels, ibogaine, indole alkaloid
Drug abuse and addiction are associated with major medical and social problems worldwide. In view of the unsatisfactory efficacy of currently available drugs to treat addiction, the search for new potentially anti-addictive compounds is of outstanding importance.
In preclinical studies on animals, ibogaine, an indole alkaloid derived from the root bark of the African shrub Tabernanthe iboga, has shown promising anti-addictive properties (Glick & Maisonneuve 1998; Alper 2001): ibogaine attenuates opioid withdrawal signs and reduces the self-administration of a variety of drugs including opioids, cocaine, nicotine, and alcohol. The inhibition of dopamine release in the nucleus accumbens as essential part of the brain’s reward systems offers an explanation for ibogaine’s anti-addictive actions. The underlying molecular mechanisms may involve interactions with neurotransmitter transporters as well as opioid and glutamate receptors, effects that have been observed at ibogaine concentrations between 0.1 and 30 µM (Glick & Maisonneuve 1998; Alper 2001).
In humans, ibogaine has a long history of use as a medicinal and ceremonial agent in West Central Africa. Besides its own psychoactive properties, anecdotal evidence suggests that ibogaine is also anti-addictive in humans, because it alleviates drug craving and impedes relapse of drug use (Mash et al. 1998; Alper 2001). In 1993, the U.S. Food and Drug Administration (FDA) approved a clinical trial in humans to study the effects of ibogaine. The National Institute on Drug Abuse (NIDA), however, decided not to fund this study because of safety concerns (Alper 2001). In spite of its status as a banned substance in the U.S. and some European countries, ibogaine is legal in most of the world. Currently, it is often used as an anti-addiction drug in alternative medicine (Vastag 2005).
Since ibogaine interacts with numerous different cellular and molecular targets (Glick & Maisonneuve 1998; Mash et al. 1998; Alper 2001), its potential to generate adverse effects is significant. Besides the expected neurotoxic actions, ibogaine also affects the cardiovascular system (Alper 2001). In both animals and humans, high doses of ibogaine decrease the heart rate. Alarming are several reported cases of sudden deaths with unclear cause after ibogaine use, which have been hypothesised to be related to cardiac arrhythmias. In accordance with this hypothesis, a severely prolonged QT interval of the electrocardiogram (ECG), associated with ventricular tachyarrhythmias, was observed in a 31-year-old woman after she had taken a single dose of ibogaine (Hoelen, Spiering & Valk 2009).
Here, we provide first experimental evidence on the mechanism by which ibogaine may generate life-threatening cardiac arrhythmias: inhibition of human ERG (hERG) potassium channels in the heart. Conducting the rapid component of the delayed rectifier potassium current IKr, hERG channels are crucial for the repolarisation phase of cardiac action potentials (Sanguinetti & Tristani-Firouzi 2006). hERG current reduction, either due to genetic defects or blockade by drugs, delays cardiac repolarisation resulting in QT interval prolongation and in an increased risk for torsade de pointes arrhythmias and sudden cardiac death. Consequently, hERG channel blockade has become a common reason for drug failure in preclinical safety trials (Redfern et al. 2003; Sanguinetti & Tristani-Firouzi 2006).
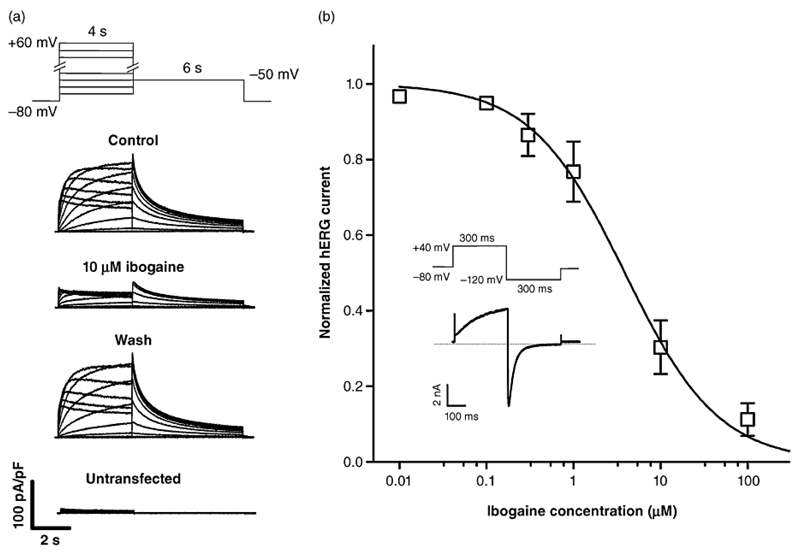
We show in Fig. 1 that ibogaine reduces currents through hERG channels, heterologously expressed in TSA-201 cells, in a reversible and concentration-dependent manner. The ibogaine concentration required to inhibit the current by 50 % (IC50) was 4 µM. Drugs with little or no margin between their therapeutic plasma concentrations and their IC50 values for hERG inhibition delay cardiac repolarisation (Redfern et al. 2003). Ibogaine plasma concentrations in humans are in the low micromolar range after single oral doses of 500-1000 mg (Mash et al. 1998; Mash et al. 2000), doses that are typically employed to treat drug addicts (10-25 mg/kg of body weight) (Mash et al. 1998; Mash et al. 2000; Alper 2001). Hence, our finding of hERG channel blockade by low micromolar ibogaine concentrations explains the previously published case report of QT interval prolongation after ibogaine intake (Hoelen et al. 2009). Moreover, drugs that have their IC50 values for hERG inhibition in the range of their therapeutic plasma concentrations, such as astemizole, cisapride, and terodiline, have been withdrawn from the market because of their high propensity to cause torsade de pointes arrhythmias (Redfern et al. 2003). As the same holds true for ibogaine, the use of this indole alkaloid in humans is highly risky. Ibogaine derivatives with reduced propensity to block hERG channels but preserved anti-addictive properties need to be developed.
Figure 1. hERG channel blockade by ibogaine.
A) Typical whole cell currents through hERG potassium channels heterologously expressed in a TSA-201 cell. Currents elicited by the pulse protocol on top under control conditions, after steady-state block with 10 µM ibogaine, and after wash out are displayed. In addition, typical endogenous currents of an untransfected TSA cell are shown. hERG currents were recorded at 22 ± 1.5°C using the whole-cell patch clamp technique as in our earlier work (Zebedin et al. 2008). In this study, the hERG channel blocker sotalol was used to validate the expression system employed. B) Concentration-response curve for the reduction of hERG tail currents by ibogaine. The inset shows the pulse protocol applied at a frequency of 1 Hz. Tail current peaks are plotted against ibogaine concentrations. Values are means ± SD (n = 4-17). The solid line represents a sigmoidal fit to the data points; Hill coefficient: 0.81 ± 0.04; IC50 value: 3.9 ± 0.3 µM.
Acknowledgements
Ibogaine was kindly donated by Sacrament of Transition (Maribor, Slovenia). This work was funded by the Austrian Science Fund (FWF, P19352 and P23060 to KH). There are no conflicts of interest.
Footnotes
Authors Contribution
XK, SB, WS and KH were responsible for the study concept and design. XK and MK acquired the electrophysiological data and assisted with data analysis. KH, SB, and XK interpreted the findings. KH drafted the manuscript. XK, MK, SB, and WS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- 1.Alper KR. Ibogaine: a review. Alkaloids Chem Biol. 2001;56:1–38. doi: 10.1016/s0099-9598(01)56005-8. [DOI] [PubMed] [Google Scholar]
- 2.Glick SD, Maisonneuve IS. Mechanisms of antiaddictive actions of ibogaine. Ann N Y Acad Sci. 1998;844:214–226. [PubMed] [Google Scholar]
- 3.Hoelen DW, Spiering W, Valk GD. Long-QT syndrome induced by the antiaddiction drug ibogaine. N Engl J Med. 2009;360:308–309. doi: 10.1056/NEJMc0804248. [DOI] [PubMed] [Google Scholar]
- 4.Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn WL, Sanchez-Ramos J. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann N Y Acad Sci. 1998;844:274–292. [PubMed] [Google Scholar]
- 5.Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, Singleton EG, Mayor M. Ibogaine: complex pharmacokinetics, concerns for safety, and preliminary efficacy measures. Ann N Y Acad Sci. 2000;914:394–401. doi: 10.1111/j.1749-6632.2000.tb05213.x. [DOI] [PubMed] [Google Scholar]
- 6.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 8.Vastag B. Addiction research. Ibogaine therapy: a 'vast, uncontrolled experiment'. Science. 2005;308:345–346. doi: 10.1126/science.308.5720.345. [DOI] [PubMed] [Google Scholar]
- 9.Zebedin E, Koenig X, Radenkovic M, Pankevych H, Todt H, Freissmuth M, Hilber K. Effects of duramycin on cardiac voltage-gated ion channels. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:87–100. doi: 10.1007/s00210-007-0248-5. [DOI] [PubMed] [Google Scholar]