Abstract
Glossopharyngeal insufflation (lung packing) is a common maneuver among experienced apnea divers by which additional air is pumped into the lungs. It has been shown that packing may compromise cardiovascular homeostasis. We tested the hypothesis that the packing-mediated increase in intrathoracic pressure enhances the baroreflex-mediated increase in muscle sympathetic nerve activity (MSNA) in response to an exaggerated drop in cardiac output (CO). We compared changes in hemodynamics and MSNA (peroneal microneurography) during maximal breath-holds without and with prior moderate packing (0.79 ± 0.40 liters) in 14 trained divers (12 men, 2 women, 26.7 ± 4.5 yr, body mass index 24.8 ± 2.4 kg/m2). Packing did not change apnea time (3.8 ± 1.0 vs. 3.8 ± 1.2 min), hemoglobin oxygen desaturation (−17.6 ± 12.3 vs. −18.7 ± 12.8%), or the reduction in CO (1 min: −3.65 ± 1.83 vs. −3.39 ± 1.96 l/min; end of apnea: −2.44 ± 1.33 vs. −2.16 ± 1.44 l/min). On the other hand, packing dampened the early, i.e., 1-min increase in mean arterial pressure (MAP, 1 min: 9.2 ± 8.3 vs. 2.4 ± 11.0 mmHg, P < 0.01) and in total peripheral resistance (relative TPR, 1 min: 2.1 ± 0.5 vs. 1.9 ± 0.5, P < 0.05) but it augmented the concomitant rise in MSNA (1 min: 28.0 ± 11.7 vs. 39.4 ± 12.7 bursts/min, P < 0.001; 32.8 ± 16.4 vs. 43.9 ± 14.8 bursts/100 heart beats, P < 0.01; 3.3 ± 2.1 vs. 4.8 ± 3.2 au/min, P < 0.05). We conclude that the early sympathoactivation 1 min into apnea after moderate packing is due to mechanisms other than excessive reduction in CO. We speculate that lower MAP despite increased MSNA after packing might be explained by vasodilator substances released by the lungs. This idea should be addressed in future studies.
Keywords: baroreflex, breath-hold diving, chemoreflex, sympathetic nervous system
trained apnea divers hold their breath for several minutes. When they perform end-inspiratory apnea sympathetic vasomotor tone is increased right from the beginning presumably through baroreflex mechanisms (20, 33, 34). The sympathetic outflow remains fairly constant until involuntary diaphragmatic contractions begin. Afterward, asphyxic conditions build up (9) such that arterial oxygen saturation may decrease to <50% while carbon dioxide partial pressure increases substantially (16). During this period, chemoreflexes massively increase sympathetic vasomotor tone. We found vasoconstrictor muscle sympathetic nerve activity (MSNA) to be already higher in divers during early apnea compared with control subjects (20). In that work, we hypothesized that divers take a deeper breath before apnea than control subjects, causing higher intrathoracic pressures, which results in greater reduction of cardiac output (CO) and generates more pronounced baroreflex-mediated sympathetic activation. The additional peripheral vasoconstriction in turn might be instrumental in conserving oxygen. Some divers perform a maneuver called glossopharyngeal insufflation (glossopharyngeal inhalation, lung packing). After deep inspiration pumplike action of the cheeks, tongue, pharynx, and larynx advance additional air into to lungs, which increases total lung capacity by up to 47% (31), raises intrapulmonary and transpulmonary pressures (31), and compresses the whole (30) or left heart (41) and large thoracic blood vessels (13, 30). We tested the hypothesis that packing enhances the early baroreflex-mediated increase in MSNA. This may result from a more excessive decline in CO compared with apnea without packing. The added baroreflex involvement may persist throughout apnea culminating in even greater increases in MSNA at the end of apnea. Thus packing may serve as a model to explore the hemodynamic and autonomic alterations with profoundly increased intrathoracic pressure, which is of relevance for the safety of apnea divers using packing as well as for patients on artificial ventilation or with pulmonary hypertension.
METHODS
Study population.
We recruited 16 active apnea divers who were able to perform lung packing. In two subjects we were unable to find or maintain a satisfying recording site within the nerve. Thus only 14 divers (12 men, 2 women, 26.7 ± 4.5 yr, body mass index 24.8 ± 2.4) were included in the statistical analysis. All participants were healthy nonsmokers and ingested no medications. The experiments were designed and conducted according to the Declaration of Helsinki. The ethical committee of the University of Split School of Medicine approved the study, and written informed consent was obtained.
Study design.
The study was done in a prospective, randomized, and crossover fashion.
Protocol.
The participants arrived at the laboratory in the postabsorptive state and had abstained from caffeine for at least 12 h. After emptying the bladder they were instrumented including searching for a suitable MSNA recording site. The subjects remained in the supine position throughout the experiments. At first, calibration curves relating lung volume and thoracic electrical impedance were determined. Volunteers performed at least two bouts of maximal static dry apnea with and without packing as the control apnea in randomized order. The apneas were preceded by 2-min baselines and followed by a few minutes of recovery. Participants were requested to perform a maximal inspiration without prior hyperventilation eventually followed by moderate packing to avoid low-output syncope. Immediately afterward a noseclip was applied.
Measurements.
Heart rate (HR) was determined using a standard electrocardiogram. Arterial blood pressure was measured noninvasively using continuous finger-pulse photoplethysmography (Finometer, Finapres Medical Systems, Arnhem, Netherlands) and calibrated against brachial arterial pressure. In eight divers CO was assessed by impedance cardiography (Cardioscreen, Medis, Ilmenau, Germany) and in six divers by Modelflow continuous cardiac output monitoring (35). Total peripheral resistance (TPR) was calculated as the ratio between mean arterial pressure (MAP) and CO. Arterial oxygen saturation (SpO2) was followed continuously by pulse oximetry (Poet II, Criticare Systems, Waukesha, WI) with the probe placed on the middle finger. A pneumatic chest belt was used to register thoracic and abdominal movements. In eight divers we assessed the packed volume from the relationship between instantaneous intrathoracic air volume and thoracic electrical impedance. The individual relationships were recorded during stepwise inhalation of air from a spirometer (Harvard Apparatus, Student model, Holliston, MA) interrupted by a short pause at each inhalation step with a closed glottis and relaxed respiratory muscles to ensure appropriate levels of intrathoracic pressure during impedance measurements.
For determination of MSNA we employed the technique of microneurography (55). We obtained multiunit recordings of postganglionic sympathetic nerve activity with unipolar tungsten microelectrodes inserted selectively into muscle nerve fascicles of the right peroneal nerve in the popliteal space. Nerve activity was amplified with a total gain of 100,000, band-pass filtered (0.7–2.0 kHz), rectified, and integrated with a time constant of 0.1 s to get mean voltage neurograms for detection and quantification of MSNA bursts.
Analysis.
Analog signals of the parameters were digitized and stored on a computer. The data files were processed by use of a program written by one of the authors (A. Diedrich) that is based on PV-WAVE (Visual Numerics, San Ramon, CA) as previously described (21). We analyzed the changes of two particular periods during apnea against the preceding 2-min baseline: the last 20 s of the first minute (“early apnea”) and the last 20 s of apnea (“late apnea”). The first period represents a quite stable stage between the Valsalva-like responses at the beginning of apnea and the subsequent stage of increasing asphyxia. The second period represents the maximum asphyxic stage achieved by the subject. Analysis periods of 20 s were chosen as a compromise between stable averages and sufficient time resolution (21).
MSNA was expressed as the number of bursts per minute [burst frequency (bursts/min)], as the number of bursts per 100 heart beats [burst incidence (bursts/100 heart beats)], as well as by means of total activity, i.e., the cumulated area under the sympathetic bursts in the mean voltage neurogram per minute expressed as arbitrary units (au). For estimation of packing volume we used linear regression analysis applied to the calibration data relating intrathoracic air volume and thoracic impedance. The validity of assessing intrathoracic volume changes by transthoracic impedance measurement has been shown previously (43).
Statistics.
All values are given as means ± SD. Two-tailed paired t-tests were used to compare data with and without packing. We assessed linear association between variables by Pearson correlation analysis. A value of P < 0.05 was considered statistically significant. Prism 4 for Windows (GraphPad Software, San Diego, CA) has been used for statistical analyses.
RESULTS
Subjects' characteristics are given in Table 1. Early and late responses to both modes of apnea are presented in Tables 2 and 3 for the whole group of divers. Figure 1 shows original recordings related to an apnea with prior glossopharyngeal insufflation. Figure 2 compares original recordings of respiration and MSNA in a typical subject at three points in time during control and packed apnea. Individual and group changes in hemodynamics and MSNA at the early and late stage of apnea are presented in Fig. 3, A and B, respectively. In Fig. 4 the relationship between apnea duration and oxygen desaturation of the hemoglobin is depicted. Note that packing did not shift the relationship.
Table 1.
Subjects' characteristics
| Parameter, unit | Mean ± SD |
|---|---|
| Age, yr | 26.7 ± 4.5 |
| Sex, M/F | 12/2 |
| BMI, kg/m2 | 24.8 ± 2.4 |
| CO, l/min | 8.6 ± 1.4 |
| SAP, mmHg | 128 ± 14 |
| MAP, mmHg | 94 ± 10 |
| DAP, mmHg | 73 ± 13 |
| TPR, dyn · s/cm5 | 960 ± 203 |
| HR, beats/min | 65 ± 5 |
| MSNA, bursts/min | 19.3 ± 9.6 |
| MSNA, bursts/100 heart beats | 26.1 ± 13.3 |
| MSNA, au/min | 1.2 ± 0.8 |
M, male; F, female; BMI, body mass index; CO, cardiac output, SAP, MAP, DAP, systolic, mean, and diastolic arterial pressure, respectively; TPR, total peripheral resistance; HR, heart rate; MSNA, muscle sympathetic nerve activity; au, arbitrary units.
Table 2.
Responses to end-inspiratory apnea without packing (control apnea)
| Parameter, unit | Baseline | Δ1 min | P | ΔEnd of Apnea | P |
|---|---|---|---|---|---|
| SpO2, % | 99.1 ± 1.3 | 0.0 ± 0.0 | 0.23 | −17.6 ± 12.3 | <0.001 |
| CO, l/min | 8.6 ± 1.5 | −3.7 ± 1.8 | <0.0001 | −2.4 ± 1.3 | <0.0001 |
| MAP, mmHg | 98.2 ± 11.7 | 9.2 ± 8.3 | <0.01 | 40.4 ± 9.4 | <0.0001 |
| TPR, relative | 1.0 ± 0.0 | 2.1 ± 0.5 | <0.0001 | 2.2 ± 0.6 | <0.0001 |
| HR, beats/min | 76.5 ± 8.0 | 4.9 ± 13.6 | 0.20 | −10.5 ± 13.2 | <0.05 |
| MSNA, bursts/min | 19.2 ± 11.0 | 28.0 ± 11.8 | <0.0001 | 28.9 ± 16.2 | <0.0001 |
| MSNA, bursts/100 heart beats | 26.2 ± 15.2 | 32.8 ± 16.4 | <0.0001 | 53.4 ± 24.9 | <0.0001 |
| MSNA, au/min | 1.2 ± 1.0 | 3.3 ± 2.1 | <0.0001 | 13.1 ± 11.8 | <0.01 |
Values are means ± SD. SpO2, oxygen saturation of hemoglobin. For TPR, Δ1-min and Δend-apneic values are relative to baseline. P values are calculated against preapneic baseline.
Table 3.
Responses to apnea after glossopharyngeal insufflation (lung packing)
| Parameter, unit | Baseline | Δ1/min | P | ΔEnd of Apnea | P |
|---|---|---|---|---|---|
| SpO2, % | 99.1 ± 0.9 | 0.1 ± 0.6 | 0.70 | −18.7 ± 12.8 | 0.64 |
| CO, l/min | 8.6 ± 2.1 | −3.4 ± 2.0 | 0.53 | −2.2 ± 1.4 | 0.59 |
| MAP, mmHg | 100.6 ± 12.6 | 2.4 ± 11.0 | <0.01 | 34.1 ± 17.3 | 0.20 |
| TPR, relative | 1.0 ± 0.0 | 1.9 ± 0.5 | <0.05 | 1.9 ± 0.5 | <0.05 |
| HR, beats/min | 76.6 ± 7.8 | 8.2 ± 16.2 | 0.27 | −7.0 ± 18.8 | 0.43 |
| MSNA, bursts/min | 19.4 ± 9.6 | 39.4 ± 12.7 | <0.001 | 31.6 ± 12.8 | 0.35 |
| MSNA, bursts/100 heart beats | 25.9 ± 13.4 | 43.9 ± 14.8 | <0.01 | 52.7 ± 17.2 | 0.90 |
| MSNA, au/min | 1.3 ± 0.8 | 4.8 ± 3.2 | <0.05 | 13.1 ± 14.2 | 0.99 |
Values are means ± SD. For TPR, Δ1-min and Δend-apneic values are relative to baseline. P values are calculated against responses to control apnea without glossopharyngeal insufflation.
Fig. 1.
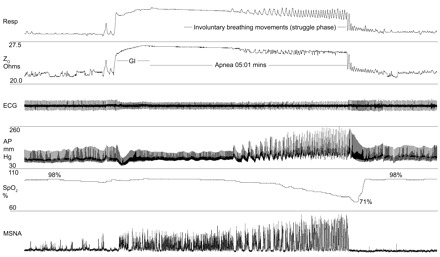
Original recordings of the respiratory belt signal (Resp), thoracic basal impedance (Z0), electrocardiogram (ECG), arterial blood pressure (AP), oxygen saturation (SpO2) as measured at the finger, and muscle sympathetic nerve activity (MSNA) in 1 diver. Note that the deflections in the top traces are caused by involuntary breathing movements during the struggle phase of apnea. These movements do not cause external gas exchange.
Fig. 2.
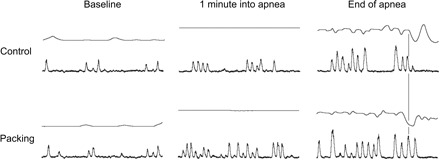
Original recordings of respiration and MSNA in 1 subject. Each example lasts for 20 s. Control: end-inspiratory apnea without glossopharyngeal insufflation (control apnea without packing). Packing: apnea with packing. The vertical line marks termination of apnea. Deflections in the respiratory signal before cessation of apnea are caused by involuntary breathing movements without external gas exchange (column “End of apnea” upper traces in the rows “Control” and “Packing”). Note the stronger sympathetic activation during the easy-going phase of apnea (last 20 s of the first minute are depicted). Furthermore, sympathetic activation increases toward the end of apnea. However, there is no difference in maximal MSNA. On cessation of apnea MSNA is abruptly suppressed.
Fig. 3.
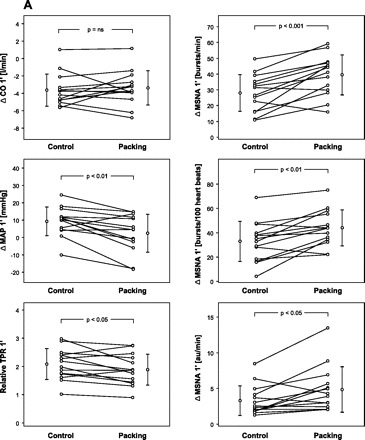
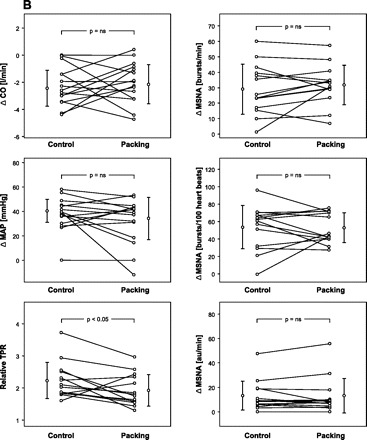
Comparison of changes (Δ) in hemodynamics and sympathetic vasoconstrictor activity at the early (A) and late stage of apnea (B). CO, cardiac output; MAP, mean arterial pressure; TPR, total peripheral resistance.
Fig. 4.
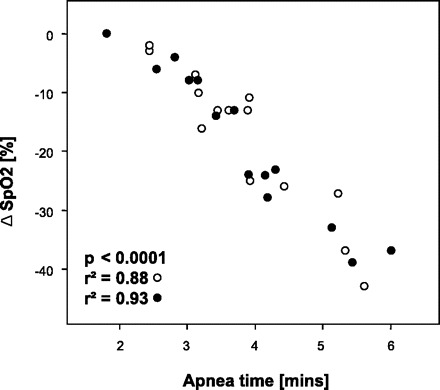
Relationships between apnea duration and hemoglobin desaturation. ○, Apnea without glossopharyngeal insufflation (packing); ●, apnea with packing. Note the overlap between the 2 conditions.
Except for early SpO2 and HR all given parameters changed as measured at the end of the first minute as well as at the end of apnea. The longest individual apnea times without and with packing were 05:37 and 06:01 min, respectively. Although packing did elevate total lung capacity by 0.79 ± 0.40 liters (range 0.50–1.74 liters, P < 0.001) it did not change apnea time (3.8 ± 1.0 vs. 3.8 ± 1.2 min), hemoglobin oxygen desaturation (see also Fig. 4), or the reduction in CO. The reductions in CO as measured by impedance cardiography and Modelflow were similar. HR responses to apnea were highly variable among subjects with changes between −20 and +36 beats/min as has been noticed previously (13). Additional packing did not change HR responses. On the other hand, packing dampened the early increases in MAP and relative TPR although it augmented the concomitant rise in all three MSNA measures. The diminished increase in TPR after packing in the early stage of apnea compared with control breath-holding was preserved throughout the apnea. Compared with baseline, however, TPR remained nearly doubled until the end of apnea. Late levels of the remaining parameters did not differ between control apnea and apnea with packing.
DISCUSSION
This work is the first to present the hemodynamic effects of moderate lung packing in relation to central vasoconstrictor sympathetic outflow. The main result of the study is that packing augments the early increase in MSNA compared with control apnea. The additional MSNA increase with packing may be mediated by several mechanisms, e.g., the arterial baroreflex due to an excessive decrease in CO, by differences in blood gases, by greater unloading of cardiopulmonary baroreceptors, or by superimposed vasodilator mechanisms.
The present study supports previous findings showing moderately elevated MSNA during the easy-going phase of dry apnea followed by tremendous sympathetic activation in the struggle phase (20). Moreover, the data confirm reduced CO during end-inspiratory apnea (17, 20). However, the present study shows that CO is not diminished further on packing as had been expected. On the other hand, in agreement with our hypothesis the maneuver led to an increase in vasoconstrictor nerve firing during the easy-going phase. The increase was more than 40% stronger than that during control apnea. In contrast, TPR and arterial pressure were lower after packing compared with control apnea by a small but statistically significant amount. The discrepancy supports the hypothesis that a packing-related vasodilator mechanism may be involved.
Hypoxia has direct peripheral vasodilator effects (27, 47, 54) and reduces vasoconstrictor responses to sympathetic stimuli (19). Moreover, it has been suggested that hypercapnia impedes vasoconstrictor transduction (47). However, these effects cannot explain the paradox because, first, notable asphyxic conditions are not present after only 1 min of apnea and, second, blood gases can be expected to be very similar during the two apnea modes (see Fig. 4).
Possible candidates causing vasodilation on lung packing are nitric oxide (NO) and prostacyclin (PGI2). NO contributes to the level of MSNA (49). NO synthases are present in the respiratory system in epithelial, endothelial, and neuronal cells (58). Distension of the lungs increases NO release (4, 39, 52). The molecule has a very short half-life of ∼0.1 s in the human circulation (25). Thus it is quite unlikely that authentic NO released by the lungs can act as vasodilator in the periphery. However, NO-like bioactivity can be transported in the circulation with the potential to be converted back to NO (32, 42). Nitrate/nitrite (32), NO-modified proteins like nitrosylhemoglobin (7), S-nitrosohemoglobin (22), and S-nitrosoalbumin (51, 59) have been shown to contribute to this NO pool. All these NO species are considerably longer-lived than authentic NO and can vasodilate the vasculature, in part by releasing NO (22). For example, S-nitrosoalbumin has a half-life of several minutes in rats (59).
Pulmonary tissue is able to produce other vasoactive substances. The secretion of prostacyclin (PGI2) into the blood is also increased on stretch (15, 50, 57) and shear stress as with increasing pressure gradient across the pulmonary vascular bed (56). As it is not inactivated during passage through the lungs (14, 18), PGI2 is a prostaglandin with a half-life of several minutes (12, 40). It exerts pulmonary (8) as well as systemic (10) vasodilation. Infusion of PGI2 in man decreases peripheral vascular resistance and arterial pressure. Increased muscle blood flow suggests that PGI2 acts predominantly on the resistance vessels (53). On the other hand, PGI2 has been shown to also constrict vessels. Whether it acts as a vasodilator or as a vasoconstrictor depends on the species as well as the vascular bed being studied. Human saphenous veins are contracted by PGI2 (29, 45, 48). Venous tone greatly determines cardiac preload and, hence, CO (38). Together with its potency to increase cardiac contractility (26) these features could have contributed to the maintained CO.
Activity of sympathetic efferents is adjusted by baroreflexes originating in the arterial as well as in the low-pressure system. End-inspiratory apnea is likely to unload cardiopulmonary baroreceptors by elevating intrathoracic pressure. Diminished firing of these low pressure receptors results in reflex vasoconstriction (1, 3, 23, 33). Certainly, vasoconstrictor activity, as assessed by MSNA, is an important factor in this regard contributing substantially to the doubling in TPR (see Fig. 3, A and B). Furthermore, concurrent constriction of capacitance vessels (38) undoubtedly helped to preserve CO. It may be speculated that glossopharyngeal inhalation unloads cardiopulmonary baroreceptors to an even greater extent than end-inspiratory apnea, which also might play a role in the greater activation of central sympathetic outflow (34). However, this mechanism cannot explain the lessened increase in TPR after packing.
The amount of additional lung volume gained by packing may have competing consequences with regard to oxygen stores and hemodynamic stability. Moderate packing in our experiments elevated intrapulmonary gas volume by <1 liter as opposed to a maximum of >4 liters in another study (31). It is possible that such small increments in oxygen stores remain undetected in terms of desaturation (Fig. 4) and breath-hold time, whereas apnea durations are prolonged with packing volumes of >1.5 liters (37). Nonetheless, our data show that even moderate packing is sufficient to exert notable sympathetic counterregulation, which masks hemodynamic alterations to some extent, i.e., most likely arterial pressure would have decreased markedly without sympathetic counterregulation. It has been suggested that packing volumes above 2 liters may aggravate the hemodynamic pattern, which is similar in part to what can be seen in patients suffering from pulmonary hypertension (13, 41, 44). Glossopharyngeal inhalation may lead to presyncope and syncope (2, 36, 46). Based on these facts, it can be argued that there are volume ranges with different hemodynamic profiles and that with exaggerated packing hemodynamic stability is reduced, which may result in pre/syncope.
Regarding the struggle phase of apnea the putative vasodilator mechanism seems to stay in effect as judged by the slightly but significantly lower TPR at the end of packed apnea. On the other hand, MSNA was similar to control apnea. Hence, the vasodilator effect may be offset partially by other, e.g., humoral, mechanisms “unloading” the sympathetic system. Nevertheless, the increase in MSNA was tremendous as has been shown previously, and it is presumably driven by chemoreflex mechanisms (5, 6, 11) together with baroreflex resetting to a higher operational pressure (20). On average, the increase was ∼20-fold and it seemed not to be influenced by moderate packing. Moreover, the abrupt silence in vasoconstrictor outflow immediately after resumption of breathing was not changed by lung packing (data not shown).
Limitations.
The most important limitation of our study was that CO has been determined indirectly. Impedance cardiography and Modelflow continuous CO monitoring are commonly used as noninvasive methods allowing for continuous recording of CO on a beat-by-beat basis. The difficulties in measuring correct absolute values are balanced by the high time resolution, which is helpful in monitoring the dynamic apnea situation. Moreover, the crossover design of the study made it less susceptible to the methodological drawbacks. Nevertheless, studies using (more) direct methods for CO estimation are necessary to validate our findings.
The volume of additional air that has been pushed into the lungs by packing was assessed indirectly and the calculations were performed only after the experiments. Thus we did not provide quantitative feedback to the divers to control for cut-off volumes during the packing maneuver. Instead, they were instructed to only moderately fill their lungs to avoid so-called “overpacking.” This subjective approach may have contributed to the variability in circulatory and autonomic responses to packing.
We did not measure expired gas concentrations either before the apneas or afterward, when the subjects resumed breathing. However, we do not think that differences in blood gas tensions were confounders, because we requested the divers to prepare equally for the apnea bouts. We did not measure vasoactive substances in blood. Future studies may scrutinize whether NO, PGI2, adenosine, other direct or indirect vasodilator substances, cotransmitters, or reflexes (24, 28, 47) may be responsible for the additional vasoconstrictor nerve activity after glossopharyngeal insufflation.
Conclusions.
We conclude that end-inspiratory apneas with additional packing exhibit greater sympathoactivation than apneas without the maneuver. Furthermore, our data suggest that the increased sympathetic response is not the result of excessive reduction in CO. We speculate that the additional MSNA may be a baroreflex-mediated response to a yet-undefined vasodilator mechanism. The question of whether vasodilator substances released by the lungs are involved should be addressed in future studies. The divers in our study performed moderate packing maneuvers. Our findings cannot be extrapolated to excessive packing, which may very well provoke dramatic hemodynamic consequences and subsequent syncope.
In summary, our results show that glossopharyngeal insufflation can be used as a model to explore the hemodynamic and autonomic alterations with profoundly increased intrathoracic pressure, which may be clinically important, e.g., in patients on artificial ventilation (57) or with pulmonary hypertension (13, 41, 44).
GRANTS
The Croatian Ministry of Science, Education and Sports (Grants No 216-2160133-0330 and 216-2160133-0130) supported the work. The Deutsche Forschungsgemeinschaft supported J. Tank and J. Jordan. The German Aerospace Center (DLR) supported K. Heusser and J. Tank.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the divers for their participation.
REFERENCES
- 1. Abboud FM, Eckberg DL, Johannsen UJ, Mark AL. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. J Physiol 286: 173–184, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson JP, Liner MH, Jonsson H. Asystole and increased serum myoglobin levels associated with “packing blackout” in a competitive breath-hold diver. Clin Physiol Funct Imaging 29: 458–461, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Baily RG, Prophet SA, Shenberger JS, Zelis R, Sinoway LI. Direct neurohumoral evidence for isolated sympathetic nervous system activation to skeletal muscle in response to cardiopulmonary baroreceptor unloading. Circ Res 66: 1720–1728, 1990. [DOI] [PubMed] [Google Scholar]
- 4. Berg JT, Deem S, Kerr ME, Swenson ER. Hemoglobin and red blood cells alter the response of expired nitric oxide to mechanical forces. Am J Physiol Heart Circ Physiol 279: H2947–H2953, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Breskovic T, Ivancev V, Banic I, Jordan J, Dujic Z. Peripheral chemoreflex sensitivity and sympathetic nerve activity are normal in apnea divers during training season. Auton Neurosci 154: 42–47, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Breskovic T, Valic Z, Lipp A, Heusser K, Ivancev V, Tank J, Dzamonja G, Jordan J, Shoemaker JK, Eterovic D, Dujic Z. Peripheral chemoreflex regulation of sympathetic vasomotor tone in apnea divers. Clin Auton Res 20: 57–63, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108: 279–287, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Wet CJ, Affleck DG, Jacobsohn E, Avidan MS, Tymkew H, Hill LL, Zanaboni PB, Moazami N, Smith JR. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg 127: 1058–1067, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Dejours P. Hazards of hypoxia during diving. In: Physiology of Breath-Hold Diving and the Ama of Japan Papers, edited by Rahn H. Washington, DC: National Academy of Sciences-National Research Council, 1965, p. 183–193. [Google Scholar]
- 10. Duffy SJ, Tran BT, New G, Tudball RN, Esler MD, Harper RW, Meredith IT. Continuous release of vasodilator prostanoids contributes to regulation of resting forearm blood flow in humans. Am J Physiol Heart Circ Physiol 274: H1174–H1183, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Dujic Z, Ivancev V, Heusser K, Dzamonja G, Palada I, Valic Z, Tank J, Obad A, Bakovic D, Diedrich A, Joyner MJ, Jordan J. Central chemoreflex sensitivity and sympathetic neural outflow in elite breath-hold divers. J Appl Physiol 104: 205–211, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Dusting GJ, Moncada S, Vane JR. Recirculation of prostacyclin (PGI2) in the dog. Br J Pharmacol 64: 315–320, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eichinger M, Walterspacher S, Scholz T, Tetzlaff R, Puderbach M, Tetzlaff K, Kopp-Schneider A, Ley S, Choe K, Kauczor HU, Sorichter S. Glossopharyngeal insufflation and pulmonary hemodynamics in elite breath hold divers. Med Sci Sports Exerc 42: 1688–1695, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Eling TE, Ally AI. Pulmonary biosynthesis and metabolism of prostaglandins and related substances. Environ Health Perspect 55: 159–168, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellsworth ML, Gregory TJ, Newell JC. Pulmonary prostacyclin production with increased flow and sympathetic stimulation. J Appl Physiol 55: 1225–1231, 1983. [DOI] [PubMed] [Google Scholar]
- 16. Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol 84: 254–271, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Ferrigno M, Hickey DD, Liner MH, Lundgren CE. Cardiac performance in humans during breath holding. J Appl Physiol 60: 1871–1877, 1986. [DOI] [PubMed] [Google Scholar]
- 18. Hawkings HJ, Smith JB, Nicolaou KC, Eling TE. Studies of the mechanisms involved in the fate of prostacyclin (PGI2) and 6-keto-PGF1alpha in the pulmonary circulation. Prostaglandins 16: 871–884, 1978. [DOI] [PubMed] [Google Scholar]
- 19. Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin. III. Effect of hypoxia and hypocapnia. J Clin Invest 49: 1252–1265, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heusser K, Dzamonja G, Tank J, Palada I, Valic Z, Bakovic D, Obad A, Ivancev V, Breskovic T, Diedrich A, Joyner MJ, Luft FC, Jordan J, Dujic Z. Cardiovascular regulation during apnea in elite divers. Hypertension 53: 719–724, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Heusser K, Tank J, Diedrich A, Engeli S, Klaua S, Kruger N, Strauss A, Stoffels G, Luft FC, Jordan J. Influence of sibutramine treatment on sympathetic vasomotor tone in obese subjects. Clin Pharmacol Ther 79: 500–508, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974. [DOI] [PubMed] [Google Scholar]
- 24. Joyner MJ, Dietz NM. Sympathetic vasodilation in human muscle. Acta Physiol Scand 177: 329–336, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Kelm M, Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res 66: 1561–1575, 1990. [DOI] [PubMed] [Google Scholar]
- 26. Kisch-Wedel H, Kemming G, Meisner F, Flondor M, Kuebler WM, Bruhn S, Koehler C, Zwissler B. The prostaglandins epoprostenol and iloprost increase left ventricular contractility in vivo. Intensive Care Med 29: 1574–1583, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Lahana A, Costantopoulos S, Nakos G. The local component of the acute cardiovascular response to simulated apneas in brain-dead humans. Chest 128: 634–639, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Lepori M, Sartori C, Duplain H, Nicod P, Scherrer U. Interaction between cholinergic and nitrergic vasodilation: a novel mechanism of blood pressure control. Cardiovasc Res 51: 767–772, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Levy JV. Contractile responses to prostacyclin (PGI2) of isolated human saphenous and rat venous tissue. Prostaglandins 16: 93–97, 1978. [DOI] [PubMed] [Google Scholar]
- 30. Lindholm P, Nyren S. Studies on inspiratory and expiratory glossopharyngeal breathing in breath-hold divers employing magnetic resonance imaging and spirometry. Eur J Appl Physiol 94: 646–651, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Loring SH, O'Donnell CR, Butler JP, Lindholm P, Jacobson F, Ferrigno M. Transpulmonary pressures and lung mechanics with glossopharyngeal insufflation and exsufflation beyond normal lung volumes in competitive breath-hold divers. J Appl Physiol 102: 841–846, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Macefield VG, Gandevia SC, Henderson LA. Neural sites involved in the sustained increase in muscle sympathetic nerve activity induced by inspiratory capacity apnea: a fMRI study. J Appl Physiol 100: 266–273, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Macefield VG, Wallin BG. Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J Auton Nerv Syst 53: 148–156, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Mathews L, Singh RK. Cardiac output monitoring. Ann Card Anaesth 11: 56–68, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Novalija J, Lindholm P, Loring SH, Diaz E, Fox JA, Ferrigno M. Cardiovascular aspects of glossopharyngeal insufflation and exsufflation. Undersea Hyperb Med 34: 415–423, 2007. [PubMed] [Google Scholar]
- 37. Overgaard K, Friis S, Pedersen RB, Lykkeboe G. Influence of lung volume, glossopharyngeal inhalation and PetO2 and PetCO2 on apnea performance in trained breath-hold divers. Eur J Appl Physiol 97: 158–164, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther 90: 179–230, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Persson MG, Lonnqvist PA, Gustafsson LE. Positive end-expiratory pressure ventilation elicits increases in endogenously formed nitric oxide as detected in air exhaled by rabbits. Anesthesiology 82: 969–974, 1995. [DOI] [PubMed] [Google Scholar]
- 40. Pirich C, Efthimiou Y, O'Grady J, Sinzinger H. Hyperalphalipoproteinemia and prostaglandin I2 stability. Thromb Res 88: 41–49, 1997. [DOI] [PubMed] [Google Scholar]
- 41. Potkin R, Cheng V, Siegel R. Effects of glossopharyngeal insufflation on cardiac function: an echocardiographic study in elite breath-hold divers. J Appl Physiol 103: 823–827, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, Kelm M. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: experimental and clinical study on the fate of NO in human blood. Circ Res 91: 470–477, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Sandberg KL, Lindstrom DP, Krueger ED, Sundell H, Cotton RB. Measurement of tidal volume during high frequency ventilation by impedance plethysmography. Pediatr Res 23: 253–256, 1988. [DOI] [PubMed] [Google Scholar]
- 44. Scherhag A, Pfleger S, Grosselfinger R, Borggrefe M. Does competitive apnea diving have a long-term risk? Cardiopulmonary findings in breath-hold divers. Clin J Sport Med 15: 95–97, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Schuller-Petrovic S, Siedler S, Kern T, Meinhart J, Schmidt K, Brunner F. Imbalance between the endothelial cell-derived contracting factors prostacyclin and angiotensin II and nitric oxide/cyclic GMP in human primary varicosis. Br J Pharmacol 122: 772–778, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seccombe LM, Chung SC, Jenkins CR, Frater CJ, Mackey DW, Pearson MA, Emmett L, Peters MJ. Lung perfusion and chest wall configuration is altered by glossopharyngeal breathing. Eur Respir J 36: 151–156, 2010. [DOI] [PubMed] [Google Scholar]
- 47. Simmons GH, Minson CT, Cracowski JL, Halliwill JR. Systemic hypoxia causes cutaneous vasodilation in healthy humans. J Appl Physiol 103: 608–615, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Sinzinger H, Fitscha P. Prostacyclin (PGI2) contracts normal and varicose human saphenous veins. Vasa 13: 228–230, 1984. [PubMed] [Google Scholar]
- 49. Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol 501: 671–675, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skinner SJ, Somervell CE, Olson DM. The effects of mechanical stretching on fetal rat lung cell prostacyclin production. Prostaglandins 43: 413–433, 1992. [DOI] [PubMed] [Google Scholar]
- 51. Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 89: 7674–7677, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stromberg S, Lonnqvist PA, Persson MG, Gustafsson LE. Lung distension and carbon dioxide affect pulmonary nitric oxide formation in the anaesthetized rabbit. Acta Physiol Scand 159: 59–67, 1997. [DOI] [PubMed] [Google Scholar]
- 53. Szczeklik J, Szczeklik A, Nizankowski R. Haemodynamic changes induced by prostacyclin in man. Br Heart J 44: 254–258, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamisier R, Norman D, Anand A, Choi Y, Weiss JW. Evidence of sustained forearm vasodilatation after brief isocapnic hypoxia. J Appl Physiol 96: 1782–1787, 2004. [DOI] [PubMed] [Google Scholar]
- 55. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 56. van Grondelle A, Worthen GS, Ellis D, Mathias MM, Murphy RC, Strife RJ, Reeves JT, Voelkel NF. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J Appl Physiol 57: 388–395, 1984. [DOI] [PubMed] [Google Scholar]
- 57. von Bethmann AN, Brasch F, Nusing R, Vogt K, Volk HD, Muller KM, Wendel A, Uhlig S. Hyperventilation induces release of cytokines from perfused mouse lung. Am J Respir Crit Care Med 157: 263–272, 1998. [DOI] [PubMed] [Google Scholar]
- 58. Vonk-Noordegraaf A, van Wolferen SA, Marcus JT, Boonstra A, Postmus PE, Peeters JW, Peacock AJ. Noninvasive assessment and monitoring of the pulmonary circulation. Eur Respir J 25: 758–766, 2005. [DOI] [PubMed] [Google Scholar]
- 59. Warnecke A, Luessen P, Sandmann J, Ikic M, Rossa S, Gutzki FM, Stichtenoth DO, Tsikas D. Application of a stable-isotope dilution technique to study the pharmacokinetics of human 15N-labelled S-nitrosoalbumin in the rat: possible mechanistic and biological implications. J Chromatogr B Analyt Technol Biomed Life Sci 877: 1375–1387, 2009. [DOI] [PubMed] [Google Scholar]


