Cardiomyopathies are clinically heterogeneous disorders that impair heart function. Although cardiomyopathies can occur at any age, pediatric cardiomyopathies in particular have an annual incidence of 1.13 cases in 100,000 children younger than 18 years of age and 8.34 cases per 100,000 infants (1). Pediatric cardiomyopathies have both genetic and nongenetic causes. Genetic etiologies of cardiomyopathy in the pediatric population are more diverse than the adult population because they encompass not only a majority of the cardiomyopathy genes described in adults, but additional syndromic, metabolic, and neuromuscular genetic causes as well. Expanded use of genetic testing has increased the diagnostic yield from ~30% to 76% of pediatric cardiomyopathy cases (2). Nevertheless, nearly 25% of cases remain idiopathic with unknown, possibly genetic, causes.
In this issue of the Journal, Almomani et al. (3) report ALPK3 as a novel gene in human pediatric cardiomyopathy. Using a combination of homozygosity mapping, whole exome sequencing, and candidate gene screening, the group identified homozygous premature stop codon mutations in ALPK3 and, from immunohistological observations of heart tissue, suggest potential mechanisms for further exploration. First, homozygosity mapping was combined with whole exome sequencing in 2 consanguineous families with idiopathic pediatric cardiomyopathy to identify potential disease-causing genomic regions. A homozygosity overlap region (3.1 Mb) was shared by both families. Next, whole exome variants from 1 affected individual in each family were filtered for variants within the shared homozygosity region; only the ALPK3 gene demonstrated homozygous-damaging variants in both families and was selected by the investigators as the putative disease gene. Sequencing of ALPK3 in 60 unrelated individuals with pediatric cardiomyopathy revealed a homozygous premature stop codon mutation in another patient, providing a third consanguineous family for study.
The affected child from the first family died within hours of birth, and 1 affected child in the second family died within a week. Post-mortem evaluation of their hearts showed a complex dilated cardiomyopathy (DCM) phenotype with severe cardiomegaly, biventricular dilation, subendocardial fibroelastosis, and absence of fatty infiltration. One other child (age 11 years) from the third family had a severe hypertrophic cardiomyopathy (HCM) phenotype. Furthermore, heterozygous relatives had variable clinical features ranging from normal heart to HCM. Immunohistochemistry of intercalated disc proteins in tissue from the first heart demonstrated reduced signal for plakoglobin and desmoplakin; interestingly, to our knowledge, this is the first report of intercalated disc remodeling in a pediatric patient with a DCM phenotype. Indeed, a previously reported Alpk3-deficient mouse model from Van Sligtenhorst et al. (4) supports these findings. The Alpk−/− mouse developed a similar complex phenotype with features of DCM and HCM, left and right ventricular dilation, hypertrophy, systolic dysfunction, myofibrillar disarray, absence of fibrosis, and—remarkably—histological observations of indistinct and irregular intercalated discs (Figure 1) (4).
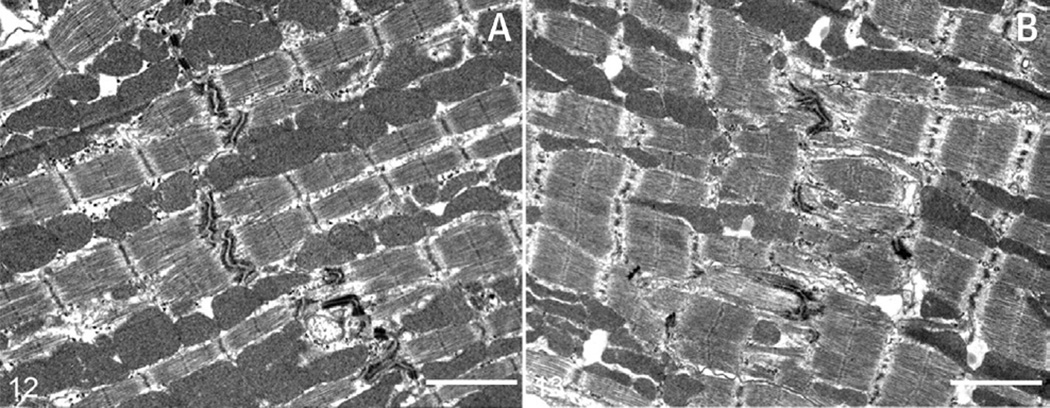
FIGURE 1. Transmission Electron Microscopy of Alpk3-Deficient Murine Hearts.
As found in the human hearts by Almomani et al. (3), the structure of intercalated discs is altered: in 24-week-old wild-type mice (A), intercalated discs are electron dense and tend to align uniformly with intercalated discs in adjacent myofibrils, whereas in Alpk3−/− mice (B), desmosomes have reduced electron density, are fragmented, and are improperly aligned with adjacent cardiomyocytes (bars = 2 µm). Reprinted with permission from Van Sligtenhorst et al. (4).
ALPK3 encodes the alpha-kinase 3 protein that is thought to play a role in cardiomyocyte differentiation, possibly by acting as a transcriptional regulator. Expression studies in mouse embryos and adult tissues have shown that Alpk3, also known as Midori, is expressed in the fetal heart as well as the adult heart and skeletal muscle, and it localizes to the nucleus where it may regulate transcription (5). Because Alpk3-deficient mice exhibit indistinct intercalated discs, Alpk3 may be necessary for their proper formation (4). Intercalated discs comprise adherens junctions, gap junctions, and desmosomes, which are collectively important for electromechanical coupling. Remodeling of intercellular junctions is a well-studied area of heart disease and heart failure; the absence of desmosomal proteins at the cell membrane, as shown by Almomani et al. (3) with reduced plakoglobin, has been previously reported in arrhythmogenic cardiomyopathy (AC) (6). Plakoglobin, in particular, plays a dual role as both a structural protein of the desmosome and a signaling molecule in the Wnt/β-catenin pathway.
The major finding of this investigation was the discovery of a novel gene linked to pediatric cardiomyopathy, supported by a previously reported mouse model. Although this study adds another gene to the growing list of those linked to pediatric (and adult) cardiomyopathy, it also raises profound questions about the pathogenic role of ALPK3 in cardiomyopathy, its causal mechanisms, and its potential mechanistic overlap with AC. Among these questions are the following:
Are ALPK3 nonsense mutation carriers at risk? Although cardiac examination of 8 heterozygous family members in the study did not reveal signs of cardiomyopathy, 2 heterozygous carriers in the third family previously had a diagnosis of HCM. In this case, the potential role of ALPK3 in adult-onset cardiomyopathy remains to be defined.
Do ALPK3 homozygous nonsense mutations cause intercalated disc remodeling in all patients? As is unfortunately the case with human tissue samples, only 1 sample was available for immunohistochemistry in this study. Although that sample showed the absence of immunohistochemical signal for plakoglobin and desmoplakin, we must keep in mind that it was only a single sample. Access to additional samples of patients with similar ALPK3 mutations will contribute to this hypothesis.
What is the mechanism of ALPK3 nonsense mutations? Although the previous Alpk3-deficient mouse model (4) developed cardiomyopathy similar to the patients presented in this study, it differed in its myocyte disarray and lack of fibrosis. Although the patients in this study all had homozygous premature stop codon mutations, no protein work was done to demonstrate either a loss-of-function or a dominant-negative model for the 3 mutations. Expression of a truncated ALPK3 protein could explain the differences in clinical manifestation between the patients and the mouse model.
What is the role of ALPK3 in the developing heart? Almomani et al. (3) speculate that ALPK3 might regulate expression of cardiac transcription factors, such as HEY and HAND proteins, which could have downstream implications in cardiomyocyte differentiation and maturation. These patients present with a severe, early form of cardiomyopathy, suggesting that understanding this disease mechanism could provide insight into the earliest mechanisms that can be perturbed to lead to cardiomyopathy. However, the putative interactions of ALPK3 are only speculated based on conserved alpha-kinase domains, so further experiments are needed to confirm this assertion.
If intercalated disc remodeling is a characteristic of homozygous ALPK3 pediatric cardiomyopathies, what potential mechanistic overlap, if any, might this have with AC? Loss of plakoglobin signal at the cardiomyocyte cell membrane has become a hallmark of AC, where it has been shown to translocate to the nucleus and act as an inhibitor of the Wnt/βcatenin signaling pathway (6,7). Additionally, plakoglobin translocation has been shown to control cardiac progenitor cell fate (8), which has implications for AC in fibroadipogenesis, but which could potentially have implications in heart development as well. Although significantly more research would need to be done to assert this potential connection, including quantifying plakoglobin protein expression in ALPK3 patients, its potential implications are intriguing.
In summary, Almomani et al. (3) have used homozygosity mapping to identify a novel pediatric cardiomyopathy gene, ALPK3, supported by an already established mouse model. Early, severe onset of cardiomyopathy in autosomal recessive patients suggests a role for ALPK3 in early developmental stages of the heart, possibly through aberrant transcriptional regulation, with the potential for cardiomyopathy to develop in heterozygous carriers later in life. Further work with this gene and its role in heart development will provide insight into its molecular mechanism in cardiomyopathy.
Acknowledgments
The authors are supported by National Institutes of Health grants 1R01HL116906 and 1R01HL109209.
REFERENCES
- 1.Wilkinson JD, Landy DC, Colan SD, et al. The pediatric cardiomyopathy registry and heart failure: key results from the first 15 years. Heart Fail Clin. 2010;6:401–413. vii. doi: 10.1016/j.hfc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindel SJ, Miller EM, Gupta R, et al. Pediatric cardiomyopathy: importance of genetic and metabolic evaluation. J Card Fail. 2012;18:396–403. doi: 10.1016/j.cardfail.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almomani R, Verhagen JMA, Herkert JC, et al. Biallelic truncating mutations in ALPK3 cause severe pediatric cardiomyopathy. J Am Coll Cardiol. 2016;67:515–525. doi: 10.1016/j.jacc.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 4.Van Sligtenhorst I, Ding ZM, Shi ZZ, et al. Cardiomyopathy in alpha-kinase 3 (ALPK3)-deficient mice. Vet Pathol. 2012;49:131–141. doi: 10.1177/0300985811402841. [DOI] [PubMed] [Google Scholar]
- 5.Hosoda T, Monzen K, Hiroi Y, et al. A novel myocyte-specific gene Midori promotes the differentiation of P19CL6 cells into cardiomyocytes. J Biol Chem. 2001;276:35978–35989. doi: 10.1074/jbc.M100485200. [DOI] [PubMed] [Google Scholar]
- 6.Asimaki A, Tandri H, Huang H, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Gras E, Lombardi R, Giocondo MJ, et al. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardi R, da Graca Cabreira-Hansen M, Bell A, et al. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2011;109:1342–1353. doi: 10.1161/CIRCRESAHA.111.255075. [DOI] [PMC free article] [PubMed] [Google Scholar]