Abstract
Aims
This study investigated the serum levels of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in a group of chronic ketamine abusers in comparison to healthy controls. The correlations between the serum BDNF, NGF level with the subjects’ demographic, pattern of ketamine use were also examined.
Methods
93 subjects who met the criteria of ketamine dependence and 39 healthy subjects were recruited. Serum BDNF and NGF levels were assayed by enzyme-linked immunosorbent assay (ELISA). Psychopathological symptoms were assessed using Positive and Negative Syndrome Scale (PANSS), Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI).
Results
Both serum levels of BDNF and NGF were significant lower in the ketamine users compared to the healthy control subjects (9.50 ± 6.68 versus 14.37 ± 6.07 ng/ml, p = 0.019 for BDNF; 1.93 ± 0.80 versus 2.60 ± 1.07 ng/ml, p = 0.011 for NGF). BDNF level was negatively associated with current frequency of ketamine use (r = −0.209, p = 0.045).
Conclusions
Both BDNF and NGF serum concentrations were significantly lower among chronic ketamine users than among health controls.
Keywords: Ketamine, Brain-derived neurotrophic factor, Nerve growth factor
1. Introduction
Ketamine, a derivative of phencyclidine (PCP), was first successfully synthesized by the American chemist Calvin Stevens in 1962. Recreational use of ketamine became popular in Europe and the United States and other countries after its hallucinogenic effects were discovered in 1970. The main pharmacologically action of ketamine is as a non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor. Ketamine has central excitatory and inhibitory effects, as well as anti-anxiety, narcotic, hallucinogenic and generally psychotomimetic effects. A low dose of ketamine produces sedative and analgesic effects, while a high dose leads to dissociative anesthesia. The hallucinogenic effect is thought to underlie the ketamine abuse (Wolff and Winstock, 2006). Ketamine is known as “k powder” in China where several epidemiological surveys carried out in urban areas have reported that the percentage of ketamine abusers among all drug addicts had increased from 21.5% in 2001 to 40% in 2009 (Chen et al., 2009; Lian et al., 2005; Liu et al., 2003; Wang et al., 2008). In 2004, ketamine was classified as a psychotropic substance in Schedule I in China (F.D.A., 2004).
Both animal and human studies have shown that ketamine can impair cognitive function, such as episodic memory, semantic memory, working memory, executive function and procedural learning (Curran and Monaghan, 2001; Morgan et al., 2004a, 2004b, 2006b, 2010). Brain-derived neurotrophic factor (BDNF) is an important neurotrophic factor associated with cognitive function, learning and memory as well as synaptic plasticity (Bekinschtein et al., 2008; Binder and Scharfman, 2004). BDNF is mainly synthesized in brain tissues, primarily distributed in the hippocampus, cerebral cortex, striatum, basal forebrain, hypothalamus, brainstem and cerebellum (Aid et al., 2007). In recent years, BDNF has also been found in the peripheral nervous system, as well as in other organs, such as the ovaries, lung, heart, platelet and skeletal muscle (Balkowiec and Katz, 2000). Nerve growth factor (NGF) is another kind of neurotrophic factor, which is widely distributed in vivo. NGF may play an important role in promoting the development of the nervous system; maintaining neuronal growth, survival and differentiation; and influencing synaptic plasticity (Yuen et al., 1996).
Human and animal studies have implied that ketamine use is associated with changes in serum BDNF and NGF levels, although results differ across studies. Studies related to human ketamine users are limited. Ricci et al. (2011) reported an elevation of serum BDNF in 17 chronic ketamine abusers. However, the sample size was small and it is difficult to draw a conclusion. In this study we explored the level of serum BDNF and NGF concentrations in a group of chronic, treatment-seeking ketamine abuse inpatients as compared to health controls. The correlations between the serum BDNF, NGF level with the demographic, drug use characteristics were also examined.
2. Methods
2.1. Participants
Ketamine abusers were recruited from Guangzhou Brain hospital and Guangzhou Baiyun voluntary drug rehabilitation hospital. Healthy controls were recruited through advertisements. All the participants underwent a semi-structured interview to assess sociodemographic characteristics psychopathological status, and substance use. Among ketamine users, the average interval between the interview and last ketamine use was 8.16 ± 4.51 days. Urine was collected to confirm self-report psychoactive drug use. The inclusion criteria for ketamine abusers were: (1) subjects met the criteria of substance dependence according to DSM-IV-TR; (2) no other substances dependence other than tobacco; (3) no other substances use other than alcohol and tobacco for at least 6 months; and (4) age between 16 and 45 years old. Inclusion criteria for healthy subjects included: (1) no axis | diagnosis according to DSM-IV-TR criteria; (2) no familial history (including first- or second-degree relatives) of psychiatric disorders; and (3) age range match with the ketamine group (16–45 years old). Participants were excluded if they had: (1) any known organic diseases or (2) history of head trauma with loss of consciousness, or (3) any unstable physical illnesses, or (4) impairments of color vision or hearing. The study was approved by the Institutional Ethics Committee and written informed consents were signed by participants themselves or their guardians. A total of 93 ketamine users and 39 healthy subjects were recruited in the present study.
Clinical symptoms among ketamine users were evaluated with the Positive and Negative Syndrome Scale (PANSS; He and Zhang, 2000; Kay et al., 1987) administered by two trained psychiatrists with 3 or more years of clinical experience. The intraclass correlation coefficient (ICC) between raters on the PANSS was 0.954. In addition, ketamine users were asked to complete Beck Depression Inventory (BDI, 13-item; Beck and Beamesderfer, 1974) and the Beck Anxiety Inventory (BAI; Beck et al., 1988) which assessed their depressive and anxiety symptoms during the two weeks before they were hospitalized.
2.2. Blood sampling
Venous blood sample was drawn from each subject using standard venipuncture technique. The average days of blood drawing since last ketamine use was 9.34 days ± 5.97 days. Serum was obtained by centrifuged at 4000 rpm for 15 min, then aliquoted was stored at −80 °C until assay.
2.3. Measurement of serum BDNF and NGF levels
Serum BDNF and NGF levels were measured by ELISA using commercially available kits respectively (BDNF Emax Immunoassay System, Promega, USA; NGF Emax Immunoassay System, Promega, USA) according to the manufacturer’s instructions. The minimal detection limits were 15.6 pg/ml for BDNF and 7.8 pg/ml for NGF respectively. The BDNF Emax Immunoassay System and NGF Emax Immunoassay System typically offered less than 3% cross-reactivity with other related neurotrophic factors (NT-3 and NT-4) at 100 ng/ml and 10 ng/ml respectively. All samples were assayed in duplicate. BDNF and NGF levels were determined by absorbance at 450 nm wave length using optical density values against standard curves calibrated with known amounts of proteins.
2.4. Statistical analysis
All the data input were double entry, with verification using EpiData software, version 3.1 (a free software released by the non-profit organization “The EpiData Association” Odense, Denmark). All statistical analyses were performed using SPSS version 21.0 for windows. Chi-square test was used to examine the difference of sex, alcohol consumption and tobacco smoking variables between ketamine users and healthy controls. Spearman’s rank order correlation was applied for non-normally distributed data to investigate the correlations between neurotrophin levels, levels of ketamine use among users. A covariance analysis, with alcohol consumption and cigarettes smoking as covariate in univariate general linear model, was implemented to compare the serum neurotrophic factors levels across the two groups. For all statistical tests, a two-tailed p < 0.05 was considered to be significant.
3. Results
3.1. Demographic characteristics of ketamine users and healthy controls
A total of 93 ketamine abusers and 39 healthy subjects were recruited. The demographic characteristics of the two groups are summarized in Table 1. There were no significant differences in age, gender, or years of education between the two groups although alcohol consumption and cigarette smoking were higher among ketamine users (p value < 0.01).
Table 1.
Clinical and demographic characteristics of ketamine users and healthy subjects.
| Ketamine users (N = 93) |
Healthy subjects (N = 39) |
|
|---|---|---|
| Age (years) | 25.56 ± 4.61 | 24.77 ± 4.75 |
| Gender (male/female) | 87/6 | 34/5 |
| Years of education | 10.91 ± 2.56 | 11.54 ± 1.70 |
| Age of first ketamine use (year) | 19.75 ± 5.02 | |
| Duration of ketamine use (month)a | 70.78 ± 34.52 | |
| Duration of dependence (month)b | 31.57 ± 18.77 | |
| Current frequency of ketamine use (days per week)c | 6.12 ± 1.58 | |
| Current average doses of ketamine consumption (gram per day) | 3.16 ± 3.02 | |
| Use of other psychoactive compoundsd | ||
| Never use | 20 (21.51%) | |
| Methamphetamine | 27 (29.03%) | |
| MDMA | 66 (70.97%) | |
| Codeine hydrochloride | 13 (13.98%) | |
| Cannabis | 22 (23.66%) | |
| Cocaine | 1 (1.08%) | |
| Heroin | 2 (2.15%) | |
| PANSS | ||
| Positive symptom subscale | 8.01 ± 1.82 | |
| Negative symptom subscale | 13.10 ± 3.74 | |
| General psychopathology subscale | 24.23 ± 5.09 | |
| Total score | 45.34 ± 8.70 | |
| BDI | 12.69 ± 6.08 | |
| BAI | 15.10 ± 8.37 | |
| Alcohol | ||
| Drink frequentlye | 27 (29%)** | 5 (12.8%) |
| Not drink frequentlyf | 66 (71%)** | 34 (87.2%) |
| Tobacco | ||
| Smoke frequentlyg | 88 (94.6%)** | 19 (48.7%) |
| Not smoke frequentlyh | 5 (5.4%)** | 20 (51.3%) |
Data are the mean ± standard deviation.
Data about use of other psychoactive compounds, alcohol consumption and tobacco smoking are the amount of cases (percentage).
N, number of subjects included in the study; M, male; F, female; MDMA 3,4-methylenedioxymethamphetamine.
Total months of ketamine use from the first time till now.
Total months of ketamine use from dependent till now.
Frequency of ketamine use in recent one month.
Use of other psychoactive compounds in one’s life span, alcohol and tobacco excluded.
Drink no less than once a week.
Including abstinence, drinking less than once a week and never drinking.
Smoke no less than 3 days per week.
Including cessation, smoking less than 3 days per week and never smoking.
p value < 0.01.
In the ketamine group, the positive symptom, negative symptom and general psychopathology scores of PANSS were 8.01 ± 1.82, 13.10 ± 3.74 and 24.23 ± 5.09 respectively. The BDI score was 12.69 ± 6.08, which indicated mild to severe depressive symptoms (Beck and Beamesderfer, 1974). The BAI score was 15.10 ± 8.37, which indicated low levels of anxiety symptoms (Beck et al., 1988).
3.2. Serum BDNF and NGF concentrations
As shown in Fig. 1, BDNF serum levels in ketamine users and healthy subjects were 9.50 ± 6.68 and 14.37 ± 6.07 ng/ml respectively (F = 5.619, p = 0.019), while NGF levels were 1.93 ± 0.80 vs. 2.60 ± 1.07 ng/ml. BDNF (F = 6.618, p = 0.011).
Fig. 1.
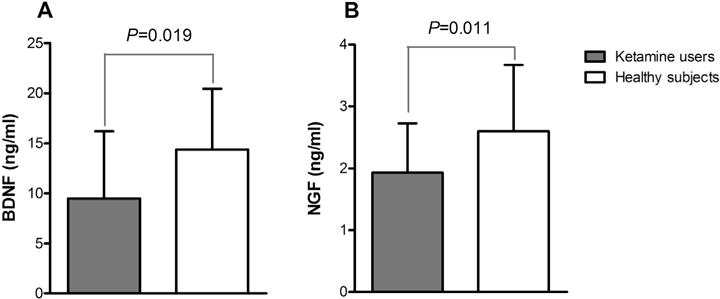
Comparisons of BDNF (A) and NGF (B) serum levels in ketamine users and healthy subjects: (A) BDNF serum level in ketamine users was significantly lower than that in healthy subjects (p = 0.019); (B) NGF level in ketamine users was significantly lower than that in healthy subjects (p = 0.011).
3.3. Correlation of serum BDNF/NGF with demographic and drug use characteristics
Correlations between BDNF and NGF concentrations and different clinical measures (Table 2), showed that there was a negative association between current frequency of ketamine use and BDNF level (r = −0.209, p = 0.045). In addition, the current average dose of ketamine consumption per day of use displayed a marginally significantly negative association with BDNF (r = −0.2, p = 0.056).
Table 2.
Association between BDNF and NGF concentration and clinical situation of ketamine use.
| BDNF(ng/ml) | NGF(ng/ml) | |
|---|---|---|
| Gender | −0.131 | 0.08 |
| Age | −0.069 | 0.078 |
| Education | −0.036 | 0.089 |
| Age of first ketamine use | −0.055 | 0.066 |
| Duration of ketamine usea | −0.154 | −0.005 |
| Duration of dependenceb | −0.041* | −0.071 |
| Current frequency of ketamine usec | −0.209 | −0.112 |
| Current average doses of ketamine consumption | −0.2 | −0.109 |
| Days since last used | 0.087 | 0.038 |
| Alcohol consumption | −0.008 | 0.028 |
| Tobacco smoking | 0.047 | 0.188 |
Total months of ketamine use from the first time till now.
Total months of ketamine use from beginning to depended till now.
Frequency of ketamine use in recent one month.
Days of blood drawing since last ketamine use.
p value < 0.05.
4. Discussion
This study investigated BDNF and NGF serum levels in chronic ketamine users in comparison with a sample of healthy controls and found serum BDNF and NGF concentrations were both lower in chronic ketamine users who were hospitalized for treatment of ketamine related disorders than in controls.
Controversies have existed regarding whether ketamine causes changes of neurotrophic factors with chronic use in both animals and humans. In animal studies it has been reported that BDNF levels in the frontal lobe and the cerebellum of rats increased after receiving intraperitoneal injection of ketamine at 1 ml/100 g body weight daily for 5 days, while NGF level increased in the cerebellum but decreased in the striatum (Becker et al., 2008). Another study, conducted in rat pups showed that repeated exposure to ketamine led to accelerated neurodegeneration and increased BDNF levels in developing brain tissues (Ibla et al., 2009). Also it was showed that ketamine prevented the increase of hippocampal BDNF caused by novel object recognition training (Goulart et al., 2010). Ricci et al. (2011) found that serum BDNF level was higher in 17 chronic ketamine users as compared to 11 healthy control subjects while NGF level was not different. In the present study we found serum BDNF and NGF levels were lower in chronic, treatment-seeking ketamine users than in health controls. Participants in previous studies were most likely to have used lower doses of ketamine at lower frequencies and probably did not meet criteria for ketamine abuse or dependence. In the present study, in contrast, ketamine users were chronic, severe, treatment-seeking inpatients who met the full diagnostic criteria for ketamine associated disorders. The discrepancy in NGF and BDNF findings may reflect these differences in dosage/frequency and duration of ketamine use.
The average interval between the last ketamine use and blood sampling was 9.34 ± 5.97 days. Considering that the half-life of ketamine is about 1–2 h (Morgan and Curran, 2012), the possibility that the lower BDNF and NGF levels reflect a withdrawal response can not be totally excluded. But as no obvious withdrawal symptoms were observed in this group of ketamine users and the usual time until withdrawal symptoms appear was 0.5–3 days (Wang et al., 2009), it is more likely that the lower neurotrophin levels were a consequence of chronic ketamine use, rather than a withdrawal response.
Most users enrolled in this study also had used psychoactive drugs other than ketamine. The usage of such compounds might also have affected the results. But the inclusion criteria limited entrants to those with no other reported substance dependencies other than tobacco, and no other substances use other than alcohol and tobacco for at least 6 months. So confounding is likely to be limited and it is reasonable to consider the lower observed levels of BDNF and NGF as primarily resulting from chronic ketamine use.
Spearman’s rank order correlation analysis showed that the serum BDNF level was negatively associated with the frequency of ketamine use, i.e., more frequent ketamine use was associated with the lower serum BDNF levels, suggesting a possible dose response effect in the modulation of BDNF level.
The mechanisms by which ketamine influence the BDNF level have been investigated intensively. Some studies have shown that the blockade of NMDA by ketamine and the activation of AMPA receptors mediate the increase of BDNF level relevant to ketamine administration (Yang et al., 2012). At the same time, other studies reported that blockade of NMDA receptors inhibits the synthesis of BDNF (Hansen et al., 2004; Semba et al., 2006; Snigdha et al., 2011) mediated by depressed activity of extracellular signal-regulated kinase 1/2 (ERK1/2) and attenuate levels of phosphorylated cAMP responsive element binding protein (CREB; Hansen et al., 2004). Thus the modulation of BDNF level triggered by blockade of NMDA receptors, by drugs such as ketamine, might be a bi-directional pathway under different circumstances.
Since BDNF and NGF play an important role in the neuron development and cognitive functions, it is intriguing to hypothesize that the reduced serum BDNF and NGF level observed in chronic ketamine abusers might play a role in ketamine-related functional impairments. The untoward effects of chronic ketamine use should be more emphasized.
Although the present study employed a relatively large sample of chronic ketamine abusers several limitations should be noticed. Firstly, only serum BDNF and NGF levels were measured which do not necessarily reflect the level of these neurotrophic factors in central nervous system. Nevertheless, it has been observed that BDNF can cross blood-brain barrier via a high-capacity saturable transport system in both directions (Pan et al., 1998) and a positive correlation has been found between serum and cortical BDNF levels (Karege et al., 2002) as well as between blood and hippocampal BDNF levels (Klein et al., 2011). Secondly, patients in the ketamine user group were on antipsychotic medicines, which added complexity to the interpretation of the results since antipsychotics (Chlan-Fourney et al., 2002; Lipska et al., 2001; Pillaia and Mahadik, 2006), as well as antidepressants (Castrén and Rantamäki, 2010; Chena et al., 2001; Coppella et al., 2003; Shimizua et al., 2003), can have an impact on the neurotrophin levels in blood or brain. Thirdly, our samples were mostly consisted of inpatients, suggesting that the severity of ketamine dependence in our patients is likely to be more serious than that in community samples. Thus, hospitalization bias should be taken into account and outpatient ketamine-dependent subjects will need to be investigated to determine whether these findings in this study can be generalized to ketamine addicts living in the community. Nonetheless, our present findings may provide some insight into levels of BDNF and NGF in chronic ketamine abusers.
In conclusion, our study demonstrated that the serum BDNF and NGF concentrations were lower in chronic ketamine-dependent patients than that in healthy control subjects and that the decrement of BDNF level was correlated with high frequency of ketamine intake as well as with positive and negative symptoms.
Acknowledgments
We would like to thank all the staffs of ward 1 and 2 in Guangzhou Baiyun voluntary drug rehabilitation hospital for the invaluable assistance. We are also grateful to Xiaomei Zhong, Xinni Luo, Xinru Chen and Ripeng Li, Wu Fengchun for their supports. We thank Bin Sun for help on statistical analysis.
Role of funding sources
This work was supported by grants to Ni Fan from National Natural Science Foundation of China (No: 81300959), National Key Clinical Program of China in Psychiatry to Guangzhou Brain Hospital (No. 201202001), grants from Guangzhou Municipal Health Bureau (No. 20131A011091) and Guangzhou Municipal Key Discipline in Medicine to Guangzhou Brain Hospital (No. GBH2014-ZD03).
Footnotes
Contributors
Ni Fan and Yuping Ning designed the study and wrote the protocol. Xiaoyin Ke recruited the subjects, undertook the statistical analysis. Ni Fan and Xiaoyin Ke wrote the manuscript. Yi Ding and Chao Zhou contributed to the subjects recruiting, data input. Ke Xu and Hongbo He helped on data analysis and manuscript revision. Minling Zhang conducted ELISA experiments. Xuefeng Deng, Xifan Zhang, Daping Wang and Yuping Liu helped enrolling volunteers. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Schwegler H, Roskoden T. Expression of mRNA of neurotrophic factors and their receptors are significantly altered after sub-chronic ketamine treatment. Med Chem. 2008;4:256–263. doi: 10.2174/157340608784325124. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Chen H, Fan C, Du J, Sun H, Zhao M. DSM-IV axle I diagnoses analysis in 506 patients with substance dependence. Chin J Drug Depend. 2009;18:200–202. [Google Scholar]
- Chena B, Dowlatshahia D, MacQueena GM, Wanga JF, Young LT. Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chlan-Fourney J, Ashe P, Nylen K, Juorio AV, Li XM. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 2002;954:11–20. doi: 10.1016/s0006-8993(02)03215-8. [DOI] [PubMed] [Google Scholar]
- Coppella AL, Peia Q, Zetterström TSC. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001;96:749–760. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- F.D.A., C. Notice on further strengthening the management of ketamine released by China Food and Drug Administration. Capital Med. 2004;15:24. [Google Scholar]
- Goulart BK, de Lima MN, de Farias CB, Reolon GK, Almeida VR, Quevedo J, Kapczinski F, Schroder N, Roesler R. Ketamine impairs recognition memory consolidation and prevents learning-induced increase in hippocampal brain-derived neurotrophic factor levels. Neuroscience. 2010;167:969–973. doi: 10.1016/j.neuroscience.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, Ikonomidou C. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16:440–453. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang M. The Chinese norm and factor analysis of PANSS. Chin J Clin Psychol. 2000;8:65–69. [Google Scholar]
- Ibla JC, Hayashi H, Bajic D, Soriano SG. Prolonged exposure to ketamine increases brain derived neurotrophic factor levels in developing rat brains. Curr Drug Saf. 2009;4:11–16. doi: 10.2174/157488609787354495. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knud-sen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Lian Z, Liu Z, Liu R, Sun G, Mu Y, Lu X, Cao J. Epidemiological survey of ketamine in China. Chin J Drug Depend. 2005;14:280–283. [Google Scholar]
- Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Mu Y, Lian Z, Zhao C, Liu Z. Analysis of ketamine use in drug abusers. Chin J Drug Depend. 2003;12:52–54. [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004a;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend. 2004b;75:301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Rossell SL, Pepper F, Smart J, Blackburn J, Brandner B, Curran HV. Semantic priming after ketamine acutely in healthy volunteers and following chronic self-administration in substance users. Biol Psychiatry. 2006;59:265–272. doi: 10.1016/j.biopsych.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pillaia A, Terry AV, Jr, Mahadik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophr Res. 2006;82:95–106. doi: 10.1016/j.schres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Ricci V, Martinotti G, Gelfo F, Tonioni F, Caltagirone C, Bria P, Angelucci F. Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmacology (Berl) 2011;215:143–148. doi: 10.1007/s00213-010-2121-3. [DOI] [PubMed] [Google Scholar]
- Semba J, Wakuta M, Suhara T. Different effects of chronic phencyclidine on brain-derived neurotrophic factor in neonatal and adult rat brains. Addict Biol. 2006;11:126–130. doi: 10.1111/j.1369-1600.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Shimizua E, Hashimotoa K, Okamuraa N, Koikea K, Komatsua N, Kumakiria C, Nakazatoa M, Watanabea H, Shinodaa N, Okadaa S-I, Iyoa M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC, McLean SL, Shemar GK, Cruise L, Shahid M, Henry B. Phencyclidine (PCP)-induced disruption in cognitive performance is gender-specific and associated with a reduction in brain-derived neurotrophic factor (BDNF) in specific regions of the female rat brain. J Mol Neurosci. 2011;43:337–345. doi: 10.1007/s12031-010-9447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Xu J, Pang Z, Hu W. Clinical analysis of the patients with ketamine induced mental disorders. Chin J Drug Depend. 2009;18:56–59. [Google Scholar]
- Wang Y, Zhang Y, Lian Z, Sun G, Bao Y, Liu Z. Epidemiological characteristics of three new drugs abuse in Beijing. Chin J Drug Depend. 2008;17:445–454. [Google Scholar]
- Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhou Z, Yang C. AMPA receptor potentially participates in the mediation of the increased brain-derived neurotrophic factor following chronic ketamine use. Psychopharmacology (Berl) 2012;220(243) doi: 10.1007/s00213-011-2538-3. author reply 245. [DOI] [PubMed] [Google Scholar]
- Yuen EC, Howe CL, Li Y, Holtzman DM, Mobley WC. Nerve growth factor and the neurotrophic factor hypothesis. Brain Dev. 1996;18:362–368. doi: 10.1016/0387-7604(96)00051-4. [DOI] [PubMed] [Google Scholar]


