Abstract
Exercise-induced muscle fatigue has been shown to be the consequence of peripheral factors that impair muscle fiber contractile mechanisms. Central factors arising within the central nervous system have also been hypothesized to induce muscle fatigue, but no direct empirical evidence that is causally associated to reduction of muscle force-generating capability has yet been reported. We developed a simulation model to investigate whether peripheral factors of muscle fatigue are sufficient to explain the muscle force behavior observed during empirical studies of fatiguing voluntary contractions, which is commonly attributed to central factors. Peripheral factors of muscle fatigue were included in the model as a time-dependent decrease in the amplitude of the motor unit force twitches. Our simulation study indicated that the force behavior commonly attributed to central fatigue could be explained solely by peripheral factors during simulated fatiguing submaximal voluntary contractions. It also revealed important flaws regarding the use of the interpolated twitch response from electrical stimulation of the muscle as a means for assessing central fatigue. Our analysis does not directly refute the concept of central fatigue. However, it raises important concerns about the manner in which it is measured and about the interpretation of the commonly accepted causes of central fatigue and questions the very need for the existence of central fatigue.
Keywords: central fatigue, motor units, interpolated twitch, voluntary drive
the sources of exercise-induced muscle fatigue have been debated since the early 1900s. There is general agreement on the influence of peripheral factors on muscle fatigue, i.e., those that develop within the muscle and impair the muscle fiber contractile mechanism, such as metabolite accumulation during prolonged exercise (see, e.g., Bergström et al. 1967; Hermansen et al. 1967; Pernow and Saltin 1971). These contractile impairments can be measured as changes in the amplitude and duration of the elicited muscle force twitch (Adam and De Luca 2003, 2005; Burke 1981; Macintosh et al. 1994; Vandervoort et al. 1983).
In addition to the influence of peripheral factors of muscle fatigue, some studies have put forth the notion of “central fatigue,” i.e., a limitation in muscle performance caused by central factors. These would arise within the central nervous system (CNS) and diminish the voluntary drive to the motoneuron pool of a muscle. Potential sources include failure of the motor cortex (see, e.g., Gandevia et al. 1996; Todd et al. 2007) and the influence of afferent inputs at the spinal level (see, e.g., Gandevia 2001). Mosso (1904) was the first to draw attention to this concept, but others have followed (e.g., Bellemare and Bigland-Ritchie 1987; Gandevia 2001; Reid 1927). Today, central fatigue is commonly accepted as a physiological phenomenon that plays a relevant role in muscle fatigue and, according to Bigland-Ritchie et al. (1983) and Gandevia (2001), protects muscles against excessive effort.
However, unlike the directly observable and verifiable influence of peripheral factors of muscle fatigue, direct empirical evidence of central fatigue has yet to be revealed. Suggestive evidence is typically reported by measuring the so-called interpolated twitch, i.e., the additional force elicited by supramaximal electrical stimulation delivered to a nerve or muscle during a voluntary contraction. This method was first introduced by Merton (1954). The amplitude of the interpolated twitch is believed to represent the proportion of available muscle force generation capacity that remains unused during a voluntary contraction and, in a corresponding fashion, to provide an indication of the level of voluntary drive to a muscle. An exercise-induced increase in the amplitude of the interpolated twitch during maximal efforts is customarily accepted as evidence of decreasing voluntary drive that is representative of central fatigue, according to Gandevia (2001) and Taylor et al. (2009), among others, but this approach has been questioned by De Haan et al. (2009) and Herzog (2009), among others, who have argued that the interpolated twitch does not provide an accurate measure of voluntary drive.
An important weakness of this method is the lack of consideration given to the influence of well-established peripheral factors of muscle fatigue. Although such influence has been known for over half a century, we found only one study, by Schillings et al. (2003), that attempted to take into account the influence of peripheral factors on central fatigue assessed with the twitch interpolation technique.
We designed the present study to investigate whether peripheral factors of muscle fatigue are sufficient to explain the modifications in muscle force that are observed during fatiguing contractions and that are commonly attributed to central fatigue. We tested this hypothesis by developing a model that calculates the force produced by a muscle during voluntary isometric contractions and during electrical stimulation applied to a muscle or to the nerve supplying a muscle.
We found that a model that includes only peripheral factors of muscle fatigue is able to replicate empirical results from studies of fatiguing contractions sustained at submaximal force levels without requiring the involvement of central factors. We also identified weakness and dubiousness in the reliability of the interpolated twitch as a measure of central fatigue during maximal voluntary efforts.
METHODS
Force Model
We approached the problem by using our previously developed model for the simulation of motor unit firing behavior and muscle force during voluntary isometric contractions (Contessa and De Luca 2013; De Luca and Contessa 2015). The model was modified to include the simulation of motor unit firing behavior and muscle force produced from electrical stimulation of a muscle or nerve. For a complete description of the original and modified model, see Contessa and De Luca (2013) and the appendix.
The block diagram of the modified force model is shown in Fig. 1. It includes two sources of excitation that are the inputs to the model: the voluntary (Fig. 1A1) and the elicited (Fig. 1A2) input excitation. The voluntary input excitation represents the combined excitation from the CNS and from the peripheral nervous system to the motoneuron pool of a muscle during voluntary contractions. The elicited input excitation represents the excitation delivered by electrical stimulation to either a muscle or a nerve supplying a muscle. Motor units are activated as a result of both excitation sources. Their firing behavior (Fig. 1C) depends on the interaction between the motor unit action potentials (MUAPs) generated as a result of voluntary (Fig. 1B1) and elicited (Fig. 1B2) input excitation, as described in the appendix. Motor unit firings are converted into motor unit force twitches (Fig. 1D). Their amplitude is modeled to vary as a function of time and motor unit firing rate to introduce the effect of peripheral fatigue and the nonlinear relation between force and firing rate (Bawa and Stein 1976). For details, see Contessa and De Luca (2013). The individual motor unit force twitches are convolved with the motor unit firing trains to calculate the motor unit forces, which are then summed to produce the output force generated by the muscle (Fig. 1E). The simulated output force is calibrated in percentage of the maximal voluntary contraction (MVC) force, i.e., the force produced when all motor units are activated at maximal voluntary input excitation. A force feedback loop is implemented to simulate muscle force sustained at fixed target force levels (Fig. 1F).
Fig. 1.
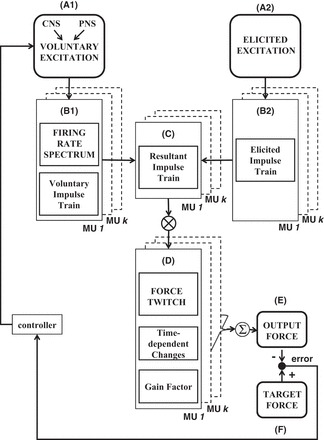
Model schematic. The force model has 2 inputs: the voluntary input excitation (A1) and the elicited input excitation (A2). The former represents the sum of all excitatory and inhibitory inputs from the central nervous system (CNS) and from the peripheral nervous system (PNS) to all the motor units in the pool of a muscle. The latter represents the effect of electrical stimulation to a muscle or nerve supplying a muscle. The voluntary input excitation determines the firing behavior and impulse trains of motor units (MUs) during voluntary contractions (B1). The voluntary impulse trains interact with the elicited impulse trains (B2) if motor units are concurrently activated by the elicited input excitation. The resultant impulse trains (C) include the combined effect of both excitation sources. They are convolved with the time-dependent and firing rate-dependent motor unit force twitches (D) to compute the force contribution of the active motor units. Motor unit forces are summed to obtain the muscle output force (E), which is compared with the target force (F). The tracking error between output and target force is used to adjust the input excitation. See text for additional details.
Simulated Contraction Protocols
With the model, we investigated the influence of peripheral factors of muscle fatigue on the simulated muscle force and on the amplitude of the interpolated twitch in the first dorsal interosseous (FDI) muscle. We simulated three protocols of voluntary contractions with different involvements of peripheral factors of muscle fatigue: 1) brief constant-force contractions, 2) maximal-effort contractions, and 3) repeated submaximal contractions. Electrical stimulation of the muscle or nerve supplying the muscle was simulated during the contractions to measure the interpolated twitch. Each simulated contraction protocol was repeated 10 times, and data are presented in results as means ± SD of the results from the 10 repetitions. The contraction protocols are described below.
Brief constant-force contractions.
We performed two sets of simulations to gain insights on the effect of force level and stimulation intensity on the amplitude of the interpolated twitch in the absence of peripheral fatigue. In the first set, voluntary force was sustained for 4 s at a level ranging from 0% to 100% MVC in different contractions, and a single maximal electrical stimulus was simulated during the task. In the second set, voluntary force was maintained at 100% MVC for 4 s, and the intensity of an electrical stimulus simulated during the task was varied from 0 to maximal in different contractions. Electrical stimulation was modeled to activate motor units either in random order or in the order of inverse physiological recruitment, i.e., progressively lower-threshold motor units were activated with increasing stimulation intensity (refer to appendix for additional details).
Maximal-effort contractions.
Based on the protocol described by Bigland-Ritchie et al. (1982), we simulated a maximal voluntary effort sustained for 60 s, during which muscle force decreased from 100% MVC at the beginning to 60% MVC at the end of the task. Peripheral fatigue was modeled as a linear time-varying decrease in the amplitude of the motor unit force twitches that replicated the empirically observed reduction in muscle force. We ensured that no central fatigue developed by constraining the voluntary input excitation to remain maximal throughout the task. Every 10 s, the contraction was interrupted to calculate the resting twitch, i.e., the force produced when electrically stimulating the muscle in the absence of voluntary contraction. Before each break, a single maximal electrical stimulus was also superimposed on the simulated voluntary force.
Repeated submaximal contractions.
Based on empirical studies of submaximal contractions reported in the literature (Eichelberger and Bilodeau 2007; Lloyd et al. 1991; Smith et al. 2007), we simulated a series of repeated contractions sustained at 20% MVC until the endurance limit, i.e., until the simulated force could no longer be sustained within 5% of the required target level. The 20% MVC target level was chosen because we had modeled the effect of peripheral fatigue on the amplitude of the motor unit force twitches in the FDI muscle during contractions at this force level in a previous study (Contessa and De Luca 2013). Briefly, the amplitude was increased linearly over time in the first 60 s of the contraction series (potentiation phase) and subsequently decreased over time. For details, see Contessa and De Luca (2013). Every 30 s during the task, brief maximal efforts of 3-s duration were simulated. Single maximal electrical stimuli were delivered during the maximal efforts, to calculate the interpolated twitch, and in between contractions, to calculate the resting twitch. We ensured that no central fatigue developed by constraining the voluntary input excitation to remain maximal during the simulated maximal efforts. The voluntary input excitation was allowed to vary during the submaximal task in order to compensate for the reduction in muscle force generation capacity caused by peripheral fatigue. In this way, the muscle force was maintained at the required 20% MVC target level (for details see Contessa and De Luca 2013).
In our model the voluntary input excitation was constrained to remain at maximal level during simulated maximal efforts in order to ensure that no central fatigue developed. It is important to note that the simulated voluntary input excitation does not represent only the influence of descending inputs from the CNS to the motoneuron pool of a muscle. It includes both descending inputs from the CNS and peripheral inputs from the peripheral nervous system, both of which combine to affect the firing behavior of the motoneurons in a muscle. Therefore, it effectively represents the level of voluntary drive to a muscle (see Contessa and De Luca 2013 and appendix for details).
Quantification of Voluntary Drive
During the simulated contractions, we quantified the level of voluntary drive to the FDI muscle with two parameters that are commonly used in studies of central fatigue: the voluntary activation index (Merton 1954) and the central activation ratio (Kent-Braun and Le Blanc 1996). They are graphically depicted in Fig. 2, A and B, respectively.
Fig. 2.
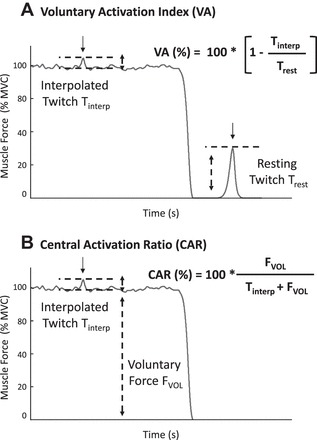
Quantification of voluntary drive. Graphic representation of 2 measures of voluntary drive: the voluntary activation index (VA; A) and the central activation ratio (CAR; B). A maximal stimulus is delivered during a voluntary contraction, and the additional force elicited by the stimulation is referred to as the interpolated twitch, or Tinterp. For the computation of VA, an additional force twitch is elicited by electrical stimulation on the muscle at rest (in the absence of concurrent voluntary contraction) and is referred to as the resting twitch, or Trest. FVOL is the magnitude of the muscle force generated during voluntary contraction. Arrows indicate the time of electrical stimulation.
The voluntary activation index is calculated as
| (1) |
where Tinterp is the interpolated twitch and Trest is the resting twitch.
The central activation ratio is calculated as
| (2) |
where FVOL is the muscle force produced during a voluntary effort.
Voluntary drive is considered to be maximal when electrical stimulation does not elicit additional force over a voluntary contraction (Tinterp = 0, voluntary activation index = 100%, central activation ratio = 100%).
RESULTS
Brief Constant-Force Contractions
Figure 3A presents the results of the simulated contractions sustained at constant-force levels ranging from 0% to 100% MVC with a superimposed maximal stimulus. Figure 3, B and C, present the results of the simulated contractions sustained at 100% MVC with superimposed electrical stimuli delivered at stimulation intensities ranging from 0 to maximal. Electrical stimulation, mimicking that applied directly to the nerve, activated motor units in the order of inverse physiological recruitment (Fig. 3B). That mimicking electrical stimulation applied directly to the muscle activated motor units in random order (Fig. 3C). Figure 3, left, shows the muscle force simulated at increasing force level and stimulation intensity. Figure 3, right, shows the amplitude of the interpolated twitch, the voluntary activation index, and the central activation ratio, calculated as a function of increasing force level and stimulation intensity.
Fig. 3.
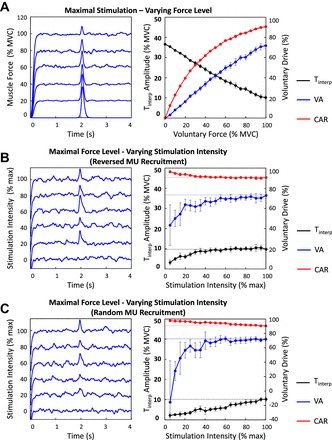
Amplitude of the interpolated twitch and voluntary drive as a function of voluntary force and stimulation intensity. Left: A: voluntary muscle force simulated at force levels increasing from 0% to 100% maximal voluntary contraction (MVC) with superimposed single maximal stimulus. B and C: voluntary muscle force simulated at 100% MVC with superimposed single stimulus elicited at stimulation intensities increasing from 0% to maximal. Electrical stimulation was modeled to activate motor units in order of inverse physiological recruitment (B) and in random order (C). Right: amplitude of Tinterp, VA, and CAR as a function of increasing voluntary force (A) and stimulation intensity (B and C). Values are displayed as averages ± SD of the estimates from 10 repetitions of the simulated protocol.
As the level of voluntary force increased from 0% to 100% MVC, the amplitude of the interpolated twitch decreased, whereas the voluntary activation index and the central activation ratio increased (see Fig. 3A, right). The two measures of voluntary drive never reached maximal level, not even when muscle activation was maximal (100% MVC simulated force). These data indicate that the maximal voluntary force that can be generated with the FDI is lower than the total amount of force that the muscle can produce when electrically stimulated.
As the level of stimulation intensity increased from 0 to maximal, the amplitude of the interpolated twitch increased (see Fig. 3, B and C, right). The two measures of voluntary drive displayed opposite trends: the voluntary activation index increased while the central activation ratio decreased with increasing stimulation intensity (see Fig. 3, B and C, right). At any stimulation intensity, the amplitude of the interpolated twitch was lower when electrical stimulation activated motor units in random order rather than in the order of inverse physiological recruitment (compare Fig. 3, B and C, right). This finding is expected because the higher-threshold, higher-amplitude force twitch motor units always contribute to the elicited force when motor units are activated in the order of inverse physiological recruitment. However, when motor units are activated in the order of inverse physiological recruitment the amplitude of the interpolated twitch tends to saturate with increasing stimulation intensity as may be seen in Fig. 3B. This feature indicates that, as progressively lower-threshold, lower-amplitude force twitch motor units are activated by increasing stimulation intensity, they contribute a lesser amount to the amplitude of the interpolated twitch. This saturation phenomenon does not occur during increasing electrical stimulation in which higher-threshold, higher-amplitude force twitch motor units are activated in random order (see Fig. 3C). At low stimulation intensities (<50% of maximal), the amplitude of the interpolated twitch was close to the magnitude of the fluctuations in the simulated muscle force when electrical stimulation activated motor units in random order, as shown in Fig. 3B, left. See Fig. 3 and Table 1 for details.
Table 1.
Amplitude of interpolated twitch and voluntary drive as function of voluntary force and stimulation intensity
| Maximal stimulation intensity | ||||||
| Voluntary force, % MVC | 0% | 5% | 25% | 50% | 75% | 100% |
| Tinterp, % MVC | 36.7 ± 0 | 35.5 ± 0.1 | 28.7 ± 0.5 | 21.3 ± 0.8 | 14.6 ± 0.8 | 10.2 ± 1.1 |
| VA, % | 3.3 ± 0.3 | 21.8 ± 1.4 | 42.0 ± 2.3 | 60.2 ± 2.3 | 72.1 ± 2.9 | |
| CAR, % | 12.5 ± 0.1 | 47.0 ± 0.5 | 69.9 ± 0.8 | 83.5 ± 0.8 | 90.6 ± 0.9 | |
| Maximal voluntary force and reversed MU recruitment | ||||||
| Stimulation intensity, % max | 0% | 5% | 25% | 50% | 75% | 100% |
| Tinterp, % MVC | 3.3 ± 1.2 | 7.3 ± 1.1 | 9.6 ± 0.9 | 9.9 ± 0.9 | 9.9 ± 1.1 | |
| VA, % | 43.7 ± 20.2 | 63.2 ± 5.4 | 67.7 ± 3.1 | 71.4 ± 2.5 | 73.2 ± 2.9 | |
| CAR, % | 96.8 ± 1.1 | 93.2 ± 0.9 | 91.3 ± 0.8 | 91.1 ± 0.7 | 91.1 ± 0.9 | |
| Maximal voluntary force and random MU recruitment | ||||||
| Stimulation intensity, % max | 0% | 5% | 25% | 50% | 75% | 100% |
| Tinterp, % MVC | 2.2 ± 1.1 | 3.5 ± 0.8 | 5.5 ± 1.2 | 8.1 ± 1.2 | 10.2 ± 1.1 | |
| VA, % | −15.4 ± 57.6 | 62.7 ± 8.6 | 68.6 ± 6.6 | 70.5 ± 4.4 | 72.3 ± 2.9 | |
| CAR, % | 97.9 ± 1.1 | 96.6 ± 0.8 | 94.8 ± 1.1 | 92.5 ± 1.0 | 90.8 ± 0.9 |
Values are averages ± SD of the estimates from 10 repetitions of the simulated protocol for amplitude of the interpolated twitch (Tinterp), voluntary activation index (VA), and central activation ratio (CAR) as a function of increasing voluntary force (top) and stimulation intensity (middle and bottom). Electrical stimulation was modeled to activate motor units in the order of inverse physiological recruitment (middle) and in random order (bottom).
MU, motor unit. MVC, maximal voluntary contraction.
Note that the central activation ratio produced higher estimates of voluntary drive than the voluntary activation index. These two measures of voluntary drive were based on the magnitude of voluntary maximal force and on the amplitude of a single elicited resting twitch, respectively. However, the amplitude of a single elicited resting twitch is always lower than the magnitude of voluntary maximal force produced by the summation of numerous motor unit force twitches. In this circumstance, it follows that our estimates of voluntary drive calculated with the central activation ratio are higher than those calculated with the voluntary activation index. Also note that the voluntary activation index obtained from our simulated data is lower (between 70% and 80% in nonfatiguing conditions) than that reported in some empirical studies for the FDI muscle (93–95% in Eichelberger and Bilodeau 2007). This difference likely derives from the use of electrical tetanic stimulation, which produces an elicited resting force higher than that produced by a single simulated electrical stimulus, in the empirical studies.
Maximal-Effort Contractions
Figure 4A shows the muscle force and the voluntary input excitation from one repetition of the simulated maximal-effort contraction during which peripheral fatigue developed; arrows indicate the time of simulated electrical stimulation. Means ± SD of the amplitude of the interpolated twitch, voluntary activation index, and central activation ratio calculated from the 10 repetitions of the simulated tasks are shown in Fig. 4B. The voluntary activation index and central activation ratio from all repetitions of the contraction protocol are displayed in Fig. 4C.
Fig. 4.
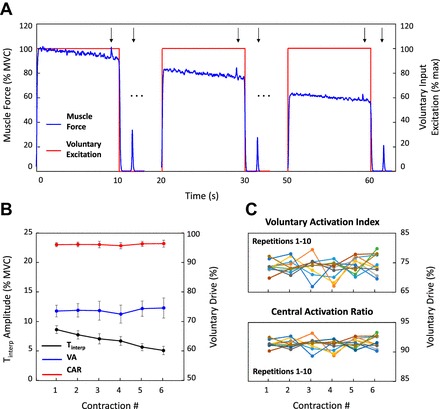
Simulated maximal voluntary efforts. A: muscle force generated during the simulation of a maximal effort sustained for 60 s during which peripheral fatigue developed. Peripheral fatigue was modeled as a time-varying decrease in the amplitude of the motor unit force twitches. The contraction was interrupted every 10 s, and a single maximal stimulus was simulated before each break superimposed on the maximal voluntary force and during each break on the muscle at rest. Voluntary input excitation remained maximal throughout the simulated protocol. Arrows indicate the time of simulated electrical stimulation. B: amplitude of Tinterp, VA, and CAR as a function of contraction number. Values are displayed as averages ± SD of the estimates from 10 repetitions of the simulated protocol. C: VA and CAR as a function of contraction number for all repetitions of the simulated task.
The effect of peripheral fatigue is evident from the progressive decrease in the simulated voluntary muscle force from 100% to 60% MVC during the task (Fig. 4A). Central fatigue did not develop, as evidenced by the fact that the voluntary input excitation remained maximal throughout the task (Fig. 4A). The amplitude of the interpolated twitch decreased significantly (P < 0.05, 2-tailed 2-sample t-test) over time (Fig. 4B). The voluntary activation index and central activation ratio did not vary (P = 0.46 and P = 0.45, respectively, 2-tailed 2-sample t-test) at the end of the contraction (Fig. 4B). Considerable variability was observed among repetitions of the simulated task, with voluntary drive increasing over time in some repetitions and decreasing in others (Fig. 4C). See Fig. 4 and Table 2 for details.
Table 2.
Simulated maximal voluntary efforts
| Repetition No. |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Average | |
| Voluntary muscle force, % MVC (first-last contraction) | 92.1-57.8 | 93.0-58.0 | 91.2-57.4 | 91.5-57.4 | 92.4-57.7 | 92.2 ± 0.6-57.7 ± 0.3 |
| Tinterp, % MVC (first-last contraction) | 8.7-4.7 | 9.1-6.2 | 7.9-6.3 | 8.9-5.3 | 7.6-4.6 | 8.8 ± 0.7-5.3 ± 0.7* |
| VA, % (first-last contraction) | 73.8-77.6 | 72.8-70.8 | 76.3-69.8 | 73.1-74.4 | 77.2-78.0 | 73.8 ± 1.9-74.8 ± 3.4 |
| CAR, % (first-last contraction) | 91.3-92.5 | 91.0-90.4 | 92.1-90.1 | 91.1-91.5 | 92.4-92.6 | 91.3 ± 0.6-91.6 ± 1.0 |
Data from 5 repetitions of the simulated contraction protocol are presented (repetitions 1–5) as well as averages ± SD of the estimates from all 10 repetitions of the simulated protocol for voluntary muscle force, amplitude of Tinterp, VA, and CAR calculated at the beginning (after ∼10 s, first contraction) and at the end (after ∼60 s, last contraction) of a simulated maximal voluntary effort during which peripheral fatigue developed. Peripheral fatigue was modeled as a time-varying decrease in amplitude of motor unit force twitches. Voluntary input excitation was maintained at maximal levels.
Statistically significant change at the end of the contraction protocol (P < 0.05, 2-tailed 2-sample t-test).
Repeated Constant-Force Contractions
Figure 5 presents the results of the simulated submaximal contractions sustained at 20% MVC with intermittent superimposed brief maximal efforts. The simulated muscle force and the voluntary input excitation during the first, middle, and last contractions of the series are shown in Fig. 5A for one repetition of the simulated protocol; arrows indicate the time of simulated electrical stimulation. The amplitude of the interpolated twitch and the voluntary activation index and central activation ratio during the task are shown in Fig. 5, B and C, respectively.
Fig. 5.
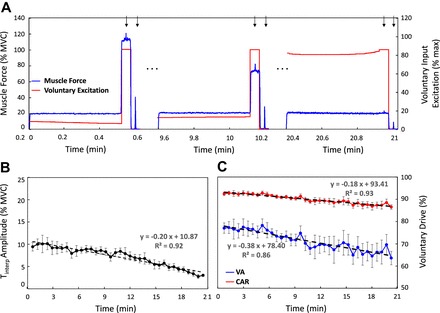
Repeated submaximal voluntary contractions. A: simulated muscle force during the first, middle, and last contractions of a series of tasks sustained at 20% MVC during which peripheral fatigue developed and repeated until the endurance limit. Peripheral fatigue was modeled as a time-varying decrease in the amplitude of the force twitch of the active motor units. A 3-s maximal effort was simulated at the end of each repetition. A single maximal stimulus was simulated on the superimposed maximal effort and on the muscle at rest between contractions. Voluntary input excitation increased over time in order to compensate for the decrease in the amplitude of the motor unit force twitches (i.e., peripheral fatigue) and maintain the 20% MVC target force level (see text for details). Voluntary input excitation remained at maximal level during every maximal task to exclude the development of central fatigue. Arrows indicate the time of simulated electrical stimulation. B: amplitude of Tinterp as a function of time. C: VA and CAR as a function of time. Values are displayed as averages ± SD of the estimates from 10 repetitions of the simulated protocol.
As the muscle force generation capacity decreased over time, i.e., peripheral fatigue developed, the voluntary input excitation needed to sustain the 20% MVC target force level increased in subsequent contractions (see Fig. 5A). It reached maximal level at the endurance limit (after 21 min). At this time, the simulated maximal effort did not produce additional force over the 20% MVC force level. The muscle force simulated during the brief maximal efforts decreased over time, as evident from the progressive decrease in the highest voluntary muscle force level produced in subsequent contractions (see Fig. 5A). This phenomenon can be attributed entirely to peripheral factors, since central fatigue did not develop during the task. (Note that the voluntary input excitation during brief maximal efforts remained maximal throughout the fatiguing contraction series, as may be seen in Fig. 5A). The amplitude of the interpolated twitch, the voluntary activation index, and the central activation ratio decreased significantly (P < 0.05, 2-tailed 2-sample t-test) at the endurance limit (Fig. 5, B and C). Both measures of voluntary drive decreased even though we ensured that no central fatigue developed. See Fig. 5 and Table 3 for details.
Table 3.
Repeated submaximal voluntary contractions
| First Contraction | Middle Contraction | Last Contraction | |
|---|---|---|---|
| Tinterp, % MVC | 9.5 ± 1.0 | 8.2 ± 0.9 | 3.1 ± 0.3* |
| VA, % | 77.0 ± 2.3 | 70.3 ± 3.3 | 63.7 ± 3.0* |
| CAR, % | 92.5 ± 0.7 | 89.8 ± 1.0 | 86.6 ± 1.0* |
Values are averages ± SD of the estimates from 10 repetitions of the simulated protocol for amplitude of Tinterp, VA, and CAR in the first, middle, and last contractions of a series of simulated contractions sustained at 20% MVC during which peripheral fatigue developed and repeated until the endurance limit. Peripheral fatigue was modeled as a time-varying decrease in the amplitude of the force twitch of the active motor units.
Statistically significant change at the end of the simulated contraction protocol (P < 0.05, 2-tailed 2-sample t-test).
DISCUSSION
We performed a series of simulations to gain a better understanding of the attributes commonly assigned to central fatigue and their relation with peripheral fatigue. We identified two major concerns that question the cogency of the parameters used to measure the degree of central fatigue. One relates to the influence of peripheral factors on parametric measures of voluntary drive and central fatigue. The other relates to the reliability of parametric measures of voluntary drive and central fatigue that are typically reported in the literature.
Influence of Peripheral Factors on Interpolated Twitch and Measures of Voluntary Drive and Central Fatigue
During simulated contractions sustained at submaximal force levels, the behavior of parameters used to measure central fatigue could be explained solely by the influence of peripheral factors. As shown in Fig. 5C, the characteristics used to measure the level of voluntary drive to the muscle decreased over the course of a simulated submaximal contraction protocol that included only peripheral factors of muscle fatigue. This finding indicates that central factors are not required to reproduce the decrease in voluntary drive that is typically reported in empirical studies of fatiguing submaximal contractions (Eichelberger and Bilodeau 2007; Lloyd et al. 1991; Smith et al. 2007; Søgaard et al. 2006). Consequently, reports of a decrease in voluntary drive during submaximal efforts should be interpreted carefully, and their implications concerning the presence of central fatigue need to be scrutinized with caution and objectivity.
While peripheral factors alone were sufficient to replicate a decrease in voluntary drive during simulated submaximal contractions, the same effect was not observed during simulated maximal efforts. As shown in Fig. 4B, the parameters used to measure the level of voluntary drive to the muscle did not vary, on average, during simulated maximal efforts that included only peripheral factors of muscle fatigue. Also, peripheral factors alone did not replicate the increase in the amplitude of the interpolated twitch that is sometimes reported in empirical studies of sustained maximal voluntary efforts (Gandevia et al. 1996). These observations do not imply that central factors are required to produce a decrease in voluntary drive or an increase in the amplitude of the interpolated twitch during fatiguing maximal efforts. As described in the next paragraph, measures of voluntary drive and central fatigue are prone to variability and methodological errors that may lead to deceiving results.
In our simulations, peripheral factors of muscle fatigue were modeled to influence only the amplitude and not the time duration of the motor unit force twitches. This choice was motivated by the lack of agreement and data in the literature as to the behavior of the force twitch duration during muscle fatigue. For instance, muscle force twitch duration during fatiguing contractions was reported to increase by Bigland-Ritchie et al. (1983), to decrease by Vøllestad et al. (1997), and to remain constant by Binder-MacLeod and MacDermond (1993). Data on the time-varying adaptations in the duration of individual motor unit force twitches are also scarce (see Contessa and De Luca 2013 for details). In the presence of this ambiguity, how the durations of the force twitch of individual motor units change cannot be established; thus we kept the time duration fixed.
The influence of peripheral factors on the shape of the motor unit force twitches raises a fundamental concern about using the interpolated twitch, which is constructed with the force twitches of the motor units that are excitable during the fatigue-inducing voluntary contraction. This methodology, which has been used consistently over the past six decades, contains a fundamental dichotomy wherein a parameter influenced by peripheral fatigue is used to measure the presence and degree of central fatigue.
Reliability of Interpolated Twitch as Measure of Voluntary Drive and Central Fatigue
Our simulation analysis demonstrated that the amplitude of the interpolated twitch is highly variable and cannot provide a reliable indication of voluntary drive. The reliability of the interpolated twitch as a measure of central fatigue is also weakened by erroneous assumptions and methodological difficulties. Both issues are discussed below.
Variability in measures of voluntary drive and central fatigue.
On average, the measured level of voluntary drive to the muscle did not vary during the 10 repetitions of the simulated maximal effort that included only peripheral factors of muscle fatigue. However, when considering individual repetitions of the simulated protocol, contrasting fatigue-dependent patterns in the level of voluntary drive to the muscle were observed. As shown in Fig. 4C, voluntary drive both increased and decreased over time in different repetitions of the simulations. These data indicate that the interpolated twitch produces highly variable estimates of voluntary drive that may lead to erroneous identification of central fatigue.
Note that in the simulation results presented in Fig. 4 none of the parameters of the model was altered in the various repetitions of the simulation. Therefore, the variability observed in the estimates of voluntary drive was solely a consequence of the physiological synaptic noise included in the model that characterizes motor unit firing behavior and that influences the summation of their force twitches. In actual empirical conditions other factors, such as the level of stimulation intensity, the number and type of stimulated motor units, or the level of voluntary input excitation, may introduce even greater variability in the estimates, as discussed by Arampatzis et al. (2007), Babault (2009), Herzog (2009), Folland and Williams (2007), and Oskouei et al. (2003), among others. As an example, consider that only some studies (Bilodeau 2006; McKenzie et al. 1992; Schillings et al. 2003; Todd et al. 2003) but not others (Bigland-Ritchie et al. 1978; Schillings et al. 2007) have observed exercise-induced central fatigue during voluntary fatiguing contractions. Contrasting patterns of voluntary drive are also commonly observed when comparing different subjects, muscles, or contraction protocols.
We investigated the influence of stimulation intensity on the amplitude of the interpolated twitch in Fig. 3, B and C. Submaximal stimulation intensities activate only a portion of the motor units available in the muscle and lead to considerable variability in the results depending on the number and type of stimulated motor units. They also lead to low-amplitude interpolated twitches that may be difficult to distinguish from ordinary fluctuations of voluntary muscle force. The pragmatic challenges of delivering electrical stimulation during maximal efforts and accurately calculating the increment in force introduce an additional level of complexity and uncertainty to the measurement of central fatigue.
The high degree of variability in the measures of voluntary drive observed both in our simulations and in empirical studies confirms the technical difficulties that hinder the investigation of central fatigue. It also indicates that current measures of voluntary drive cannot be reliably applied to measure or identify central fatigue.
Methodological concerns on use of interpolated twitch as measure of voluntary drive and central fatigue.
The amplitude of the interpolated twitch is used to measure the proportion of available voluntary muscle force that cannot be accessed during voluntary contractions. As previously indicated by Herzog (2009), this method is based on the erroneous assumption that muscles are controlled to exert their full force potential. However, it is now known that during voluntary contractions the high-threshold motor units do not produce fully fused forces, not even during maximal efforts (De Luca and Contessa 2015; De Luca and Erim 1994; Hu et al. 2014), whereas electrically stimulated high-threshold motor units can be excited to fire at rates greater than those that occur naturally and tetanize their forces, producing greater total muscle force. Additionally, the simultaneous stimulation of motor units induces synchronized firings, causing a localized summation of their force twitches that leads to greater interpolated twitch. It follows that the amplitude of the interpolated twitch is not a reliable indicator of the level of voluntary drive to a muscle. This is evident in Fig. 3, where we show that maximal electrical stimulation always produces an increment over voluntary muscle force, i.e., a visible interpolated twitch, even during simulated maximal voluntary efforts.
Another concern relates to the procedure for measuring voluntary drive and central fatigue during maximal voluntary efforts. Proper execution of maximal efforts is challenging and requires extensive training and motivation. Even so, increased perceived effort due to developing peripheral fatigue may induce subjects to intentionally decrease the contraction strength in an attempt to ease the task or prolong the contraction duration. This behavior would lead to reductions in voluntary drive that could be erroneously interpreted as the occurrence of central fatigue. The challenges of performing genuine maximal efforts may also provide an explanation for the inconsistent reports in the literature. For instance, previous studies using the interpolated twitch have reported that muscles can (Bigland-Ritchie et al. 1986; Gandevia and McKenzie 1988; Merton 1954; Todd et al. 2003) and cannot (Belanger and McComas 1981; Bigland-Ritchie et al. 1992; Dowling et al. 1994; Lloyd et al. 1991) be activated maximally during maximal voluntary efforts.
These concerns that relate to the use of the interpolated twitch as a measure of voluntary drive and central fatigue are valid without regard to the method used to elicit the interpolated twitch. For instance, over the past 20 years transcranial magnetic stimulation (TMS) has been applied to measure central fatigue. This methodology originated from the presumption that supraspinal sources of central fatigue derive from failure of the motor cortex to provide adequate descending drive to the motoneuron pool of a muscle (see, e.g., Gandevia et al. 1996; Todd et al. 2007). Briefly, the motor cortex is stimulated with TMS during maximal voluntary efforts, and fatigue-induced changes in the amplitude of the interpolated twitch elicited by TMS are measured. This methodology is similar to that previously described for electrical stimulation. Therefore, evidence of central fatigue derived with the use of TMS-elicited interpolated twitch is subject to the same drawbacks and methodological errors that affect the measurement of the electrically elicited interpolated twitch.
Does the Notion of Central Fatigue Fulfill a Need?
We have discussed challenges and confounding factors that raise concerns regarding commonly accepted measures of voluntary drive and central fatigue. Even if we assume that central fatigue develops during fatiguing voluntary contractions, it would have only a minor influence on force performance. During fatiguing tasks, most (at least 80%–90%) of the observed force loss is commonly attributed to peripheral factors (Gandevia et al. 1996; Schillings et al. 2003, 2007; Smith et al. 2007), and the reported decrease in voluntary drive is typically very low, ranging from 5% to 10% (Eichelberger and Bilodeau 2007; Gandevia et al. 1996; Lloyd et al. 1991). The weakness of these observations raises questions about the impact of central factors on muscle fatigue.
Despite the minor effect of central fatigue, this phenomenon has been ascribed the functional purpose of protecting muscles from excessive fatigue and contractile failure (Gandevia 2001; St Clair Gibson et al. 2001). In a practical sense, these neural protective mechanisms are likely not necessary. According to recent understanding, it appears that the control of motoneurons has not evolved to maximize the force that can be produced by a muscle (De Luca and Contessa 2012, 2015; Hu et al. 2014). We and others (e.g., De Luca et al. 1982a; Holobar et al. 2009; Masakado et al. 1995; Person and Kudina 1972; Seyffarth 1940; Stock et al. 2012) have shown that the control of motor unit firing rates is organized in an inverse hierarchical arrangement referred to as the “onion-skin” scheme (De Luca and Erim 1994), in which earlier-recruited motor units have greater firing rates than later-recruited ones during voluntary tasks. Even at maximal force level, higher-threshold motor units never reach the firing rates needed to produce fused force and muscles do not exert their full force potential.
In conclusion, our analysis does not directly refute the concept of central fatigue. However, it raises troubling questions about the interpretation of the proposed causes of central fatigue, and it shows that the attributes assigned to central fatigue can be explained solely by peripheral factors. Consequently, observations of central fatigue should be interpreted with scrutiny and abundant objectivity.
After six decades of discussion, it still remains for empirical evidence supporting the existence of central fatigue to be offered. Until then, the notion of central fatigue remains an unsubstantiated conjecture that has not strengthened its position in neurophysiology.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R43 NS-077526 and a grant from the Neuromuscular Research Foundation.
DISCLOSURES
Carlo J. De Luca is the President of the Neuromuscular Research Foundation and the President of Delsys Inc.
AUTHOR CONTRIBUTIONS
Author contributions: P.C. and C.J.D.L. conception and design of research; P.C. and A.P. performed experiments; P.C. and A.P. analyzed data; P.C., A.P., and C.J.D.L. interpreted results of experiments; P.C. and A.P. prepared figures; P.C. and C.J.D.L. drafted manuscript; P.C., A.P., and C.J.D.L. edited and revised manuscript; P.C. and C.J.D.L. approved final version of manuscript.
Appendix: SIMULATION MODEL: EFFECT OF VOLUNTARY AND ELICITED INPUT EXCITATION
Effect of Voluntary Input Excitation
The voluntary input excitation, ϕ, is based on the concept of common drive (De Luca et al. 1982b), which describes a common excitation driving the firing behavior of all the motor units in the motoneuron pool of a muscle. It ranges from ϕ = 0, when no motor unit is active and no force is produced, to ϕ = 1, the maximal level of voluntary input excitation required to produce the maximal voluntary contraction (MVC) force.
The first dorsal interosseous (FDI) muscle is represented by 120 motor units as described by Feinstein et al. (1955). Motor units are activated when ϕ is greater than or equal to their recruitment threshold value, τ, which ranges over 0 < τ < 67%, as identified by De Luca and Hostage (2010). The firing rate of each active motor unit increases as a negative exponential with increasing voluntary input excitation and is inversely related to the motor unit recruitment threshold. This hierarchical inverse relationship between voluntary input excitation and motor unit firing rate forms a firing rate spectrum, which we refer to as the “onion skin.” In this spectrum, lower-threshold motor units have greater firing rates than higher-threshold ones at any time and force during voluntary contractions (see De Luca et al. 1982a; De Luca and Contessa 2012; De Luca and Erim 1994 for details). The motor unit firing rates are translated into time-varying impulse trains, to which noise is added by modeling the interpulse interval (IPI) between two adjacent firings of a motor unit as a random variable with Gaussian distribution and a coefficient of variation of 20% (Clamann 1969; Macefield et al. 2000; Moritz et al. 2005; Nordstrom et al. 1992). For more details, see Contessa and De Luca (2013).
Effect of Elicited Input Excitation
The elicited input excitation, ε, determines the percentage of motor units that are activated by electrical stimulation. It ranges from ε = 0, when no motor unit is activated, to ε = 1, the maximal level of elicited input excitation that activates all the motor units in the motoneuron pool of the muscle. The model allows the simulation of electrical stimulation delivered either to the motor point over a muscle or to the nerve supplying a muscle. In the first case, motor units are activated in random order with uniform probability distribution as indicated by the empirical evidence presented by Knaflitz et al. (1990) and Gregory and Bickel (2005). In the second case, motor units are activated in inverse order of physiological recruitment, i.e., in the order of decreasing recruitment threshold τ, as suggested by the empirical studies of Bergquist et al. (2011, 2012).
When a motoneuron is activated by elicited input excitation, the motor unit action potential (MUAP) generated at the stimulation site travels in two directions: toward the muscle in a time tp (orthodromic elicited MUAP) and toward the neuron body and away from the muscle in a time tic (antidromic elicited MUAP). Orthodromic and antidromic elicited MUAPs are shown in Fig. A1. If the motoneuron is concurrently active as a result of voluntary input excitation, voluntary MUAPs are also generated at the motoneuron body and travel toward the muscle fibers in a time tp + tic. They are also shown in Fig. A1. The interaction between voluntary and elicited MUAPs is modeled according to the work of Crago and Makowski (2014). Briefly, three types of interactions can occur: collision block, stimulation failure, and phase resetting. These are graphically depicted in Fig. A1. Collision block occurs when the antidromic elicited MUAP collides with a voluntary MUAP. Collision block prevents the antidromic elicited MUAP from reaching the motoneuron body and averts the firing that would be otherwise generated by the voluntary MUAP upon arrival at the muscle. Only the orthodromic elicited MUAP generated at the stimulation site reaches the muscle after a time tp and results in a motor unit firing. Stimulation failure occurs when the elicited input excitation is delivered during the motoneuron refractory period tr after a voluntary MUAP has traveled past the stimulation site. The transition of a voluntary MUAP depolarizes the motoneuron axon and prevents the generation of elicited MUAPs for the time tr. Thus the elicited input excitation fails to produce a response and only the voluntary MUAP reaches the muscle causing the motor unit to fire. Phase resetting refers to a delay in the generation of voluntary MUAPs at the motoneuron body after the arrival of an antidromic elicited MUAP. This event resets the motoneuron body to its resting potential and delays the generation of voluntary MUAPs. A motor unit firing is generated as a result of the orthodromic elicited MUAP.
Fig. A1.
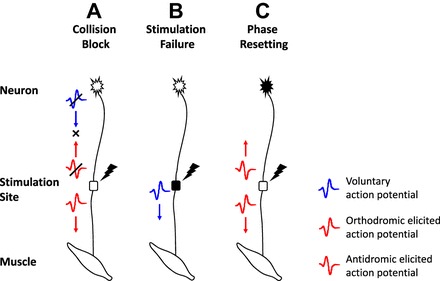
Interaction between voluntary and elicited firings. Graphic representation of the interactions between voluntary and elicited action potentials: collision block (A), stimulation failure (B), and phase resetting (C). Refer to text for additional details.
Following the work of Jami and Petit (1975) and of Dengler et al. (1988), the motor unit axonal conduction velocity was taken to range between 40 and 62 m/s and to vary linearly with the logarithm of the amplitude of the motor unit force twitches. The distance between the spinal cord and the ulnar nerve and between the spinal cord and the FDI muscle was taken to be 0.72 m and 0.8 m, respectively (Crago et al. 2014). Using these values, tic and tp ranged between 12.9 and 20 ms and between 0.3 and 2 ms, respectively. The motoneuron refractory period tr was fixed at 2 ms for all motor units (Borg 1983, 1984).
REFERENCES
- Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol 90: 2919–2927, 2003. [DOI] [PubMed] [Google Scholar]
- Adam A, De Luca CJ. Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol 99: 268–280, 2005. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Mademli L, De Monte G, Walsh M. Changes in fascicle length from rest to maximal voluntary contraction affect the assessment of voluntary activation. J Biomech 40: 3193–3200, 2007. [DOI] [PubMed] [Google Scholar]
- Babault N. The interpolated twitch to determine voluntary activation in various conditions. J Appl Physiol 107: 360, 2009. [DOI] [PubMed] [Google Scholar]
- Bawa P, Stein RB. Frequency response of human soleus muscle. J Neurophysiol 39: 788–793, 1976. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol Respir Environ Exerc Physiol 51: 1131–1135, 1981. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Bigland-Ritchie B. Central components of diaphragmatic fatigue assessed by phrenic nerve stimulation. J Appl Physiol 62: 1307–1316, 1987. [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Clair JM, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J Appl Physiol 110: 627–637, 2011. [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Wiest MJ, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: quadriceps femoris. J Appl Physiol 113: 78–89, 2012. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand 71: 140–150, 1967. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol 61: 421–429, 1986. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol 50: 313–324, 1983. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RH. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med 54: 609–614, 1978. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Kukulka CG, Lippold OC, Woods JJ. The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. J Physiol 330: 265–278, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Thomas CK, Rice CL, Howarth JV, Woods JJ. Muscle temperature, contractile speed, and motoneuron firing rates during human voluntary contractions. J Appl Physiol 73: 2457–2461, 1992. [DOI] [PubMed] [Google Scholar]
- Bilodeau M. Central fatigue in continuous and intermittent contractions of triceps brachii. Muscle Nerve 34: 205–213, 2006. [DOI] [PubMed] [Google Scholar]
- Binder-MacLeod SA, McDermond LR. Changes in the force-frequency relationship of the human quadriceps femoris muscle following electrically and voluntarily induced fatigue. Phys Ther 72: 95–104, 1993. [DOI] [PubMed] [Google Scholar]
- Borg J. Effects of prior activity on the conduction in single motor units in man. J Neurol 46: 317–321, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Refractory period of single motor nerve fibres in man. J Neurol Neurosurg Psychiatry 47: 344–348, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology, and functional organization. In: Handbook of Physiology. The Nervous System. Motor Control Bethesda, MD: Am Physiol Soc, 1981, sect. 1, vol. II, p. 345–422. [Google Scholar]
- Clamann HP. Statistical analysis of motor unit firing patterns in a human skeletal muscle. Biophys J 9: 1233–1251, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contessa P, De Luca CJ. Neural control of muscle force: indications from a simulation model. J Neurophysiol 109: 1548–1570, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Makowski NS. Alteration of neural action potential patterns by axonal stimulation: the importance of stimulus location. J Neural Eng 11: 056016, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Makowski NS, Cole NM. Contributions to muscle force and EMG by combined neural excitation and electrical stimulation. J Neural Eng 11: 056022, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan A, Gerrits KH, de Ruiter CJ. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 355–357, 2009. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Biomechanical benefits of the Onion-Skin motor unit control scheme. J Biomech 48: 195–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104: 1034–1046, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler R, Stein RB, Thomas CK. Axonal conduction velocity and force of single human motor units. Muscle Nerve 11: 136–145, 1988. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Konert E, Ljucovic P, Andrews DM. Are humans able to voluntarily elicit maximum muscle force? Neurosci Lett 179: 25–28, 1994. [DOI] [PubMed] [Google Scholar]
- Eichelberger TD, Bilodeau M. Central fatigue of the first dorsal interosseous muscle during low-force and high-force sustained submaximal contractions. Clin Physiol Funct Imaging 27: 298–304, 2007. [DOI] [PubMed] [Google Scholar]
- Feinstein B, Lindegârd B, Nyman E, Wohlfart G. Morphologic studies of motor units in normal human muscles. Acta Anat (Basel) 23: 127–142, 1955. [DOI] [PubMed] [Google Scholar]
- Folland JP, Williams AG. Methodological issues with the interpolated twitch technique. J Electromyogr Kinesiol 17: 317–327, 2007. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Activation of human muscles at short muscle lengths during maximal static efforts. J Physiol 407: 599–613, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85: 358–364, 2005. [PubMed] [Google Scholar]
- Hermansen L, Hultma E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand 71: 129–139, 1967. [DOI] [PubMed] [Google Scholar]
- Herzog W. Twitch interpolation represents muscle activation in a qualitative manner only. J Appl Physiol 107: 360–361, 2009. [DOI] [PubMed] [Google Scholar]
- Holobar A, Farina D, Gazzoni M, Merletti R, Zazula D. Estimating motor unit discharge patterns from high-density surface electromyogram. Clin Neurophysiol 120: 551–562, 2009. [DOI] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, Suresh NL. Motor unit firing rate patterns during voluntary muscle force generation: a simulation study. J Neural Eng 11: 026015, 2014. [DOI] [PubMed] [Google Scholar]
- Jami L, Petit J. Correlation between axonal conduction velocity and tetanic tension of motor units in four muscles of the cat hind limb. Brain Res 96: 114–118, 1975. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 19: 861–869, 1996. [DOI] [PubMed] [Google Scholar]
- Knaflitz M, Merletti R, De Luca CJ. Inference of motor unit recruitment order in voluntary and electrically elicited contractions. J Appl Physiol 68: 1657–1667, 1990. [DOI] [PubMed] [Google Scholar]
- Lloyd AR, Gandevia SC, Hales JP. Muscle performance, voluntary activation, twitch properties and perceived effort in normal subjects and patients with the chronic fatigue syndrome. Brain 114: 85–98, 1991. [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AG, Howell JN, Bigland-Ritchie B. Discharge behavior of single motor units during maximal voluntary contractions of a human toe extensor. J Physiol 528: 227–234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh BR, Grange RW, Cory CR, Houston ME. Contractile properties of rat gastrocnemius muscle during staircase fatigue and recovery. Exp Physiol 79: 59–70, 1994. [DOI] [PubMed] [Google Scholar]
- Masakado Y, Akaboshi K, Nagata M, Kimura A, Chino N. Motor unit firing behavior in slow and fast contractions of the first dorsal interosseous muscle of healthy men. Electroencephalogr Clin Neurophysiol 97: 290–295, 1995. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Bigland-Ritchie B, Gorman RB, Gandevia SC. Central and peripheral fatigue of human diaphragm and limb muscles assessed by twitch interpolation. J Physiol 454: 643–656, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Mosso A. Fatigue. London: Swan Sonnenschein, 1904. [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka ME. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouei MA, Van Mazijk BC, Schuiling MH, Herzog W. Variability in the interpolated twitch torque for maximal and submaximal voluntary contractions. J Appl Physiol 95: 1648–1655, 2003. [DOI] [PubMed] [Google Scholar]
- Pernow B, Saltin B. Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol 3l: 416–422, 1971. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972. [DOI] [PubMed] [Google Scholar]
- Reid C. The mechanism of voluntary muscular fatigue. Br Med J 2: 545–546, 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillings ML, Hoefsloot W, Stegeman DF, Zwarts MJ. Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur J Appl Physiol 90: 562–568, 2003. [DOI] [PubMed] [Google Scholar]
- Schillings ML, Kalkman JS, Janssen HM, van Engelen BG, Bleijenberg G, Zwarts MJ. Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol 118: 292–300, 2007. [DOI] [PubMed] [Google Scholar]
- Seyffarth H. The Behaviour of Motor-Units in Voluntary Contraction. Oslo: Jacob DybWads, 1940. [Google Scholar]
- Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol 103: 560–568, 2007. [DOI] [PubMed] [Google Scholar]
- Søgaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol 573: 511–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair Gibson A, Lambert ML, Noakes TD. Neural control of force output during maximal and submaximal exercise. Sports Med 31: 637–650, 2001. [DOI] [PubMed] [Google Scholar]
- Stock WS, Beck TW, Defreitas JM. Effects of fatigue on motor unit firing rate versus recruitment threshold relationship. Muscle Nerve 45: 100–109, 2012. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Olsen HB, Sjøgaard G, Søgaard K. Voluntary activation of trapezius measured with twitch interpolation. J Electromyogr Kinesiol 19: 584–590, 2009. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol 102: 1756–1766, 2007. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551: 661–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA, Quinlan J, McComas AJ. Twitch potentiation after voluntary contraction. Exp Neurol 81: 141–152, 1983. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Sejersted I, Saugen E. Mechanical behavior of skeletal muscle during intermittent voluntary isometric contractions in humans. J Appl Physiol 83: 1557–1565, 1997. [DOI] [PubMed] [Google Scholar]


