Summary
The development of more effective vaccines against Mycobacterium tuberculosis (Mtb) remains a major goal in the effort to reduce the enormous global burden of disease caused by this pathogen. Whole-cell vaccines based on live mycobacteria with attenuated virulence represent an appealing approach, providing broad antigen exposure and intrinsic adjuvant properties to prime durable immune responses. However, designing vaccine strains with an optimal balance between attenuation and immunogenicity has proven to be extremely challenging. Recent basic and clinical research efforts have broadened our understanding of Mtb pathogenesis and created numerous new vaccine candidates that are designed to overcome different aspects of immune evasion by Mtb. In this review, we provide an overview of current efforts to create improved vaccines against tuberculosis based on modifications of live attenuated mycobacteria. In addition, we discuss the use of such vaccine strains as vectors for stimulating protective immunity against other infectious diseases and cancers.
Keywords: Mycobacterium tuberculosis, immune evasion, live vaccine, mycobacterial antigens, auxotroph, attenuation, pro-apoptotic, autophagy, phagosome
Introduction
The genus Mycobacterium, includes both saprophytic and pathogenic species, consists of more than 100 species (1) (http://www.bacterio.net/mycobacterium.html). In this review we will focus on just three species, M. tuberculosis (Mtb), M. bovis, and M. smegmatis, which comprise the basis for the majority of live vaccines currently under development. Mtb and M. bovis are closely related, slow growing mycobacteria that are highly pathogenic in humans, and are the etiological agents of human and cattle tuberculosis (TB), respectively (2). M. smegmatis, in contrast, is a fast growing saprophytic mycobacterium that does not cause disease in immunocompetent humans, and lacks many of the specific adaptations that impart virulence to Mtb and M. bovis (3). The only vaccine currently available for human TB is the M. bovis Bacillus Calmette-Guèrin (BCG) vaccine, originally developed in the early 20th century by Calmette and Guèrin by using approximately 230 passages of M. bovis in culture between 1908 and 1921 to produce an attenuated live vaccine strain (4). Following its initial development, BCG was distributed to many global sites and further cultivation to produce multiple distinct daughter strains. These strains are named accordingly to the country, city, or laboratory in which they were produced, giving rise to designations such as BCG Pasteur, Danish, Russia, Tice, Connaught, Glaxo, Tokyo and many more. Although these BCG substrains were derive from the same common ancestor, they have been shown to harbor significant differences at the genomic level, at least some of which are likely to alter the specificity and quality of the immune responses that they stimulate following vaccination (5). Comparative genomic analyses have demonstrated multiple regions of difference (RD) that represent deletions that are present in one or more of the BCG substrains when compared to the genomes of parental virulent M. bovis or Mtb. Some of these contain virulence-related genes, including examples that encode type VII secretion systems, sigma factors and members of the PE_PGRS family of proteins (6, 7). The absence of the genes contained in some of these regions of difference, in particular RD1, is responsible for the marked reduction of virulence of BCG (8, 9), and its ability to be used as a live vaccine to induce protective immunity without causing disease.
As a vaccine, BCG offers partial protection against leprosy and childhood extra-pulmonary TB. This, along with its comparative safety and ease of production, accounts for its very widespread use with more than 3.5 billion people having been vaccinated to date. However, BCG has been found to provide inconsistent and often inadequate protection against pulmonary TB, and the level of attenuation of the organism is not always sufficient for immunocompromised patients (10). There is also concern that expression of important mycobacterial antigens might have been lost during the attenuation process that converted virulent M. bovis into the BCG vaccine (11, 12). On the other hand, it is likely that many virulence-related genes still present in BCG interfere with the optimal stimulation of anti-mycobacterial immunity and the induction of long lasting protective immunity (13). The genomes of Mtb and BCG each contain approximately 4000 genes, with large numbers of these apparently contributing to intracellular survival and virulence as well as numerous mechanisms for adaptation and immune evasion in the mammalian host (14, 15). Although further engineering of live mycobacteria to achieve improved safety and immunogenicity is a complex undertaking, it is also likely that many opportunities exist for improving on the results obtained with BCG.
Manipulation of the host immune response by Mtb
The tight co-evolution between Mtb and its human host has shaped this organism into an extremely effective pathogen, with some estimates suggesting that as many as 1 out of every 3 humans currently harbor latent infection (16). Mtb is an obligate intracellular pathogen that is transmitted mainly by inhalation of aerosolized infectious droplets. After entering the lung alveoli, Mtb is taken up by alveolar macrophages where it immediately begins to manipulate the host innate immune responses (for reviews see (17)). Initial important events in this process include uptake by the macrophage through specific surface receptors, the inhibition of phagosome-lysosome fusion, neutralization of reactive oxygen and nitrogen species and further adaptations to the environment of the phagosome. Like many other intracellular pathogens, neutralizing the normally harsh lumen of the phagolysosome is a priority for Mtb, and is essential for its establishment and eventual persistence in the host (for reviews see (18, 19)). Mtb stalls phagosome maturation by inhibiting the fusion of the Mtb-containing phagosome with lysosomes (20, 21). Once in the phagosome, the bacterium activates genes such as ureC that neutralize the acidic pH of this compartment (22). Other bacterial genes, such as sodA, are involved in reducing the levels of reactive oxygen species in the infected cell (23). These and other mechanisms not only assist Mtb in dealing with the immediate consequences of the innate immune response against intracellular infection, but also interfere with stimulation of critically important adaptive immunity by blocking antigen presentation pathways (24), autophagy (25), the induction of apoptotic cell death (26), and the manipulation of regulatory T cell responses (27, 28). All of these effects are believed to contribute to suboptimal priming of mycobacteria-specific T cells (29), leading to an adaptive immune response that fails to clear the pathogen, and may actually promotes its survival through the formation of encapsulating granuloma.
Given the extensive immune evasion programs of Mtb, which are likely replicated to a great extent by BCG, any potential route to develop a more effective vaccine must take into account these immune evasion strategies of Mtb, either by overcoming or disabling them. Although still incomplete, our understanding of the mechanisms of disruption of normal host cell processes by Mtb has progressed to the point that has enabled attempts at rational design of strains that more effectively engage the immune system at various levels. This can be done mainly through precise deletion or insertion of genes in Mtb or BCG, using methods that are now highly developed. As illustrated schematically in Figure 1 and detailed in the text that follows, many of the current approaches to novel vaccines for prevention of disease from Mtb infection involve modifying the immune evasion strategies of live mycobacteria. In addition, approaches have been developed to reduce or eliminate the ability of genetically modified mycobacteria to persist or replicate after introduction into human hosts, which provides improvements in the safety and tolerability of new live vaccine strains. The application of these approaches to new vaccine strains currently under development or in clinical testing is reviewed below.
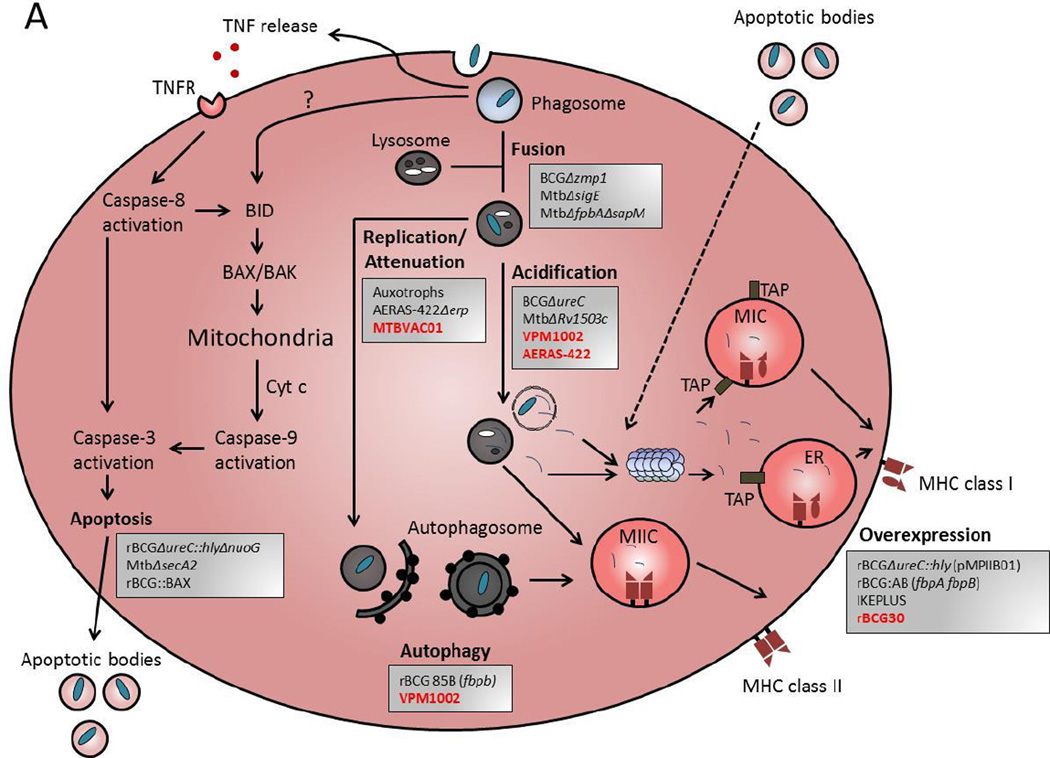
Figure 1. Targeting mechanisms of immune evasion by Mycobacteria to develop novel live attenuated mycobacteria vaccines.
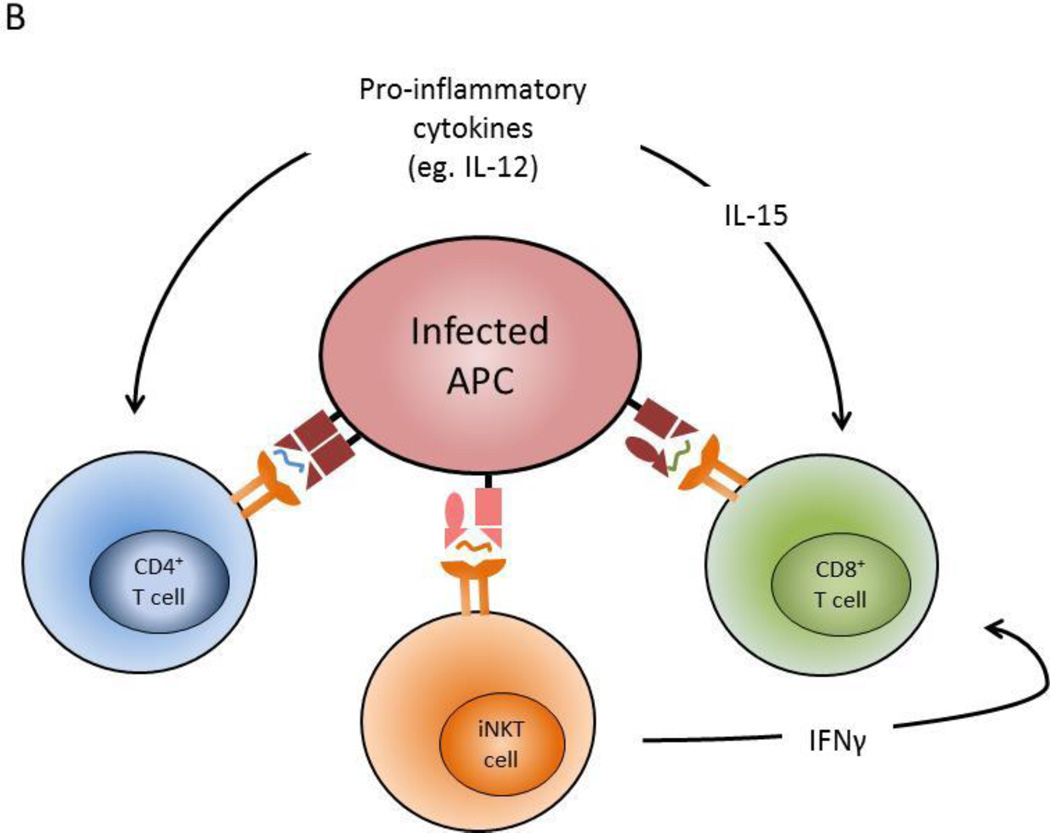
Live mycobacteria vaccine strains have been engineered either by deletion or insertion of genes to enhance the immune response. (A) An antigen presenting phagocyte (DC or macrophage) is illustrated schematically to illustrate host cell processes that are disrupted or manipulated during infection with live mycobacteria. Vaccines are listed in grey boxes and are grouped according to the intended mechanism(s) to achieve improved efficacy against TB. Red font indicates vaccines in in clinical trials, and black font indicates those in pre-clinical development. The six intended mechanisms of the vaccines shown are: 1) Improved phagosome-lysosome fusion, 2) increase attenuation / decrease replication of the bacilli, 3) increase acidification of the bacteria containing vacuoles, 4) induction of apoptosis and 5) autophagy, and 6) overexpression of mycobacteria antigens. (B) The direct mechanisms by which modified vaccine strains enhance antigen presenting cell function lead to enhanced activation of adaptive immune effector cells, including CD4+ and CD8+ T cells, and NKT cells. Also, some vaccine strains can modulate cytokines which can have a pronounce effect on the immune response to improve vaccine efficacy.
New live BCG or tuberculosis vaccines in recent clinical trials
To date, three well-studied modified BCG strains (rBCG30, VPM1002, and AERAS-422) have progressed through multiple levels of preclinical assessment and have entered into clinical testing (30–32). In addition, one novel vaccine based on a live, attenuated form of Mtb (MTBVAC01) has progressed to this level (32). Each of these has been conceived primarily as a novel pre-exposure vaccine to replace the current BCG vaccines for use in newborns. The properties of these next generation vaccine candidates and the status of their clinical evaluations are briefly summarized below.
rBCG30 vaccine strain (rBCGfbpB+)
The first live mycobacteria vaccine that was specifically designed to improve on the current BCG vaccine was rBCG30, a recombinant BCG strain transformed with a plasmid encoding overexpression of the immunodominant antigen 85B (Ag85B) encoded by fbpB (33, 34). Antigen 85B is part of the Ag85 complex that consists of Ag85A, Ag85C, and Ag85D (35, 36). Like other Ag85 proteins in the complex, Ag85B has been characterized to have multiple biological activities, including binding to fibronectin (37, 38) and elastin (39), enzymatic activity important for cell wall structure (i.e., mycolic acid transferase) (40), and is an immunodominant antigen in both mouse and human infections (for reviews see (41)). Another recently documented feature of Ag85B is its ability to induce autophagy in host phagocytic cells (42). It is likely that Ag85B overexpression has multiple effects on the immune response, although its ability to induce strong CD4+ T cell responses associated with interferon-γ secretion provided the main rationale for the design of rBCG30.
Assessment of rBCG30 in a variety of animal models showed distinct improvements in immunogenicity and vaccine efficacy in direct comparisons to standard BCG immunization. In particular, studies in guinea pigs showed rBCG30 strains constructed in both the Tice and Connaught parental strains to be more immunogenic compared to their corresponding unmodified strains, and this corresponded with better protection against Mtb challenge (34, 43). A phase I clinical trial established the safety of the rBCG30 vaccine in healthy adults, and its intended enhanced stimulation of immune responses to Ag85B in humans (33). However, current work on clinical development of rBCG30 as a substitute for standard BCG in humans is presently on hold, as the focus of the field has shifted to more attenuated and broadly immunogenic candidate vaccines (for reviews see (31)). Preclinical work continues with development of related rBCG30 strains that also incorporate additional attenuating mutations, such as a deletion in the mycobactin synthesis pathway (ΔmbtB), which is defective in iron acquisition (44). This and other forms of modified rBCG30 are of interest as potentially safer and more efficacious vaccines for use in neonatal populations with high prevalence of HIV infection or other types of immunosuppression.
VPM1002 modified BCG vaccine strain (rBCG ΔureC::hly)
The recombinant BCG VPM1002 vaccine was engineered to expresses the Listeria monocytogenes pore-forming protein listeriolysin O (LLO) encoded by the hly gene, which mediates the perforation of the phagosomal membrane. The design of this strain was based on the idea that expression of LLO should allow mycobacterial antigens to more efficiently access the host cell cytosol. This should lead to increased processing of antigens in the MHC class I antigen presentation pathway, and augmented CD8+ cytolytic T cell priming. In addition, LLO expression also appears to induce apoptosis in infected macrophages, which indirectly increases CD8 T+ cell responses by cross-priming through an alternate mechanism. Another feature of the VPM1002 strain is the deletion of the ureC encoding a urease that is involved in the inhibition of phagosome acidification. This modification improves the membrane disrupting activity of LLO, which is optimal at pH5.5 (22). In addition, stronger acidification of phagosomes in infected cells could also improve processing of protein antigens and their presentation by MHC class II to CD4+ T cells, such as was shown in the case of Ag85B in infected macrophages (45). Studies in mice have also suggested that immunization with VPM1002 leads to greater expansion of IL-17 producing T cells compared to standard BCG vaccination, although the reasons for this are not clear (46).
The properties of the BCG ΔureC::hly strain have been well characterized in cultured macrophages and mouse models (22), and the clinical development of VPM1002 as a potential second generation vaccine to replace BCG has been extensively reviewed by Kaufmann et al. (30, 31). Phase I clinical studies in healthy adults have been completed in 80 volunteers in Germany and 24 volunteers in Bloemfontein, South Africa, showing immunogenicity and safety that were judged sufficient for proceeding with clinical evaluation in newborn infants (47). A phase IIa trial in HIV-unexposed newborn infants in South Africa is currently in progress and shows that VPM1002 is as safe as BCG and elicits similar type of immunity reported in the phase I studies (30) (ClinicalTrials.gov identifier NCT01479972), and a phase IIb trial in HIV infected newborns is also being considered (48). The improved safety and enhanced immunogenicity of VPM1002 has prompted interest to test it as a potential agent for treatment of bladder cancer to replace BCG, which is currently licensed for use as a treatment for nonmuscle invasive bladder cancer. Although the precise anti-tumor mechanism of BCG is not fully known, it is believed that induction of strong Th1 immunity by BCG and the preferential uptake of the bacilli by tumor cells are factors that contributes to tumor immunity (49). Clinical trials to assess VPM1002 for treatment of bladder cancer have been planned but are not yet initiated (ClinicalTrials.gov identifier NCT02371447).
AERAS-422 modified BCG vaccine strain (rBCGΔureC::pfoA Rv3407+fbpA+fbpB+)
The rBCG vaccine AERAS-422 combines the rationales of both the VPM1002 and rBCG30 vaccine strains. AERAS-422 overexpresses the immunodominant antigens Ag85A (fbpA), Ag85B (fbpB), and a latently expressed TB antigen Rv3407. Instead of the L. monocytogenes LLO, the perforin perfringolysin O (PFO) of Clostridium perfringens has been introduced to disrupt phagosomal membrane. The PFO cassette was inserted into ureC to remove the urease activity of the AERAS-422 vaccine strain, even though the PFO was engineered to maintain porin activity at neutral pH (for reviews see (31)). Studies in mouse models clearly showed the vaccine strain was attenuated, has enhanced immunogenicity for responses to the overexpressed BCG antigens, and elicited protection against Mtb challenge superior to standard BCG vaccination (50). However, clinical development of AERAS-422 as a novel TB vaccine was recently terminated during a phase I clinical trial due to occurrence of reactivation of varicella-zoster virus leading to shingles in two vaccinated healthy control subjects (31, 51). The cause of this unexpected adverse effect remains unclear, but it suggests the possibility of potentially significant immunosuppression as a result of vaccination with AERAS-422. This will likely need to be studied further in animal model systems before the possibility of resuming clinical trials can be considered.
Attenuated M. tuberculosis MTBVAC01 vaccine strain (Mtb ΔphoP ΔfadD26)
The MTBVAC01 vaccine strain was constructed in the background of the virulent Mycobacterium tuberculosis strain MT103 isolated from an immunocompetent TB patient (52). Due to the guidelines established by the World Health Organization (WHO) which require a minimum of two independent strongly attenuating for live vaccine strains derived from Mtb, the MTBVAC01 vaccine strain contains stable unmarked deletions of both the phoP and fadD26 genes (52). PhoP is a transcriptional regulator of a two-component system involved in the regulation of approximately 2% of the M. tuberculosis genome, including genes responsible for responses to hypoxia and other types of stress, lipid metabolism, respiration and virulence (53). The other attenuating feature of MTBVAC01 is the deletion of the fadD26 gene, which encodes an acyl-CoA synthase required for lipid metabolism and the biosynthesis of phthiocerol dimycocerosates (DIMs), a component of the mycobacterial cell wall required for intracellular survival (54, 55). Preclinical data in animal models showed that the MTBVAC01 vaccine strain was as good as BCG in terms of safety, and induced better levels of protection following virulent Mtb challenge (52). The MTBVAC01 vaccine entered a phase I clinical trial at the end of 2013 which is currently ongoing (ClinicalTrials.gov identifier NCT02013245). Further development of the MTBVAC01 includes the hyper-attenuated MTBVAC Δerp mutant (56). The Mtb erp gene encodes for the exported repetitive protein (ERP), a secreted antigen found on the bacterial surface. The Mtb Δerp mutant fails to replicate in murine macrophages and is attenuated in mice (57). The MTBVAC Δerp− mutant strain achieved higher attenuation than MTBVAC01 while maintaining a similar level of immunogenicity in mice (56).
New and developing approaches for live mycobacteria vaccines
Out of the four vaccines described above, only the VPM1002 has proceeded into phase II trials, while the rest have either been halted or delayed due to potential safety concerns or the need for further optimization. The complexity of Mtb evasion and subversion of host immunity may require more complex modifications of live mycobacteria to generate vaccines that can fully recruit protective adaptive immunity against subsequent exposure. This would likely include further modifications of live vaccine strains to enable phagosome maturation, and to promote cellular processes that enhance antigen presentation such as apoptosis and autophagy. In addition, a range of other possible modifications are being considered, including some that modulate pro-inflammatory cytokines, enhance the formation of memory T cells, or make relevant mycobacterial antigens more available as targets for immune response through a variety of different mechanisms. New candidate vaccine strains based on some of these principles are briefly described below.
Auxotrophy mutations
The deletion of essential biosynthetic genes to create auxotrophic mutants is one of the most straightforward and effective ways to reduce the virulence of Mtb or BCG to negligible levels. One gene that has been extensively targeted for this is leuD, which encodes isopropyl malate isomerase, an essential enzyme for leucine biosynthesis. Mutants that are completely deficient in the activity of leuD are fully viable when grown in culture medium supplemented with leucine, but are completely unable grow and persist in vivo in mice (58). Other auxotrophy mutations with similar properties are deletions of genes for pantothenate synthesis (panC and panD), and the lysA gene required for lysine biosynthesis. Typically, strains with deletion mutations in any of these genes are highly attenuated for growth even in severely immunodeficient SCID mice, greatly surpassing the level of attenuation of BCG.
A great challenge in the development of live vaccines is finding the optimal balance between attenuation and immunogenicity. While most of the auxotrophic mutants of Mtb or BCG show complete attenuation of virulence, they may have subtle differences in their levels of stimulation of durable protective immunity. For example, immunization of mice with an MtbΔleuD auxotroph gave a level of protection against virulent Mtb challenge that appeared to be inferior to standard BCG vaccination (59). On the other hand, vaccination with an MtbΔpanCD auxotroph gave protective immunity to virulent Mtb challenge at levels comparable in BCG immunization (60). Such differences in immunogenicity may be due to subtle variations in the degree of initial replication or persistence of the different auxotrophs. However, since the correlates of protective immunity against Mtb infection remain unknown, it has been difficult to determine which of the multiple possible auxotrophic mutations are optimal for providing the best balance of attenuation and immunogenicity. This is an important point that needs to be resolved, since the incorporation of at least two independent and non-reverting auxotrophic mutations is generally recognized as the best approach to generating a truly safe live Mtb or BCG vaccine strain for use in immunocompromised subjects. Double auxotroph mutants of Mtb have performed extremely well in terms of safety in animal models, and in most cases induce protective immunity similar to standard BCG (61–64). The introduction of additional mutations to enhance immunogenicity into double auxotrophic strains provides an attractive approach for generating extremely safe, improved live Mtb vaccines. For example, introduction of the ΔsecA2 mutation into an auxotrophic or double auxotrophic strain of Mtb has been shown to provide a pro-apoptotic effect with enhanced CD8+ T cell priming and enhanced protection against Mtb challenge (65–67).
Antigen overexpression
Following on the pioneering studies carried out with rBCG30, more sophisticated efforts have gradually developed for improving the immunogenicity of BCG by forced overexpression of one or more mycobacterial antigens. The enhanced safety and immunogenicity of BCGΔureC::hly (VPM1002) prompted its use as the basis for further genetic modification by this approach. This has led to the creation of the vaccine strain rBCGΔureC::hly (pMPIIB01) that constitutive overexpresses three mycobacteria antigens (Rv1733c, Rv2659c, and Rv3407) associated with latent Mtb infection (68, 69). By including these antigens, the pMPIIB01 vaccine can potentially enhance immune recognition of dormant mycobacteria and prevent TB reactivation. Although both the VPM1002 and the rBCGΔureC::hly (pMPIIB01) protected mice from Mtb challenge similarly at early time points, the latter was superior at a later time point (200 days post challenge) (68), which suggests the temporal features of Mtb antigen expression is an important consideration for vaccine design.
Another modified BCG strain based on the antigen overexpression principle is rBCG:AB, which is a BCG China strain engineered to constitutively express both Ag85A and Ag85B encoded by the fbpA and fbpB genes, respectively. The rBCG:AB vaccine gave better protection as compared to vaccination with rBCG:A or rBCG:B strains overexpressing only one of the two antigens (70). Thus, targeting multiple immunodominant Mtb antigens as opposed to a single immunodominant antigen may have advantageous effects. As stated in the review by da Costa et al. (71), it is important to determine if the rBCG:AB can also enhance the establishment of functional long lasting memory.
Enhancement of phagosome maturation
Several novel mutations in BCG and Mtb have been identified that improve phagosome maturation, potentially improving processing of the bacteria to provide better antigen presentation. One example that has been studied on the BCG background is the BCGΔzmp1 strain, in which deletion of the Rv0198c gene leads to deficiency of the zinc metalloprotease 1 (Zmp1). The importance of Zmp1 in phagosome maturation was initially highlighted by studies implicating it in the degradation of cellubrevin (VAMP3), a v-SNARE molecule involved in fusion and trafficking of endosomal vesicles (72). Other studies to elucidate how Zmp1 stalls phagosome maturation have shown that Zmp1 overexpression in both rMtb and rBCG prevents inflammasome activation in infected cells by inhibiting both caspase 1 and IL-1β activities (73). As a vaccine strain, the BCGΔzmp1 is more immunogenic compared to standard BCG and induces stronger memory responses in mice (74) and bovine models (75). BCGΔzmp1 is attenuated in SCID mice, suggesting a favorable safety profile. In addition, it induced better protection than standard BCG vaccination following Mtb challenge in guinea pigs (76).
Screening of Mtb mutant libraries have identified additional Mtb mutants with defects in their ability to arrest phagosome acidification and inhibit phagosome-lysosome fusion (77). This function appears to be central to the mycobacterial immune evasion strategy, and the coordinated activity of multiple nonredundant pathways appear to be involved in controlling this aspect of the host cell environment. Several specific genes contributing to this mechanism have been deleted from Mtb, and the resulting mutants are of interest for novel vaccine design. One example involves deletion of the Mtb sigma factor σE (SigE; Rv1221), which regulates a complex network of gene expression that includes pathways important for maintaining cell wall stability such as mycolic acid biosynthesis. It was discovered that the MtbΔsigE mutant is more susceptible to stress, either from SDS or due to growth inside macrophages, and induces stronger innate immune responses including increased activation of Toll-like receptors 1 and 2, increased pro-inflammatory cytokines, chemokines and prostaglandins. The MtbΔsigE mutant show significant attenuation of virulence in BALB/c and nude mouse models. Immunogenicity of MtbΔsigE was superior to that of BCG, and in turn provided better protection after Mtb challenge (78). Further analysis shows that MtbΔsigE was not capable of arresting phagosome maturation as shown by an increase in phagosome-lysosome fusion (79). The mechanism for this is unclear, although it is possible that it relates to the important role of SigE in regulating cell wall integrity and effects on the display of surface lipids of Mtb such as ManLAM that are able to stall phagosome maturation (80).
Another mutant of Mtb that has been found to be deficient for inhibition of phagosome-lysosome fusion is the MtbΔfbpA strain, with a deletion of the gene encoding Ag85A. This strain failed to grow in nutrient poor media and inside macrophages (81), and was attenuated in mice (82). Further analysis revealed that the MtbΔfpbA vaccine strain remained latent in immunized mice and revived after 300 days, possibly due to declining immune function of the host animals due to aging (82). However, the MtbΔfpbA mutant only partially inhibited phagosome lysosome fusion (83). In order to augment this relatively weak phenotype, a further deletion was made in the sapM gene, which encodes an acid phosphatase that has been found to interfere with phosphotidylinositol 3-phosphate signaling and subsequent phagolysosome fusion. The double knockout MtbΔfpbAΔsapM_mutant was more attenuated, had increased phagosome-lysosome fusion and was more immunogenic in mice than the MtbΔfpbA or the MtbΔsapM single mutants (84).
Although further attenuation may be required to guarantee safety, this double mutant of Mtb is a potentially interesting candidate for further vaccine development.
A transposon library screen of Mtb identified Rv1503c, which encodes an enzyme involved in cell wall glycolipid syntheses, to also play a role in the inhibition of phagosome maturation. An MtbΔRv1503c mutant accumulated the acyl sulfoglycolipids Ac3SGL and Ac4SGL, which are thought to be among the relevant stimuli that drive phagosome maturation and acidification (85). This emphasizes the importance of mycobacterial glycolipids as mechanisms for evading the host immune response (86, 87), and points to additional pathways that may be targeted to improve immunogenicity of modified vaccine strains.
Promoting apoptosis
As discussed earlier, Mtb and BCG actively inhibit pathways leading to apoptosis of infected cells, and this eliminates a major alternate pathway for antigen processing and presentation. Several genetic modifications of these mycobacteria have been shown to restore apoptotic death of infected macrophages. One example is the deletion of the Mtb nuoG gene, which encodes a subunit of the type I NADH dehydrogenase complex that suppresses reactive oxygen species in macrophages and inhibits the induction of TNF-mediated apoptosis (88). The MtbΔnuoG mutant was less virulent than the wild type Mtb and induced apoptosis, and these effects of nuoG deletion are also seen in BCG. Currently, development of a nuoG deleted mutant in the background of rBCG with deletion of a urease gene and expression of the pore forming listeriolysin O (rBCGΔureC::hlyΔnuoG ) is underway (89).
A phenotype similar to nuoG deletion has also been observed for secA2 deletion in Mtb, although not in BCG. The Sec-dependent protein export pathway in Mtb not only consists of the essential housekeeping SecA1, but also a non-essential secA2 for secretion of a relatively small set of virulence-related proteins. The MtbΔsecA2 mutant showed moderate attenuation of virulence, but gave a major improvement in immunogenicity that was correlated with its increased ability to induce apoptosis of infected phagocytes. This phenotype was attributed to diminished secretion of the enzymes SodA (superoxide dismutase A) and KatG (catalase-peroxidase), leading to increased accumulation of superoxide radicals and hydrogen peroxide (90). Consistent with this, a Mtb mutant with reduced SodA production was found to be attenuated and more susceptible to killing by hydrogen peroxide (23), similar to a MtbΔsecA2 mutant (90). Vaccination studies using the MtbΔsecA2 mutant revealed this strain to be more effective in priming antigen specific CD8+ T cells, resulting in better protection against Mtb challenge as compared to BCG in both the murine and the guinea pig models (91).
Another approach for upregulating apoptosis induction is the expression of specific apoptosis promoting proteins in recombinant vaccine strains. One example is the rBCG::BAX strain, which expresses the pro-apoptotic protein BAX, a Bcl-2 family protein that induces apoptosis by triggering cytochrome c release from the mitochondria (92). Indeed, rBCG::BAX induced more apoptosis in both in vitro macrophage experiments and in vivo mouse models. Splenocytes from immunized mice showed a stronger BCG specific immune responses in rBCG::BAX as compared to those in the wild type BCG group (93). Improving safety and testing for protection against Mtb challenge are the next steps for the development of this vaccine strain.
Increasing autophagy
Autophagy has been increasingly recognized in recent years as an important alternate mechanism for delivering pathogen-derived antigens to the MHC class I and II presentation pathways. It appears likely that Mtb and BCG both encode programs that interfere with autophagy in infected cells, thus providing another immune evasion strategy that may compromise vaccine induced immune responses. Paradoxically, it has been found that the mycobacterial Ag85B protein actually promotes autophagy through mechanisms that are not yet understood. Thus, forced over-expression of Ag85B in BCG represents one effective approach to enhance autophagy induction, and this has been found to improve antigen presentation and increase protective immunity against Mtb challenge (42). Similarly, the VPM1002 vaccine strain (rBCGΔureC::hly) mentioned above which expresses a pore forming toxin that creates leakage of phagosomal contents into the cytosol has also been found serendipitously to increase autophagy. This appears to be due to its ability to activate the AIM2 inflammasome, probably through the release of bacterial DNA into the cytosol (94). The identification of genes encoding mycobacterial effectors that actively block autophagy is an area of active research, and this should open up new avenues for improving immunogenicity of novel live vaccine strains.
Production and modulation of cytokines
Cytokines are proteins secreted by immune cells to communicate and influence each other in order to establish a robust and functional immune response (95). Shortly after internalizing pathogens such as Mtb, infected macrophages and dendritic cells begin to secrete pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-12 (96). Cytokines such as IL-12 are important for the activation of naïve T cells, thus bridging together the innate and adaptive immune responses (97). The induction of a long-term immunological memory is important in generating an ideal and optimal adaptive immune response especially in the context of vaccine development, and it has been shown that the cytokine IL-15 is important for the maintenance of memory CD8+ T cells (98). Besides the interplay between antigen presenting cells and T cells, other immune cells, such as NKT and NK cells can also be involved in the establishment of the adaptive immune response through secretion of pro-inflammatory cytokines. Here we briefly discuss several approaches to manipulating the cytokine milieu induced by live vaccine strains that have shown potential promise.
One example is the generation of Mtb strains that enhance production of IL-12 by infected phagocytes, as this is a key cytokine for the priming of adaptive immunity that appears to be actively suppressed by BCG and Mtb infection (99). A genetic screen identified the Mtb mmaA4 gene, which encodes a methyl transferase involved in mycolic acid synthesis, as a factor involved in the suppression of both IL-12p40 and TNF by infected macrophages (99, 100). Deletion of the mmaA4 gene from either Mtb or BCG significantly augmented the production of these cytokines by macrophages. However, immunization of mice with BCGΔmmaA4 did not induce better protection following Mtb challenge than standard BCG immunization. Nevertheless, administration of this strain to mice in a formulation with cationic liposomes did result in improved protection from Mtb challenge and stronger induction of multifunctional CD4+ T cells (101). Thus, restoration of IL-12 production may be a viable strategy for enhancing vaccines, but only in combination with other factors.
Another strategy is to actually engineer secretion of functional cytokines by the mycobacteria itself. An example of this is provided by a novel rBCG strain expressing IL-15 fused to the secreted Ag85B protein. Overexpressing Ag85B in rBCG to provide this antigen for augmenting T cell responses has been described above as a rationale for improving vaccination. By fusing murine IL-15 to Ag85B in the rBCG-Ag85B-IL15 strain, a live vaccine combining enhanced antigen delivery with modification of the cytokine milieu was created. Given its normal functions in supporting T cell proliferation and maintenance of memory T cells, it was thought that providing IL-15 in this way during vaccine-induced responses would improve long term protective immunity. In fact, mice immunized with the rBCG-Ag85B-IL15 developed stronger BCG-specific CD8+ and CD4+ memory T cell responses compared to rBCG-Ag85B. Vaccination with rBCG-Ag85B-IL15 also provided enhanced protection against Mtb challenge as compared to rBCG-Ag85 vaccination (102). Interestingly, it has been shown that administration of free recombinant IL-15 during Mtb infection did not enhance Mtb specific memory T cell responses (103), possibly due to rapid clearance of the cytokine in the host. However in the case of rBCG-A85B–IL15, the slow and continuous release of the cytokine by the rBCG might overcome this problem and thus contribute to generation of BCG-specific memory T cell populations (102). These findings with IL-15 provide an early proof-of-concept for this approach, and this method could readily be extended to many other cytokines of interest (104).
Another approach to inducing an effective cytokine milieu during T and B cell priming following vaccination is through the activation of NKT and NK cells. NKT cells are a specialized group of T cells which recognized glycolipid compounds bound to the CD1d molecule. They are a highly attractive candidate to modulate because of the availability of specific activating compounds (105). Upon TCR ligation by CD1d-glycolipid complexes, NKT cells can rapidly release a variety of cytokines, and induce secondary activation of other leukocytes including dendritic cells, NK cells, B cells and T cells, thus amplifying the immune response (105). A potential disadvantage of using NKT cell activating glycolipids as vaccine antigens is their tendency to induce NKT cell anergy as well as toxicity, especially in the liver (106–108). However, recent work has shown that these problems can be circumvented by incorporating NKT cell activating glycolipids directly into live bacterial vaccine strains (105, 109, 110). When tested in the context of TB infection, BCG modified in this way with NKT cell activating glycolipids confers better protection against Mtb challenge than unmodified BCG (110). This appears to be due at least in part to an enhancing effect of NKT cell activation on the priming of CD8+ T cells specific for mycobacterial antigens.
Vaccine strains based on M. smegmatis
Mycobacterium smegmatis is a fast growing non-pathogenic mycobacterium often used in laboratories for genetic manipulation. The ease of genetic manipulation of this organism, and its extremely low potential for causing disease, makes it attractive as a potential live vaccine vector. For example, the envelope protein (Env) of HIV (111) and several Mtb antigens such as Ag85C, MPT51, and HspX (112) have been expressed in M. smegmatis and to be immunogenic in mice when delivered in this context (111, 112). In addition, M. smegmatis expresses many homologues of Mtb antigens, and significant levels of immunologic crossreactivity exist between the two species (113, 114). In an unmodified form, M. smegmatis has not performed well as a vaccine against Mtb in animal models, generally inducing protective immunity that is lower than that achieved with standard BCG vaccination. However, genetically modified forms of M. smegmatis have recently been developed that show promising improvements. For example, transposon mutants of M. smegmatis that have enhanced protein secretion have been isolated, and these show improved priming of T cell responses against specific secreted protein antigens produced by the bacteria (115). Another interesting candidate vaccine strain was constructed by deletion of the chromosomal region encoding the ESX-3 type VII secretion system in M. smegmatis. This region is implicated in iron acquisition, intracellular persistence and virulence in pathogenic mycobacteria such as Mtb and is substantially conserved in M. smegmatis. Deletion of the genes encoding ESX-3 along with introduction of the homologous genes from Mtb led to the creation of a M. smegmatis strain designated IKEPLUS, which demonstrated powerful induction of anti-mycobacterial immunity when injected into mice (116). The mechanism for this effect appears to involve priming of polyfunctional CD4+ T cells that cross-react with endogenous Mtb antigens. Further studies of IKEPLUS may help to establish M. smegmatis as a viable platform for construction of live vaccine strains.
Other applications of live mycobacteria vaccines
Based on safety, ease of production and strong adjuvant effects of BCG, there has long been interest in using it as a vector for vaccines or immunotherapies to address diseases other than Mtb infection. Other targets have included major chronic infections such as HIV and malaria, as well as cancer. Recent advances such as those made on expression of HIV antigens in recombinant BCG have helped to increase interest in this approach (117). Concomitant infection with HIV and Mtb is a major global health problem and it has been reported recently that one third of Mtb-related deaths are attributable to HIV infection, most likely due to the increased incidence of reactivation of latent TB (118). Against this backdrop, the possibility of a safely attenuated recombinant BCG or Mtb strain that expresses HIV antigens to induce immunity against both pathogens is extremely appealing. Multiple efforts toward this goal have been attempted, and in some cases studies of immunogenicity and safety have been initiated in rodent and non-human primate models (117, 119, 120). This approach remains an active area of preclinical investigation, and it is unclear at this time whether it ultimately will find an application in the effort to create effective vaccines for prevention or control of HIV infection.
Similarly for malaria, it has been shown that expression of Plasmodium falciparum antigens in rBCG can induce malaria specific immune responses (121–123). One antigen that has been studied in this context is the MSP-1 protein, a surface antigen on P. falciparum that is a major target for antibodies (124). A mutant form of the C-terminal 19 kDa fragment of MSP-1 was expressed in BCG (rBCG016), and immunization of mice with this strain induced antibodies that recognized the parasite surface and blocked P. falciparum invasion of human erythrocytes in vitro. Although this and other related studies represent early stages in development of potential BCG based malaria vaccines (122), they point to the possibility of the rational use of rBCG as a blood-stage malaria vaccine candidate.
Interest in the use of BCG for cancer immunotherapy has an extremely long history, going back to the first half of the twentieth century. In 1976, BCG was used for the first time in the treatment of superficial bladder cancer by instillation directly into the bladder (125, 126). This approach has been proven effective, and is still used as a primary therapy to prevent recurrence after transurethral resection of tumors of non-muscle invasive bladder cancer (127, 128). The anti-tumoral activity of intravesical BCG includes the innate susceptibility of tumor cells to be infected by intracellular bacteria (129), and the binding of BCG to fibronectin present in the malignant but not on the normal urothelial cells that are usually covered with glycosaminoglycans (130). After internalization, the mechanism of BCG vaccine in anti-tumor immunity may be similar to the pathology of mycobacteria infection of the lung, including secretion of pro-inflammatory cytokines and chemokines, and recruitment of lymphocytes (131), macrophages, dendritic cells, neutrophils, and NK cells (132) leading to the formation of a BCG induced granuloma in the bladder wall. It is unclear whether modification of BCG by any of the approaches currently considered might further improve its efficacy in bladder cancer. However, the live rBCG vaccine strain VPM1002 mentioned earlier is currently being entered into clinical trials to test its efficacy against bladder tumor. In addition, modified forms of BCG or other attenuated mycobacteria, remain of potential interest as vectors for delivering tumor associated antigens or for general immunostimulation in cancer patients.
Expert commentary and five-year view
With the emergence of drug resistant strains of Mtb and the HIV epidemic, better vaccines for prevention and control of tuberculosis are urgently needed. Given that the original BCG vaccine strain was developed almost a century ago, it is astonishing that this remains the only vaccine for tuberculosis that is currently approved for use in humans. This fact is even more sobering given that multiple studies have shown that as a vaccine, the protective efficacy of BCG against adult pulmonary tuberculosis is often poor and varies greatly according to geographic region and poor identified factors (133, 134). Despite the obvious challenges, the potential advantages of live mycobacterial vaccines continue to inspire efforts in this field. Great progress has been made in developing approaches to safely attenuating live mycobacteria, especially through precise gene deletions that result in auxotrophy for multiple nutrients or essential metabolites that are unavailable in the tissues of the vaccinated host. Using this approach, it is very likely that BCG or even Mtb strains that are significantly safer than the current BCG vaccine can now be produced for use in immunosuppressed individuals. In addition, methods for stable expression of foreign antigens in BCG or attenuated Mtb also continue to advance, and this may ultimately enable the use of live mycobacterial vaccine strains as vectors for vaccination against other infections such as HIV and malaria. Basic research remains the most important tool in pushing forward new frontiers in vaccine design, which are hoped to lead to eventual breakthroughs for controlling tuberculosis. Identification of the key protective mycobacterial antigens, improved methods for genetic modification of mycobacteria, rational approaches to attenuation and new understanding of adjuvants have all contributed to new concepts in vaccine design. An enormous amount of information has accumulated on Mtb infection since the introduction of BCG vaccination, both in terms of our understanding of the pathogen and the host response against it. It seems likely that application of this new knowledge will eventually lead us to a better live mycobacterial vaccine strain that will replace BCG, but the realization of this goal is still not clearly within our grasp.
A variety of different approaches are currently being pursued in the effort to make better TB vaccines, and these include the use of selected Mtb antigens delivered using a range of different vectors or in the form of purified proteins. However, the live vaccine strain remains a preferred option for a number of very relevant reasons. Experience in the vaccine field has generally shown that live-attenuated organism vaccines, such as BCG, are superior to killed or subunit vaccines for conferring durable immune responses. Live whole cell vaccines also provide the broadest possible coverage of the antigenic repertoire of a pathogen, and have inherent adjuvant properties that can be extremely effective for enhancing the magnitude, potency and durability of immune responses. However, the lack of success to date in producing a clearly superior replacement for BCG indicates that we are still hampered by an incomplete understanding of the mechanisms or correlates of protective immunity to Mtb infection. For example, deletion of the RD regions removed several virulence genes and made BCG safe for use as a vaccine. However, this also removed genes encoding immunodominant antigens, which could account for the suboptimal level of protection confers by the BCG vaccine against Mtb. The importance of these immunodominant antigens in the context of protective immunity are also not well understood since most Mtb (135) and BCG (12) human T cell epitopes are highly conserved, and thus appear not to be responsive to immune pressure to generate escape variants. Furthermore, the lack of animal models that can truly represent the human population puts strong emphasis on human clinical trials, which require enormous outlay of resources, time and effort. This creates an unavoidable bottleneck in vaccine development, and severely slows the pace of development and testing of new vaccine candidates.
Going forward in the search for an improved live-attenuated vaccine strain to replace BCG, there are a number of obvious issues that must be resolved. Perhaps the most basic of these is whether a new live vaccine strain should be built on an existing BCG strain, or instead created de novo from M. tuberculosis. Since BCG was derived from M. bovis, it is extremely similar but not identical to pathogen it is intended to vaccinate against. The significance of the differences between BCG and M. tuberculosis in this regard remain unclear. Common sense would suggest that it might be better to use an attenuated version of the actual pathogen for vaccination, since this should provide an immunization that is more precisely matched to the subsequent challenge. Although the great majority of expressed proteins are highly conserved between BCG and Mtb, there nevertheless are easily discernible differences in the immunological targets presented by the two species. In fact, on recent study has highlighted this by showing that approximately 23% (358/1530) of the currently known human T cell epitopes of Mtb are missing from BCG (12). The vast majority of these missing epitopes are contained within the deleted genomic regions (RDs) of BCG, although a few amino acid substitutions have also been indentified in known antigens such as Ag85B. This may have significant implications for some of the candidate vaccines that incorporate overexpression of Ag85B, such as the rBCG30, rBCG:AB, and rBCG fbpB vaccines described in this review. Nevertheless, in spite of the potential significant antigenic differences between BCG and Mtb, an obvious advantage of BCG is its general acceptance as a safe vaccine and the vast experience that has accumulated on its use in many human populations. This makes regulatory approval, testing and production significantly more straightforward. Thus, the issue of whether to pursue a modified BCG versus a novel attenuated Mtb-based strain to replace the current BCG vaccine remains unsettled, and both approaches will likely continue to proceed in parallel.
Table 1.
Live attenuated mycobacteria vaccines
| Vaccines | Mechanisms | Status | References |
|---|---|---|---|
| Overexpression | |||
| rBCG30 (fbpB+) / ΔmbtB | Ag85B | rBCG30: Clinical Phase I ΔmbtB: Pre-clinical |
33,34,43,44 |
| rBCGΔureC::hly (pMPIIB01) | Reactivation in Mtb: Rv1733c, Rv2659c, Rv3407 | Pre-clinical | 68,69 |
| rBCG:AB | Ag85A and Ag85B | Pre-clinical | 70 |
| M. smegmatis high secretors | Enhanced protein secretion mutant | Discovery | 115 |
| IKEPLUS | Mtb ESX3 region | Pre-clinical | 116 |
| AERAS-422 (rBCGΔureC::pfoA Rv3407+fbpA+fbpB+) |
Early and latent antigens | Clinical Phase I (Terminated) |
31,50,51 |
| Phagosome maturation | |||
| BCGΔzmp1 | Zinc metalloprotease: inflammasome | Pre-clinical | 72–76 |
| MtbΔsigE | Sigma factor σE: cell wall lipids | Pre-clinical | 78–80 |
| MtbΔfpbAΔsapM | Ag85A: phagolysosome fusion SapM: PI3P signaling |
Pre-clinical | 81–84 |
| Phagosome acidification | |||
| Mtb ΔRv1503c | TDP-4-oxo-6-deoxy-D-glycolipids: acyl sulfoglycolipids | Pre-clinical | 85 |
| Pro apoptotic | |||
| rBCGΔureC::hlyΔnuoG | (nuoG) type I NADH dehydrogenase: inhibits TNF mediated apoptosis | Pre-clinical | 88,89 |
| MtbΔsecA2 | Secretion of sodA | Pre-clinical | 23,90,91 |
| rBCG::BAX | Apoptosis regulator BAX | Pre-clinical | 92,93 |
| Pro autophagic | |||
| rBCG fbpB | Ag85B | Pre-clinical | 42 |
| VPM1002 (BCGΔureC::hly) | Activation of AIM2 inflammasome | Clinical Phase IIa | 22,31,94 |
| Modulate cytokines | |||
| rBCG-Ag85B-IL15 | T cell proliferation | Pre-clinical | 102,104 |
| Glycolipid adjuvant incorporation | NK and NKT cells | Pre-clinical | 105,109,110 |
| Attenuated / Auxotrophs | |||
| MTBVAC01 (MtbΔphoPΔfadD26) | Transcriptional regulator, acyl-CoA synthase | Clinical Phase I | 52–57 |
| MtbΔleuD | Isopropyl malate isomerase: leucine biosynthesis | Pre-clinical | 58,59 |
| MtbΔpanCD | Pantothenate biosynthesis | Pre-clinical | 60 |
| MtbΔlysA | Lysine biosynthesis | Pre-clinical | 62 |
| MtbΔlysAΔpanCD | Lysine and pantothenate biosynthesis | Pre-clinical | 61,63 |
| MtbΔlysAΔsecA2 | Lysine biosynthesis and sodA secretion | Pre-clinical | 65–67 |
| MtbΔleuDΔpanCD | Leucine and pantothenate biosynthesis | Pre-clinical | 64 |
| BCGΔmmaA4 | Methoxy mycolic acid synthase 4 | Pre-clinical | 99–101 |
Key issues.
The costs and efforts required for clinical trials impose severe limitations on the number of vaccines that can be tested. This issue is compounded by the lack of clearly predictive animal models for testing protective efficacy of candidate vaccines against Mtb challenge.
Basic research to better understand the pathogenesis of Mtb and its mechanisms for evading host immunity remains critically important for refining vaccine design and selecting the best vaccine candidates for clinical trials.
Research development in recent years, including identification of mycobacterial antigens, improved methods for genetic modification of mycobacteria, rational approaches to attenuation and new understanding of adjuvants, have all contributed to new concepts in vaccine design. These advances have accelerated the creation of new candidate vaccine strains that show promise in various animal models.
Live Mycobacteria vaccines provide a broad antigen exposure and intrinsic adjuvant properties to prime durable immune responses. However, due to the complexity of Mtb pathogenesis, it is often difficult to achieve a balance between attenuation and immunogenicity for improved vaccine efficacy.
Efforts to improve live mycobacterial cell vaccines against TB can be applied to other infections and diseases such as HIV, malaria, and cancer, further consolidating efforts in designing vaccines against various diseases.
Acknowledgments
The authors were supported by grants from the U.S. Department of Health and Human Services – National Institutes of Health (P01AI063537, R01AI093649) and the U.S. Department of Health and Human Services – National Institutes of Health – National Institute of General Medical Sciences (UO1GM111849). LJ Carreño received a fellowship from the Pew Charitable Trusts.
Footnotes
Financial and competing interests disclosure
SA Porcelli is paid as a consultant for Vaccinex, a biotechnology company with commercial interest in modified live bacterial vaccines. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
* Of interest
** Of considerable interest
- 1.Gao B, Gupta RS. Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol Mol Biol Rev. 2012;76:66–112. doi: 10.1128/MMBR.05011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Campos S, Smith NH, Boniotti MB, Aranaz A. Overview and phylogeny of Mycobacterium tuberculosis complex organisms: Implications for diagnostics and legislation of bovine tuberculosis. Res Vet Sci. 2014;97(Suppl):S5–S19. doi: 10.1016/j.rvsc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Bange FC, Collins FM, Jacobs WR., Jr Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber Lung Dis. 1999;79:171–180. doi: 10.1054/tuld.1998.0201. [DOI] [PubMed] [Google Scholar]
- 4.Sakula A. BCG: who were Calmette and Guerin? Thorax. 1983;38:806–812. doi: 10.1136/thx.38.11.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Zhang Y, Zheng H, Pan Y, Liu H, Du P, Wan L, Liu J, Zhu B, Zhao G, Chen C, Wan K. Genome sequencing and analysis of BCG vaccine strains. PLoS One. 2013;8:e71243. doi: 10.1371/journal.pone.0071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST. Comparative genomics of the mycobacteria. Int J Med Microbiol. 2000;290:143–152. doi: 10.1016/S1438-4221(00)80083-1. [DOI] [PubMed] [Google Scholar]
- 7.Joung SM, Ryoo S. BCG vaccine in Korea. Clin Exp Vaccine Res. 2013;2:83–91. doi: 10.7774/cevr.2013.2.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostowy S, Cleto C, Sherman DR, Behr MA. The Mycobacterium tuberculosis complex transcriptome of attenuation. Tuberculosis (Edinb) 2004;84:197–204. doi: 10.1016/j.tube.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A. Immunological conditions of children with BCG disseminated infection. Lancet. 1995;346:581. doi: 10.1016/s0140-6736(95)91421-8. [DOI] [PubMed] [Google Scholar]
- 11.Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C, Inwald JK, Golby P, Garcia JN, Hewinson RG, Behr MA, Quail MA, Churcher C, Barrell BG, Parkhill J, Cole ST. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007;104:5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Copin R, Coscolla M, Efstathiadis E, Gagneux S, Ernst JD. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG) Vaccine. 2014;32:5998–6004. doi: 10.1016/j.vaccine.2014.07.113. • Sequencing analysis revealed several immunodominant antigens such as Ag85B and PstS1 mutated in BCG strains as compared to Mtb, therefore impacting the number of T cell epitopes.
- 13.Agger EM, Andersen P. A novel TB vaccine; towards a strategy based on our understanding of BCG failure. Vaccine. 2002;21:7–14. doi: 10.1016/s0264-410x(02)00447-4. [DOI] [PubMed] [Google Scholar]
- 14.Ehrt S, Rhee K, Schnappinger D. Mycobacterial genes essential for the pathogen’s survival in the host. Immunol Rev. 2015;264:319–326. doi: 10.1111/imr.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrellad MA, Klepp LI, Gioffre A, Sabio y Garcia J, Morbidoni HR, de la Paz Santangelo M, Cataldi AA, Bigi F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4:3–66. doi: 10.4161/viru.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 17.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 18.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 19.Russell DG, VanderVen BC, Lee W, Abramovitch RB, Kim MJ, Homolka S, Niemann S, Rohde KH. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe. 2010;8:68–76. doi: 10.1016/j.chom.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon AH, Hart PD, Young MR. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980;286:79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- 21.Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: phthiocerol dimycocerosate and the attenuation indicator lipid. Infect Immun. 1974;9:150–158. doi: 10.1128/iai.9.1.150-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, Bancroft GJ, Reyrat JM, van Soolingen D, Raupach B, Kaufmann SH. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, Lakey DL, Bochan MR, Kernodle DS. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164:2213–2219. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 24.Baena A, Porcelli SA. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens. 2009;74:189–204. doi: 10.1111/j.1399-0039.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deretic V. Autophagy in tuberculosis. Cold Spring Harb Perspect Med. 2014;4:a018481. doi: 10.1101/cshperspect.a018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porcelli SA, Jacobs WR., Jr Tuberculosis: unsealing the apoptotic envelope. Nat Immunol. 2008;9:1101–1102. doi: 10.1038/ni1008-1101. [DOI] [PubMed] [Google Scholar]
- 27.Boer MC, van Meijgaarden KE, Joosten SA, Ottenhoff TH. CD8+ regulatory T cells, and not CD4+ T cells, dominate suppressive phenotype and function after in vitro live Mycobacterium bovis-BCG activation of human cells. PLoS One. 2014;9:e94192. doi: 10.1371/journal.pone.0094192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semple PL, Binder AB, Davids M, Maredza A, van Zyl-Smit RN, Dheda K. Regulatory T cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with tuberculosis. Am J Respir Crit Care Med. 2013;187:1249–1258. doi: 10.1164/rccm.201210-1934OC. [DOI] [PubMed] [Google Scholar]
- 29. Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. • Identified delayed T cell priming by Mtb as an important virulence mechanism to interfere with host immunity and enable the adaptation of the pathogen to the host.
- 30.Kaufmann SH, Cotton MF, Eisele B, Gengenbacher M, Grode L, Hesseling AC, Walzl G. The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev Vaccines. 2014;13:619–630. doi: 10.1586/14760584.2014.905746. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Curr Opin Biotechnol. 2012;23:900–907. doi: 10.1016/j.copbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Whole Mycobacteria Cell Vaccines for Tuberculosis Summary G. Developing whole mycobacteria cell vaccines for tuberculosis: Workshop proceedings, Max Planck Institute for Infection Biology, Berlin, Germany, July 9, 2014. Vaccine. 2015;33:3047–3055. doi: 10.1016/j.vaccine.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 33.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97:13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohara N, Kitaura H, Hotokezaka H, Nishiyama T, Wada N, Matsumoto S, Matsuo T, Naito M, Yamada T. Characterization of the gene encoding the MPB51, one of the major secreted protein antigens of Mycobacterium bovis BCG, and identification of the secreted protein closely related to the fibronectin binding 85 complex. Scand J Immunol. 1995;41:433–442. doi: 10.1111/j.1365-3083.1995.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 36.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo CJ, Bell H, Hsieh CL, Ptak CP, Chang YF. Novel mycobacteria antigen 85 complex binding motif on fibronectin. J Biol Chem. 2012;287:1892–1902. doi: 10.1074/jbc.M111.298687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naito M, Ohara N, Matsumoto S, Yamada T. The novel fibronectin-binding motif and key residues of mycobacteria. J Biol Chem. 1998;273:2905–2909. doi: 10.1074/jbc.273.5.2905. [DOI] [PubMed] [Google Scholar]
- 39.Kuo CJ, Ptak CP, Hsieh CL, Akey BL, Chang YF. Elastin, a novel extracellular matrix protein adhering to mycobacterial antigen 85 complex. J Biol Chem. 2013;288:3886–3896. doi: 10.1074/jbc.M112.415679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 41.Huygen K. The Immunodominant T-Cell Epitopes of the Mycolyl-Transferases of the Antigen 85 Complex of M. tuberculosis. Front Immunol. 2014;5:321. doi: 10.3389/fimmu.2014.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71:1672–1679. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tullius MV, Harth G, Maslesa-Galic S, Dillon BJ, Horwitz MA. A Replication-Limited Recombinant Mycobacterium bovis BCG vaccine against tuberculosis designed for human immunodeficiency virus-positive persons is safer and more efficacious than BCG. Infect Immun. 2008;76:5200–5214. doi: 10.1128/IAI.00434-08. • Further development of the rBCG30 modified BCG vaccine strain to enhance its attenuation.
- 45.Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J Exp Med. 2001;194:1421–1432. doi: 10.1084/jem.194.10.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis. 2011;204:1573–1584. doi: 10.1093/infdis/jir592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grode L, Ganoza CA, Brohm C, Weiner J, 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31:1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. Global Tuberculosis Report. WHO; 2014. [Google Scholar]
- 49.Begnini KR, Buss JH, Collares T, Seixas FK. Recombinant Mycobacterium bovis BCG for immunotherapy in nonmuscle invasive bladder cancer. Appl Microbiol Biotechnol. 2015;99:3741–3754. doi: 10.1007/s00253-015-6495-3. [DOI] [PubMed] [Google Scholar]
- 50.Sun R, Skeiky YA, Izzo A, Dheenadhayalan V, Imam Z, Penn E, Stagliano K, Haddock S, Mueller S, Fulkerson J, Scanga C, Grover A, Derrick SC, Morris S, Hone DM, Horwitz MA, Kaufmann SH, Sadoff JC. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 51.Kupferschmidt K. Infectious disease. Taking a new shot at a TB vaccine. Science. 2011;334:1488–1490. doi: 10.1126/science.334.6062.1488. [DOI] [PubMed] [Google Scholar]
- 52.Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, Fernandez C, Parra A, Cardona PJ, Vilaplana C, Ausina V, Williams A, Clark S, Malaga W, Guilhot C, Gicquel B, Martin C. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31:4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalo-Asensio J, Mostowy S, Harders-Westerveen J, Huygen K, Hernandez-Pando R, Thole J, Behr M, Gicquel B, Martin C. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One. 2008;3:e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 55.Infante E, Aguilar LD, Gicquel B, Pando RH. Immunogenicity and protective efficacy of the Mycobacterium tuberculosis fadD26 mutant. Clin Exp Immunol. 2005;141:21–28. doi: 10.1111/j.1365-2249.2005.02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solans L, Uranga S, Aguilo N, Arnal C, Gomez AB, Monzon M, Badiola JJ, Gicquel B, Martin C. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine. 2014;32:5192–5197. doi: 10.1016/j.vaccine.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 57.Berthet FX, Lagranderie M, Gounon P, Laurent-Winter C, Ensergueix D, Chavarot P, Thouron F, Maranghi E, Pelicic V, Portnoi D, Marchal G, Gicquel B. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science. 1998;282:759–762. doi: 10.1126/science.282.5389.759. [DOI] [PubMed] [Google Scholar]
- 58.McAdam RA, Weisbrod TR, Martin J, Scuderi JD, Brown AM, Cirillo JD, Bloom BR, Jacobs WR., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR, Jr, Bloom BR. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR., Jr A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med. 2002;8:1171–1174. doi: 10.1038/nm765. [DOI] [PubMed] [Google Scholar]
- 61.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L, Wei H, Letvin NL, Frothingham R, Haynes BF, Chen ZW, Jacobs WR., Jr Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavelka MS, Jr, Chen B, Kelley CL, Collins FM, Jacobs Jr WR., Jr Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2003;71:4190–4192. doi: 10.1128/IAI.71.7.4190-4192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR., Jr Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun. 2005;73:1196–1203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampson SL, Mansfield KG, Carville A, Magee DM, Quitugua T, Howerth EW, Bloom BR, Hondalus MK. Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate. Vaccine. 2011;29:4839–4847. doi: 10.1016/j.vaccine.2011.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hinchey J, Jeon BY, Alley H, Chen B, Goldberg M, Derrick S, Morris S, Jacobs WR, Jr, Porcelli SA, Lee S. Lysine auxotrophy combined with deletion of the SecA2 gene results in a safe and highly immunogenic candidate live attenuated vaccine for tuberculosis. PLoS One. 2011;6:e15857. doi: 10.1371/journal.pone.0015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain P, Hsu T, Arai M, Biermann K, Thaler DS, Nguyen A, Gonzalez PA, Tufariello JM, Kriakov J, Chen B, Larsen MH, Jacobs WR., Jr Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio. 2014;5:e01245–e01214. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranganathan UD, Larsen MH, Kim J, Porcelli SA, Jacobs WR, Jr, Fennelly GJ. Recombinant pro-apoptotic Mycobacterium tuberculosis generates CD8+ T cell responses against human immunodeficiency virus type 1 Env and M. tuberculosis in neonatal mice. Vaccine. 2009;28:152–161. doi: 10.1016/j.vaccine.2009.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reece ST, Nasser-Eddine A, Dietrich J, Stein M, Zedler U, Schommer-Leitner S, Ottenhoff TH, Andersen P, Kaufmann SH. Improved long-term protection against Mycobacterium tuberculosis Beijing/W in mice after intra-dermal inoculation of recombinant BCG expressing latency associated antigens. Vaccine. 2011;29:8740–8744. doi: 10.1016/j.vaccine.2011.07.144. • Further development of the VPM1002 to enhance immunogenicity by targeting latent antigens of Mtb.
- 69.Schuck SD, Mueller H, Kunitz F, Neher A, Hoffmann H, Franken KL, Repsilber D, Ottenhoff TH, Kaufmann SH, Jacobsen M. Identification of T-cell antigens specific for latent mycobacterium tuberculosis infection. PLoS One. 2009;4:e5590. doi: 10.1371/journal.pone.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C, Fu R, Chen Z, Tan K, Chen L, Teng X, Lu J, Shi C, Fan X. Immunogenicity and protective efficacy of a novel recombinant BCG strain overexpressing antigens Ag85A and Ag85B. Clin Dev Immunol. 2012;2012:563838. doi: 10.1155/2012/563838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.da Costa AC, Nogueira SV, Kipnis A, Junqueira-Kipnis AP. Recombinant BCG: Innovations on an Old Vaccine. Scope of BCG Strains and Strategies to Improve Long-Lasting Memory. Front Immunol. 2014;5:152. doi: 10.3389/fimmu.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fratti RA, Chua J, Deretic V. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J Biol Chem. 2002;277:17320–17326. doi: 10.1074/jbc.M200335200. [DOI] [PubMed] [Google Scholar]
- 73.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansen P, Fettelschoss A, Amstutz B, Selchow P, Waeckerle-Men Y, Keller P, Deretic V, Held L, Kundig TM, Bottger EC, Sander P. Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin Vaccine Immunol. 2011;18:907–913. doi: 10.1128/CVI.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khatri B, Whelan A, Clifford D, Petrera A, Sander P, Vordermeier HM. BCG Deltazmp1 vaccine induces enhanced antigen specific immune responses in cattle. Vaccine. 2014;32:779–784. doi: 10.1016/j.vaccine.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 76.Sander P, Clark S, Petrera A, Vilaplana C, Meuli M, Selchow P, Zelmer A, Mohanan D, Andreu N, Rayner E, Dal Molin M, Bancroft GJ, Johansen P, Cardona PJ, Williams A, Bottger EC. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine. 2015;33:1353–1359. doi: 10.1016/j.vaccine.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 77.Stewart GR, Patel J, Robertson BD, Rae A, Young DB. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 2005;1:269–278. doi: 10.1371/journal.ppat.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hernandez Pando R, Aguilar LD, Smith I, Manganelli R. Immunogenicity and protection induced by a Mycobacterium tuberculosis sigE mutant in a BALB/c mouse model of progressive pulmonary tuberculosis. Infect Immun. 2010;78:3168–3176. doi: 10.1128/IAI.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casonato S, Provvedi R, Dainese E, Palu G, Manganelli R. Mycobacterium tuberculosis requires the ECF sigma factor SigE to arrest phagosome maturation. PLoS One. 2014;9:e108893. doi: 10.1371/journal.pone.0108893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Armitige LY, Jagannath C, Wanger AR, Norris SJ. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect Immun. 2000;68:767–778. doi: 10.1128/iai.68.2.767-778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Copenhaver RH, Sepulveda E, Armitige LY, Actor JK, Wanger A, Norris SJ, Hunter RL, Jagannath C. A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential. Infect Immun. 2004;72:7084–7095. doi: 10.1128/IAI.72.12.7084-7095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katti MK, Dai G, Armitige LY, Rivera Marrero C, Daniel S, Singh CR, Lindsey DR, Dhandayuthapani S, Hunter RL, Jagannath C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saikolappan S, Estrella J, Sasindran SJ, Khan A, Armitige LY, Jagannath C, Dhandayuthapani S. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS One. 2012;7:e36198. doi: 10.1371/journal.pone.0036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brodin P, Poquet Y, Levillain F, Peguillet I, Larrouy-Maumus G, Gilleron M, Ewann F, Christophe T, Fenistein D, Jang J, Jang MS, Park SJ, Rauzier J, Carralot JP, Shrimpton R, Genovesio A, Gonzalo-Asensio JA, Puzo G, Martin C, Brosch R, Stewart GR, Gicquel B, Neyrolles O. High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog. 2010;6:e1001100. doi: 10.1371/journal.ppat.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arbues A, Lugo-Villarino G, Neyrolles O, Guilhot C, Astarie-Dequeker C. Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids. Front Cell Infect Microbiol. 2014;4:173. doi: 10.3389/fcimb.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller JL, Velmurugan K, Cowan MJ, Briken V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010;6:e1000864. doi: 10.1371/journal.ppat.1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR, Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:453–464. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 91.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, Morris SL, Jacobs WR, Jr, Porcelli SA. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 93.Li G, Liu G, Song N, Kong C, Huang Q, Su H, Bi A, Luo L, Zhu L, Xu Y, Wang H. A novel recombinant BCG-expressing pro-apoptotic protein BAX enhances Th1 protective immune responses in mice. Mol Immunol. 2015;66:346–356. doi: 10.1016/j.molimm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 94. Saiga H, Nieuwenhuizen N, Gengenbacher M, Koehler AB, Schuerer S, Moura-Alves P, Wagner I, Mollenkopf HJ, Dorhoi A, Kaufmann SH. The Recombinant BCG DeltaureC::hly Vaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. J Infect Dis. 2015;211:1831–1841. doi: 10.1093/infdis/jiu675. • Further characterization of the VPM1002 modified BCG vaccine strain identifies mechanisms of its imporved immunogenicity.
- 95.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–7041. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 97.Ma X. TNF-alpha and IL-12: a balancing act in macrophage functioning. Microbes Infect. 2001;3:121–129. doi: 10.1016/s1286-4579(00)01359-9. [DOI] [PubMed] [Google Scholar]
- 98.Richer MJ, Pewe LL, Hancox LS, Hartwig SM, Varga SM, Harty JT. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. 2015 doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dao DN, Sweeney K, Hsu T, Gurcha SS, Nascimento IP, Roshevsky D, Besra GS, Chan J, Porcelli SA, Jacobs WR. Mycolic acid modification by the mmaA4 gene of M. tuberculosis modulates IL-12 production. PLoS Pathog. 2008;4:e1000081. doi: 10.1371/journal.ppat.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolibab K, Derrick SC, Jacobs WR, Morris SL. Characterization of an intracellular ATP assay for evaluating the viability of live attenuated mycobacterial vaccine preparations. J Microbiol Methods. 2012;90:245–249. doi: 10.1016/j.mimet.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Derrick SC, Dao D, Yang A, Kolibab K, Jacobs WR, Morris SL. Formulation of a mmaA4 gene deletion mutant of Mycobacterium bovis BCG in cationic liposomes significantly enhances protection against tuberculosis. PLoS One. 2012;7:e32959. doi: 10.1371/journal.pone.0032959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang C, Yamada H, Shibata K, Maeda N, Yoshida S, Wajjwalku W, Ohara N, Yamada T, Kinoshita T, Yoshikai Y. Efficacy of recombinant bacille Calmette-Guerin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J Infect Dis. 2008;197:1263–1274. doi: 10.1086/586902. [DOI] [PubMed] [Google Scholar]
- 103.Lazarevic V, Yankura DJ, DiVito SJ, Flynn JL. Induction of Mycobacterium tuberculosis-specific primary and secondary T-cell responses in interleukin-15-deficient mice. Infect Immun. 2005;73:2910–2922. doi: 10.1128/IAI.73.5.2910-2922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toe JG, Pellegrini M, Mak TW. Promoting immunity during chronic infection--the therapeutic potential of common gamma-chain cytokines. Mol Immunol. 2013;56:38–47. doi: 10.1016/j.molimm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 105. Venkataswamy MM, Ng TW, Kharkwal SS, Carreno LJ, Johnson AJ, Kunnath-Velayudhan S, Liu Z, Bittman R, Jervis PJ, Cox LR, Besra GS, Wen X, Yuan W, Tsuji M, Li X, Ho DD, Chan J, Lee S, Frothingham R, Haynes BF, Panas MW, Gillard GO, Sixsmith JD, Korioth-Schmitz B, Schmitz JE, Larsen MH, Jacobs WR, Jr, Porcelli SA. Improving Mycobacterium bovis bacillus Calmette-Guerin as a vaccine delivery vector for viral antigens by incorporation of glycolipid activators of NKT cells. PLoS One. 2014;9:e108383. doi: 10.1371/journal.pone.0108383. • Modification of live recombinant BCG with glycolipid adjuvants to enhance its performance as a Mtb vaccine and as a vaccine vector for other infections.
- 106.Leung B, Harris HW. NKT cells in sepsis. Clin Dev Immunol. 2010 doi: 10.1155/2010/414650. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sullivan BA, Kronenberg M. Activation or anergy: NKT cells are stunned by alpha-galactosylceramide. J Clin Invest. 2005;115:2328–2329. doi: 10.1172/JCI26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh M, Quispe-Tintaya W, Chandra D, Jahangir A, Venkataswamy MM, Ng TW, Sharma-Kharkwal S, Carreno LJ, Porcelli SA, Gravekamp C. Direct incorporation of the NKT-cell activator alpha-galactosylceramide into a recombinant Listeria monocytogenes improves breast cancer vaccine efficacy. Br J Cancer. 2014;111:1945–1954. doi: 10.1038/bjc.2014.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, Reddington F, Besra GS, Jacobs WR, Jr, Porcelli SA. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu JS, Peacock JW, Vanleeuwen S, Hsu T, Jacobs WR, Jr, Cayabyab MJ, Letvin NL, Frothingham R, Staats HF, Liao HX, Haynes BF. Generation of mucosal anti-human immunodeficiency virus type 1 T-cell responses by recombinant Mycobacterium smegmatis. Clin Vaccine Immunol. 2006;13:1204–1211. doi: 10.1128/CVI.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Junqueira-Kipnis AP, de Oliveira FM, Trentini MM, Tiwari S, Chen B, Resende DP, Silva BD, Chen M, Tesfa L, Jacobs WR, Jr, Kipnis A. Prime-boost with Mycobacterium smegmatis recombinant vaccine improves protection in mice infected with Mycobacterium tuberculosis. PLoS One. 2013;8:e78639. doi: 10.1371/journal.pone.0078639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maura RB, Férnandez S, Reyes G, Perez JL, Reyes F, de los Angeles García M, Fariñas M, Infante JF, Tirado Y, Puig A. Evaluation of the potential of Mycobacterium smegmatis as vaccine candidate against tuberculosis by in silico and in vivo studies. VacciMonitor. 2010;19:20–26. [Google Scholar]
- 114.Rodriguez L, Tirado Y, Reyes F, Puig A, Kadir R, Borrero R, Fernandez S, Reyes G, Alvarez N, Garcia MA, Sarmiento ME, Norazmi MN, Perez Quinoy JL, Acosta A. Proteoliposomes from Mycobacterium smegmatis induce immune cross-reactivity against Mycobacterium tuberculosis antigens in mice. Vaccine. 2011;29:6236–6241. doi: 10.1016/j.vaccine.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 115.Taylor N, Bahunde F, Thompson A, Yu JS, Jacobs WR, Jr, Letvin NL, Haynes BF, Lee S. Enhanced priming of adaptive immunity by Mycobacterium smegmatis mutants with high-level protein secretion. Clin Vaccine Immunol. 2012;19:1416–1425. doi: 10.1128/CVI.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, Chen M, Kim J, Lukose R, Chan J, Orme IM, Porcelli SA, Jacobs WR., Jr A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med. 2011;17:1261–1268. doi: 10.1038/nm.2420. • A novel M. smegmatis based vaccine against Mtb conferred strong protective immunity against TB, suggesting alternate platforms for constructing novel live vaccine strains.
- 117.Hart BE, Asrican R, Lim SY, Sixsmith JD, Lukose R, Souther SJ, Rayasam SD, Saelens JW, Chen CJ, Seay SA, Berney-Meyer L, Magtanong L, Vermeul K, Pajanirassa P, Jimenez AE, Ng TW, Tobin DM, Porcelli SA, Larsen MH, Schmitz JE, Haynes BF, Jacobs WR, Jr, Lee S, Frothingham R. Stable Expression of Lentiviral Antigens by Quality-Controlled Recombinant BCG Vectors. kClin Vaccine Immunol. 2015 doi: 10.1128/CVI.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 119.Leung NJ, Aldovini A, Young R, Jarvis MA, Smith JM, Meyer D, Anderson DE, Carlos MP, Gardner MB, Torres JV. The kinetics of specific immune responses in rhesus monkeys inoculated with live recombinant BCG expressing SIV Gag, Pol, Env, and Nef proteins. Virology. 2000;268:94–103. doi: 10.1006/viro.1999.0131. [DOI] [PubMed] [Google Scholar]
- 120.Jensen K, Ranganathan UD, Van Rompay KK, Canfield DR, Khan I, Ravindran R, Luciw PA, Jacobs WR, Jr, Fennelly G, Larsen MH, Abel K. A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques. Clin Vaccine Immunol. 2012;19:1170–11781. doi: 10.1128/CVI.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nurul AA, Norazmi MN. Immunogenicity and in vitro protective efficacy of recombinant Mycobacterium bovis bacille Calmette Guerin (rBCG) expressing the 19 kDa merozoite surface protein-1 (MSP-1(19)) antigen of Plasmodium falciparum. Parasitol Res. 2011;108:887–897. doi: 10.1007/s00436-010-2130-5. [DOI] [PubMed] [Google Scholar]
- 122.Teo WH, Nurul AA, Norazmi MN. Immunogenicity of recombinant BCG-based vaccine expressing the 22 kDa of serine repeat antigen (SE22) of Plasmodium falciparum. Trop Biomed. 2012;29:239–253. [PubMed] [Google Scholar]
- 123.Zheng C, Xie P, Chen Y. Recombinant Mycobacterium bovis BCG producing the circumsporozoite protein of Plasmodium falciparum FCC-1/HN strain induces strong immune responses in BALB/c mice. Parasitol Int. 2002;51:1–7. doi: 10.1016/s1383-5769(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 124.Uthaipibull C, Aufiero B, Syed SE, Hansen B, Guevara Patino JA, Angov E, Ling IT, Fegeding K, Morgan WD, Ockenhouse C, Birdsall B, Feeney J, Lyon JA, Holder AA. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J Mol Biol. 2001;307:1381–1394. doi: 10.1006/jmbi.2001.4574. [DOI] [PubMed] [Google Scholar]
- 125.Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008;179:53–56. doi: 10.1016/j.juro.2007.08.122. [DOI] [PubMed] [Google Scholar]
- 126.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 127.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, Roupret M, European Association U. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 128.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS, Jr, Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 129.Huang G, Redelman-Sidi G, Rosen N, Glickman MS, Jiang X. Inhibition of mycobacterial infection by the tumor suppressor PTEN. J Biol Chem. 2012;287:23196–23202. doi: 10.1074/jbc.M112.351940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durek C, Brandau S, Ulmer AJ, Flad HD, Jocham D, Bohle A. Bacillus-Calmette-Guerin (BCG) and 3D tumors: an in vitro model for the study of adhesion and invasion. J Urol. 1999;162:600–605. doi: 10.1016/s0022-5347(05)68633-8. [DOI] [PubMed] [Google Scholar]
- 131.Ratliff TL, Gillen D, Catalona WJ. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. 1987;137:155–158. doi: 10.1016/s0022-5347(17)43909-7. [DOI] [PubMed] [Google Scholar]
- 132.Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, Jocham D, Ratliff TL, Bohle A. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 133.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, Snell L, Mangtani P, Adetifa I, Lalvani A, Abubakar I. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zodpey SP, Shrikhande SN. The geographic location (latitude) of studies evaluating protective effect of BCG vaccine and it’s efficacy/effectiveness against tuberculosis. Indian J Public Health. 2007;51:205–210. [PubMed] [Google Scholar]
- 135.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]