In many animal societies where hierarchies govern access to reproduction, the social rank of individuals is related to their age and weight1–5 and slow-growing animals may lose their place in breeding queues to younger ‘challengers’ who grow faster than they do5,6. The threat of being displaced may be expected to favour the evolution of competitive growth strategies, where individuals increase their own rate of growth in response to increases in the growth of potential rivals. While growth rates have been shown to vary in relation to changes in the social environment in several vertebrates including social fish2,3,7 and mammals8, it is not yet known whether individuals increase their growth rates in response to increases in the growth of particular reproductive rivals. Here we show that, in wild Kalahari meerkats (Suricata suricatta), subordinates of both sexes respond to experimentally induced increases in the growth of same-sex rivals by raising their own growth rate and food intake. In addition, when individuals acquire dominant status, they show a secondary period of accelerated growth whose magnitude increases if the difference between their own weight and that of the heaviest subordinate of the same sex in their group is small. Our results show that individuals adjust their growth to the size of their closest competitor and raise the possibility that similar plastic responses to the risk of competition may occur in other social mammals, including domestic animals and primates.
Recent studies have revealed the extent to which aspects of the social environment can affect growth in several vertebrates. In some social fish, the risk of conflict with dominant individuals reduces the growth rates of subordinates2,3,7 while, in some mammals, prenatal growth increases in response to physiological stress levels in pregnant mothers in high-density environments8. However, studies have not yet investigated whether adolescents or adults can adjust their growth rates in relation to changes in the size of specific rivals who may displace them in reproductive queues. In many cooperatively breeding mammals, subordinates of both sexes queue for reproductive opportunities in breeding groups, sometimes for several years5,9. Rank in these queues is usually determined by relative age and weight, and previous research has produced some evidence of strategic adjustments in growth. In mole-rats and meerkats, adult females that acquire the dominant breeding position commonly show a period of secondary growth10–12 which may allow them to increase their fertility or consolidate their status5,13. Here, we describe experiments that investigate whether subordinate meerkats queuing for breeding opportunities also engage in competitive growth.
Meerkats live in groups of 3–50 individuals where 90% of reproduction is monopolised by a single dominant pair5. Subordinates of both sexes contribute to costly cooperative activities, including pup-feeding, babysitting and raised-guarding14. Within groups, subordinates of the same sex are ranked in a hierarchy based on age and weight15. If the breeding female dies, the oldest and heaviest subordinate typically replaces her, and subordinate females occasionally displace breeders5. Unlike females, most males leave their natal groups voluntarily when they are 2–4 years old in small parties of two to six individuals, and attempt to displace males in other groups5,16. If they are successful, the oldest and heaviest male usually assumes the breeding position5,16. Data presented here are derived from a twenty-year study of wild meerkats that has encompassed more than sixty groups in which all individuals were recognisable. Most individuals were trained to climb onto electronic balances and were weighed three times a day (dawn; after three hours of foraging; and dusk) on approximately ten days a month throughout their lives5. Changes in the weight of individuals between the beginning and end of morning foraging sessions provide a measure of their food intake.
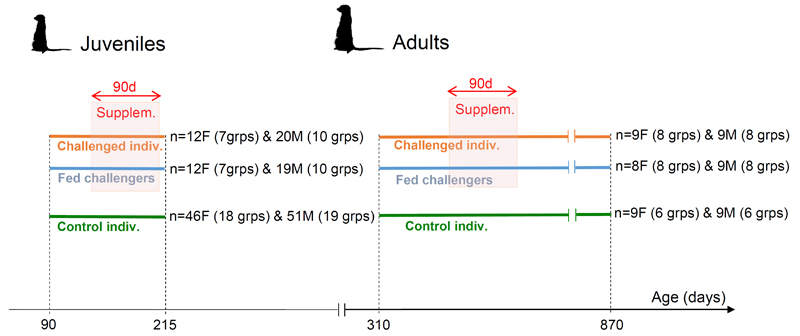
Using 14 groups of habituated meerkats, we manipulated the growth of subordinates of both sexes by provisioning particular individuals and measuring effects on the growth and food intake of individuals of the same sex immediately above them in the age-related hierarchy. We identified pairs of same-sex littermates belonging to two distinct age classes: juveniles (aged 4–7 months), who had recently reached nutritional independence (n=12 female and 19 male litters from 12 groups), and young adults (aged 12–24 months), who had reached sexual maturity and were able to compete for any breeding vacancies that occurred5 (n=8 female and 9 male litters from 14 groups). In each pair, we fed the lighter individual, later referred to as the ‘challenger’, with half a hard-boiled egg twice per day for three months. We subsequently compared the growth of unfed littermates, referred to as ‘challenged’ individuals, with those of unfed control individuals of the same age from other litters over the same period (Extended Data Figure 1).
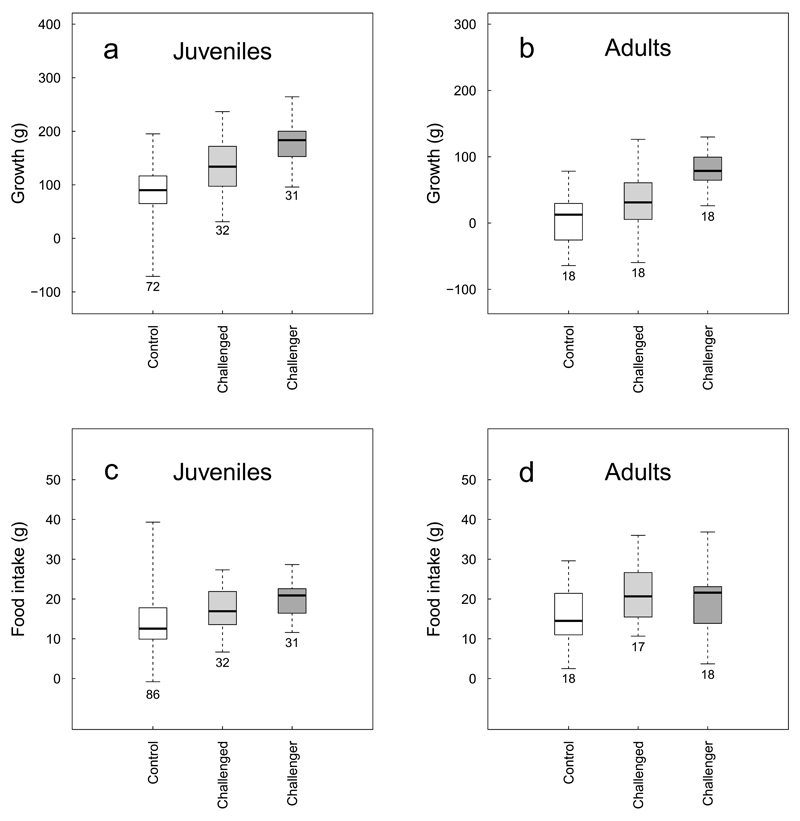
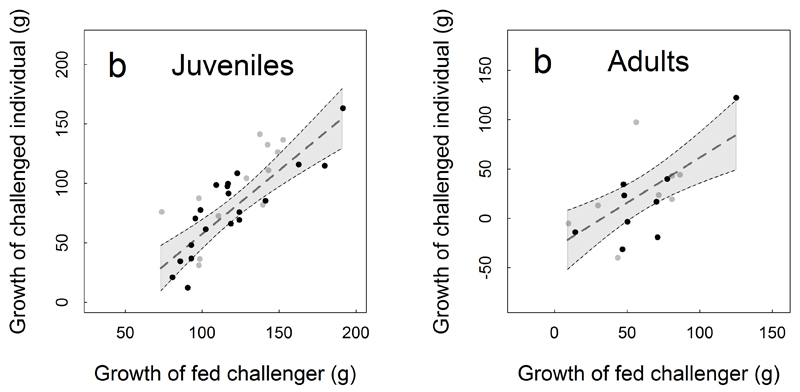
Challenged individuals of both age classes responded to increases in the growth of fed challengers by increasing their average weight (both in absolute terms and relative to controls) over the course of the experiment. Growth from the start to the mid-point of the experiment was greater in challenged than in control individuals (Figure 1a-b; juveniles: two sample Welch t-test, n=32 challenged and 72 control individuals, t=4.17, P<10-4; adults: n=18 challenged and 18 age- and sex-matched control individuals, paired t-test, t=2.10, df=17, P=0.050), generating a difference in the average weight of challenged and control individuals halfway through the experiment (juveniles: n=32 challenged and 83 control individuals, 504.3±68.2g vs. 438.5±73.2g, two-sample Welch t-test, t=4.54, P<10-4, adults: pairwise weight difference=40.7±51.06g, paired t-test, t=3.38, df=17, P=0.003). Differences in growth were, however, no longer detectable in the second half of the experiment (Juveniles: n=27 challenged and 74 control individuals, two-sample Welch t-test, t=0.22, P=0.825; adults: paired t-test, t=-24.23, df=17, P=0.059), suggesting that challenged individuals may not be capable of sustaining accelerated growth over extended periods. In both age classes, the growth of challenged individuals over the first half of the experiment was positively correlated with the growth of their fed challenger (Extended Data Figure 2, Extended Data Table 1), suggesting that challenged individuals adjusted their growth response to the growth of their rival. Increases in the growth of challenged individuals were associated with increases in food intake: food intake was greater for challenged than for control individuals in the first half of the experiment (Figure 1c-d, juveniles: n=32 challenged and 86 control individuals, two-sample Welch t-test, t=2.17, P=0.033, adults: paired t-test: t=2.80, df=16, P=0.013), but not in the second half (Juveniles: n=29 challenged and 83 control individuals, two-sample Welch t-test, t=1.19, P=0.240; adults: paired t-test: t=-0.16, df=16, P=0.876).
Figure 1. Competitive growth in subordinates.
Boxplots showing the growth (individual weight difference between the start and mid-point of the experiment) (panels a, b) and food intake (average morning weight gain in the first half of experiment) (panels c, d) of unfed, ‘challenged’ individuals (light grey boxes) and of their fed ‘challengers’ (dark grey boxes) relative to control individuals (white boxes) in juveniles (panels a, c) and adults (panels b, d). Whiskers comprise all data points. Numbers below the boxes indicate the number of individuals.
Social mechanisms other than cooperative growth could conceivably contribute to increases in the growth of challenged animals, but we were unable to find any evidence that this was the case. It is unlikely that potential increases in the contributions of fed challengers to cooperative activities in the first half of experiment reduced the contributions of challenged animals and so increased their weight gain. First, juveniles contribute little to cooperative activities, so accelerated growth in challenged juveniles cannot be mediated by changes in cooperative behaviour. Second, challenged adults maintained their investment in raised-guarding and pup-feeding in the same period relative to control animals (Wilcoxon signed-rank paired-test, raised-guarding: V=52, df=17, P=0.156, pup-feeding: V=30, df=14, P=0.095). Finally, adult fed challengers increased their contributions to raised guarding but not to pup-feeding (Wilcoxon signed-rank paired-test: raised-guarding: V=143, df=17, P=0.013, pup-feeding: V=67, df=14, P=0.719).
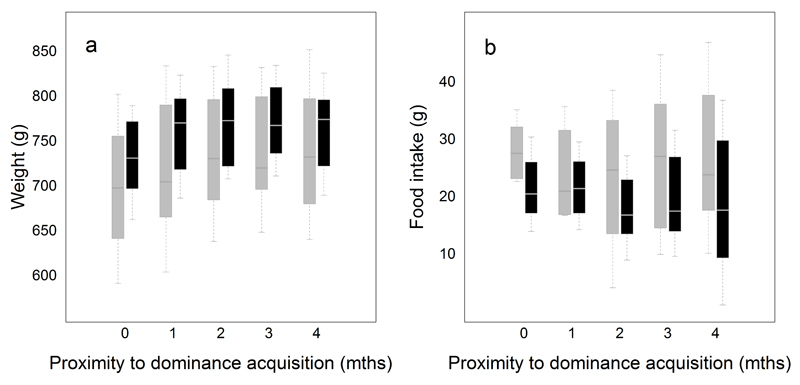
Additional analyses suggest that adults that acquire dominant positions may also adjust their growth rates in a strategic fashion. In both sexes, the lifetime breeding success of dominant meerkats depends on the length of time they hold the dominant position5 which, in females, increases with the difference between their own weight and the weight of the heaviest subordinate of the same sex5. Since subordinates engage in competitive growth, we examined whether individuals that have recently acquired the dominant position adjust the magnitude of their subsequent increase in weight to the relative weight of their closest rival. We first analysed whether newly dominant males and females increase their growth rate following dominance acquisition by comparing their weight in the month prior to dominance acquisition and in the four months following dominance acquisition. New dominants of both sexes increased in weight after acquiring dominance (analysis of variance with repeated measures, effect of month post-dominance acquisition on weight: F4,184=16.81, P<10-4, Figure 2a, Extended Data Figure 3a). The extent of growth following dominance acquisition did not differ between the sexes (analysis of variance with repeated measures, interaction between sex and month post-dominance acquisition: F4,184=1.22, P=0.31) and primarily occurred in the two months following dominance acquisition (see Extended Data Table 2 for the results of the post-hoc tests). This growth response may not solely reflect improved access to resources, as food intake remained constant in both sexes during the same period (analysis of variance with repeated measures, effect of month post-dominance acquisition on food intake: F4,112=0.34, P=0.850, and interaction between sex and month post-dominance acquisition: F4,112=0.09, P=0.986, Extended Data Figure 3b).
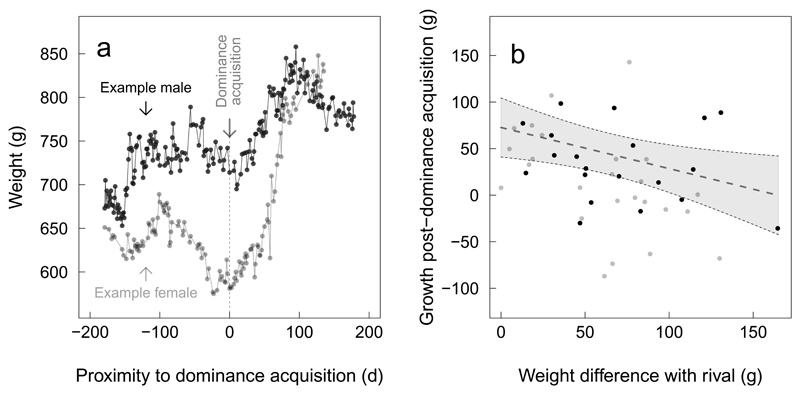
Figure 2. Competitive growth in dominants.
Panel a: example growth trajectories of a male and female during their transition to dominance. Panel b: adjustment of growth following dominance acquisition in response to social competition in 20 males and 25 females. Dots show the raw values (grey for females, black for males) of dominant weight gain within the 150 days following dominance acquisition as a function of weight difference to the heaviest same-sex subordinate (measured at dominance acquisition). The dotted line shows the predicted values of the linear model (results presented in Extended Table 3) and standard deviations of the predicted values are delineated by shaded areas.
The growth of new dominants in the five months following dominance acquisition was more pronounced when the heaviest same-sex subordinate was closer to their own weight at the time of dominance acquisition (Linear Model, estimate±SD=-0.76±0.27, F1,36=7.69, P<0.01, Figure 2b and Extended Data Table 3). There was no significant sex difference in this accelerated growth (Extended Data Table 3). Rapid post-dominance growth exacerbated existing weight differences between dominants and same-sex subordinates, with the result that most established dominants were the heaviest individual of their sex in their group (females: 58% of groups, males: 68%). While similar periods of growth after dominance acquisition in female naked mole-rats have been interpreted as a way of enhancing fecundity11,12,17, the presence of strategic growth adjustments to the relative size of rivals in dominant meerkats of both sexes suggests that these increases may serve to consolidate their status and prolong their breeding tenure5,13.
Our findings suggest that subordinates can track changes in the growth and size of potential competitors, perhaps using physical contact as well as visual, vocal or olfactory cues, and react by adjusting their own growth. While the physiological correlates of increased growth rates in challenged individuals are not yet known, hormonal changes associated with heightened threat of competition may increase growth and food intake. Acceleration in growth following dominance acquisition is probably associated with the sudden lifting of reproductive suppression and a re-orientation of life-history strategy. The hormonal profile of dominant meerkats is distinct from that of subordinates, with higher plasmatic levels of oestradiol and progesterone in breeding females and of cortisol in breeders of both sexes10,18,19. Sex steroids are known to regulate the production of critical actors in the insulin/growth factor pathway in the mammalian reproductive tract and associated tissues20, which may result in the up-regulation of anabolic genes involved in growth. Strategic increases in growth rates could be constrained by energy and fitness costs21. Allocation of additional resources to growth by challenged individuals may depress immune function and reduce longevity as a result of increases in oxidative stress and telomere shortening22 while increases in time spent foraging may raise predation risk, which is high in meerkats23.
Our results suggest that competitive growth may represent an important component of the developmental strategy of individuals. Recognition of this process may alter classic perspectives on mechanisms of social competition, which frequently suggest that the phenotype of interacting individuals determines the outcome of competitive interactions rather than vice versa. As reproductive queues are widespread in social mammals and the size and weight of individuals often affect their status and breeding success24, competitive growth may occur in many other social species, possibly including domestic mammals, nonhuman primates and humans.
Methods
Study site and population
Data were collected between 1996 and 2013 as part of a long-term study of wild meerkats at the Kuruman River Reserve, South Africa. The site experiences a hot-wet season (October–April) and a cold-dry season (May–September), with extensive interannual variation in rain23. Rainfall was measured daily (mm) using a standard gauge27. Details regarding the site and population are published elsewhere5,14,23.
Meerkats were habituated to humans and individually recognizable due to dye marks. Groups were visited about three times a week, so life-history events (births, deaths, emigrations, changes in dominance) were known to an accuracy of about three days5,14. Pregnancy status was inferred from parturition date and affects female weight from the midpoint of gestation, lasting approximately 70 days25. Females were considered pregnant from 40 days prior to parturition or from the first day of detectable pregnancy in cases where abortions occurred. Dominant individuals were identified by their behaviour towards group-mates4,5. They scent-marked more frequently than subordinates, and asserted their dominance over others by anal marking, by rubbing them with their chin, and more rarely by attacking and biting them. Changes in dominance were immediately recognizable, as they were often preceded by a short period (hours to days) of intense fighting, and were accompanied by dramatic changes in behaviour in the contesting individuals. Previous genetic work has shown the absence of incestuous matings within groups4. If all immigrant males die, a natal male may become socially dominant in his group. Natal dominant males do not mate-guard the dominant female, who is often their mother, and regularly conduct extraterritorial forays for mating opportunities26. These males (77/166 dominant males in our dataset) have been excluded from analyses.
Weight measures
Individuals were trained to climb onto a laboratory balance in return for drops of water or crumbs of hard-boiled egg, allowing us to record body weight to an accuracy of 1g (Figure 1a). Although individuals were often weighed three times a day, we only used data collected in the morning right after emergence from the burrow and before foraging, to avoid noise created by variation in foraging success throughout the day27. Food intake, or morning weight gain, was calculated as the difference between weight collected before foraging activity started, and weight collected after about three hours of foraging10.
Cooperative behaviour
Three cooperative activities are regularly performed by male and female meerkats14: (1) babysitting newborn pups, where an individual stays at the burrow while the rest of the group forages, (2) feeding pups that are old enough to join foraging trips (approximately 1–3 months old), and (3) raised-guarding, where an individual ceases foraging and climbs to a raised position to watch out for potential dangers. The occurrence of babysitting, pup-feeding and raised-guarding was recorded ad libitum as events during observation sessions, allowing quantification of relative rates of helping per individual, i.e. the number of occurrences of one cooperative behaviour performed by one individual relative to the total number of occurrences of that behaviour in the group over a given time period.
Competitive growth experiment
From 2010 to 2013, we conducted a set of 3-month feeding experiments on adults aged 310–870 days and on juveniles aged 111–215 days to investigate whether unfed littermates (challenged individuals) would increase their growth rate in response to experimentally elevated growth rates of their fed siblings (challengers). We identified pairs containing at least two same-sex littermates and fed the individual that was lightest (or as heavy as its sibling) when the experiment started (mean weight difference (±SD) in juveniles: 9.8±30.6g, in adults: 29.9±28.2g). The fed individuals received half an egg twice daily four times a week during three months. Competitive growth has never been described previously, so no prior information was available for power analyses to establish adequate sample sizes. For 17 fed adults including 8 females, the shortest feeding bout lasted 55 days and the mean±SD feeding duration was 84±11 days. For 31 fed juveniles including 12 females, the shortest feeding bout lasted 21 days and the mean±SD feeding duration was 76±21 days. For one adult female litter and one juvenile male litter, there were three same-sex siblings and the two lightest individuals were very close in weight (i.e., their average weight difference was lower than 10g in the 15 days preceding the experiment); one of them was fed, and the two unfed siblings were included in the cohort of challenged individuals. Experiments were interrupted when a pregnancy was detected in an experimental female (fed or unfed), and corresponding data were excluded from analysis. In other cases where the experiment was aborted (e.g., if an individual disappeared), data collected during the shortened period were included in analyses; note that for three juvenile dyads, food supplementation lasted respectively 21, 23 and 26 days, so these individuals were excluded from all calculations related to measures describing the second half of the experiment. Observations and weighing sessions were not subjected to blinding, because weight gained by fed individuals during the experiment was often detectable by observers.
Statistical analysis
Competitive growth in subordinates
In order to investigate the effect of feeding individuals on the growth of their unfed same-sex littermate, we first calculated the growth and food intake, averaged over the first or the second half of the experiment for challenged individuals, challengers and control individuals. Growth was calculated as the individual difference between weight recorded immediately prior to the start of the experiment and at the mid-point of the experiment (45 days), or as the individual change in weight from the mid-point to the end of the experiment (90 days). Food intake, calculated in terms of morning weight gain, was averaged for each individual, over days 5–45 of the experiment (the first four days were excluded to allow for potential adjustments in challenged individuals) and then over experimental days 45–90. We compared these measures across challenged and control individuals using two-sample Welch t-tests (for juveniles) and paired t-tests (for adults) after checking that variance was homogeneous across groups using Levene tests (P>0.05 in all cases). We focus on the contrast between challenged and control individuals: significantly higher growth in challenged individuals over controls would provide experimental evidence for competitive growth, defined as an elevated increase in growth in response to the challenge of a fed rival. Control individuals were selected as any individual from the population during the experimental period (2010–2013) that had a lighter same-sex littermate in his/her group at the age at which supplemental feeding commenced in experimental groups (120 days in juveniles, one year in adults), in order to match criteria used to identify unfed individuals in experimental dyads (Extended Data Figure 1). In adults, where heterogeneity in the age at the start of the experiment was considerable (361–772 days, mean±SD=496.7±112.9 days), each challenged individual was matched to the same-sex individual of the control cohort that was closest in age (differences in birth dates between challenged individuals and their matched control were small: 2–32 days, mean+SD=11.2±8.4) and present in the population at the time of the experiment. Matching each experimental individual with a same-age and same-sex control in this way allows us to control for environmental variation that may otherwise introduce noise when comparing the weight and growth of individuals that underwent a supplementation at different time periods (e.g. during the dry versus the wet season). Individual weight prior to the experiment was averaged across the 15 days preceding the experiment, weight at mid-point was averaged across days 45–60 of the experiment, and weight at the end of the experiment was averaged across experimental days 90–105.
It was not possible to select such matched control individuals in juveniles, however, as there was no control litter born shortly before or after experimental litters in several cases. Small age differences can introduce important noise when comparing weights among juveniles, because growth rates are relatively high between four and seven months of age, compared to later ages27. In the juvenile cohort, age at the start of the experiment was very homogeneous (range: 111–128 days of age, mean±SD=122.3±4.7), so matching experimental dyads with control individuals by age was deemed less necessary. Individual weight records were averaged across 95–110 days of age (before experiment); 170–185 days of age (after ca. 45 days of experiment); and 215–230 days of age (after ca. 90 days of experiment), and growth was calculated between these time points.
We further ran a linear model investigating the relationship between the growth of challenged individuals and the growth of their fed challenger to test whether the growth responses of challenged individuals were adjusted to the weight gain of their fed challenger. Growth was the response variable, and was calculated as the weight difference between the start and the mid-point of the experiment (since the above analyses suggested that competitive growth was highest at this time). Explanatory variables included sex, age at start of experiment, and cumulated rainfall in the past nine months, which was previously found to influence the growth of individual meerkats27. Results and sample sizes are presented in Extended Data Table 1 and Extended Data Figure 2.
Changes in cooperative activities during experimental periods
We investigated the influence of the experiment on pup-feeding and raised-guarding rates in the adult cohort only, because helping is rare before six months of age14. We did not consider babysitting because less than half of the experimental groups exhibited babysitting during the experiment. For each observation session, we measured the observed proportion of raised-guarding events performed by the focal individual relative to the total number of events recorded for the group. We then calculated individual deviation from the proportion expected under the null hypothesis, where each individual contributes equally, calculated as the inverse of the number of helpers in the group. We averaged this deviation across all observation sessions for each individual during the first half of the experiment (10–120 sessions per individual, median=19). Thus, mean deviation gives an indication of the extent of cooperative behaviour relative to average contributions in the group: individuals with a larger, more positive deviation have higher cooperative behaviour. We compared the mean deviations between challenged individuals and their matched controls using paired Wilcoxon signed-rank tests, as the response variable was not normally distributed. We used the same approach to test for differences in individual contributions to pup-feeding between challenged and control individuals.
Growth after the acquisition of dominant status
When investigating changes in weight following dominance acquisition, we considered individuals that maintained dominance for at least six months, to avoid biasing the sample towards short and unstable tenures. We averaged weight records for each individual (n=42 females and 30 males) across the 30 days preceding dominance acquisition (labelled “month 0”) and then across days 0–30; 30–60; 60–90 and 90–120 following dominance acquisition (respectively labelled “months 1, 2, 3 and 4”). Weights recorded during pregnancies were excluded. We then retained only individuals with no missing data in any of these five one-month blocks (n=21 females and 27 males) to ensure a balanced design. Thus, we could evaluate the significance of weight differences between one-month blocks using a repeated measures analysis of variance with multiple factors. Factors included sex, proximity to dominance acquisition (with 5 levels: month 0, 1, 2, 3 and 4), and the interaction between sex and proximity to dominance acquisition, to test if the temporal dynamics of post-dominance growth differed between males and females. Post-hoc tests were conducted using paired t-tests with adjusted p-values to compare within-individual changes in weight before dominance acquisition to each of the four months after acquisition; as well as between each month of the four month period following acquisition of dominance. A Bonferroni correction was applied to correct for multiple testing. These results are presented in Extended Data Figure 3a and Extended Data Table 2.
We compared changes in food intake (measured as morning weight gain) following dominance acquisition using the same approach. As described above, we retained only individuals with no missing data in any of the five one-month blocks (n=9 females and 21 males) to evaluate the significance of differences in food intake between one-month blocks using a repeated measures analysis of variance with multiple factors. As above, factors included were sex, proximity to dominance acquisition and their interaction. These results are illustrated by Extended Data Figure 3b.
Dominant growth adjustments to the weight of the heaviest subordinate
To investigate the effect of competition on growth following dominance acquisition, we ran a Linear Model, with weight gain within 150 days following dominance acquisition (calculated as weight 150 days after dominance acquisition minus weight at dominance acquisition, each averaged across all weights for 10 days prior to and after the time-point of interest) as our response variable. We focused on a five-month period after dominance acquisition, because previous models had revealed that growth rates were elevated in the four months following dominance acquisition. We included all new dominant females that retained dominance for longer than six months and had at least one subordinate female in their group that was older than six months when they became dominant. Six months is the age of the youngest female who ever reached dominance. Weights recorded during pregnancies were excluded. We included all new dominant males that had at least one non-natal subordinate male in their group that was older than six months when they became dominant. Natal subordinate males were not considered as rivals because they hardly ever reproduce or fight for dominance4. Explanatory variables included sex, rainfall (averaged over the 150 days following dominance acquisition), a sinusoidal term describing season of dominance acquisition27, age at dominance acquisition, time since dominance acquisition (ranging from 0 to 150 days), absolute weight difference with the same-sex rival (i.e., heaviest subordinate at the time of dominance acquisition). In addition, the interaction between sex and absolute weight difference with the same-sex rival tested whether the effect of the weight difference to the main rival differed between sexes. We used the absolute value of weight difference because graphical exploration of the data suggested that dominant growth rates increase when their rival is either slightly heavier or slightly lighter, but not when their rival is much lighter or much heavier. In cases where a rival is much heavier but failed to win fights over dominance, he or she may have poor competitive abilities for other reasons and may not represent a threat to the dominant. The results and sample sizes are presented in Extended Data Table 3.
All statistical analyses were run with R 3.1.328, and all tests were two-sided.
Extended Data
Extended Data Figure 1. Diagram depicting the experimental design.
Juvenile experiments were conducted from 15/12/2010 to 19/08/2012, and adult experiments from 28/03/2011 to 20/07/2013. Each horizontal line represents longitudinal weight data collected from an experimental group. Thick orange lines represent unfed, challenged individuals and blue lines represent fed challengers. Thick green lines represent control individuals, which were animals of the same sex and age-range from the same population over the same period (2010–2013). Red boxes indicate the 3-month experimental windows of food supplementation, which spanned different time periods for different dyads (allowing us to disentangle experimental effects from environmental and seasonal effects on weight) and, for the adult experiment, occurred any time between 310 and 870 days of age. F: female, M: male. Note that the x-axis is not drawn to scale, to facilitate comparison of the design between the juvenile and adult cohorts. The meerkat icon was downloaded from PhyloPic: http://phylopic.org, with credit to Michael Keesey.
Extended Data Figure 2. Relationship between the growth of the challenged individual and the growth of its fed challenger in juveniles (a) and adults (b).
Thirty-two juvenile and 17 adult experimental pairs were included. Growth was calculated as the individual weight difference between the start and mid-point of the experiment. Dots show the raw values (grey for females, black for males). The dotted line shows the predicted values of the linear model (results presented in Extended Table 1) and standard deviations of the predicted values are delineated by shaded areas.
Extended Data Figure 3. Changes in weight (a) and food intake (b) in new dominant females (grey boxes, n=42) and males (black boxes, n=30).
Boxplots show the raw values, averaged for each individual during the month preceding dominance acquisition (labelled ‘0’), as well as during the 1st, 2nd, 3rd and 4th months post-dominance acquisition (respectively labelled ‘1’, ‘2’, ‘3’ and ‘4’). Whiskers show all data points that are no further away from the box than half the interquartile range.
Extended Data Table 1. Results of linear models investigating the relationship between the growth of challenged individuals and their fed challengers in juveniles and adults.
The response variable is the growth of the challenged individual, calculated as the individual weight difference between the start and mid-point of the experiment. The juvenile model includes 12 females and 20 males and the value of the model adjusted R2 is 0.65. The adult model includes 8 females and 9 males and the value of the model adjusted R2 is 0.61. Est.: Estimate, SD: standard deviation.
| Variable | Est. | SE | DF | F-value | P-value |
|---|---|---|---|---|---|
| JUVENILES | |||||
| Growth of fed challenger (g) | 1.068 | 0.17 | 27 | 39.43 | <10-4 |
| Sex | -14.178 | 8.50 | 27 | 2.78 | 0.107 |
| Age | 0.726 | 0.94 | 27 | 0.59 | 0.448 |
| Rainfall | 0.012 | 0.09 | 27 | 0.02 | 0.897 |
| ADULTS | |||||
| Growth of fed challenger (g) | 0.916 | 0.24 | 12 | 14.72 | 0.002 |
| Sex | 6.143 | 13.99 | 12 | 0.19 | 0.668 |
| Age | -0.164 | 0.06 | 12 | 7.16 | 0.020 |
| Rainfall | 0.205 | 0.08 | 12 | 7.19 | 0.020 |
Extended Data Table 2. Results of the posthoc paired t-tests investigating temporal changes in weight following dominance acquisition.
Pairwise comparison tests were conducted after the repeated measures ANOVA to compare within-individual changes in weight between the month preceding dominance acquisition (labelled ‘0’) and the four months (labelled ‘1’ to ‘4’) following dominance acquisition, as well as between each of the four months post-dominance acquisition. A Bonferroni correction was applied to correct for multiple testing.
| Proximity of dominance acquisition (months) | |||||
|---|---|---|---|---|---|
| df=47 for all tests | |||||
| 1 | 2 | 3 | 4 | ||
| Proximity dominance acquisition (months) | |||||
| 0 | t=4.34, p<0.001 | t=5.83, p<10-4 | t=7.28, p<10-4 | t=5.09, p<10-4 | |
| 1 | – | t=3.52, p<0.001 | t=3.94, p=0.003 | t=2.63, p=0.115 | |
| 2 | – | – | t=0.90, p=1.000 | t=0.14, p=1.000 | |
| 3 | – | – | – | t=0.78, p=1.000 | |
| 4 | – | – | – | – | |
Extended Data Table 3. Results of the linear model investigating changes in body weight within 150 days following dominance acquisition in relation to absolute weight difference with the heaviest same-sex subordinate.
This analysis includes 25 females and 20 males. The value of the model adjusted R2 is 0.21. Est.: Estimate, SD: standard deviation, and F-value: F-statistic of an F-test.
| Variable | Est. | SE | DF | F-value | p-value |
|---|---|---|---|---|---|
| Age at dominance acquisition (days) | -0.030 | 0.02 | 36 | 2.59 | 0.117 |
| Sex (reference: female) | -5.541 | 28.75 | 36 | 0.04 | 0.848 |
| Rainfall (mm) | -0.270 | 0.11 | 36 | 5.65 | 0.023 |
| Seasonality | 5.425 | 11.04 | 36 | 0.24 | 0.626 |
| Weight gap with main rival (g) | -0.758 | 0.27 | 36 | 7.69 | 0.009 |
| Sex : weight gap with main rival | 0.597 | 0.39 | 36 | 2.29 | 0.139 |
Acknowledgements
We are extremely grateful to the many volunteers, field managers, PhD students and post-docs who have contributed to data collection over the past 15 years, and to D. Gaynor, I. Stevenson, P. Roth, J. Samson, R. Millar, E. Cameron, J. du Toit and M. Haupt for invaluable support. We are grateful to M. Manser for her contribution to the organization of the Kalahari Meerkat Project (KMP). We also thank Dom Cram for comments on previous drafts, and Andrew Bateman, Alexandre Courtiol, and Mick Crawley for statistical advice. Northern Cape Conservation and the Kotze family kindly provided permission to work in the Kalahari. Our work was approved by the Animal Ethics Committee of the University of Pretoria (Project number: EC010-13). The KMP is supported and organized by the Universities of Cambridge and Zurich. This research was supported by NERC (Grant RG53472) and the ERC (Grant 294494).
Footnotes
Author contributions
EH implemented the analysis and drafted the results; THCB, SE and MB planned the experiments which were conducted by NT and other members of the Kalahari Meerkat Project; EH, SE, MB and THCB wrote the paper.
Reprints and permissions information is available at http://www.nature.com/reprints/index.html.
References and Notes
- 1.Hoogland JL. The black-tailed prairie dog: social life of a burrowing mammal. University of Chicago Press; Chicago: 1995. [Google Scholar]
- 2.Buston PM. Social hierarchies: size and growth modification in clownfish. Nature. 2003;424:145. doi: 10.1038/424145a. [DOI] [PubMed] [Google Scholar]
- 3.Heg D, Bender N, Hamilton WD. Strategic growth decisions in helper cichlids. Proc R Soc Lond Ser B-Biol Sci. 2004;271:S505. doi: 10.1098/rsbl.2004.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH. Factors affecting reproductive success of dominant male meerkats. Mol Ecol. 2008;17:2287. doi: 10.1111/j.1365-294X.2008.03734.x. [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH, et al. Intrasexual competition and sexual selection in cooperative mammals. Nature. 2006;444(7122):1065. doi: 10.1038/nature05386. [DOI] [PubMed] [Google Scholar]
- 6.Reeve HK, Peters JM, Nonacs P, Starks PT. Dispersal of first “workers” in social wasps: causes and implications of an alternative reproductive strategy. Proc Natl Acad Sci USA. 1998;95:13737. doi: 10.1073/pnas.95.23.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong MYL, Munday PL, Buston PM, Jones GP. Fasting or feasting in a fish social hierarchy. Curr Biol. 2008;18:R372. doi: 10.1016/j.cub.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer B, et al. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science. 2013;340:1215. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- 9.Hauber ME, Lacey EA. Bateman's principle in cooperatively breeding vertebrates: The effects of non-breeding alloparents on variability in female and male reproductive success. Integr Comp Biol. 2005;45(5):903. doi: 10.1093/icb/45.5.903. [DOI] [PubMed] [Google Scholar]
- 10.Russell AF, et al. Adaptive size modification by dominant female meerkats. Evolution. 2004;58:1600. doi: 10.1111/j.0014-3820.2004.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 11.Young AJ, Bennett NC. Morphological divergence of breeders and helpers in wild damaraland mole-rat societies. Evolution. 2010;64:3190. doi: 10.1111/j.1558-5646.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 12.Dengler-Crish CM, Catania KC. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J Exp Biol. 2007;210:4351. doi: 10.1242/jeb.009399. [DOI] [PubMed] [Google Scholar]
- 13.Clutton-Brock T. Structure and function in mammalian societies. Phil Trans R Soc B. 2009;364:3229. doi: 10.1098/rstb.2009.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clutton-Brock TH, et al. Evolution and development of sex differences in cooperative behavior in meerkats. Science. 2002;297:253. doi: 10.1126/science.1071412. [DOI] [PubMed] [Google Scholar]
- 15.Thavarajah NK, Fenkes M, Clutton-Brock TH. The determinants of dominance relationships among subordinate females in the cooperatively breeding meerkat. Behaviour. 2014;151:89. [Google Scholar]
- 16.Doolan SP, Macdonald DW. Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricatta) in the south-western Kalahari. J Zool. 1996;240:59. [Google Scholar]
- 17.O'Riain MJ, et al. Morphological castes in a vertebrate. Proc Natl Acad Sci USA. 2000;97:13194. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson AA, et al. Hormonal correlates of dominance in meerkats (Suricata suricatta) Horm Behav. 2004;46(2):141. doi: 10.1016/j.yhbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Young AJ, Monfort SL, Clutton-Brock TH. The causes of physiological suppression among female meerkats: a role for subordinate restraint due to the threat of infanticide? Horm Behav. 2008;53:131. doi: 10.1016/j.yhbeh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Dantzer B, Swanson EM. Mediation of vertebrate life histories via insulin-like growth factor-1. Biol Rev. 2012;87:414. doi: 10.1111/j.1469-185X.2011.00204.x. [DOI] [PubMed] [Google Scholar]
- 21.Arendt JD. Adaptive intrinsic growth rates: An integration across taxa. Q Rev Biol. 1997;72:149. [Google Scholar]
- 22.Metcalfe NB, Monaghan EP. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 23.Clutton-Brock TH, et al. Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. J Anim Ecol. 1999;68:672. [Google Scholar]
- 24.Clutton-Brock T, Huchard E. Social competition and selection in males and females. Philosophical Transactions of the Royal Society B-Biological Sciences. 2013;368:20130074. doi: 10.1098/rstb.2013.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp SP, English S, Clutton-Brock TH. Maternal investment during pregnancy in wild meerkats. Evol Ecol. 2013;27:1033. [Google Scholar]
- 26.Young AJ, Spong G, Clutton-Brock TH. Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc R Soc B. 2007;274:1603. doi: 10.1098/rspb.2007.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.English S, Bateman AW, Clutton-Brock TH. Lifetime growth in wild meerkats: incorporating life history and environmental factors into a standard growth model. Oecologia. 2012;169:143. doi: 10.1007/s00442-011-2192-9. [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]