Abstract
A network of brain regions involving the ventral inferior frontal gyrus/anterior insula (vIFG/AI), presupplementary motor area (pre‐SMA) and basal ganglia has been implicated in stopping impulsive, unwanted responses. However, whether this network plays an equal role in response inhibition under different sensorimotor contexts has not been tested systematically. Here, we conducted an fMRI experiment using the stop signal task, a sensorimotor task requiring occasional withholding of the planned response upon the presentation of a stop signal. We manipulated both the sensory modality of the stop signal (visual versus auditory) and the motor response modality (hand versus eye). Results showed that the vIFG/AI and the preSMA along with the right middle frontal gyrus were commonly activated in response inhibition across the various sensorimotor conditions. Our findings provide direct evidence for a common role of these frontal areas, but not striatal areas in response inhibition independent of the sensorimotor contexts. Nevertheless, these three frontal regions exhibited different activation patterns during successful and unsuccessful stopping. Together with the existing evidence, we suggest that the vIFG/AI is involved in the early stages of stopping such as triggering the stop process while the preSMA may play a role in regulating other cortical and subcortical regions involved in stopping. Hum Brain Mapp 35:2119–2136, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: prefrontal cortex, human, cognitive control, stop‐signal task, fMRI
INTRODUCTION
Cognitive control refers to the ability for an individual to change quickly his/her response under certain behavioral contexts, including inhibition of inappropriate, impulsive, or habitual responses. Many daily life situations call for such need of response inhibition; for examples, stop stepping into the street when a car is quickly approaching, prevent speaking improper words in public, withhold looking towards welding spark, etc. A deficit in response inhibition can cause inconvenience and impact on quality of life, which is commonly associated with a variety of neurological and psychiatric disorders including Obsessive and Compulsive disorder, Attention‐Deficit Hyperactivity disorder and substance abuse [Chamberlain and Sahakian, 2007; Groman, et al., 2009].
The stop‐signal task and the go/no‐go task are typical behavioral paradigms used for studying response inhibition in the laboratory setting. These tasks require motor responses to a more frequently presented go signal and occasional suppression of the prepotent response when a less frequent stop/no‐go signal appears [Logan and Cowan, 1984; Verbruggen and Logan, 2008]. Neuroimaging studies using these paradigms have repeatedly shown that a cortico‐subcortical network including frontal, parietal, and temporal cortices and basal ganglia is involved in response inhibition, with the frontoinsular cortex being emphasized to play a more critical role [Aron and Poldrack, 2006; Boecker et al., 2011; Boehler et al., 2010; Cai and Leung, 2011, 2011; Chikazoe et al., 2009a; Curtis et al., 2005; Garavan et al., 1999; Hampshire et al., 2010; Konishi et al., 1999; Li et al., 2006; Sharp et al., 2010; Tabu et al., 2012]. Most prior studies, however, investigated response inhibition of a single sensorimotor association, particularly visually guided stopping of hand movements. Few investigated neural substrates of response inhibition using other sensory signal (auditory and tactile) or other effectors (eye, mouth, and foot). Thus, while a set of common brain regions have been implicated for response inhibition across behavioral contexts, it is unclear whether findings from studying one type of sensorimotor association can be generalized to other types. It is also unclear whether these regions are involved in countermanding per se or other more general cognitive processes such as target and response monitoring.
Some investigators examined the neural substrates of response inhibition by varying the effector. Leung and Cai [2007] found activations in the bilateral ventral inferior frontal gyrus/anterior insula (vIFG/AI) during the inhibition of both hand and eye movements cued by a visual stop signal. That study had several limitations. First, because the same visual stop signal was used for signaling the inhibition of hand and eye movements, it is unclear whether the common activation across motor modalities should be attributed to the stopping process, the process of detecting the same visual stop signal, or both. The same potential confound goes for two other studies, in which the vIFG/AI was shown to be commonly activated during inhibition of hand and foot movements [Tabu et al., 2012] and during inhibition of hand and vocal responses [Xue et al., 2008]. Second, error trials were not identified in the Leung and Cai study as eye movements were not recorded in the magnet. Third, the nature of response execution was not well matched across the motor modalities: the saccadic eye movements were typically triggered, by go signals presented in the peripheral visual fields, in a more reflexive way whereas the hand movements (button‐presses) were typically generated in a more volitional way. To resolve these issues in this study, we presented the stop signals in two different sensory domains (visual versus auditory), used an eye tracker to record eye movements during scanning, and placed the go signals at the fovea.
We designed a stop‐signal task with 2 × 2 sensorimotor associations. By pairing a hand/eye effector with a visual/auditory stop signal, we made four sensorimotor associations, including Hand‐Visual (HV), Hand‐Auditory (HA), Eye‐Visual (EV), and Eye‐Auditory conditions (EA; Fig. 1a). The different sensorimotor conditions were organized in blocks with randomized go and stop trials. Based on our previous work [Cai and Leung, 2011; Leung and Cai, 2007], we predicted that the bilateral vIFG/AI, along with the right middle frontal gyrus (MFG) and the presupplementary motor area (preSMA), would be activated during response inhibition across four different sensorimotor associations. We also predicted that the bilateral vIFG/AI would be involved in an early stage of stopping and would be activated during both successful and unsuccessful stopping as shown in an earlier report [Cai and Leung, 2011]. We also expected that the modality‐specific sensorimotor areas such as the visual/auditory sensory areas, frontal eye fields and hand motor area would show differential responses comparing the two types of stop signal and the two effectors.
Figure 1.
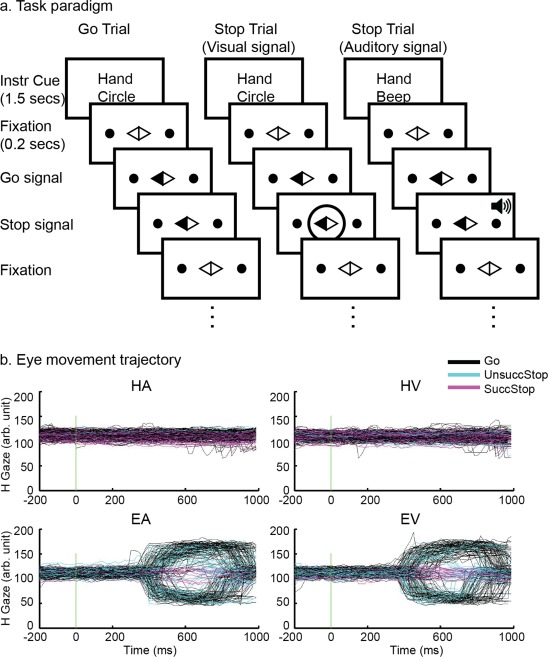
(a) Stop‐signal task paradigm illustration: Each experiment run consists of multiple miniblocks. Each block began with a cue (e.g., “Hand Circle,” “Hand Beep,” “Eye Circle,” and “Eye Beep”), indicating the task condition (e.g., “HV,” “HA,” “EV,” and “EA”). The cue was presented for 1.5 s with a warning beep at the end, followed by a fixation (a diamond at the center with two black dots on the periphery) and a sequence of Go and Stop trials. Every 2.7–5.3 s, the left or right part of the diamond turned to black (a Go signal), indicating a left or right response. This is a Go trial. Occasionally, a Stop signal was presented shortly after the Go signal, requiring one to cancel the prepared response. If the cue was “Circle,” the Stop signal was a circle presented around the diamond. If the cue was “Beep,” the Stop signal was a beep. (b) Eye movement trajectory illustration of one subject. Each color line represents the horizontal movement trajectory of the right eye of the subject in a single trial. The color of the line codes trial type: Go: black; UnsuccStop: cyan; SuccStop: magenta. The vertical line at time point 0 on x‐axis indicates the onset of Go signal. The y‐axis indicates horizontal gaze coordinate (arbitrary unit). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
METHODS
Participants
Twenty‐three healthy young adults (age range: 19–37 yrs, 12 females) were recruited from the Vanderbilt university campus and local community in Nashville. None reported a history of neurological/psychiatric disorders or a history of drug abuse. All participants had normal or corrected to normal vision. All participants provided written consent, which is approved by the local Institutional Review Board. Four participants were excluded in the group analysis because of large head movement during scanning, which left 19 subjects in the final analysis.
Stop‐Signal Task
The stop‐signal task was a two (sensory modalities: visual versus auditory) × 2 (motor modalities: hand versus eye) design, forming four different conditions: HV, HA, EV, and EA (Fig. 1a). On each trial, a white diamond shape fixation was displayed at the center of the screen and two white dots (targets) were displayed (one on the left and one on the right side, 4.4° of eccentricity). Subjects were asked to look at the diamond and put their right index fingers between the left and right buttons during the time when they did not need to make any responses. After 200 ms, either the left or right part of the central diamond turned black (i.e., Go signal). Subjects were required to press either the left or the right button using their right index finger in the hand conditions (HV and HA) or make a saccadic eye movement to the left or the right dot in the eye conditions (EV and EA). Participants were told to make no eye movement in the hand conditions and no hand response in the eye conditions. We used foveal Go signals rather than peripheral Go signals (e.g., a dot appears on the left/right) to assure similar voluntary control demand in both hand and eye conditions. This is important because people tend to make reflexive saccades to periphery visual stimuli but no equivalent mechanism can be established for hand movements. Occasionally (30% of all trials), a circle at the center (i.e., visual Stop signal) or a beep (900 Hz; i.e., auditory Stop signal) was presented shortly after a Go signal. The Stop signal lasted for 300 ms. Subjects were told to make no response when either stop signal was presented. The interval between the Go signal and Stop signal is the Stop‐signal delay (SSD). To balance the difficulty in stopping hand versus eye movements, four 4 SSDs were chosen separately for each effector with the goal of obtaining stop accuracies that range from 0 to 100% at similar intervals. The exact SSD values were determined based on previous studies and our preliminary tests [Band et al., 2003; Boucher et al., 2007; Leung and Cai, 2007]. The SSDs for the hand conditions were 10, 110, 210, and 310 ms and the SSDs for the eye conditions were 10, 90, 180, and 270 ms. The SSDs were randomly assigned, at equal chance, to the stop trials of each condition. The sequences of go and stop trials were randomly generated using the “optseq” algorithm, which was designed to increase the sensitivity of detecting BOLD signal change among task conditions [Dale, 1999] (http://surfer.nmr.mgh.harvard.edu/optseq).
Each experiment run had eight task blocks, two for each condition. The order of task blocks was counterbalanced. At the beginning of each block, an instruction cue (“hand”/“eye” and “circle”/“beep”) was presented with a warning beep (500 Hz) for 2 s, followed by 15 continuous trials. The ITI was 1.7, 2.3, or 4.3 seconds. The resting interval between two adjacent blocks was 16, 18, or 20 s.
Localizer Tasks
Two localizer tasks were used to identify the brain areas involved in executing hand/eye movements and perceiving visual/auditory signals. Both localizer runs consisted of eight task blocks. In the hand and eye localizer tasks, the block instruction was “hand” or “eye,” respectively. The paradigm was similar to the stop‐signal task except without the stop signal. Subjects made button‐presses using their right index finger in the hand task or make saccades to the left/right dot in the eye task. In the visual and auditory localizer tasks, the block instruction was “circle” or “beep,” respectively. Subjects made button‐presses whenever they saw a circle in the center in the “circle” blocks or whenever they heard a beep in the “beep” blocks.
Procedure and Apparatus
Each participant was well practiced before the scanning session. Subjects first practiced one run of each localizer task (5 min each). Afterward, they were trained to perform three runs of the stop signal task (7 min each). Speedy and accurate response was emphasized during the training and throughout the fMRI experiment. Subjects were required to achieve 90% accuracy for go trials and about 50% accuracy for stop trials in the hand task. Eye movement data was not acquired during the training session.
During the scanning session, each subject performed eight runs of stop‐signal task and one run of each localizer task. Subjects did the localizer tasks either at the beginning or the end of the experiment. The running order of tasks was counterbalanced across subjects.
Visual stimuli were rear‐projected onto a screen positioned at the back of the head coil. Subjects viewed the visual stimuli through a mirror mounted on the head coil. Stimuli presentation was controlled and response data were collected with E‐prime (version 2.0.1.109, Psychology Software Tools, Pittsburgh, PA) running on a computer with a Windows XP operation system. A response box interfaced with the computer through the parallel port was used for collecting manual responses.
Oculomotor Recording and Analysis
A long‐range optic eye tracker (Applied Sciences Laboratories, Bedford, MA) was used to record eye position in the scanner at a sampling rate of 60 Hz. The camera was at the back of the magnet bore opening and the illumination beam was targeted adjacent to the right side of the projection screen. Only the right eye was monitored. Nine‐point calibration and drift correction was applied at the very beginning of scanning and before each run if necessary. Eye‐position recording started at the beginning of each run. Figure 1b shows eye movement trajectory from one subject on different trial types (i.e., go, unsuccessful stop and successful stop) across the four task conditions (i.e., HA, HV, EA, and EV).
Eye data was exported using EYENAL (Applied Sciences Laboratories, Bedford, MA). Saccades were detected by a two‐step procedure using in‐house software. Fixations were identified using the criteria that at least six continuous data points are within a 0.5° radius circle of the center of these data points. The onset and offset of a fixation were the time tag of the first and last point of the fixation. A saccade was defined as a shift between two continuous fixations. The onset of a saccade was the offset of the last fixation and the offset of a saccade was the onset of the next fixation.
SSRT Estimation
According to the Race Model [Logan and Cowan, 1984], the estimation of stop signal reaction time (SSRTs) was based on the inhibition function (the probability of responding on stop trials as a function of SSDs) and distribution of the reaction time (RT) on go trials. SSRT was calculated using the integration method: SSRT = T‐SSD, where T is the time point when the integration of go RT distribution equals to the proportion of unsuccessful stop trials. To minimize estimation bias introduced by extreme SSDs [Band et al., 2003], only estimated values from the SSDs that produced stop accuracies of 25–75% were averaged as the SSRT.
Image Acquisition
All scans were carried out on a Philips 3 T Achieva system with an eight‐channel SENSE head coil (Cleveland, OH). Head movement was minimized using foam padding and a tape across the forehead. We first collected a series of high‐resolution structural 3D images (T1‐weighted, 3D turbo field echo, 176 sagittal slices, slice thickness = 1 mm, TR/TE = 9.9/4.6 ms, matrix = 256 × 256, FOV = 25 × 25 cm). Ten series of functional images were acquired parallel to the anterior‐posterior commissural (AC‐PC) line using a standard T2*‐sensitive gradient‐recalled single shot echo planar pulse (EPI) sequence (33 axial slices, 5 mm thick, interleaved, TR/TE = 2000/30 ms, Matrix = 80 × 80, FOV = 24 × 24 cm, and Flip angle = 79°).
Image Data Preprocessing
First, for quality control, we screened EPI runs with significant image ghosting and motion artifacts. Second, the first four EPI images in each run were discarded to allow T2* signal to reach equilibrium. Third, the remaining EPI images were corrected for differences in slice acquisition time and head motion. Runs of translational motion ≥3 mm or rotational motion is ≥1.5° were excluded. Fourth, a mean image volume was generated from the realigned images and the mean image was normalized to the Montreal Neurological Institute (MNI) EPI template, using a 12‐parameter affine registration followed by a series of nonlinear transformations [Friston, 1995]. The normalization parameters were then applied to all realigned EPI images. Finally, all EPI images were spatially smoothed with a Gaussian kernel of 8 mm at full‐width at half maximum and were high‐pass filtered with a cutoff of 1/128 Hz. Above processing were carried out by MRIcro (http://www.mccauslandcenter.sc.edu/mricro) and Statistical Parametric Mapping version 2 (SPM2, Welcome Department of Imaging Neuroscience, University College London, http://www.fil.ion.ucl.ac.uk/spm/).
Image Data Modeling
Blood oxygenation level‐dependent (BOLD) responses to specific events of each task condition were estimated using the general linear model (GLM) [Friston, 1995]. For the stop‐signal tasks (i.e., HA, HV, EA, and EV), the following events were modeled: cue, correct Go trials (Go), successful Stop trials (SuccStop), unsuccessful Stop trials (UnsuccStop), and trials of no interest for each condition. Go was defined as go trials on which the correct response was made within 1 s after the go signal. SuccStop was defined as stop trials on which no response was detected within 1 s after the go signal. UnsuccStop was defined as stop trials on which a motor response was made within 1 s after the go signal. Trials of no interest refer to incorrect Go trials, hand trials on which subjects made saccades, or eye trials on which the saccadic eye movement cannot be identified because of blink or system noise. All vectors were convolved with a canonical hemodynamic response function and then entered as regressors in the GLM. To eliminate artifacts caused by task‐related motion, six motion parameters were entered as covariates. This procedure was demonstrated to increase the signal‐to‐noise ratio and improve task effects estimated using the GLM [Johnstone et al., 2006]. For the localizer tasks, blocks were modeled as the following vectors: hand, eye, auditory, and visual. Each block vector was constructed using the onset and duration of the block.
Voxel‐Wise Image Data Analysis
Estimated parameters (beta values) were calculated and assigned to each voxel for each event (or each block) for each task condition for each participant using the GLM (the first‐level analysis). T tests were applied at the group level for contrasts of interests (the second‐level analysis). A threshold of P < 0.05 (FDR corrected) was used to generate the contrast maps.
Furthermore, we applied conjunction analysis to identify cortical regions commonly activated across task conditions. To compare with our previous work [Leung and Cai, 2007], SuccStop and UnsuccStop were grouped as Stop. We first generated the contrast of Stop vs. Go for each task condition (P < 0.001, uncorrected) and then applied conjunction analysis to identify voxels that were suprathreshold across all conditions [Friston, et al., 1995; Nichols et al., 2005]. We also conducted conjunction analysis using SuccStop‐Go contrasts to examine common activation involved in successful stopping across all conditions. Because only half of the Stop trials were SuccStop trials, we applied a lower threshold for each contrast (P < 0.005 uncorrected) in the SuccStop‐Go conjunction to make a fair comparison with the Stop‐Go conjunction. Thus, the essential threshold was P < 0.001^4 for the Stop‐Go conjunction and P < 0.005^4 for the SuccStop‐Go conjunction.
Region of Interest Analysis
In our previous study [Leung and Cai, 2007], several prefrontal regions were activated in stopping hand and eye movements, including the bilateral vIFG/AI (left: x = −42, y = 12, and z = −6; right: x = 42, y = 18, and z = −6), the right MFG (x = 36, y = 48, and z = 21) and the preSMA (x = 9, y = 18, and z = 54). We generated four ROIs using the coordinates of these prefrontal regions to examine whether they were commonly involved in stopping across four sensorimotor associations in this study.
We also examined regions that are known to be specialized in sensory and motor processing. By contrasting the auditory versus visual blocks in the localizer task, we identified cortical regions involved in detecting the stop signal. As expected, we found activation in the bilateral superior temporal gyrus (STG) for processing the auditory signal and the bilateral middle occipital gyrus (MOG) for processing the visual signal (P < 0.05, FDR corrected, Fig. 2a). The motor localizer task showed that the left M1, premotor and SMA were activated in generating hand responses whereas the bilateral frontal eye field (FEF), SEF, inferior parietal lobule (IPL), and superior parietal lobule were activated in making saccadic eye movements (P < 0.05, FDR corrected, and Fig. 2b). Some of these regions such as the SMA, left premotor cortex, and IPL showed greater activation during both hand and eye localizer tasks (P < 0.05, FDR corrected, Fig. 2b). Sensorimotor ROIs include the bilateral STG (left: x = −60, y = −30, z = 9; right: x = 60, y = −21, and z = 12), MOG (left: x = −33, y = −87, z = −6; right: x = 36, y = −81, z = −3), left primary motor cortex (M1) (x = −36, y = −12, and z = 57), supplementary motor area (SMA) (x = −9, y = −3, and z = 57), and bilateral FEF (left: x = −27, y = −3, and z = 48; right: x = 36, y = 0, and z = 48).
Figure 2.
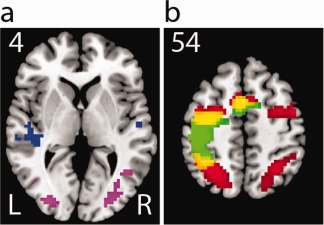
Sensorimotor activation in the localizer tasks. (a) The group contrast map of auditory and visual blocks, blue: auditory > visual; purple: visual > auditory; (b) The group map of hand and eye blocks, red: eye; green: hand, yellow: overlap; all P < 0.05 FDR corrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
All ROIs were 6‐mm radius spheres. The beta weight of each event (e.g., Go, SuccStop and UnsuccStop) of each task condition (e.g., HA, HV, EA, and EV) was derived for each ROI using Marsbar (http://marsbar.sourceforge.net/). Three‐way ANOVA was applied on the ROI data to examine main effects of sensory (Auditory versus Visual), motor (Hand versus Eye), and trial type (Go versus SuccStop versus UnsuccStop) factors as well as their interactions. Post‐hoc paired t‐tests were corrected for multiple comparisons using the Bonferroni method.
Behavior‐Brain Modulation
Since the average SSRT was significantly different among some task conditions (see Behavioral results), we conducted exploratory analysis to examine whether the regional response differences are correlated with the observed SSRT differences. More specifically, we conducted two t‐tests with the differences in SSRT included as covariates: (1) [(SuccStopEA − GoEA) − (SuccStopEV − GoEV)] with (SSRTEA − SSRTEV) as the covariate and (2) [(SuccStopHA − GoHA) − (SuccStopHV − GoHV)] with (SSRTHA − SSRTHV) as the covariate. This analysis was only applied to compare conditions with same motor modalities, such as EA vs. EV and HA vs. HV because each pair of conditions had identical sensorimotor mapping for the Go trials. This approach was taken to minimize the complexity of double subtraction. Both the contrast maps and covariate maps were thresholded at P < 0.001, uncorrected and cluster size > 9.
RESULTS
Behavioral Results
Behavioral measures are reported in Table 1. A 2 × 2 ANOVA was conducted to test for the main effects of motor and sensory factors and their interaction. Response performance on go trials were similar across conditions, with no significant main effects on accuracy and RT (P's > 0.28). For the stop accuracy, significant effects were found for the interaction between sensory and motor modalities (F[1,18] = 32.88, P < 0.001) and the main effect of sensory modality (F[1,18] = 48.62, P < 0.001). Post‐hoc analysis showed that the stop accuracy was higher with the visual stop signal than with the auditory signal (EV > EA, t[18] = 7.49, P < 0.001; and HV > HA, t[18] = 2.18, P < 0.05). Response times on unsuccessful stop trials were similar across conditions (all ps > 0.1). Consistent with the Race Model [Logan and Cowan, 1984], response times on unsuccessful stop trials were significantly shorter than those on go trials for each task condition (all ps < 0.001). For the SSRT, the main effects of motor modality (F[1,18] = 38.06, P < 0.001), sensory modality (F[1,18] = 27.38, P < 0.001) and their interaction (F[1,18] = 15.79, P < 0.001) were significant. Post‐hoc analysis showed that the SSRTs were longer for stopping eye movements than for stopping hand movements (EA > HA, t[18] = 5.85, P < 0.001; EV > HV; t[18] = 3.75, P < 0.001). Stopping saccadic eye movements also takes a longer time with the auditory stop signal than with the visual stop signal (EA > EV, t[18] = 5.03, P < 0.001).
Table 1.
Behavioral measures
| HA | HV | EA | EV | |
|---|---|---|---|---|
| Go ACC (%) | 98 (3) | 98 (3) | 98 (1) | 98 (2) |
| Go RT (ms) | 503 (43) | 497 (38) | 513 (45) | 511 (37) |
| UnsuccStop RT (ms) | 470 (34) | 465 (34) | 465 (36) | 474 (40) |
| Stop ACC (%):SSD1a | 85 (19) | 95 (9) | 72 (18) | 88 (12) |
| Stop ACC (%):SSD2a | 78 (18) | 90 (13) | 57 (24) | 87 (18) |
| Stop ACC (%):SSD3a | 58 (22) | 56 (26) | 48 (23) | 68 (23) |
| Stop ACC (%):SSD4a | 17 (16) | 10 (9) | 19 (16) | 29 (20) |
| SSRT (ms) | 274 (28) | 271 (18) | 349 (41) | 296 (32) |
a SSD1, 2, 3, and 4 were 10, 110, 210, and 310 ms in HA and HV tasks and 10, 90, 180, and 270 ms in EA and EV tasks.
Sensorimotor‐Independent Activation During Response Inhibition
To identify cortical regions that were commonly activated in stopping, we applied conjunction analysis to the Stop versus Go contrasts from the four sensorimotor conditions. Figure 3a shows the suprathreshold voxels across all four stop‐go contrasts in the frontal cortex, including the bilateral vIFG/AI, right MFG, SFG, and pre‐SMA. Other commonly activated regions included the IPL and superior and middle temporal gyrus. Similar common activations were found using the cross‐modality stop‐go contrasts: HA and EV conjunction or HV and EA conjunction.
Figure 3.
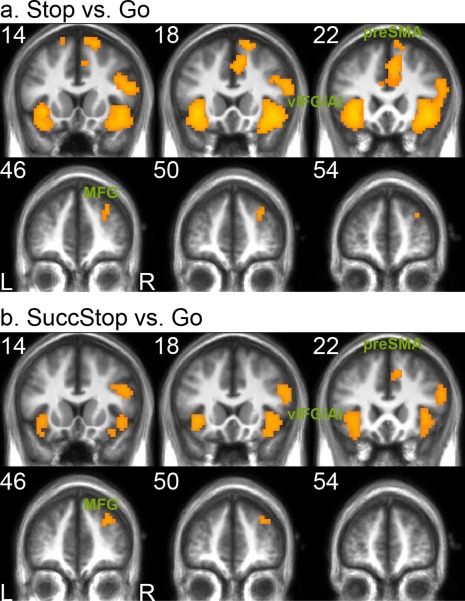
Sensorimotor‐independent activation during response inhibition. (a) The conjunction analysis of Stop‐Go contrasts across all sensorimotor conditions showed suprathreshold activation in the bilateral vIFG/AI, right MFG, and pre‐SMA. The Stop‐Go contrast in each sensorimotor condition was thresholded at P < 0.001 uncorrected and cluster size > 9. (b) The conjunction analysis of SuccStop‐Go contrasts across all sensorimotor conditions showed similar activation pattern. The SuccStop‐Go contrast in each sensorimotor condition was thresholded at P < 0.005 uncorrected and cluster size >9. Abbreviations: vIFG/AI: ventral inferior frontal gyrus/anterior insular; MFG: middle frontal gyrus; preSMA: presupplementary motor area. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To further test whether these regions are commonly involved in successful stopping, we conducted additional conjunction analysis of the SuccStop versus Go contrasts across the four sensorimotor conditions. Similar pattern of suprathreshold activation found in the prefrontal cortex, including the bilateral vIFG/AI, right MFG and preSMA (see Fig. 3b). Table 2 lists the main clusters from these conjunction analyses.
Table 2.
Conjunctive analysis across HA, HV, EA, and EV tasks
| Region | Cluster size | Z | X | Y | Z |
|---|---|---|---|---|---|
| Stop‐Go | |||||
| Right AI/IFG | 480 | 5.96 | 42 | 18 | −9 |
| Right IFG | 3.88 | 54 | 18 | 21 | |
| Right IFG | 3.58 | 48 | 27 | 21 | |
| Left AI/IFG | 243 | 5.93 | −33 | 21 | −6 |
| Right IPL | 83 | 4.61 | 63 | −42 | 24 |
| Right IPL | 3.58 | 63 | −39 | 42 | |
| Right ACC | 164 | 4.58 | 6 | 27 | 30 |
| Right preSMA | 3.98 | 9 | 21 | 45 | |
| Left ACC | 3.75 | −6 | 30 | 24 | |
| Left MTG | 67 | 4.45 | −51 | −54 | 3 |
| Right MTG | 51 | 4.37 | 57 | −57 | 3 |
| Right SFG | 41 | 4.25 | 18 | 15 | 66 |
| Right SFG | 3.24 | 9 | 12 | 69 | |
| Right SFG | 3.18 | 9 | 27 | 63 | |
| Left STG | 20 | 3.74 | −63 | −48 | 15 |
| Right MTG | 9 | 3.49 | 33 | −48 | 39 |
| Left SFG | 9 | 3.43 | −15 | 9 | 66 |
| Right MFG | 12 | 3.39 | 30 | 48 | 33 |
| SuccStop‐Go | |||||
| Right MTG | 166 | 3.8 | 51 | −51 | 6 |
| Right MTG | 3.68 | 57 | −54 | 0 | |
| Right SMG | 3.34 | 63 | −39 | 39 | |
| Left AI/IFG | 102 | 3.5 | −36 | 18 | −6 |
| Left OFC | 2.99 | −33 | 24 | −18 | |
| Left OFC | 2.61 | −36 | 15 | −18 | |
| Right IFG | 115 | 3.36 | 45 | 33 | 0 |
| Right AI/IFG | 3.3 | 39 | 21 | 0 | |
| Right AI/IFG | 3.21 | 45 | 15 | −6 | |
| Right IFG | 65 | 3.31 | 54 | 18 | 21 |
| Left MTG | 41 | 3.22 | −60 | −60 | −3 |
| Left MTG | 2.68 | −63 | −48 | 9 | |
| Right MFG | 23 | 3.19 | 30 | 48 | 33 |
| Right MFG | 2.89 | 27 | 42 | 27 | |
| Right preSMA | 9 | 2.86 | 9 | 21 | 42 |
Each Stop‐Go contrast thresholded at P < 0.001 uncorrected and cluster size > 9; each SuccStop‐Go contrast threshold at P < 0.005 uncorrected and cluster size > 9.
We further applied ROI analysis to examine whether these commonly activated areas exhibit any differential responses to the different trial types and sensorimotor contexts across the stop signal tasks. Figure 4 shows the beta weights of several prefrontal ROIs, including the bilateral vIFG/AI, the right MFG, and the preSMA. Overall, the four ROIs showed similar activity patterns in response to the task manipulations, with small variations (see below).
Figure 4.
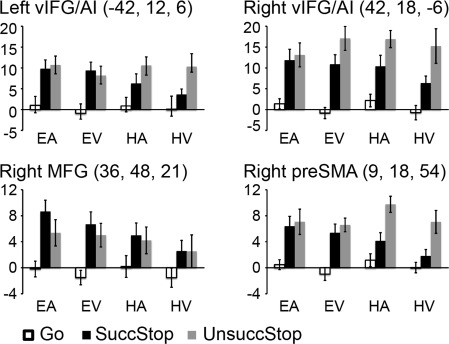
Beta weights of response inhibition ROIs. ROIs include the bilateral vIFG/AI, right MFG, and preSMA. In each bar chart, bars from left to right show the average beta weights in Go, SuccStop, and UnsuccStop trials at each sensorimotor condition. The coordinates (x, y, and z) are in MNI space. Abbreviations: vIFG/AI: ventral inferior frontal gyrus/anterior insular; MFG: middle frontal gyrus; preSMA: presupplementary motor area.
As expected, all four ROIs showed greater responses on trials requiring response inhibition in comparison to go trials, resulting in a significant main effect of trial type (all ps < 0.001). Post‐hoc t‐tests were conducted to compare activation difference among Go, SuccStop, and UnsuccStop for each task conditions for each ROI, with the Bonferroni‐corrected significance threshold for the three paired t‐tests set at P < 0.017. The bilateral vIFG/AI showed significantly greater activation on SuccStop and UnsuccStop than Go for most conditions (all ps < 0.017), except for the SuccStop versus Go comparison for HV for the left vIFG/AI (P = 0.058). The right MFG showed significantly greater activation on SuccStop and UnsuccStop than Go for most conditions (all ps < 0.017), except for the SuccStop/UnsuccStop versus Go comparisons for HV (ps = 0.048) and UnsuccStop versus Go comparison for HA (P = 0.04). The preSMA showed significantly greater activation on SuccStop and UnsuccStop than Go for most conditions (all ps < 0.017), except for the SuccStop versus Go comparison for HV (P = 0.19). While none of these ROIs showed significantly greater activation on SuccStop than UnsuccStop (all ps > 0.042), some ROIs showed greater activation on UnsuccStop than SuccStop for some conditions (bilateral vIFG/AI for HA; preSMA for HA and HV; all ps < 0.017).
We did not predict significant main effects of sensory or motor modality for these prefrontal ROIs. Indeed, none of the four ROIs showed significant main effect of motor modality, with all ps > 0.12. There was also no significant main effect of sensory modality for most ROIs (all ps > 0.05), except it was significant for the preSMA (P < 0.017, Auditory > Visual).
Intriguingly, we found significant interactions between motor and trial factors for the preSMA and the left vIFG/AI (all ps < 0.012). In particular, the preSMA showed significantly greater activation in UnsuccStop than SuccStop in hand conditions (all ps < 0.017) but not in eye conditions (all ps > 0.44). The other interactions were not significant and we did not find any three‐way interactions among the motor, sensory and trial factors (all ps > 0.1).
Sensorimotor‐related Activation During Response Inhibition
For the sensory ROIs, we examine whether visual and auditory ROIs responded to the stop signals differently under different sensorimotor contexts (e.g., HA vs. EA) and whether their responses varied by trial type (e.g., SuccStop vs. UnsuccStop). Figure 5 shows beta weights of these ROIs, including the bilateral STG and MOG.
Figure 5.
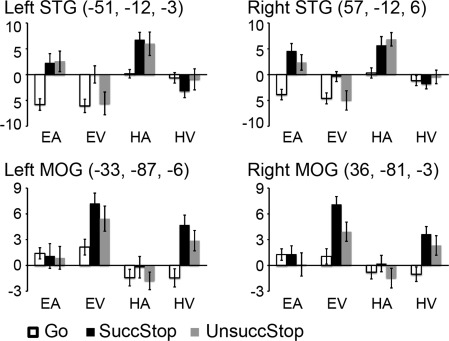
Beta weights of sensory ROIs. ROIs include the bilateral STG and MOG. In each bar chart, bars from left to right show the average beta weights in Go, SuccStop, and UnsuccStop trials for each sensorimotor condition. The coordinates (x, y, and z) are in MNI space. Abbreviations: STG: superior temporal gyrus; MOG: middle occipital gyrus.
These sensory ROIs showed significant sensory and trial type main effects (all ps < 0.001), as expected, and sensory by trial interaction (all ps < 0.006). Paired t‐tests confirmed that the bilateral STG showed significantly greater activity on SuccStop and UnsuccStop than Go for HA and EA (all ps < 0.009) while similar comparisons were insignificant for HV and EV (with all ps > 0.08, except SuccStop versus Go for EV, P < 0.004). In contrast, the bilateral MOG showed significantly greater activity on SuccStop and UnsuccStop than Go for HV (all ps < 0.009), and significantly greater activity on SuccStop versus Go (P < 0.012) but approaching significance for UnsuccStop versus Go (P = 0.027) for EV, while similar comparisons were insignificant for HA and EA (all ps > 0.1). The differences between SuccStop and UnsuccStop for each task condition did not reach significance for any of the ROIs after correction for multiple comparisons (all ps > 0.044). The main effect of motor was significant for all ROIs (all ps < 0.006; for bilateral STG, Hand > Eye; for bilateral MOG, Eye > Hand). The bilateral STG also showed significant interactions between motor modality and trial type (left: P = 0.024, right: P < 0.001) while no other interactions were significant (all ps > 0.17). The bilateral MOG showed no other significant interactions (all ps > 0.44). Taking together, these data showed that the sensory regions are modulated not only by the sensory signal but also by the response modality during the stop trials.
For the motor ROIs, we examine whether the hand motor and oculomotor control areas showed differential activity in executing and stopping responses under different sensorimotor contexts. Figure 6 shows beta weights of these ROIs, including the bilateral FEF known for controlling eye movements and the left M1 and SMA known for controlling hand movements. The right FEF and left M1 showed the expected significant main effect of motor modality (all ps < 0.001) whereas the left FEF and SMA did not (all ps > 0.14). Besides, the right FEF, left M1, and SMA showed a significant main effect of trial type (all ps < 0.006) while the left FEF did not (P = 0.26). The only significant interaction effect between motor modality and trial type was found for the left M1 (P < 0.001, all other ps > 0.34). Paired t‐tests revealed greater activity in the left M1 on Go and UnsuccStop than on SuccStop for HA and HV (all ps < 0.017). The right FEF was more active on UnsuccStop than on Go for EA (P < 0.004) and approaching significance for EV (P = 0.078). The SMA showed greater activation on UnsuccStop than on SuccStop for HA (P = 0.036) and HV (P = 0.033), though neither reached the significance threshold after multiple comparison correction. No significant differences were found for any other paired comparisons for the other task conditions for these ROIs (all ps > 0.1).
Figure 6.
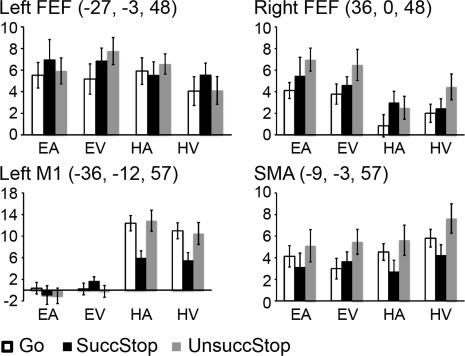
Beta weights of motor ROIs. ROIs include the bilateral FEF, left M1, and SMA. In each bar chart, bars from left to right show the average beta weights in Go, SuccStop, and UnsuccStop trials for each sensorimotor condition. The coordinates (x, y, and z) are in MNI space. Abbreviations: FEF: frontal eye field; SMA: supplemental motor area.
Besides, none of these motor ROIs showed a significant main effect of sensory modality (all ps > 0.19), interaction between sensory and trial (all ps > 0.33), or interaction among the three factors (all ps > 0.11). There were significant interactions between motor and sensory for left FEF (P < 0.02) and left M1 (P < 0.05) but not for others (all ps > 0.12).
Behavior‐Brain Modulation
By covarying out the modulation effect of SSRT difference between task conditions, the bilateral auditory cortices (i.e., STG) showed significantly greater activation in the SuccStop‐Go contrast for HA than that for HV whereas bilateral visual cortex (i.e., MOG) showed greater activation in the SuccStop‐Go contrast for HV than that for HA (see Fig. 7a). Similar effects in the sensory cortices were found in the comparison between EA and EV (see Fig. 7b). Besides, the response differences in the right IFG, MFG, and SFG between HA and HV were significantly and positively correlated with the SSRT difference between the two conditions (see Fig. 7a), whereas only the activity difference in the right IFG between EA and EV was significantly and positively correlated with the corresponding SSRT difference (see Fig. 7b). Activation clusters from the contrast maps and covariate maps are reported in Table 3.
Figure 7.
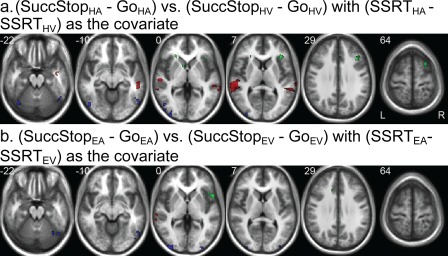
Behavior‐brain modulation. (a) Group contrast of (SuccStopHA − GoHA) vs. (SuccStopHV − GoHV) with (SSRTHA − SSRTHV) as the covariate shows dominant regional difference in auditory/visual cortex between HA and HV after the modulation effect of SSRT difference is removed (red: HA > HV; blue: HV > HA). SSRT difference is positively correlated with regional difference in right IFG, MFG, and SFG (green). (b) Group contrast of (SuccStopEA − GoEA) vs. (SuccStopEV − GoEV) with (SSRTEA − SSRTEV) as the covariate shows dominant regional difference in auditory/visual cortex between HA and HV after the modulation effect of SSRT difference is removed (red: EA > EV; blue: EV > EA). SSRT difference is positively correlated with regional difference in right IFG (green). All ps < 0.001, uncorrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Behavior‐brain modulation analysis
| Region | Cluster | Z | X | Y | Z |
|---|---|---|---|---|---|
| [(SuccStopHA − GoHA)–(SuccStopHV − GoHV)] with (SSRTHA − SSRTHV) as the covariate | |||||
| HA > HV | |||||
| Left STG | 563 | 4.79 | −54 | −38 | 14 |
| Left STG | 4.56 | −58 | −18 | 2 | |
| Left SMG | 4.35 | −66 | −46 | 10 | |
| Right STG | 52 | 4.52 | 42 | −6 | −20 |
| Right STG | 3.54 | 50 | −6 | −18 | |
| Right MTG | 204 | 4.24 | 52 | −36 | −6 |
| Right SMG | 3.87 | 48 | −38 | 6 | |
| Right SMG | 3.73 | 58 | −40 | 6 | |
| Right STG | 12 | 3.59 | 70 | −30 | 6 |
| HV > HA | |||||
| Right MOG | 105 | 4.36 | 54 | −66 | −8 |
| Right ITG | 4.04 | 48 | −60 | −18 | |
| Right MOG | 150 | 4.11 | −48 | −66 | −18 |
| Left MOG | 3.91 | −48 | −70 | −4 | |
| Left MOG | 143 | 3.91 | −40 | −88 | −6 |
| Left MOG | 3.8 | −32 | −94 | −6 | |
| Left MOG | 3.79 | −48 | −84 | 2 | |
| Left FG | 26 | 3.77 | −38 | −66 | ‐24 |
| Regions positively correlated with SSRT difference between HA vs. HV | |||||
| Right IFG | 31 | 3.96 | 60 | 6 | 12 |
| Brainstem | 12 | 3.83 | 4 | −30 | −36 |
| Right MFG | 71 | 3.81 | 40 | 22 | 32 |
| Left Accumbens | 21 | 3.79 | −6 | 6 | −6 |
| Left Caudate | 3.55 | −10 | 10 | 0 | |
| Right IFG | 39 | 3.75 | 36 | 28 | 8 |
| Right AI/IFG | 3.32 | 36 | 24 | 0 | |
| Right ACC | 11 | 3.65 | 4 | 28 | 32 |
| Right AI/IFG | 17 | 3.64 | −24 | 30 | 0 |
| Right SFG | 64 | 3.56 | 26 | 12 | 64 |
| Right SFG | 3.52 | 24 | 14 | 56 | |
| Right AG | 12 | 3.4 | 56 | −62 | 40 |
| Right AG | 3.38 | 56 | −56 | 46 | |
| [(SuccStopEA − GoEA)–(SuccStopEV − GoEV)] with (SSRTEA − SSRTEV) as the covariate | |||||
| EA > EV | |||||
| Left STG | 15 | 4.72 | −68 | −38 | 0 |
| Left STG | 13 | 3.43 | −66 | −26 | −2 |
| Right Amygdala | 10 | 3.42 | 22 | −12 | −14 |
| EV > EA | |||||
| Left MOG | 108 | 4.64 | −38 | −94 | −2 |
| Left ITG | 29 | 4.2 | −48 | −62 | −14 |
| Left MOG | 18 | 4.14 | −56 | −66 | −6 |
| Right FG | 80 | 3.85 | 46 | −68 | −22 |
| Right MOG | 3.79 | 54 | −64 | −12 | |
| Right MOG | 3.44 | 52 | −76 | −6 | |
| Right MOG | 31 | 3.74 | 32 | −94 | 4 |
| Right SOG | 19 | 3.65 | 30 | −70 | 34 |
| Right FG | 17 | 3.61 | 32 | −66 | −22 |
| Right MOG | 9 | 3.42 | −42 | −80 | −4 |
| Regions positively correlated with SSRT difference between EA vs. EV | |||||
| Right Opeculum | 21 | 4.07 | 60 | −18 | 12 |
| Right IFG | 42 | 3.9 | 52 | 12 | 0 |
| Right AI/IFG | 3.21 | 42 | 20 | 0 | |
| Left ACC | 14 | 3.81 | −14 | 26 | 26 |
All ps < 0.001, uncorrected and cluster size > 9.
DISCUSSION
To study response inhibition apart from specific sensorimotor effects and general control process, we measured BOLD response while human participants performing the stop‐signal task of four different sensorimotor associations. Our findings show that not only the bilateral vIFG/AI, but also the right MFG and preSMA are central for response inhibition, and their involvement is independent of the modality of both the stop signal (auditory or visual) and the response effector (hand or eye). These prefrontal regions showed similar level of activity during successful and unsuccessful stopping across the sensorimotor conditions, except the preSMA was more activated in unsuccessful than successful stopping of hand responses. Thus, these prefrontal regions are likely involved in the earlier stages of the stopping process, but they may play different roles through different basal ganglia circuits [Duann et al., 2009; King et al., 2012]. Indeed, we did not find any common activation in the basal ganglia system, which is not too surprising as the neural circuits for the control of hand and eye movements do not overlap [Alexander et al., 1986].
Frontoinsular Cortex and Response Inhibition
This study replicated our previous findings [Leung and Cai, 2007] by showing almost identical conjunctive activation patterns in stopping hand and eye movements, while this study goes further by revealing that the activation of bilateral vIFG/AI, right MFG, and preSMA during response inhibition is independent of the sensorimotor context. The factorial task design produced four task conditions with different demands in overt sensory processing and motor control but similar demand in cognitive processes required by countermanding. Our behavioral observations confirmed that performances are mostly similar across the four conditions. (Although the subjects were less accurate on stopping eye movements with the auditory stop signal, similar prefrontal activation patterns were observed for both EA and EV conditions; data not shown.) Thus, the common prefrontal activations cannot be attributed to specific overt sensory processing or specific motor control, but rather revealing their tight association with response inhibition. Moreover, our finding of a significant correlation between the differences in prefrontal responses and the differences in SSRT across task conditions further supports a significant role of these prefrontal regions in response inhibition.
Among the prefrontal areas, the vIFG/AI has been particularly implicated in response inhibition though its exact functional role is still in debate. Human brain lesion and TMS studies revealed that damage or temporary disruption of the right IFG produces behaviorally relevant deficit in response inhibition [Aron et al., 2003; Chambers et al., 2006]. However, some evidence suggests that the involvement of the right IFG can be associated with visual target detection instead [Hampshire et al., 2010]. The IFG, however, is a large heterogeneous structure and its different divisions may serve different roles in cognitive control [Cai and Leung, 2011; Chikazoe et al., 2009a]. It has been suggested that the dorsal IFG/IFJ is more involved in detecting infrequent visual stimuli [Chikazoe et al., 2009a] whereas the ventral‐posterior IFG (including AI) plays a potential role in an early stage of response inhibition, such as initiating the stop process, rather than stopping per se [Cai and Leung, 2011]. In fact, the center of the vIFG/AI cluster from our conjunction analysis was localized in the ventral‐posterior parts of the IFG. Moreover, this study is in agreement with previous findings in showing that the ventral‐posterior IFG was equally activated in successful and unsuccessful stopping [Cai and Leung, 2011; Tabu et al., 2012]. This supports the notion that the vIFG/AI is important in the stopping process but not in directly countermanding the unwanted motor response. We suggest the vIFG/AI is likely to play a role in an early stage of response inhibition, such as initiating the stop process, which is necessary but not sufficient for successful stopping.
Early engagement of the vIFG/AI during response inhibition is also supported by other lines of evidence. One study [Zhang and Li, 2012] showed increased BOLD activity in a frontoparietal network including IFG during both successful and unsuccessful stopping but much earlier BOLD response onset during successful than unsuccessful stopping. This work suggests that what really matters to the successfulness of stopping is the timing rather the intensity of the IFG activation. An earlier onset of the IFG activation may lead to earlier initiation of the stop process and hence, may increase the likelihood of successful stopping. The finding of IFG activation during the preparatory phase of response inhibition also provides converging evidence for the role of the IFG in an early stage of response inhibition [Aron et al., 2007; Chikazoe et al., 2009b; Hu and Li, 2012; Jahfari et al., 2010].
While the AI is frequently found activated in cognitive control tasks, its role in cognitive control has not received much attention and was barely discussed. Numerous within‐subject studies and meta‐analysis studies have identified AI activation in different contexts of cognitive control [Buchsbaum et al., 2005; Derrfuss et al., 2004; Duncan and Owen, 2000; Eckert et al., 2009; Levy and Wagner, 2011; Liu et al., 2004; Nee et al., 2007; Neumann et al., 2008; Rubia et al., 2001; Simmonds et al., 2008; Swick et al., 2011; Wager et al., 2005]. Since experimental manipulation of cognitive control often involves a behaviorally salient event, many have suggested a plausible role of the AI in saliency detection [Downar et al., 2001, 2002; Harsay et al., 2012; Metereau and Dreher, 2012; Nelson et al., 2010; Seeley et al., 2007; Wiech et al., 2010]. This interpretation is in line with the observation of comparable activation of AI on both unsuccessful and successful stop trials, if not more in unsuccessful than successful stop trials, observed in this study as well as in our previous studies [Cai and Leung, 2011, 2011]. This is because the stop signal is a behaviorally salient signal on both unsuccessful and successful stop trials, and probably more salient in the former situation than the latter. In consistent with the saliency detection theory, Ide et al. [2013] has identified the AI among other cortical regions being positively modulated by how unexpected the stimuli are in the stop‐signal task. Furthermore, there is some evidence showing that the AI may even have a more direct influence on the behavioral outcome. One previous study reported a correlation between activity in the left AI and stopping speed, which can be influenced by the efficiency of stop‐signal detection [Boehler et al., 2012]. One reason why the AI has long been neglected and less discussed in cognitive control studies is that the activations found in AI and IFG are typically parts of a large activation cluster, with the AI activation considered as an extension from the IFG activation. Although this study cannot distinguish them, we speculate that the AI and the vIFG have rather different roles in response inhibition, with the former more involved in saliency detection and the latter in triggering stopping process.
Other Prefrontal Areas and Response Inhibition
Although our finding of greater activation in the preSMA in correspondence to UnsuccStop than SuccStop seems pointing to its role in error processing, the existing literature shows a more complicated picture. First, a great number of fMRI, lesion and TMS studies showed that the preSMA plays an important, if not critical, role in successful stopping [Aron and Poldrack, 2006; Aron et al., 2007; Boecker et al., 2011; Boehler, et al., 2010; Cai et al., 2012; Cai and Leung, 2011; Chen et al., 2009; Chevrier et al., 2007; Chikazoe et al., 2009b; Curtis et al., 2005; Floden and Stuss, 2006; Hampshire et al., 2010; Leung and Cai, 2007; Nachev et al., 2007; Sharp et al., 2010; Tabu et al., 2012]. Second, the preSMA has been implicated in many other cognitive control functions such as task switch, configuration or preparation [Brass and von Cramon, 2002; Dove et al., 2000; Luks et al., 2007; Rushworth et al., 2002; Swann et al., 2012]. Consistent with the literature, we found that the preSMA is commonly activated during stopping both eye and hand movements. The fact that the preSMA is more activated in unsuccessful than successful stopping on the hand task but not on the eye task suggests that the preSMA, or at least the part we examined, is engaged in cognitive control processes associated with unsuccessful stopping rather than general error monitoring. This could be interpreted in at least two ways. First, most UnsuccStop trials relative to SuccStop trials are accompanied with longer SSDs, calling for faster and more urgent stopping needs. Supporting evidence showed that faster stopping is correlated with both greater activation in the preSMA [Li et al., 2006] and stronger structural connectivity between the preSMA and subthalamic nucleus [Forstmann et al., 2012]. Moreover, microstimulation of a parallel region for oculomotor control, the supplementary eye fields, was shown to facilitate stopping [Stuphorn and Schall, 2006]. Second, the increased activation on UnsuccStop trials could be related to the effort for behavioral adjustment following stopping failure, including response slowing [Li et al., 2008; Verbruggen and Logan, 2008]. It has been shown that the preSMA is involved in response slowing [Aron et al., 2007; Jahfari et al., 2010] and the connections between the preSMA and striatum contribute to regulating response tendency [Forstmann et al., 2010]. Moreover, different types of postdecision neurons in the supplementary eye fields are associated with behavioral reinforcement or modulation [Stuphorn et al., 2000]. We cannot dissociate these two accounts in this study because of the limited temporal resolution of fMRI. Nonetheless, it is possible that increasing stopping efficiency and inducing response slowing (both closely associated with stopping failure) are both necessary for optimizing stopping performance; and such behavioral adjustments are likely achieved by modulating the brain regions involved in response slowing, likely through the preSMA and basal ganglia circuit. Interestingly, we found worse stopping performance and greater preSMA activation in association with the auditory task than in the visual task. Therefore, the preSMA activation pattern in our study seems to be more in line with its potential role in adjusting the stopping performance.
It is also important to point out that the medial superior frontal cortex, a larger cortical region including but not limited to the preSMA, SMA, and ACC, has previously been associated with various cognitive control processes [Ridderinkhof et al., 2004; Rushworth et al., 2004]. A recent study [Zhang et al., 2012] showed that the human medial superior frontal cortex could be divided into multiple functional subregions, including the anterior/posterior preSMA and SMA, by their distinct intrinsic cortical/subcortical connectivity patterns during resting state fMRI. These distinctions suggest that the different medial superior frontal areas support different cognitive functions. In particular, the anterior preSMA, where the center of our preSMA ROI locates, is highly connected with lateral prefrontal regions and the caudate nucleus. Perhaps, this portion of the preSMA is involved in the control of behavioral adjustment as part of the prefrontal‐striatal circuit. Interestingly, another study [Ide and Li, 2011] identified Granger causal influence from the SMA to the IFG during stopping manual responses. As the IFG is tightly associated with response inhibition, it implies a broader engagement of the medial superior frontal areas in leading behavioral adjustment. Taken together, the existing evidence converges on that the human medial superior frontal cortex comprises multiple functional subregions, which may have different computational roles in subserving cognitive control function during response inhibition.
Similar to the vIFG/AI, the activation of the right MFG did not significantly differentiate between successful and unsuccessful stopping. It is possible that the right MFG plays a role during the early stage of the stop process just like the vIFG/AI. However, the right MFG is not typically associated with the stop‐signal task, while it is more often reported in studies using the Go/No‐Go task [Swick et al., 2011]. This is probably because the standard stop‐signal task involves only a single sensorimotor condition and has minimum working memory demand. The right MFG has been closely linked with working memory [Funahashi, 2006; Levy and Goldman‐Rakic, 2000; Petrides, 2000]. In this study and previous studies [Leung and Cai, 2007], it is possible that the changes in sensorimotor mapping required the subjects to keep track of the current stop signal and its demand of response inhibition, and thus engaged the right MFG.
Sensorimotor Regions and Response Inhibition
There is a small literature on how the sensorimotor brain regions are involved in response inhibition tasks. We used the localizer tasks to identify the sensory areas activated in response to the auditory and visual stop signals, and examined their activity during response inhibition. As expected, the auditory and visual regions responded differentially to the auditory and visual stop signal, respectively. This is further confirmed by comparing contrasts of SuccStop‐Go between conditions with the same motor output but different sensory input. Neither region exhibited significantly greater activity in successful than unsuccessful stopping. A previous study by Cai and Leung [2011] found greater activation in the sensory association region (i.e., the middle temporal gyrus) during successful stopping. In that study, the detection of stop signal required precise sensory feature processing (i.e., change of orientation or color) and thus the sensory signal processing time might have influenced stop success. Since this task only required the detection of the appearance of the stop signal, the little processing time required in processing the sensory signal might not produce a significant contribution to stop success.
Our findings show that the left M1 and right FEF are respectively involved during the hand and eye go/stop trials. The response patterns of the left M1 was primarily involved in hand movement execution as expected. The response pattern of the FEF, however, was not as straightforward. It is relatively well understood that the FEF is involved in both execution and inhibition of saccadic eye movements [Brown et al., 2006, 2008; Cornelissen et al., 2002; Curtis et al., 2005; Ettinger et al., 2008; Heinen, et al., 2006]. Like the superior colliculus, the FEF has movement‐related and fixation‐related neurons [Hanes et al., 1998]. In this study, the bilateral FEF did show great activation on Go, SuccStop and UnsuccStop trials, which is consistent with a previous stop‐signal study [Curtis et al., 2005]. However, we found a main effect of motor modality for the right FEF but not for the left FEF. This suggests that the left FEF may not be unique for oculomotor control. The FEFs have been implicated in various other cognitive processes, such as temporarily maintenance of task‐related information and top‐down attentional control [Corbetta and Shulman, 2002; DiQuattro and Geng, 2011; Gaymard et al., 1999; Geier et al., 2007; Makino et al., 2004]. Further studies would be needed to delineate the differences between the left and right FEF in cognitive and response control.
Sensorimotor Effect on Behavioral Stopping
Surprisingly, the saccadic RTs and SSRTs in this study were much longer than those in the previous human countermanding studies [Boucher et al., 2007; Cabel et al., 2000; Curtis et al., 2005; Emeric et al., 2007; Kornylo et al., 2003]. The long reaction latency is a feature of voluntary saccades [Mort et al., 2003; Walker et al., 2000]. In comparison to reflexive saccades that are automatically triggered by periphery visual stimuli (e.g., a flash of the target at a periphery location), voluntary saccades are based on endogenous decisions (e.g., shifting gaze to an existing target or memorized target location). In this study, voluntary saccades were elicited by the go signal placed in the fovea (with the black portion of central diamond indicating which one of the two targets to look at). Besides, the SSRT of the EV condition was significantly shorter than that of the EA condition. Several other studies have also reported that foveal visual stop signals relative to auditory stop signals can lead to faster stopping of unwanted saccades [Armstrong and Munoz, 2003; Cabel et al., 2000; Morein‐Zamir and Kingstone, 2006]. This implies that there are potentially different control mechanisms underlying visually and auditorily guided stopping of saccadic eye movements. The advantage of the visual foveal stop signal over the auditory stop signal in stopping is likely attributed to oculomotor control mechanism involving the superior colliculus. The superior colliculus receives visual input directly from the retina and from the visual cortex and other cerebral cortex and it has movement‐ and fixation‐related neurons contributing to the initiation of saccade and fixation [Munoz and Wurtz, 1993, 1995; Pare and Hanes, 2003]. In this study, the visual stop signal is a circle presented at fovea. The appearance of foveal stimuli can lead to discharge of fixation‐related neurons, which in turn stop the voluntary saccades. Unfortunately, fMRI spatial resolution in this study was not sufficient to identify accurately activations in the superior colliculus. However, the SSRT difference between the EA and EV supports the notion that different stopping mechanisms may be involved in controlling different types of saccadic eye movements.
In conclusion, we found that the bilateral vIFG/AI and the preSMA along with the right MFG are commonly activated in response inhibition in all sensorimotor manipulations. Our finding suggests that this prefrontal network plays a common role in response inhibition regardless of the sensorimotor context. Within this network, the vIFG/AI may be particularly important for triggering the stop process, whereas the preSMA may serve a role in regulating other cortical/subcortical regions involved in stopping movements. The limitation of this study is that the paradigm is not designed to disentangle the precise functions of these prefrontal regions in response inhibition [but see Cai and Leung, 2011; Chikazoe et al., 2009a]. Future research is required to understand further how these prefrontal regions communicate with the sensorimotor structures to support the inhibitory control function.
ACKNOWLEDGMENT
The authors thank the staff of Vanderbilt University Institute of Imaging Science for their generous support. They also thank Dr. Xue Tu for assistance on programming in‐house eye movement data analysis software.
REFERENCE
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Armstrong IT, Munoz DP (2003): Inhibitory control of eye movements during oculomotor countermanding in adults with attention‐deficit hyperactivity disorder. Exp Brain Res 152:444–452. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD (2003): Horse‐race model simulations of the stop‐signal procedure. Acta Psychol 112:105–142. [DOI] [PubMed] [Google Scholar]
- Boecker M, Drueke B, Vorhold V, Knops A, Philippen B, Gauggel S (2011): When response inhibition is followed by response reengagement: An event‐related fMRI study. Hum Brain Mapp 32:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2010): Pinning down response inhibition in the brain‐conjunction analyses of the Stop‐signal task. Neuroimage 52:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2012): The influence of different Stop‐signal response time estimation procedures on behavior‐behavior and brain‐behavior correlations. Behav Brain Res 229:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher L, Stuphorn V, Logan GD, Schall JD, Palmeri TJ (2007): Stopping eye and hand movements: Are the processes independent? Percept Psychophys 69:785–801. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2002): The role of the frontal cortex in task preparation. Cereb Cortex 12:908–914. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S (2006): Inhibition and generation of saccades: Rapid event‐related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage 33:644–659. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S (2008): Isolation of saccade inhibition processes: Rapid event‐related fMRI of saccades and nogo trials. Neuroimage 39:793–804. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF (2005): Meta‐analysis of neuroimaging studies of the Wisconsin card‐sorting task and component processes. Hum Brain Mapp 25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabel DW, Armstrong IT, Reingold E, Munoz DP (2000): Control of saccade initiation in a countermanding task using visual and auditory stop signals. Exp Brain Res 133:431–441. [DOI] [PubMed] [Google Scholar]
- Cai W, Leung H‐C (2011): Cortical activity during manual response inhibition guided by color and orientation cues. Brain Res 1261:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung HC (2011): Rule‐guided executive control of response inhibition: Functional topography of the inferior frontal cortex. PLoS One 6:e20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, George JS, Verbruggen F, Chambers CD, Aron AR (2012): The role of the right pre‐supplementary motor area in stopping action: Two studies with event‐related transcranial magnetic stimulation. J Neurophysiol 108:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ (2007): The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20:255–261. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB (2006): Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18:444–455. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH (2009): Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44:537–545. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R (2007): Dissociation of response inhibition and performance monitoring in the stop signal task using event‐related fMRI. Hum Brain Mapp 28:1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K‐i, Morimoto H, Hirose S, Miyashita Y, Konishi S (2009a): Functional dissociation in right inferior frontal cortex during performance of go/no‐go task. Cereb Cortex 19:146–152. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009b): Preparation to inhibit a response complements response inhibition during performance of a stop‐signal task. J Neurosci 29:15870–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Kimmig H, Schira M, Rutschmann RM, Maguire RP, Broerse A, Den Boer JA, Greenlee MW (2002): Event‐related fMRI responses in the human frontal eye fields in a randomized pro‐ and antisaccade task. Exp Brain Res 145:270–274. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M (2005): Canceling planned action: An FMRI study of countermanding saccades. Cereb Cortex 15:1281–1289. [DOI] [PubMed] [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY (2004): Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23:604–612. [DOI] [PubMed] [Google Scholar]
- DiQuattro NE, Geng JJ (2011): Contextual knowledge configures attentional control networks. J Neurosci 31:18026–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2001): The effect of task relevance on the cortical response to changes in visual and auditory stimuli: An event‐related fMRI study. Neuroimage 14:1256–1267. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2002): A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87:615–620. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS (2009): Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29:10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2009): At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp 30:2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Pare M, Pouget P, Stuphorn V, Taylor TL, Schall JD (2007): Influence of history on saccade countermanding performance in humans and macaque monkeys. Vis Res 47:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC (2008): Decomposing the neural correlates of antisaccade eye movements using event‐related FMRI. Cereb Cortex 18:1148–1159. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT (2006): Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 18:1843–1849. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schafer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R (2010): Cortico‐striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 107:15916–15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Jahfari S, Bazin PL, Neumann J, Schafer A, Anwander A, Turner R (2012): Cortico‐subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage 60:370–375. [DOI] [PubMed] [Google Scholar]
- Friston KJ (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Friston KJ, Penny WD, Glaser DE (2005): Conjunction revisited. Neuroimage 25:661–667. [DOI] [PubMed] [Google Scholar]
- Funahashi S (2006): Prefrontal cortex and working memory processes. Neuroscience 139:251–261. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proc Natl Acad Sci USA 96:8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud‐Pechoux S, Pierrot‐Deseilligny C (1999): The frontal eye field is involved in spatial short‐term memory but not in reflexive saccade inhibition. Exp Brain Res 129:288–301. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B (2007): Circuitry underlying temporally extended spatial working memory. Neuroimage 35:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD (2009): Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev 33:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010): The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 50:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF II, Schall JD (1998): Role of frontal eye fields in countermanding saccades: Visual, movement, and fixation activity. J Neurophysiol 79:817–834. [DOI] [PubMed] [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, Ridderinkhof KR (2012): Error awareness and salience processing in the oddball task: Shared neural mechanisms. Front Hum Neurosci 6:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen SJ, Rowland J, Lee BT, Wade AR (2006): An oculomotor decision process revealed by functional magnetic resonance imaging. J Neurosci 26:13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li CS (2012): Neural processes of preparatory control for stop signal inhibition. Hum Brain Mapp 33:2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS (2011): A cerebellar thalamic cortical circuit for error‐related cognitive control. Neuroimage 54:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CS (2013): Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci 33:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR (2010): Responding with restraint: What are the neurocognitive mechanisms? J Cogn Neurosci 22:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR (2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AV, Linke J, Gass A, Hennerici MG, Tost H, Poupon C, Wessa M (2012): Microstructure of a three‐way anatomical network predicts individual differences in response inhibition: A tractography study. Neuroimage 59:1949–1959. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y (1999): Common inhibitory mechanism in human inferior prefrontal cortex revealed by event‐related functional MRI. Brain 122(Pt 5):981–991. [DOI] [PubMed] [Google Scholar]
- Kornylo K, Dill N, Saenz M, Krauzlis RJ (2003): Cancelling of pursuit and saccadic eye movements in humans and monkeys. J Neurophysiol 89:2984–2999. [DOI] [PubMed] [Google Scholar]
- Leung H‐C, Cai W (2007): Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci 27:9893–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011): Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman‐Rakic PS (2000): Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133:23–32. [DOI] [PubMed] [Google Scholar]
- Li C‐s.R, Huang C, Constable RT, Sinha R (2006): Imaging response inhibition in a stop‐signal task: Neural correlates independent of signal monitoring and post‐response processing. J Neurosci 26:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C‐sR, Yan P, Sinha R, Lee T‐W (2008): Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL (2004): Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event‐related fMRI. Neuroimage 22:1097–1106. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB (1984): On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev 91:195–327. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG (2007): Preparatory allocation of attention and adjustments in conflict processing. Neuroimage 35:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Yokosawa K, Takeda Y, Kumada T (2004): Visual search and memory search engage extensive overlapping cerebral cortices: An fMRI study. Neuroimage 23:525–533. [DOI] [PubMed] [Google Scholar]
- Metereau E, Dreher JC: Cerebral correlates of salient prediction error for different rewards and punishments. Cereb Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Morein‐Zamir S, Kingstone A (2006): Fixation offset and stop signal intensity effects on saccadic countermanding: A crossmodal investigation. Exp Brain Res 175:453–462. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C (2003): Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18:231–246. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH (1993): Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70:559–575. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH (1995): Saccade‐related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73:2313–2333. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C (2007): The role of the pre‐supplementary motor area in the control of action. Neuroimage 36 Suppl 2:T155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J (2007): Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, von Cramon DY, Lohmann G (2008): Model‐based clustering of meta‐analytic functional imaging data. Hum Brain Mapp 29:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J‐B (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–660. [DOI] [PubMed] [Google Scholar]
- Pare M, Hanes DP (2003): Controlled movement processing: Superior colliculus activity associated with countermanded saccades. J Neurosci 23:6480–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2000): The role of the mid‐dorsolateral prefrontal cortex in working memory. Exp Brain Res 133:44–54. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E (2001): Mapping motor inhibition: Conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13:250–261. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002): Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J Neurophysiol 87:2577–2592. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA (2010): Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 107:6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH (2008): Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia 46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD (2006): Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9:925–931. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD (2000): Performance monitoring by the supplementary eye field. Nature 408:857–860. [DOI] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N (2012): Roles for the pre‐supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage 59:2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U (2011): Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. Neuroimage 56:1655–1665. [DOI] [PubMed] [Google Scholar]
- Tabu H, Mima T, Aso T, Takahashi R, Fukuyama H (2012): Common inhibitory prefrontal activation during inhibition of hand and foot responses. Neuroimage 59:3373–3378. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2008): Response inhibition in the stop‐signal paradigm. Trends Cogn Sci 12:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J (2005): Common and unique components of response inhibition revealed by fMRI. Neuroimage 27:323–340. [DOI] [PubMed] [Google Scholar]
- Walker R, Walker DG, Husain M, Kennard C (2000): Control of voluntary and reflexive saccades. Exp Brain Res 130:540–544. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I (2010): Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Aron, AR, Poldrack RA (2008): Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18:1923–1932. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CS (2012): Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp 33:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS (2012): Resting‐state functional connectivity of the medial superior frontal cortex. Cereb Cortex 22:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


