Abstract
People with Parkinson's disease exhibit debilitating gait impairments, including gait slowness, increased step variability, and poor postural control. A widespread supraspinal locomotor network including the cortex, cerebellum, basal ganglia, and brain stem contributes to the control of human locomotion, and altered activity of these structures underlies gait dysfunction due to Parkinson's disease.
The ability to walk is severely impaired in people with Parkinson's disease (PD), and these impairments are associated with reduced quality of life, frequent falls, and complications from falls such as increased morbidity and mortality (6, 96). Recently, a widespread supraspinal locomotor network has been described, including premotor cortical, motor cortical, basal ganglia, cerebellar, and brain stem structures (66, 137). PD impairs structure and function in all of these locomotor regions, and these pathological and compensatory changes contribute to Parkinsonian gait, characterized by its slowness, variability, and poor postural control. In this review, we first discuss the basis for these three primary Parkinsonian gait impairments. Then, we discuss how altered structure and function of supraspinal locomotor regions contribute to gait impairments in people with PD.
Gait Impairments in People With PD
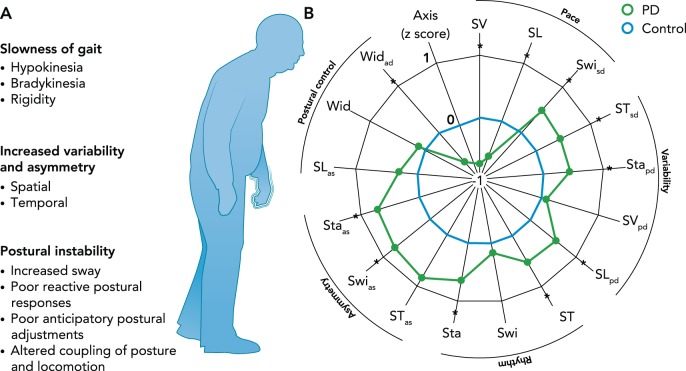
Gait impairments associated with PD can be broadly separated into continuous and transient disturbances (45). Continuous gait control problems have been recently characterized using principle component analysis as consisting of five independent domains: pace, rhythm, variability, asymmetry, and postural control (FIGURE 1B; Ref. 41). For the purposes of this review, we have consolidated these domains to three primary gait impairments: 1) gait slowness (pace, rhythm), 2) increased variability and asymmetry, and 3) poor postural control. These impairments have been shown to be relatively independent (22, 41), and we postulate each depends on partially distinct neural networks. Transient gait disturbances include occasional, context-specific festination (rapid, short steps) and freezing of gait (either akinesia or trembling of the legs) (126). In the current review, we will not discuss these transient disturbances but will refer to several recent reviews (34, 46, 102, 103).
FIGURE 1.
Continuous gait disturbances
A: continuous gait disturbances in people with PD. B: people with PD exhibit dysfunction in gait speed (pace/rhythm), variability and asymmetry, and postural control. This is depicted by a satellite plot showing deviations from control subjects (dotted line). SV, step velocity; SL, step length; Swi, swing time; ST, Step time; Sta, Stance time; Wid, Step width; sd, standard deviation (gait variability); as, asymmetry. *Differences between the control and PD group. Figure reproduced from Ref. 41 with permission from Movement Disorders.
Slowness of Gait
Gait slowness is common in people with PD (93) and can be observed throughout the course of the disease. The primary causes of gait slowness are 1) hypokinesia (reduced step size)/bradykinesia (increased step duration) and 2) rigidity/hypertonia.
Hypokinesia and bradykinesia of gait.
Parkinsonian gait is both hypokinetic (e.g., small movements such as reduced step amplitude and arm swing) and bradykinetic (e.g., slow movements such as slower step and arm swing velocity) (22, 41). However, reduced size of steps may be a more consistent contributor to slowed gait than slower steps (93). Similar to voluntary, upper extremity movement, most aspects of step kinematics and kinetics (e.g., joint angles, ground reaction forces, arm swing, etc.) are diminished in PD (92, 93). These findings suggest that, in the absence of transient disturbances such as freezing, the primary pathology of PD gait is inadequate scaling of motor output rather than the coordination of locomotor patterns (27, 28, 87). One notable exception is the increase in co-contraction of agonists and antagonists in the shank during walking (30). Hypokinesia and bradykinesia of gait is also apparent in the small and slow arm swing and lack of axial trunk rotation in people with PD, even after controlling for gait speed (156). Despite hypokinetic arm swing, coordination of arm and leg motion is largely intact in people with PD (29).
Rigidity/hypertonia of gait.
Rigidity is a specific type of hypertonicity observed in people with PD. Both axial and limb rigidity are observed in people with PD (84, 148), and both likely contribute to gait slowness. Axial tone can be measured using a device that slowly rotates different portions of the body, e.g., hips, trunk, and neck, during quiet stance (48). The resistance to these slow rotations provides a measure of passive resistance to external movements while an individual is actively maintaining stance posture. This technique shows hip, trunk, and neck tone to be elevated 30–50% in people with PD compared with age-matched control subjects (148). Axial rigidity contributes to gait slowness as hip rigidity interferes with hip hyperextension, a primary factor in step length and gait speed (3). Furthermore, excessive neck tone is related to functional mobility, such as ability to curve gait into a figure eight or roll over (40). Finally, changing gait direction with a turn is particularly slow in people with PD, even when gait speed is normal (156). Increased trunk stiffness and resistance to twisting may contribute to the more en block turning style of people with PD (55, 61) and the reduced turning speed.
People with PD also exhibit abnormal tone of the limbs, including the knees and ankles. This increased tone contributes to the flexed postural alignment of people with PD as well as gait slowness. Excessive flexor muscle actively pulls the hips, knees, and ankles into flexion, resulting in flexed spinal abnormalities, stooped posture, and reduced lower limb joint torques (58, 62). This abnormal posture also pushes the center of mass forward over the feet (125) and contributes to short, shuffling steps common in this population. In addition, excessive tonic muscle activity in ankle flexors and extensors increases co-contraction and joint stiffness (58). The elevated tone may be compensatory as it slows the speed and extent of body center of mass displacements and stabilizes joints. However, increased tone and phasic antagonist co-contraction reduces the net joint torques for control of gait and posture. Specifically, reduction in net ankle plantarflexion torque, a primary propulsive force during gait, results in smaller steps and slower gait velocity (71). Another factor that may play a role in the reduced ankle plantar flexion torque, and thus reduced gait velocity, in people with PD is a proximal distribution of joint torques from the ankle to the hip (130).
Increased Variability and Asymmetry of Gait
Gait variability includes “stride-to-stride fluctuations in walking” (53), and is consistently elevated in people with PD (41, 52). Variability in the medio-lateral and anterior posterior planes may have distinct sources. Collins and Kuo (20) demonstrated that step width variability, which occurs in the medio-lateral plane, is related to active step-to-step adjustment by the central nervous system to maintain balance during gait. This suggests that elevated lateral step variability observed in people with PD may partially reflect impaired control of lateral postural equilibrium. In contrast to medio-lateral variability, anterior-posterior variability of steps is closely related to fluctuations in self-selected walking speed. That is, anterior-posterior variability co-varies with gait speed and, in particular, the slow fluctuations in gait speed that are likely unrelated to step-to-step adjustments (20). Variability of steps is larger in people with PD in both the anterior-posterior and medio-lateral directions (52). Interestingly, increased variability of steps and reduced step length seem to be somewhat distinct phenomenon, since increased step variability appears before reduced step length, and step length is improved (increased) with levodopa, whereas variability is levodopa resistant (4, 5). These results suggest the possibility of different underlying causes for these changes in parkinsonian gait.
Excessive temporal and spatial left-right asymmetry of stepping parameters has also been consistently observed in people with PD (41, 109). For example, step length (119) and step time (41, 109) have been shown to be more asymmetric in people with PD than in healthy older adults. This likely relates to the asymmetric onset of bradykinesia and rigidity in lower limbs as well as in upper limbs. Temporal (156) and spatial (82) asymmetry of the arms during walking also occurs in people with PD. In fact, one of the earliest signs of abnormal gait in PD is asymmetric arm swing amplitude (156). Even postural control asymmetry, measured as the velocity of center of pressure movement under the left and right feet during quiet stance, has been shown to be elevated in people with PD (44). Furthermore, gait asymmetry, along with variability, has been suggested to underlie more serious gait impairments, such as freezing of gait and falls (110). Thus asymmetry represents an important and independent gait impairment in people with PD.
Poor Postural Control
Postural control involves maintaining, achieving, and restoring a state of balance during movements and posture (111). Indeed, all three components of postural control are impaired in people with PD and affect their gait.
Maintaining balance during standing or walking activities requires precise control of the head-arms-trunk (HAT) segments (145). During standing, people with PD have increased area, velocity, and jerkiness of postural sway and reduced limits of stability, defined as the maximum center of mass displacement possible without changing one's base of support. Limit of stability is especially impaired in people with PD in the backward direction (57) and is observed even in very early PD, before taking any antiparkinsonian medication (59, 86). In fact, the characteristic flexed, inflexible postural alignment of people with PD results in forward position of the body center of mass, possibly to protect against backward falling (125). Maintenance of balance during standing may worsen when on levodopa, possibly due to levodopa-induced dyskinesia (18, 22). Lateral control of balance is particularly affected in people with PD, as lateral trunk sway is especially elevated during quiet stance (1, 85) and while walking with and without obstacles (42, 134). While some amount of lateral motion is necessary for stability and optimized energetics (79), too much lateral motion is likely detrimental and is related to increased falls (120).
Achieving balance during voluntary movements such as gait initiation or fast, upper extremity movements is also impaired in people with PD. For example, anticipatory postural adjustments (APAs), defined as involuntary displacement of the center of pressure in preparation for voluntary movements, are bradykinetic in people with PD (86). A common example of an APA is the lateral and posterior movement of the center of pressure toward the swing foot before initiating a step from quiet stance. This lateral movement of the center of pressure is necessary to lift the stepping foot. The slow, small APAs observed in people with PD have been linked to delayed step initiation (76) and reduced step width, since smaller step widths permit smaller lateral weight shifts during locomotion. In fact, people with PD have difficulty scaling up the size of their lateral APAs before a step when their step width increases, which can result in inability to take a step (akinetic freezing) (116).
Small APAs may be due parkinsonian motor bradykineisa since slow movements require smaller APAs. Alternatively, APA dysfunction may be related to poor temporal coupling of posture (e.g., trunk lean) and gait (i.e., stepping). The coupling of posture and gait during walking or gait initiation is highly complex and requires precise control of the center of mass trajectory (83, 89, 146, 147). Unsurprisingly, people with PD have difficulty with posture-gait coupling (for review, see Ref. 88), and this has been suggested to contribute to gait challenges including delayed step-time and freezing of gait (65). Interestingly, APAs can be increased to normal levels when a step is initiated in response to an external cue or when on levodopa (15). Recently, it has been shown that rehabilitative interventions to assist lateral displacement of the APA may be beneficial for improving steps in people with PD (88).
Restoring balance after a slip, trip, or other external perturbation is also dysfunctional in people with PD. For example, people with PD exhibit smaller steps with larger subsequent displacements of the center of mass than healthy adults in response to large surface translations that trigger automatic compensatory stepping responses (23, 76, 131). Similarly, people with PD exhibit hypokinetic postural responses with reduced rate of rise of reactive torque in response to smaller perturbations (58, 60). The size of stepping responses to external perturbations, however, can be increased when people with PD can see their legs as they step toward a visual target, suggesting a deficit in proprioception (evaluation of limb position) and/or kinesthesia (evaluation of limb movement) (63).
The ability to quickly adapt postural responses based on task and environmental context is also altered in people with PD (16, 60). Chong and colleagues showed that, if perturbation direction was unexpectedly altered, healthy adults immediately adapted their postural responses by changing postural muscle activation patterns. People with PD, however, took several trials before exhibiting the appropriate postural response to the new perturbations. This represents a relative “inflexibility” to alter postural responses to match changes in task conditions or contexts (24). However, despite potentially slowed rates of adaptation and learning, people with PD can eventually adapt gait and stepping patterns with repetition (11, 56, 90, 118).
Summary of Gait Impairments
Slowed gait, increased variability, and poor postural control are the primary gait impairments in people with PD. While some gait impairments in people with PD are related to primary pathophysiology (such as bradykinesia), others may be compensatory in nature. For example, reduced step length and walking speed can be protective against falls as more time is spent with both feet on the ground at slower gait speeds. Similarly, while increased rigidity contributes to slowed gait (81, 99), it may also represent a compensatory mechanism to increase joint stiffness and joint stability. Age-related impairments in mobility (e.g., reduced muscle strength, reduced proprioception) also reduce the ability to control the trunk or increase walking speed. These effects are further compounded by physical inactivity and sarcopenia that are especially pronounced in people with PD (for review, see Ref. 33). Therefore, while we focus our review on the possible neural underpinnings of each gait impairment, it is important to note that compensatory mechanisms and secondary, age-related musculoskeletal impairments also contribute to gait impairments in people with PD, in addition to neurodegeneration.
Neural Underpinnings of Gait Dysfunction in People with PD
A definitive diagnosis of PD includes death of dopaminergic cells within the substantia nigra pars compacta that project to the striatum (i.e., caudate and putamen) of the basal ganglia. Often overlooked, however, is that numerous other brain regions also show altered structure and function in people with PD. For instance, areas that release acetylcholine (ACh), such as the pedunculopontine nucleus (PPN) and nucleus basalis of Meynert (nbM), and noradrenaline, such as the locus coeruleus, exhibit alpha-synuclein deposition very early in the disease (14). Cortical motor and nonmotor structures show considerable deposition later in the course of the disease. Cerebellar function is also altered in PD (149). Indeed, every critical node in the central locomotor network (66) likely plays a role in PD gait dysfunction. Table 1 summarizes studies investigating the neural activity during gait or gait-like tasks, showing reduced or increased activity in a number of brain regions (or nodes) within the locomotor/postural network in people with PD. In the following section, we will discuss how these changes in supraspinal activity may contribute to the gait dysfunction described above: gait slowness, variability/asymmetry, and impaired postural control.
Table 1.
Summary of recent imagining studies showing increased or decreased activity of supraspinal locomotor nodes during actual or imagined locomotion in people with Parkinson's disease
| Reduction of Activity in PD | Reference(s) | Increase in Activity in PD | Reference(s) |
|---|---|---|---|
| Motor | |||
| Supplementary motor area (L) | 5 | ||
| Dorsal premotor (R) | 142 | ||
| Primary motor cortex | 142 | ||
| Cortical | |||
| Precuneus (L) | 21, 51 | Temporal cortex (R) | 51 |
| Precuneus (R) | 21, 51, 142 | Insula (R) | 51 |
| Parieto-occipital region | 21, 142 | Superior frontal gyrus (L) | 51 |
| Posterior hippocampus (L) | 21 | Cingulate cortex (L) | 51 |
| Superior parietal lobule (R) | 21, 132 | ||
| Anterior cingulate cortex (R) | 21, 132 | ||
| Anterior cingulate cortex (L) | 21 | ||
| Inferior parietal lobule (R) | 142 | ||
| Occipital | 142 | ||
| Lingual gyri | 21 | ||
| Basal ganglia | |||
| Globus pallidus (R) | 105 | ||
| Putamen (R,L) | 104 | ||
| Brain stem | |||
| PPN/MLR | 21 | ||
| Cerebellum | |||
| Cerebral hemisphere (L) | 21, 51 | Cerebellar locomotor region | 51 |
| Cerebellar locomotor region | 21 |
L, left; R, right; PPN, pedunculopontine nucleus; MLR, mesencephalic locomotor region.
Neural Underpinnings of Slowness in PD Gait
Hypokinesia and bradykinesia.
Hypokinesia and bradykinesia contribute to slowed gait and may be explained partially by the long-standing “rate-model” of basal ganglia pathology in people with PD (2, 26, 43). Although this model has been adapted over time (25), it remains a commonly used model of motor dysfunction in PD and supports a critical role of the basal ganglia in scaling self-initiated movement.
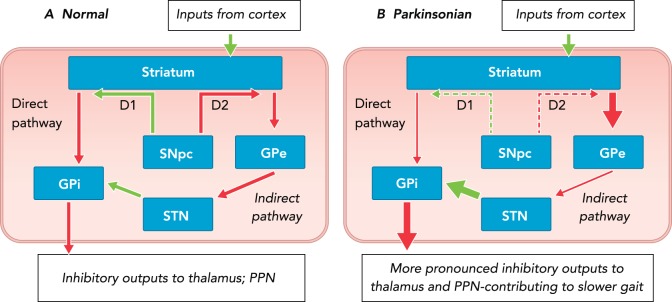
FIGURE 2 depicts the “rate model” of basal ganglia pathophysiology in people with PD. This model suggests that neural degeneration of the substantia nigra pars compacta results in increased inhibition of the globus pallidus external segment and reduced inhibition of the globus pallidus internal segment. Together, this leads to overexcitation of the globus pallidus internal segment and thus more inhibition of the thalamus and PPN. Interestingly, recent research suggests that increased inhibitory output from the pons [an area affected early in the course of PD (14)] may further exacerbate these changes by suppressing substantia nigra output (32).
FIGURE 2.
Rate model of basal ganglia dysfunction
Rate model of basal ganglia dysfunction in normal (A) and parkinsonian (B) states. Over activity of the indirect and underactivity of the direct pathways result in more inhibitory output from the basal ganglia output structures (GPi) to the thalamus and the brain stem, and ultimately reduced amplitude of movements, including gait. Green arrows represent excitatory and red arrows inhibitory projections. Arrow thickness represents the relative firing rate of projections, and dashed arrows indicate the relative reduction of the SNpc D1 and D2 dopaminergic projections to the striatum. SNpc, substantia nigra pars reticulate; GPe, globus pallidus external segment; GPi, globas pallidus internal segment; STN, subthalamic nucleus.
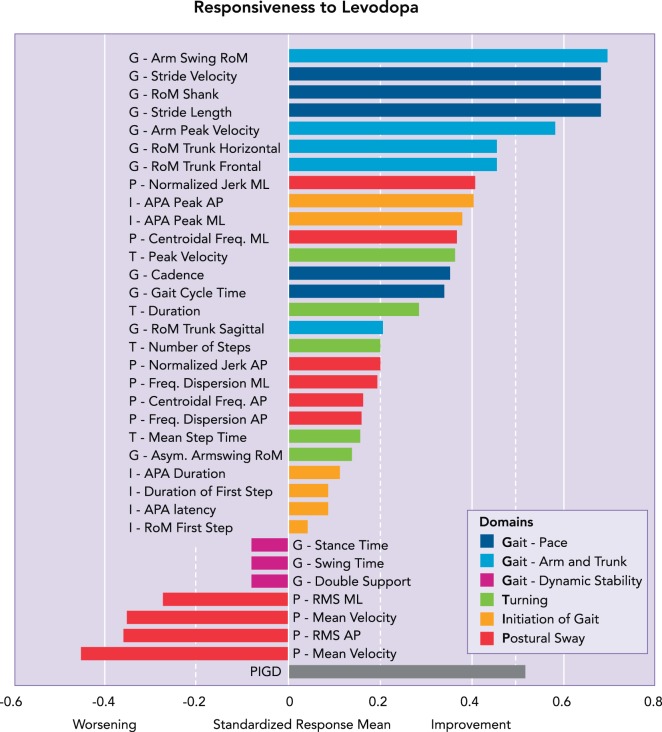
These alterations in basal ganglia output result in over-inhibition of the ventral anterior/ventral lateral thalamus and reduced excitation of cortical motor structures such as the supplementary motor area (SMA) and primary motor cortex (72, 135) (FIGURE 3). Given the importance of these motor and premotor regions for movement scaling and planning (97), the reduced excitation of the SMA could lead to hypokinetic gait, APAs, and automatic postural responses (49, 136, 139). This hypothesis is supported by the fact that gait and APA hypokinesia is improved by levodopa in the early stages of the disease. Indeed, we recently showed that hypokinesia (e.g., reduced step length, gait velocity, and arm swing) was the gait impairment most improved by levodopa. These results are shown in FIGURE 4, which depicts the effects of levodopa on different gait domains (e.g., pace, arm and trunk movement, dynamic stability, etc.) in 104 individuals with idiopathic PD. Interestingly, other measures of gait dysfunction, including gait timing and postural sway, were unaffected or even worsened, respectively, by levodopa (22).
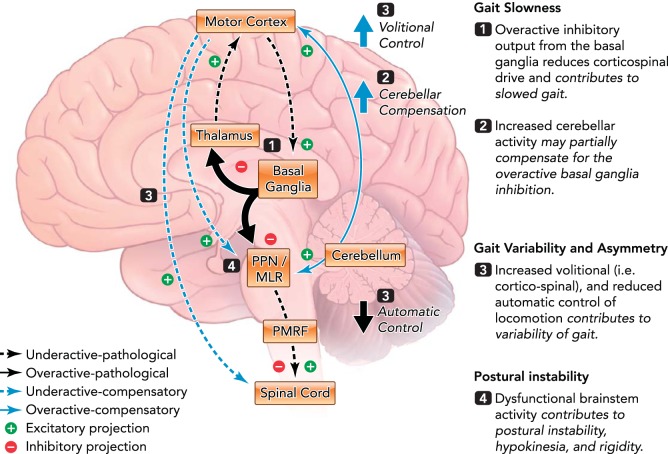
FIGURE 3.
Framework for supraspinal control of locomotion in people with PD
Alterations in activity of the basal ganglia (1) and brain stem (4) contribute to gait slowness and increased postural instability, respectively, and increased cerebellar activity may partially compensate for these alterations (2). Increased volitional control (i.e., cortico-spinal) and reduced automatic control (3) may contribute to increased gait variability and asymmetry. See text box above for more information. PPN, pedunculopontine nucleus; MLR, mesencelphalic locomotor region; PMRF, pontomedulary reticular formation; SMA, supplementary motor area.
FIGURE 4.
The dysfunction in speed, variability and asymmetry, and postural control in PD are differently affected by levodopa
Rightward (positive) values represent a positive impact of levodopa, whereas leftward (negative) values represent a negative impact of levodopa. Gait speed and size of limb movements are improved by levodopa (top). Postural sway can be worsened by levodopa (bottom). Although few variability or asymmetry variables were available in this particular analysis, asymmetry of arm swing and temporal coordination, such as double support time, were not consistently improved by levodopa. A value larger than 0.20 represents small, 0.50 moderate, and 0.80 large responsiveness. *Differences between the control and PD group. Figure reproduced from Ref. 22 with permission from Movement Disorders.
The notion that altered basal ganglia-thalamo-cortical function is related to gait slowness is supported by recent neuroimaging studies (Table 1). For example, Hanakawa and colleagues (51) showed that SMA activity was reduced in people with PD with respect to healthy adults during actual walking. More recent studies have shown that the amount of activation in the SMA during imagined walking was correlated to overground gait function in people with PD (21, 105). In addition, basal ganglia activity (105) and the availability of dopamine in the nigrostriatal system (104) are reduced in people with PD during locomotion with respect to healthy adults.
Dysfunction of locomotor network regions outside of the basal ganglia-thalamo-cortical system, including areas related to cholinergic activity, have also been implicated in gait slowness in people with PD. ACh in the brain comes from three primary sources: cholinergic interneurons in the striatum; the nbM, which is the primary source of ACh to the cortex and basal forebrain; and the PPN, which supplies cholinergic input to the thalamus and spinal cord (for review, see Ref. 154). Recent work suggests that the availability of cortical ACh, supplied by the nbM, may be specifically related to gait speed. For example, among a cohort of 125 people with PD, gait speed was not affected in people with PD with nigrostriatal denervation and normal cholinergic denervation. In contrast, gait speed was significantly reduced in individuals with both nigrostriatal and cholinergic denervation. This suggests that gait speed may be related to either cholinergic dysfunction (alone) or in conjunction with dopaminergic dysfunction (7). Furthermore, Rochester et al. (117) measured cortical cholinergic availability using transcranial magnetic stimulation, showing correlations between gait speed and step length with cortical cholinergic function. These findings suggest that impaired cortical ACh dysfunction (supplied primarily by the nbM) together with dopamine dysfunction may lead to reduced gait speed. Indeed, although the substantia nigra is commonly noted as the source of motor dysfunction in PD, the nbM is among the first structures to exhibit structural pathology in the course of PD (14). In addition to the nbM, the PPN may also play a role in hypokinesia, since PPN lesions in primates cause arm and leg hypokinesia (77, 95). Interestingly, The PPN may also play a role in akinesia, or the lack of movement. Jenkinson and colleagues (69) showed that levodopa in combination with PPN stimulation improved the mean number of movements per hour in a parkinsonian primate to normal levels, again suggesting that both dopaminergic and non-dopaminergic dysfunction are critical for movement production.
In contrast to reduced activity of cortical and brain stem areas, several investigations have shown increased activity in the cerebellum, possibly compensating for altered basal ganglia function. This compensatory hypothesis has been supported by several recent investigations (for review, see Ref. 149). A single proton emission computed tomography study during locomotion showed concomitant increased activity in midline cerebellum and decreased activity of the SMA in people with PD (51). In a follow-up study, Hanakawa and colleagues showed that, by using transverse lines on the floor (visual cues), gait in people with PD was improved and the cerebellum and lateral premotor cortex were especially active. The premotor cortex, which is partially regulated by the cerebellum, is related to externally cued movement (122). In contrast, the SMA, which receives considerable input from the basal ganglia, may preferentially contribute to internally driven movement (97). Therefore, in people with PD, the cerebellum may regulate gait using external cues, compensating for poorer internally generated movement via the basal ganglia and SMA (50). More recently, Festini and colleagues demonstrated that when off anti-Parkinson medication (levodopa), people with PD exhibit increased levels of cerebellar-whole brain and cerebellar-cerebellar connectivity. Furthermore, increased cerebellar activity was, in some cases, correlated to improved motor and cognitive performance in people with PD (35). The cerebellum plays a critical role in the generation (112) and timing (31) of movements, particularly during locomotion (67, 91), and is densely connected with the locomotor brain stem areas (such as PPN) (68), the basal ganglia (12, 13), and the cortex. Furthermore, this region has recently been shown to exhibit relatively high presynaptic cholinergic terminals, underscoring its importance within the cholinergic system (107). Thus, although the mechanism is not fully understood, increased activity of the cerebellum in PD may be an attempt to compensate for reduced activity of other structures, including the SMA. This may, however, result in overdependence on external cues (8, 51, 149) (FIGURE 3).
Hypertonicity/rigidity.
Despite their congruent role in gait slowness, hypokinesia/bradykinesia and rigidity may have somewhat distinct neural underpinnings (70). While hypo- and bradykinesia are related primarily to dysfunction of the cortico-thalamo-basal ganglia loop (see previous section), rigidity may be related to dysfunction of the interaction between the basal ganglia and deep brain structures, including the cholinergic region of the PPN (FIGURE 3). Converging animal and human experiments suggest that the PPN and MLR within the brain stem play critical roles for controlling axial postural tone. Specifically, the MLR, consisting primarily of the cuneiform nucleus, contributes to the excitatory muscle tone and rhythm-generating system (137). In animals, tonic activity of this region can elicit gait-like flexion and extension of hindlimbs. Conversely, the PPN inhibits extensor and flexor alpha motoneurons (138). Both the MLR and PPN receive considerable efferent, inhibitory signals from the globus pallidus internal segment (GPi) (137), and, as noted above, people with PD exhibit excessive inhibitory output from the GPi. Thus the elevated inhibition of the MLR in people with PD likely reduces the ability to initiate and maintain locomotion. In contrast, inhibition of the inhibitory system (i.e., the PPN) may result in hypertonus and rigidity (140). However, axial and limb rigidity are somewhat distinct due to the different pathways from dorsolateral (axial) and ventromedial (limbs) descending spinal systems (80). In fact, although levodopa is very effective in reducing limb rigidity (58), it does not reduce axial rigidity (40, 148). Thus primary dysfunction of the PPN, either prior to or secondary to nigrostriatal dysfunction, may contribute to increased axial tone.
Neural Underpinnings of Variability and Asymmetry in PD Gait
Gait variability and asymmetry in people with PD has been well characterized; however, the neural underpinnings of this impairment are not well understood. One hypothesis suggests that people with PD have difficulty controlling automatic movements and thus shift to more voluntary control of gait (8). This compensatory shift from automatic gait to voluntary stepping may result in more gait variability (19, 153) (FIGURE 3). Indeed, considerable research has shown that conscious control of typically overlearned tasks reduces performance and increases variability (153). Automatic tasks seem to rely more on subcortical structures, including the basal ganglia and brain stem, whereas voluntary tasks rely more on cortical activity and require more attention (19, 49, 140, 151, 152). People with PD exhibit larger than normal cortical activity during upper extremity motor tasks, both when the task is new and after overlearning has occurred (150, 152), suggesting more voluntary control of tasks in this population. Thus even highly overlearned tasks, such as walking, may rely more heavily on cortical structures in people with PD. This shift toward increased voluntary locomotor control may partially compensate for dysfunction of the basal ganglia and brain stem automatic pathways but may also increase variability.
Dual task paradigms provide further support of PD impairments in automatic control of gait leading to increased variability while walking (74). When completing gait with a secondary cognitive task, healthy adults typically demonstrate decrements in both the cognitive task (e.g., counting backward by 3s) and gait. These decrements in performance are noted as “dual task cost.” Because cognitive tasks are primarily supported by frontal structures and require attention, dual task cost suggests that the primary motor task (e.g., walking) also requires a level of attention and voluntary control. Interestingly, people with PD have more pronounced dual task cost during walking than age-matched adults (74). This increased dual task cost in people with PD suggests that gait requires more attentional, voluntary control than in healthy adults, resulting in increased variability (106, 108).
Gait asymmetry may be related directly to asymmetric neural dysfunction within the basal ganglia. Dopaminergic neuron loss is often asymmetric, and the side with greater dopamine loss corresponds to the opposite side with greater motor signs in humans (75, 78, 114, 141) and in animals (39). Similarly, asymmetry of rigidity and bradykinesia have been shown to relate to asymmetric limb movements during gait, asymmetric turning impairments, and asymmetric dopaminergic degeneration (144). Finally, a recent report suggests increased levodopa use may improve asymmetry of gait (41).
Neural Underpinnings of Poor Postural Control in PD Gait
Considerable research is pointing toward an important role of non-dopaminergic structures such as the PPN in postural dysfunction of people with PD (FIGURE 3). For example, trunk sway and variability in the mediolateral direction, common measures of postural control, are excessive in people with PD but do not correlate well with other PD signs, such as the UPDRS (85), and are not consistently improved by levodopa (113, 115). In fact, lateral sway during stance may be increased after taking levodopa; possibly due to reduced rigidity, with no concomitant improvement in postural control (116). Muller and colleagues recently demonstrated that thalamic cholinergic innervation, i.e., the amount of cholinergic neurons projecting from the PPN to the thalamus, was directly correlated to postural sway (94). Importantly, no correlations were observed between postural sway and cortical ACh innervation. In contrast, Rochester and colleagues showed that gait speed, but not step-width variability (a measure of postural control), was related to cortical ACh activity (117). Therefore, ACh in the thalamus, supplied primarily by the PPN, seems to be related to postural control (e.g., postural sway and sway variability) (94), whereas cortical cholinergic function, supplied by the nbM, may be related to gait speed and hypokinesia (7, 36).
Restoring balance after an external perturbation relies on cortical, basal ganglia, and brain stem structures (64). Like voluntary stepping, the hypokinetic steps in response to large, external perturbations may reflect altered basal ganglia-thalamo-cortical circuitry. However, levodopa does not improve compensatory stepping as it improves voluntary stepping, suggesting that brain regions outside of the basal ganglia, such as areas related to ACh, contribute to reactive postural control strategies (23, 76). Indeed, recent animal and human research suggests that automatic postural responses are stored in the brain stem (54, 100, 101, 133). Furthermore, ACh in the thalamus (provided primarily by the PPN) is correlated with falls in people with PD, whereas dopaminergic function is not (9). In addition, medication that improves ACh availability (donepezil and rivostigmine) may reduce falls in people with PD (17). Together, these results suggest that non-dopaminergic regions, including the brain stem, likely play a critical role for postural responses and fall prevention.
Several other converging lines of evidence support the role of PPN and ACh in postural control. Karachi et al. (73) recently showed that PPN cholinergic lesions, both with and without concomitant nigrostriatal dopaminergic losses resulted in postural instability in rhesus monkeys. Furthermore, patients with progressive supranuclear palsy show severe balance impairments and postural instability, specifically with spontaneous backward falls. Progressive supranuclear palsy is an atypical parkinsonian syndrome marked by PPN and thalamic cholinergic dysfunction and cell loss but relatively spared cortical cholinergic innervation (47, 129). Falls, a prominent outcome of postural instability, are correlated to cholinergic innervation from the PPN to the thalamus in people with PD. Bohnen and colleagues (9, 10) showed that fallers had reduced thalamic cholinergic function with respect to nonfallers, with no similar loss in nigrostriatal dopaminergic denervation. Furthermore, a recent imaging study showed that people with PD exhibited altered PPN activity during imagined walking with respect to healthy controls, providing a direct link between PPN activity and gait dysfunction in people with PD (21). Deep brain structures with dense connections to the PPN, including the pontomedulary reticular formation, have also been linked to generation of APAs and coupling of posture and gait (123). For example, recent electrophysiological studies in cats have shown that the pontomedulary reticular formation contributes to coupling of anticipatory postural adjustments and limb movements (123, 124). While the neural underpinnings of poor postural and locomotor coupling in PD is not well understood, people with PD exhibit early pathology in deep brain structures such as the pontomedulary reticular formation (14). Therefore, it is possible that this pathology contributes to the poor coupling of posture and locomotion.
Interestingly, deep brain stimulation in the subthalamic nucleus (STN) (98, 115) or GPi (115) has been shown to improve some measures of postural sway in PD, including mediolateral sway velocity. Given the intractability of postural sway to levodopa, these finding suggests that deep brain stimulation of the STN and GPi may influence non-dopaminergic pathways. Indeed, considerable bidirectional pathways between the STN and deep brain structures, such as the PPN, exist, and impaired signaling from the STN to the PPN has been suggested to be central to some postural control dysfunction in people with PD (128). In fact, a recent report by Weiss and colleagues demonstrated that STN deep brain stimulation may improve walking via direct activation of the PPN (143), further underscoring the importance of both the STN and PPN in postural control in people with PD.
Together, these reports underscore the role of structures both within and outside the basal ganglia, including the PPN, thalamus, and cerebellum, in gait dysfunction in PD. Specifically, the deep brain structures and their dense connections with the cortex, cerebellum, STN, and spinal cord contribute to gait dysfunction observed in people with PD (37, 38).
Conclusions and Future Directions
Gait impairments in people with PD include pronounced slowness, variability, and postural instability. Considerable work is unraveling the pathophysiology underlying these characteristics, demonstrating likely contributions from a number of supraspinal regions. Specifically, slowness of gait seems to be related to dysfunction of the basal ganglia-thalamo-cortical loop in conjunction with reduced cortical ACh, as well as rigidity. Gait variability may be related to a shift from automatic to voluntary control of locomotion in people with PD. Finally, postural instability seems to be related to alterations in ACh in brain stem structures such as the PPN and MLR. In addition to these changes, increased activation in the cerebellum and cortex may partially compensate for impaired activity in other brain regions but may result in overdependence on external cues.
Additional work is necessary to continue to elucidate mechanisms for gait dysfunction in people with PD. Continued investigation of neural activity during actual and imagined locomotion (for review, see Ref. 8) as well as structural and functional neural connectivity will provide further insight into how the brain is differently active in people with PD who have specific parkinsonian impairments such as freezing of gait, dyskinesia, and dystonia. Finally, new studies are focusing on the role of cognitive impairments in gait dysfunction (127). Impairments of gait and cognition may share neural underpinnings in the prefrontal cortex. For example, availability of cortical ACh may underlie attention and executive cognitive dysfunction in people with PD (154), so new trials are examining the effects of cholinesterase inhibitors at improving both cognitive and gait function in people with PD (121). Indeed, each of the gait characteristics discussed above (slowness, variability, and postural control) have been directly linked to cognitive declines, perhaps because of shared prefrontal circuitry (for review, see Ref. 155). Further work is necessary to understand how cognitive function contributes to control of locomotion and gait impairments in PD.
Footnotes
This work was supported by grants from the United States Department of Veteran's Affairs Rehabilitation Research and Development Service (Career Development Award-1: no. I01BX007080; to D.S.P.) and VA Merit Award (E1075-R; to F.B.H.), the National Institutes of Health (R01 AG-006457 29; to F.B.H.), and the Medical Research Foundation of Oregon (Early Investigator Award; to D.S.P.). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
F. B. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU. No other authors declare any conflict of interest.
Author contributions: D.S.P. and F.B.H. prepared figures; D.S.P. and F.B.H. drafted manuscript; D.S.P. and F.B.H. edited and revised manuscript; D.S.P. and F.B.H. approved final version of manuscript.
References
- 1.Adkin AL, Bloem BR, Allum JH. Trunk sway measurements during stance and gait tasks in Parkinson's disease. Gait Posture 22: 240–249, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Baker R. Measuring Walking: A Handbook of Clinical Gait Analysis. Hoboken, NJ: Wiley, 2013. [Google Scholar]
- 4.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease. Eur J Neurosci 24: 1815–1820, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Blin O, Ferrandez AM, Pailhous J, Serratrice G. Dopa-sensitive and dopa-resistant gait parameters in Parkinson's disease. J Neurol Sci 103: 51–54, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Bloem BR, van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson's disease. Adv Neurol 87: 209–223, 2001. [PubMed] [Google Scholar]
- 7.Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, Albin RL, Muller ML. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 81: 1611–1616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnen NI, Jahn K. Imaging: What can it tell us about Parkinsonian gait? Mov Disord 28: 1492–1500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 73: 1670–1676, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J Cereb Blood Flow Metab 32: 1609–1617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonsinsukh R, Saengsirisuwan V, Carlson-Kuhta P, Horak FB. A cane improves postural recovery from an unpracticed slip during walking in people with Parkinson disease. Phys Ther 92: 1117–1129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107: 8452–8456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cognitive Sci 17: 241–254, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord 12: 206–215, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Chong RK, Horak FB, Woollacott MH. Parkinson's disease impairs the ability to change set quickly. J Neurol Sci 175: 57–70, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 75: 1263–1269, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KA, Lobb BM, Nutt JG, McNames J, Horak F. Objective measurement of dyskinesia in Parkinson's disease using a force plate. Mov Disord 25: 602–608, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark DJ. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci 9: 246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins SH, Kuo AD. Two independent contributions to step variability during over-ground human walking. PLos One 8: e73597, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cremers J, D'Ostilio K, Stamatakis J, Delvaux V, Garraux G. Brain activation pattern related to gait disturbances in Parkinson's disease. Mov Disord 27: 1498–1505, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa is a double-edged sword for balance and gait in people With Parkinson's disease. Mov Disord 30: 1361–1370, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kam D, Nonnekes J, Oude Nijhuis LB, Geurts AC, Bloem BR, Weerdesteyn V. Dopaminergic medication does not improve stepping responses following backward and forward balance perturbations in patients with Parkinson's disease. J Neurol 261: 2330–2337, 2014. [DOI] [PubMed] [Google Scholar]
- 24.de Lima-Pardini AC, Papegaaij S, Cohen RG, Teixeira LA, Smith BA, Horak FB. The interaction of postural and voluntary strategies for stability in Parkinson's disease. J Neurophysiol 108: 1244–1252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord 15, Suppl 3: S237–S240, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson's disease. Brain 127: 1755–1773, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci 19: 2871–2880, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Dietz V, Michel J. Locomotion in Parkinson's disease: neuronal coupling of upper and lower limbs. Brain 131: 3421–3431, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Dietz V, Zijlstra W, Prokop T, Berger W. Leg muscle activation during gait in Parkinson's disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol 97: 408–415, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci 16: 1609–1619, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Emir UE, Tuite PJ, Oz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLos One 7: e30918, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord 23: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Fasano A, Herman T, Tessitore A, Strafella AP, Bohnen NI. Neuroimaging of freezing of gait. J Parkinsons Dis 5: 241–254, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Festini SB, Bernard JA, Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered cerebellar connectivity in Parkinson's patients ON and OFF l-DOPA medication. Front Hum Neurosci 9: 214, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitts P. Perceptual-motor skills learning. In: Categories of Human Learning, edited by Melton A. Waltham, MA: Academic, 1964, p. 243–285. [Google Scholar]
- 37.Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLos One 9: e100291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136: 2405–2418, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fornaguera J, Carey RJ, Huston JP, Schwarting RK. Behavioral asymmetries and recovery in rats with different degrees of unilateral striatal dopamine depletion. Brain Res 664: 178–188, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol 219: 430–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galna B, Lord S, Burn DJ, Rochester L. Progression of gait dysfunction in incident Parkinson's disease: impact of medication and phenotype. Mov Disord 30: 359–367, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Galna B, Murphy AT, Morris ME. Obstacle crossing in Parkinson's disease: mediolateral sway of the centre of mass during level-ground walking and obstacle crossing. Gait Posture 38: 790–794, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol 119: 1459–1474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geurts AC, Boonstra TA, Voermans NC, Diender MG, Weerdesteyn V, Bloem BR. Assessment of postural asymmetry in mild to moderate Parkinson's disease. Gait Posture 33: 143–145, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Giladi N, Horak FB, Hausdorff JM. Classification of gait disturbances: distinguishing between continuous and episodic changes. Mov Disord 28: 1469–1473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord 23, Suppl 2: S423–S425, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 74: 1416–1423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol 96: 2678–2687, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Hanakawa T. Neuroimaging of standing and walking: special emphasis on Parkinsonian gait. Parkinsonism Relat Disord 12: S70–S75, 2006. [Google Scholar]
- 50.Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Ann Neurol 45: 329–336, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain 122: 1271–1282, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Hausdorff JM. Gait dynamics in Parkinson's disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 19: 026113, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehab 2: 19, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong M, Perlmutter JS, Earhart GM. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil Neural Repair 23: 166–176, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong M, Perlmutter JS, Earhart GM. Podokinetic after-rotation in Parkinson disease. Brain Res 1128: 99–106, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Exp Neurol 193: 504–521, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol 75: 2380–2396, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson's disease using body-worn sensors. Mov Disord 28: 1544–1551, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci 111: 46–58, 1992. [DOI] [PubMed] [Google Scholar]
- 61.Huxham F, Baker R, Morris ME, Iansek R. Head and trunk rotation during walking turns in Parkinson's disease. Mov Disord 23: 1391–1397, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs JV, Dimitrova DM, Nutt JG, Horak FB. Can stooped posture explain multidirectional postural instability in patients with Parkinson's disease? Exp Brain Res 166: 78–88, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson's disease. Neuroscience 141: 999–1009, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm 114: 1339–1348, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol 215: 334–341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jahn K, Deutschlander A, Stephan T, Kalla R, Hufner K, Wagner J, Strupp M, Brandt T. Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res 171: 353–362, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 39: 786–792, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord 24: 319–328, 2009. [DOI] [PubMed] [Google Scholar]
- 69.Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport 17: 639–641, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Johnson MD, Zhang J, Ghosh D, McIntyre CC, Vitek JL. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J Neurophysiol 108: 567–577, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Judge JO, Davis RB 3rd, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci 51: M303–M312, 1996. [DOI] [PubMed] [Google Scholar]
- 72.Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections. J Neurophysiol 88: 1420–1432, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehericy S, Hirsch EC, Francois C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 120: 2745–2754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis 2012: 918719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry 52: 72–76, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.King LA, St George RJ, Carlson-Kuhta P, Nutt JG, Horak FB. Preparation for compensatory forward stepping in Parkinson's disease. Arch Phys Med Rehabil 91: 1332–1338, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kojima J, Yamaji Y, Matsumura M, Nambu A, Inase M, Tokuno H, Takada M, Imai H. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett 226: 111–114, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Kumar A, Mann S, Sossi V, Ruth TJ, Stoessl AJ, Schulzer M, Lee CS. [11C]DTBZ-PET correlates of levodopa responses in asymmetric Parkinson's disease. Brain 126: 2648–2655, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther 90: 157–174, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuypers HG. The descending pathways to the spinal cord, their anatomy and function. Prog Brain Res 11: 178–202, 1964. [DOI] [PubMed] [Google Scholar]
- 81.Kwon KY, Kim M, Lee SM, Kang SH, Lee HM, Koh SB. Is reduced arm and leg swing in Parkinson's disease associated with rigidity or bradykinesia? J Neurol Sci 341: 32–35, 2014. [DOI] [PubMed] [Google Scholar]
- 82.Lewek MD, Poole R, Johnson J, Halawa O, Huang X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson's disease. Gait Posture 31: 256–260, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech 26: 633–644, 1993. [DOI] [PubMed] [Google Scholar]
- 84.Mak MK, Wong EC, Hui-Chan CW. Quantitative measurement of trunk rigidity in parkinsonian patients. J Neurol 254: 202–209, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study. Gait Posture 36: 471–476, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mancini M, Zampieri C, Carlson-Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur J Neurol 16: 1028–1034, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci 27: 7105–7116, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mille ML, Creath RA, Prettyman MG, Johnson Hilliard M, Martinez KM, Mackinnon CD, Rogers MW. Posture and locomotion coupling: a target for rehabilitation interventions in persons with Parkinson's disease. Parkinsons Dis 2012: 754186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mille ML, Simoneau M, Rogers MW. Postural dependence of human locomotion during gait initiation. J Neurophysiol 112: 3095–3103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammadi F, Bruijn SM, Vervoort G, van Wegen EE, Kwakkel G, Verschueren S, Nieuwboer A. Motor switching and motor adaptation deficits contribute to freezing of gait in Parkinson's disease. Neurorehabil Neural Repair 29: 132–142, 2015. [DOI] [PubMed] [Google Scholar]
- 91.Mori S, Nakajima K, Mori F, Matsuyama K. Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res 143: 341–351, 2004. [DOI] [PubMed] [Google Scholar]
- 92.Morris M, Iansek R, McGinley J, Matyas T, Huxham F. Three-dimensional gait biomechanics in Parkinson's disease: evidence for a centrally mediated amplitude regulation disorder. Mov Disord 20: 40–50, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 117: 1169–1181, 1994. [DOI] [PubMed] [Google Scholar]
- 94.Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson's disease. Brain 136: 3282–3289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munro-Davies LE, Winter J, Aziz TZ, Stein JF. The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Exp Brain Res 129: 511–517, 1999. [DOI] [PubMed] [Google Scholar]
- 96.Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ, Group CS. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology 70: 2241–2247, 2008. [DOI] [PubMed] [Google Scholar]
- 97.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Nantel J, McDonald JC, Bronte-Stewart H. Effect of medication and STN-DBS on postural control in subjects with Parkinson's disease. Parkinsonism Relat Disord 18: 285–289, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Nieuwboer A, De Weerdt W, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil 20: 142–150, 1998. [DOI] [PubMed] [Google Scholar]
- 100.Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, Bloem BR, Weerdesteyn V, Geurts AC. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci 34: 275–281, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC, Bloem BR, Weerdesteyn V. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience 245: 109–120, 2013. [DOI] [PubMed] [Google Scholar]
- 102.Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol 14: 768–778, 2015. [DOI] [PubMed] [Google Scholar]
- 103.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10: 734–744, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, Torizuka T, Sakamoto M. Presynaptic and postsynaptic dopaminergic binding densities in the nigrostriatal and mesocortical systems in early Parkinson's disease: a double-tracer positron emission tomography study. Ann Neurol 46: 723–731, 1999. [DOI] [PubMed] [Google Scholar]
- 105.Peterson DS, Pickett KA, Duncan RP, Perlmutter JS, Earhart GM. Brain activity during complex imagined gait tasks in Parkinson disease. Clin Neurophysiol 125: 995–1005, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peterson DS, Plotnik M, Hausdorff JM, Earhart GM. Evidence for a relationship between bilateral coordination during complex gait tasks and freezing of gait in Parkinson's disease. Parkinsonism Relat Disord 18: 1022–1026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, Muller ML, Albin RL, Koeppe RA. In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 55: 396–404, 2014. [DOI] [PubMed] [Google Scholar]
- 108.Plotnik M, Dagan Y, Gurevich T, Giladi N, Hausdorff JM. Effects of cognitive function on gait and dual tasking abilities in patients with Parkinson's disease suffering from motor response fluctuations. Exp Brain Res 208: 169–179, 2011. [DOI] [PubMed] [Google Scholar]
- 109.Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson's disease. Exp Brain Res 181: 561–570, 2007. [DOI] [PubMed] [Google Scholar]
- 110.Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson's disease. Mov Disord 23, Suppl 2: S444–S450, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil 14: 402–406, 2000. [DOI] [PubMed] [Google Scholar]
- 112.Purzner J, Paradiso GO, Cunic D, Saint-Cyr JA, Hoque T, Lozano AM, Lang AE, Moro E, Hodaie M, Mazzella F, Chen R. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. J Neurosci 27: 6029–6036, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Revilla FJ, Larsh TR, Mani A, Duker AP, Cox C, Succop P, Gartner M, Jarrin Tejada C, Bhattacharya A. Effect of dopaminergic medication on postural sway in advanced Parkinson's disease. Front Neurol 4: 202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riederer P, Sian-Hulsmann J. The significance of neuronal lateralisation in Parkinson's disease. J Neural Transm 119: 953–962, 2012. [DOI] [PubMed] [Google Scholar]
- 115.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry 73: 267–274, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson's disease: influence of initial stance conditions. Neurosci Lett 406: 128–132, 2006. [DOI] [PubMed] [Google Scholar]
- 117.Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ. Cholinergic dysfunction contributes to gait disturbance in early Parkinson's disease. Brain 135: 2779–2788, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roemmich RT, Field AM, Elrod JM, Stegemoller EL, Okun MS, Hass CJ. Interlimb coordination is impaired during walking in persons with Parkinson's disease. Clin Biomech (Bristol, Avon) 28: 93–97, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roemmich RT, Nocera JR, Stegemoller EL, Hassan A, Okun MS, Hass CJ. Locomotor adaptation and locomotor adaptive learning in Parkinson's disease and normal aging. Clin Neurophysiol 125: 313–319, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rogers MW, Mille ML. Lateral stability and falls in older people. Exerc Sport Sci Rev 31: 182–187, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database Syst Rev 3: CD006504, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain 120: 963–976, 1997. [DOI] [PubMed] [Google Scholar]
- 123.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol 90: 3066–3086, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Schieppati M, Nardone A. Free and supported stance in Parkinson's disease. The effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain 114: 1227–1244, 1991. [DOI] [PubMed] [Google Scholar]
- 126.Schoneburg B, Mancini M, Horak F, Nutt JG. Framework for understanding balance dysfunction in Parkinson's disease. Mov Disord 28: 1474–1482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurotherapeut 11: 1057–1075, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shine JM, Moustafa AA, Matar E, Frank MJ, Lewis SJ. The role of frontostriatal impairment in freezing of gait in Parkinson's disease. Front Syst Neurosci 7: 61, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, Asahina M, Hattori T, Tanada S, Irie T. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy. Ann Neurol 46: 62–69, 1999. [PubMed] [Google Scholar]
- 130.Skinner JW, Lee HK, Roemmich RT, Amano S, Hass CJ. Execution of activities of daily living in persons with Parkinson disease. Med Sci Sports Exerc 47: 1906–1912, 2015. [DOI] [PubMed] [Google Scholar]
- 131.Smulders K, Esselink RA, De Swart BJ, Geurts AC, Bloem BR, Weerdesteyn V. Postural inflexibility in PD: does it affect compensatory stepping? Gait Posture 39: 700–706, 2014. [DOI] [PubMed] [Google Scholar]
- 132.Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain 134: 59–72, 2011. [DOI] [PubMed] [Google Scholar]
- 133.Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol 101: 1334–1350, 2009. [DOI] [PubMed] [Google Scholar]
- 134.Stegemoller EL, Buckley TA, Pitsikoulis C, Barthelemy E, Roemmich R, Hass CJ. Postural instability and gait impairment during obstacle crossing in Parkinson's disease. Arch Phys Med Rehabil 93: 703–709, 2012. [DOI] [PubMed] [Google Scholar]
- 135.Takada M, Hoshi E, Saga Y, Inoue Ki Miyachi S, Hatanaka N, Inase M, Nambu A. Organization of two cortico-basal ganglia loop circuits that arise from distinct sectors of the monkey dorsal premotor cortex (Online). In: Basal Ganglia: An Integrative View, edited by Barrios FA, Bauer C. Rijeka, Croatia: InTech, 2013. http://www.intechopen.com/books/basal-ganglia-an-integrative-view/organization-of-two-cortico-basal-ganglia-loop-circuits-that-arise-from-distinct-sectors-of-the-monk. [Google Scholar]
- 136.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev 57: 192–198, 2008. [DOI] [PubMed] [Google Scholar]
- 137.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord 28: 1483–1491, 2013. [DOI] [PubMed] [Google Scholar]
- 138.Takakusaki K, Kohyama J, Matsuyama K. Medullary reticulospinal tract mediating a generalized motor inhibition in cats: III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience 121: 731–746, 2003. [DOI] [PubMed] [Google Scholar]
- 139.Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog Brain Res 143: 231–237, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res 50: 137–151, 2004. [DOI] [PubMed] [Google Scholar]
- 141.van der Hoorn A, Bartels AL, Leenders KL, de Jong BM. Handedness and dominant side of symptoms in Parkinson's disease. Parkinsonism Relat Disord 17: 58–60, 2011. [DOI] [PubMed] [Google Scholar]
- 142.Wai YY, Wang JJ, Weng YH, Lin WY, Ma HK, Ng SH, Wan YL, Wang CH. Cortical involvement in a gait-related imagery task: comparison between Parkinson's disease and normal aging. Parkinsonism Relat Disord 18: 537–542, 2012. [DOI] [PubMed] [Google Scholar]
- 143.Weiss PH, Herzog J, Potter-Nerger M, Falk D, Herzog H, Deuschl G, Volkmann J, Fink GR. Subthalamic nucleus stimulation improves parkinsonian gait via brainstem locomotor centers. Mov Disord 30: 1121–1125, 2015. [DOI] [PubMed] [Google Scholar]
- 144.Winogrodzka A, Wagenaar RC, Booij J, Wolters EC. Rigidity and bradykinesia reduce interlimb coordination in Parkinsonian gait. Arch Phys Med Rehabil 86: 183–189, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Winter D. Biomechanics and Motor Control of Human Movement. Hoboken, NJ: John Wiley & Son's, 2009. [Google Scholar]
- 146.Winter D. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1996. [Google Scholar]
- 147.Winter DA, MacKinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res 97: 359–367, 1993. [DOI] [PubMed] [Google Scholar]
- 148.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol 208: 38–46, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain 136: 696–709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain 128: 2250–2259, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis 82: 226–234, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91: 1690–1698, 2004. [DOI] [PubMed] [Google Scholar]
- 153.Wulf G. Attention and Motor Skill Learning. Champaign, IL: Human Kinetics, 2007, p. 18–28. [Google Scholar]
- 154.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord 26: 2496–2503, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 23: 329–342; quiz 472, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry 81: 171–176, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]