Abstract
Multi-morbidity, the co-occurrence of multiple physical or psychological illnesses, is prevalent particularly among older adults. The number of Americans with multiple chronic diseases is projected to increase from 57 million in 2000 to 81 million in 2020. However, behavioral medicine and health psychology, while focusing on the co-occurrence of psychological/psychiatric disorders with primary medical morbidities, have historically tended to ignore the co-occurrence of primary medical comorbidities, such as diabetes and cancer, and their biopsychosocial implications. This approach may hinder our ecologically valid understanding of the etiology, prevention, and treatment of individual patients with multi-morbidity. In this selective review, we propose a heuristic biobehavioral framework for the etiology of multi-morbidity. More acknowledgment and systematic research on multiple, co-existing disorders in behavioral medicine is consistent with the biopsychosocial model’s emphasis on treating the “whole person,” which means not considering any single illness, its symptoms, risk factors, or mechanisms, in isolation. As systems analytics, big data, machine learning, and mixed model trajectory analyses, among others, come on-line and become more widely available, we may be able to tackle multi-morbidity more holistically, efficiently and satisfactorily.
Keywords: Multi-morbidity, co-morbidity, behavioral medicine, health psychology, biopsychosocial model, multiple chronic conditions
INTRODUCTION
“No longer can each chronic illness be considered in isolation,” (1).
“Multi-morbidity,” which refers to an individual experiencing the co-occurrence of two or more chronic diseases (including for example, asthma, arthritis, diabetes, heart disease, hypertension, cancer, and human immunodeficiency virus infection), is common, especially in older adults, that is, persons over 60 years of age (1–5). Approximately 57 million Americans had multiple chronic diseases in 2000 and the number is projected to be 81 million in 2020 (2). Such persons also exhibit rapid declines in health status and greater likelihood of disability (3,5,6,7,8). Co-occurrence of illnesses is also costly; 66% of total health care spending currently is for care of those Americans with multi-morbidity (9).
Presence of one chronic disease is often associated with a greater than expected, subsequent occurrence of other disorders (10), [e.g., diabetes with the risk of colorectal cancer (11); melanoma with death from amyotrophic lateral sclerosis (12); depression with diabetes or coronary heart disease (13, 14)]. As these examples show, the co-occurrence of multiple diseases crosses organ and system divides (10). Such clinical and epidemiological observations suggest a synergistic or activated process might underlie the emergence of multiple chronic conditions (15) that may have profound implications for research, treatment and health policy (16–18). Medical research, however has tended to adopt a “one-disease-at-a-time” approach; whereby the incidence of single diseases has been the focus until recently. This approach is being reconsidered as the proportion of the population of persons at risk or living with multiple concurrent illnesses is increasing.
Psychosomatic and behavioral medicine have had a more complicated relationship with this issue. On the one hand, the co-occurrence of psychological/psychiatric disorders (e.g., depression and anxiety) and psychosocial factors (e.g., early adversity, personality factors, socioeconomic status) with primary medical morbidities has been the focus of psychosomatic and behavioral medicine since the fields began. However, the relationship between the biobehavioral mechanisms and factors contributing to the co-occurrence or successive occurrence of multiple, primary medical conditions, for instance in patients with both diabetes and cancer or heart disease and arthritis, has not been a point of emphasis. This paper proposes a biobehavioral framework and future directions for examining the co-occurrence of multiple primary medical morbidities.
What Should We Call It?
“Multi-morbidity” is often confused with the more common term, “co-morbidity” but maintaining a distinction between them is preferable. Co-morbidity refers to the presence of other diseases in addition to an index disease (15, 19). For example, specialty care clinicians and researchers may be interested in a patient’s heart disease as a primary condition and consider any coexistent diseases as subsidiary. There are situations, especially in primary care, where someone has multiple disease conditions and no particular illness is the exclusive focus. The term, multi-morbidity, seems more appropriate for such scenarios.1
It is important to distinguish between multiple diseases whose co-occurrences would be expected from base-rate probabilities (i.e., simple multi-morbidity) versus co-occurrences exhibited at greater than expected probability (i.e., associative multi-morbidity) (26). As an example of the first kind, Prados-Torres (27) observes hypertension often co-occurs with other conditions because it is a highly prevalent disorder. In contrast, nonrandom associations of health disorders represent cases of associative multi-morbidity. This second category may help identify the patients at greatest illness risk, and encourage more frequent screening and design of special prevention programs, including targeting screening. Subsumed under associative multi-morbidity is causal multi-morbidity for which shared risk factors, genetic, physiological and psychosocial disease pathways may be operating (26). Instances of causal multi-morbidity, such as the cluster comprised of single vessel vascular disease, hypertension, diabetes and chronic renal insufficiency, should be excellent candidates for multiple levels of analysis in behavioral medicine research and receive emphasis in the present paper.2
Most of the currently available evidence on multi-morbidity relies on cross-sectional methods. This type of evidence provides a snapshot of the prevalence of pairings and triads of different diseases (4,30) but the developmental trajectories of these diseases are unclear (10, 18). The mapping of trajectories of emergence and succession of multiple morbidities has the potential to assess how close they are to each other with respect to pathogenic mechanisms (27). Valuable information already has been mined from existing cohorts, such as the combined Center for Medicare and Medicaid Services claims data and Surveillance, Epidemiology and End Results Program (SEER) resources (30), although those sources lack data about untreated and/or undetected conditions, and are restricted to older adults.
Current challenges in defining and identifying multi-morbidity, mapping multiple disease etiology and trajectories may seem daunting, especially in view of the challenges associated with studying one-condition at a time for behavioral researchers. However, as systems analytics, big data, machine learning, and mixed model trajectory analyses, among others, come on-line and become more widely available, we may be able to tackle multi-morbidity more holistically, efficiently and satisfactorily.
A Biobehavioral Framework for Multi-Morbidity Etiology
Decades of progress in behavioral medicine have identified basic mechanisms whereby behavioral and biological interactions contribute to the initiation, progression, treatment and recovery from single diseases (32,33). The same processes also likely have applicability to those with multi-morbidity. Page’s “Mosaic Theory” of hypertension (34)—positing the combined role of several factors—may be an appropriate first-step. Eventually in the area of hypertension, empirical investigations of the roles of genetics, environment, adaptive, neural, mechanical, and hormonal perturbations led to identification of common molecular and cellular events. So too, a mosaic framework may serve as a heuristic for an etiological model of multi-morbidity that eventually may lead to research that identifies a small core-set of critical processes.
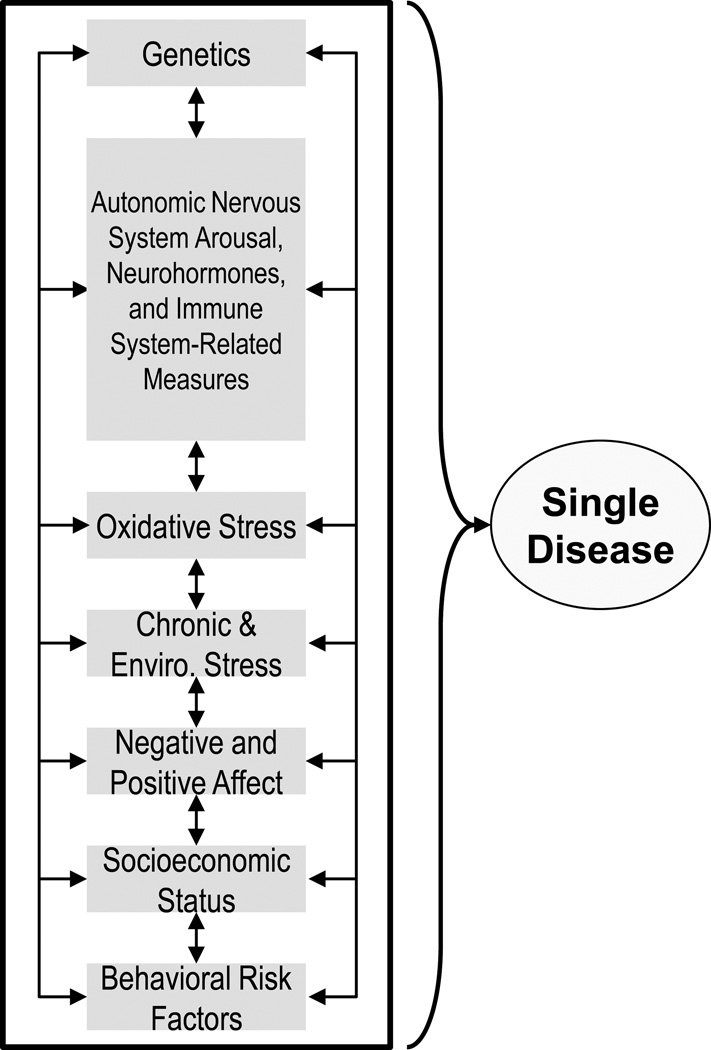
Our mosaic framework first considers factors involved in the etiology of single disease conditions and includes: (a) genetics; (b) Autonomic Nervous System arousal (e.g., heart rate variability, sympathetic nervous system, parasympathetic nervous system); (c) neurohormones (e.g., Hypothalamic-Pituitary-Adrenal Axis and catecholamines); (d) immune system-related measures (e.g., inflammation, micro-organisms, etc.); (e) oxidative stress; (f) chronic and environmental stress; (g) negative and positive affective dispositions; (h) socioeconomic status; (i) behavioral risk factors. (See Figure 1; the two-way arrows acknowledge the cross-effects or “traffic,” among these factors.) This list is not exhaustive and we recognize that the latent constructs overlap.
Figure 1.
Biopsychosocial Factors of Single Disease Etiology
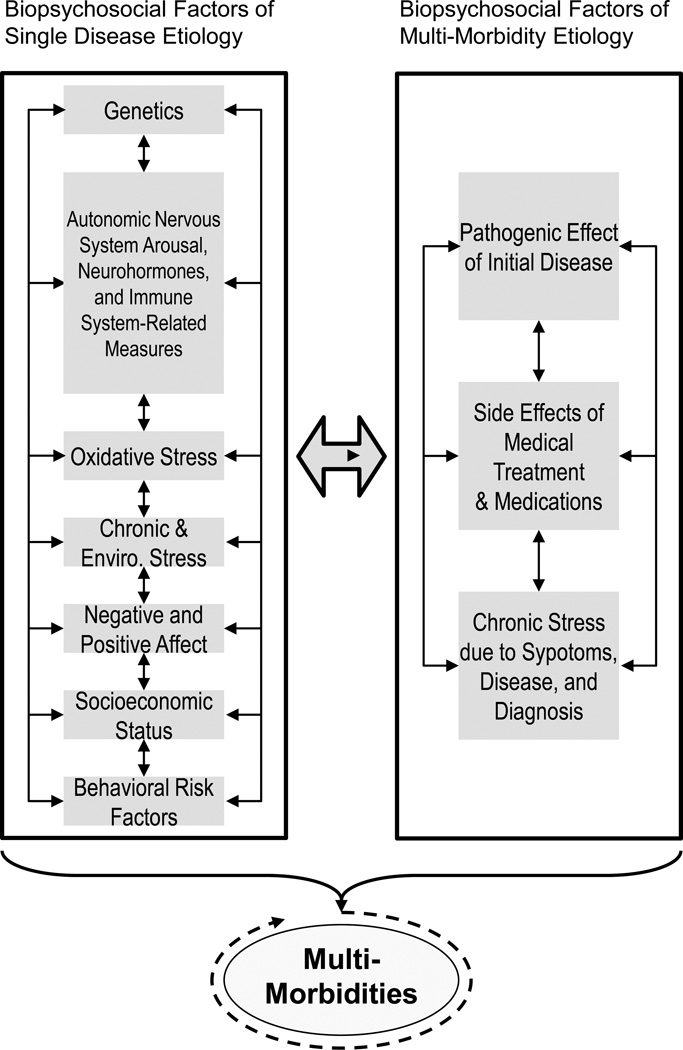
The framework for the multi-morbidity scenario depicted in Figure 2 (left panel) includes the same factors, such as shared genes, ANS arousal, neurohormones, immune-system related factors, negative and positive affective dispositions, shared behavioral risk factors, etc., as in Figure 1. In one causal multi-morbidity scenario, two or more diseases emerge more or less at the same time because of some shared subset of the factors listed in the left panel. Hypothyroidism and obesity would be one such example (35). Other causal multi-morbidity scenarios involve the development of one condition followed later by another condition (or conditions) as a function of the downstream biological effects of the initial disease, acute or chronic stress induced by diagnosis and treatment of the initial disease and/or the side-effects of medical treatment (Figure 2, Right panel). The model also recognizes that development of a second or third, disease condition may in turn produce recursive, cascading loops, all of which may amplify the biological and psychosocial insults to the system.
Figure 2.
Reciprocal Effects of Biopsychosocial Factors of Single Disease and Multi-Morbidity Etiology
(left panel) includes the same factors, such as shared genes, autonomic arousal, neurohormones, immune system-related activity, negative and positive affective dispositions, shared behavioral risk factors, etc., as in Figure 1. In one scenario, two or more diseases emerge more or less simultaneously because of a shared subset of the factors, listed in the left panel. The right panel depicts the staggered emergence of other conditions as a function of downstream biological effects of the initial disease, acute/chronic stress induced by diagnosis and treatment of the initial disease and/or the side-effects of medical treatment.
In the multi-morbidity scenario, the simultaneous or staggered emergence of two or more diseases should be more likely if they are related to the same organ systems or are caused by the same set of factors (referred to as causal multi-morbidity above). For example, our earlier examples of hypertension, single vessel vascular disease, diabetes and chronic renal insufficiency are typically classified as related comorbid conditions, whereas hypertension, asthma and gastritis are often considered unrelated (36, 37). A more systematic approach for categorizing diseases as related is based on shared genetic susceptibility or other shared pathways or mechanisms (see 38 for description of the “diseasome”—the combined set of all known disorder/disease genetic associations). An example of genetic commonality is the co-occurrence of Alzheimer’s Disease and myocardial infarction where an apolipoprotein E allele is implicated (39). A complementary approach identifies common metabolic-based defects among disease phenotypes (40). The co-occurrence of diabetes and colorectal and bladder cancers may represent, in part, shared metabolic pathways (41) (see below).
Examples of staggered emergence of diseases
The thirty percent increased risk of colorectal cancer conferred by diabetes, suggested a downstream effect of the first emerging condition on the second (11). While both diseases share similar risk factors, physical inactivity and obesity (42), diabetes and cancer appear to be positively associated even with these risks controlled (11). The downstream effect of diabetes may stem from constant exposure to hyperinsulimia, which is associated with production of insulin-like growth factor that can cause cell-proliferation and inhibit apoptosis (42,43), thereby increasing tumor growth. This is an illustration of how two or more successive diseases can result from metabolic defects—the cell’s inability to breakdown a metabolic substrate—that can have a cascading effect that leads to the coupling (or co-occurrence) of diverse diseases (40). In a metabolic disease network in which two diseases are linked, mutated enzymes associated with the disorders catalyze adjacent metabolic reactions.
Medication-Related Multi-morbidity
Worthy of special mention (see Figure 2, right panel) is the role of medical treatment and medications in contributing to multi-morbidity. Approval of new drugs by the Food and Drug Administration (FDA) requires evidence of efficacy through phase 3 clinical trials of one index condition (our emphasis). This means that possible drug-to-drug, drug-to-disease, and disease-to-disease interactions are frequently unknown and potentially life-threatening (44). Besides improving safety, more proactive measurements of any drug toxicity may provide insights about multi-morbidity. As an illustration, the recognition that anthracyclines, which are widely and effectively used for the treatment of breast cancer, increase the long-term risk of heart failure has motivated the development of oncology-cardiology partnerships and revision of clinical guidelines (45, 46). Another example is the use of pegylated interferon – alpha (IFN-α) to treat chronic hepatitis patients, which has the side-effect of inducing depression in 23 to 45% of patients (47) probably because IFN reduces serotonin levels (48), and/or activates other proinflammatory cytokines and the hypothalamic-pituitary-adrenal (HPA) axis, which can induce depression (49).
Future Directions for Research in Multi-Morbidity and Behavioral Medicine
Recognition of the prevalence of multi-morbidity and the proposed framework suggests several research directions for behavioral medicine. Below we present some illustrative examples from five general categories: (a) epidemiology; (b) autonomic imbalance; (c) animal models; (d) symptom perception, treatment-seeking and medical diagnosis; and (e) behavioral interventions and evidence-based clinical guidelines.
Clinical and Behavioral Epidemiology
Medicine, public health and behavioral medicine need large, representative longitudinal cohorts that recruit persons earlier in life (e.g., middle-age) to obtain a comprehensive picture of when multiple illnesses initially emerge and whether there are common multi-morbidity trajectories. For behavioral medicine researchers, critical questions will address the role of illness- and non-illness related stressors and cumulative psychosocial burdens or emerging behavioral disruptions associated with highly prevalent or common multi-morbidity phenotypes (50). Therefore, assessment of psychosocial variables, such as stress, social support, negative affective dispositions, etc., should be an important component of any large-scale epidemiological study of emergence and trajectory of multi-morbidity.
Autonomic and HPA Imbalance
Behavioral researchers, positing that exaggerated patterns of physiological reactivity experience “wear and tear” on tissues and organs, that eventually lead to bodily dysfunction and susceptibility to disease (51, 52), have devoted attention to identifying cardiovascular, neuroendocrine and immune reactivity to stressors (53). In studies of mental stress or naturalistic stressors with patients with existing illness, however presence of other medical disorders tends to be an exclusion criterion. As a result, we lack information about how stress-induced laboratory or ambulatory reactivity is influenced by the presence of one or more other diseases and whether reactivity has prognostic value for survival or quality of life in these common multi-morbid patients. Studies of how reactivity patterns are altered in the context of multiple diseases might be enlightening about which stress indices are most affected, and whether disease co-occurrence amplifies or decreases the magnitude of reactivity. Such results may have prognostic value as well as implicate common disease pathways.
Animal models
The use of experimental animal models of disease initiation and progression permits manipulation of many of the psychobiological processes described earlier that cannot be ethically or feasibly manipulated in humans. Study of multi-morbidity in experimental animal models is rare in behavioral medicine, in contrast to extensive research on the effects of stress on the initiation and progression of single disorders (54, 55). The development of animal models of multiple chronic conditions, incorporating behavioral theory and empirical methods, represents a significant opportunity for behavioral medicine researchers. Clearly, there are complications, such as the need to identify species for whom multiple disorders can be experimentally induced. However, the potential to study genetic, autonomic, and immune factors, as well as the variables of interest to the behavioral medicine field, as they affect development of multiple diseases using animal models would seem to have great potential.
Symptom Perception, Treatment Seeking and Medical Diagnosis
In the multi-morbidity scenario, patients may have difficulty deciding whether a new symptom is just another manifestation of their known condition, or if another disease has emerged that may need medical attention. Part of the difficulty is that common symptoms frequently cross multiple diseases, and symptoms cluster as well (56). Laypeople have commonsense or mental models of illness, acquired through socialization, modeling and folk transmission, that guide how they label somatic changes and infer that they are experiencing symptoms (57–59). As an example, some studies indicate that most people have a mental model for heart attack including such features as severe chest pain, sweating, and labored breathing (60). Perceived somatic changes that match the person's mental model are labeled as symptoms and may initiate self-care or medical treatment-seeking. Lacking a match, however the person may discount the changes as benign or minor or take a “wait-and-see” approach. People also use heuristic rules, such as “if symptoms develop gradually they are probably due to aging and not to illness,” (61) —that prompt them to discount the somatic changes and not seek care. In short, multi-morbidities that present with a complex pattern of physical changes may create uncertainty for the patient about what they are experiencing and whether there is cause for additional medical or psychological diagnosis and consequent treatment. Much more is known about the diagnosis of single diseases than multiple conditions. Often, each set of symptoms is referred to a different medical specialist, which can produce uncoordinated care and even iatrogenesis if consideration of the entire set of diseases, and their complex interplay is not managed differently than is being done in current practice.
Although research on commonsense models and illness heuristics has acknowledged that circumstances (such as aging or stressful events; 62) can complicate symptom perception and interpretation, the implications of the multi-morbidity scenario for self-care, treatment-seeking, diagnosis and treatment-planning have not been systematically explored. This is another future research direction for behavioral medicine.
Behavioral Intervention and Clinical Guidelines
Outside of the research context, evidence-based clinical guidelines tend to focus on single diseases (63), which means patients with multiple chronic diseases, and the health practitioners who treat them, have to cope with problems of coordination of care (64, 65) and conflicting guidelines (66). For example, a recommendation of physical activity, which would be helpful for chronic obstructive pulmonary disease, may be contraindicated for a patient who also has osteoarthritis of the hip. The general absence of clear clinical guidelines that do not conflict for treating the patient with multiple morbidities represents a significant gap. Some progress, however, is being made in developing and testing alternative co-morbidity-adapted protocols for patients with the most prevalent combinations of concurrent diseases (67).
There is a possibility for synergistic effects of applying a behavioral intervention to multi-morbidity. If we shift to the related area of prevention, sometimes it may be better to have multiple targets. For example, Spring, et al. (68) noted that suboptimal diet and sedentary behavior tend to cluster as risk behaviors and tested whether intervening simultaneously on both may have synergistic benefits. In a randomized clinical trial involving remote coaching supported by mobile decision support technology and financial incentives, the condition in which both diet and activity were behavioral targets fared the best. Perhaps a behavioral intervention that targets two disorders simultaneously allows the patient to build on the initial successes with one condition. Also, because behavioral interventions often target maladaptive cognitive appraisals and affective responses, which can apply to multiple illness conditions, there may be cumulative improvement.
Another hypothesis, based on the “diseasome” and human metabolic networks described earlier (38, 40), is that multiple diseases, characterized as “close,” that is have a family resemblance, based on genes, common mechanisms, interrelated metabolic pathways, or shared risk behaviors, may be more responsive to the same kinds of behavioral interventions than are “distant” co-occurring disorders. This hypothesis is worthy of future research attention.
Challenges to Studying Multi-Morbidity
Although we think it is the right time and behavioral medicine has the appropriate tools to systematically address the topic of multi-morbidities, we also appreciate that logical, empirical and logistical challenges exist. Two are described below.
Heterogeneity in Measuring Multi-morbidity
The identification of patients with multiple chronic illnesses is typically based on disease counts taken from medical records or on clinician or patient checklists (69). Different criteria are used to create counts; sometimes they are based on diseases, health problems or larger categories of “related diseases,” which may vary depending on the clinician’s mental models. The most used common illness checklists also are based on different categorical dimensions. For example, the Cumulative Illness Index (70) collects ratings of severity of impact on 14 distinct organ systems, whereas the Charlson (71) inquires about 14 to 22 distinct diseases. This means there may be considerable variability in the classification of patients with multi-morbidity. We think that advances in network medicine (38,40), based on shared genes and metabolic pathways, offer the potential to improve classification of multi-morbidity.
Diagnosis of multi-morbidity also creates challenges. Having been diagnosed and clinically managed for one condition, the probability that others will be identified may increase because of increased medical scrutiny, or ascertainment bias (18, 72). However, there may also be an opposing (negative ascertainment) bias: in some cases, diagnostic procedures for other conditions (e.g., an asymptomatic cancer) may not be administered. For example, Alzheimer’s patients may be reported to have a lower cancer malignancy rate because they are less likely to be referred for cancer screening (73).
Patient numbers
Some questions about multi-morbidity will require researchers to add new comparison groups to their experimental or observational research designs. For example, to test whether cognitive behavioral therapy is less effective, or synergistic, in hypertension management for patients who also have another chronic condition, it will be necessary to have experimental treatment, usual-care or attention-placebo arms for patients with multiple conditions and for patients with only hypertension. Besides additional labor and expense, there will be a need for more participants to attain sufficient statistical power to detect effects. It is well-known that recruitment numbers for clinical trials are far below what is desirable and several strategies are currently being tested to improve recruitment rates. Encouraging more research on patients with co-occurring illnesses probably will be seen as a significant challenge for trialists and other researchers, who currently are having problems recruiting the requisite numbers just for patients with a single condition.
There is no simple solution, but it should be emphasized that the proportion of patients with multiple disorders is expected to grow as the population ages. Also, it is ironic that one reason researchers encounter difficulties in RCT recruitment is because patients with co-occurring conditions are often excluded from trials. Although recruitment may be challenging, the upside is clinical inferences will be based on more representative samples, and interesting new insights will be attained about the usefulness of behavioral medicine interventions and mechanistic approaches to those with multi-morbidity.
Conclusions
With the aging of the population (74), the prevalence of persons with multiple health problems will only increase. Furthermore, medical advances in cancer and heart disease, as examples, have extended survival so more people will live longer with multiple chronic diseases. Neither mainstream medicine nor behavioral medicine, based mainly on a “one-disease-at-a-time” approach, has fully recognized or addressed the increasing problem of multi-morbidity of primary medical conditions. Understanding the genesis of multiple diseases, their biological and psychosocial consequences and how best to prevent and treat them underscores the need for multiple levels of analysis and transdisciplinary science. Behavioral medicine and health psychology, which incorporate both features because they subscribe to the biopsychosocial model, have much to offer for understanding and improving the well-being of patients with multi-morbidity.
Acknowledgments
The authors extend their thanks to Mary O’Connell for assisting with the figures and to Ian Kronish for reading an earlier draft. Authors, Suls and Green, received no support. Davidson’s work was supported by grants HL-101663, HL-84034, HL114924, and HL-115941 from the National Heart, Lung, and Blood Institute. Additional support was provided by contract ME-1403-12304 PCORI (Patient-Centered Outcomes Research Institute), and the New York Presbyterian Hospital. Davidson is the co-owner of MJBK, a small business that provides mhealth technology solutions to consumers. She is also the co-owner of IOHealthWorks, a small consulting services company. She is a member of the United States Preventive Services Task Force (USPSTF).
Abbreviations
- ANS
Autonomic Nervous System
- HRV
Heart Rate Variability
- SNS
Sympathetic Nervous System
- PNS
Parasympathetic Nervous System
- HPA
Hypothalamic-Adrenal-Cortical Axis
- FDA
Food and Drug Administration
- MCC
Multiple Chronic Conditions
- SEER
Surveillance and End Results Program
Footnotes
No conflicts of interest to report.
Note. The statements and opinions stated in this paper do not necessarily represent the opinions of the National Cancer Institute or views and policies of the United States Preventive Services Task Force.
Another term, multiple chronic conditions (MCC), is used in recent initiatives by the Department of Health and Human Services (20, 21), Agency for Healthcare Research and Quality (22) and the Patient-Centered Outcomes Research Institute (23) and sometimes is treated synonymously with multimorbidity. The U.S. Department of Health and Human Services designation, however, refers to both illnesses and “conditions” (e.g., “risk for falls” in the latter category) lasting a year or more and requiring ongoing medical attention and/or limit activities of daily living (24). We prefer multi-morbidity because of its focus on illness and because MCC implies a “snapshot” of the patient although in many cases one illness emerges prior to the others (10, 15). The phrase, “complex” or “complicated” patients has also been applied to multiple disease scenarios, but the ways that patients are complex or complicated is left ambiguous. Observers also have expressed concerns that these terms may lay the blame on the patient (25). The phrase “complicated patient,” as frequently used, also includes persons with psychosocial difficulties and few financial resources so it does not seem appropriate for our present purposes.
In describing multi-morbidities, we are focusing on the co-occurrence of different diagnosable physical and psychiatric/psychological disorders. Some dispositional risk factors for disease, such as social isolation, perceived stress and hostility, also tend to co-occur and may be causally related (28, 29), but they do not qualify as diagnosable medical conditions. Extended discussion of their relationship and implications for multi-morbidities is beyond the scope of this paper, but they are briefly considered in a subsequent section, “Future Directions.”
Contributor Information
Jerry Suls, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, Bethesda, MD.
Paige A. Green, Chief, behavioral and Psychological Sciences Research Branch, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, Bethesda, MD.
Karina W. Davidson, Center for Behavioral Cardiovascular Health, Department of Medicine, Columbia University Medical College, New York, NY.
References
- 2.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder MD, Weiss KD, Blumenthal D. Multiple Chronic Conditions: Prevalence, Health Consequences, and Implications for Quality, Care Management, and Costs. J Gen Intern Med. 2007;22(Suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Probst-Hensch N. Chronic age-related diseases share risk factors: Do they share pathophysiological mechanisms and does that matter? Swiss Med Wkly. 2010;140:E-1. doi: 10.4414/smw.2010.13072. [DOI] [PubMed] [Google Scholar]
- 3.Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu A, Maltais D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults Estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10 doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. http://www.un.org/esa/population/publications/WPA2009/WPA2009_WorkingPaper.pdf. [Google Scholar]
- 6.Wu S, Green A. Projection of chronic illness prevalence and cost inflation. Washington, DC: RAND Health; 2000. [Google Scholar]
- 7.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: The Women’s Health and Aging Study. J Clin Epidmiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 8.Dy SM, Pfoh ER, Salive ME, Boyd CM. Health-related quality of life and functional status quality indicators for older persons with multiple chronic conditions. J Am Geriatr Soc. 2013;61:2120–2127. doi: 10.1111/jgs.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson G. Chronic care: Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. http://www.rwjf.org/files/research/50968chroniccare.chartbook.pdf. [Google Scholar]
- 10.Vos R, van den Akker M, Boesten J, Robertson C, Metsemakers J. Trajectories of multimorbidity: exploring patterns of multimorbidity in patients with more than ten chronic health problems in life course. BMC Family Practice. 2015;16:2. doi: 10.1186/s12875-014-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer. JNCI. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DM, Curtis RE, Daugherty SE, Goedert JJ, Kuncl RW, Tucker MA. The association between cancer and amyotrophic lateral sclerosis. Cancer Control Causes. 2013;24:55–60. doi: 10.1007/s10552-012-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RJ, Freedland K, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A metaanalysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 14.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biological Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 15.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tinetti ME, Fried TR, Boyd C. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity’s many challenges: Time to focus on the needs of this vulnerable and growing population. BMJ. 2007;334:1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace RB, Salive ME. The dimension of multiple chronic conditions: where we go from here? A commentary on the Special Issue of Preventing Chronic Disease. Prev Chronic Dis. 2013;10:E59. doi: 10.5888/pcd10.130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almirall J, Fortin M. The coexistence of terms to describe the presence of multiple concurrent diseases. J Comorbidity. 2013;3:4–9. doi: 10.15256/joc.2013.3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. Washington, DC: [Accessed February 28, 2013]. Multiple Chronic Conditions-A Strategic Framework: Optimal Health and Quality of Life for Individuals with Multiple Chronic Conditions. http://www.hhs.gov/ash/initiatives/mcc/index.html. [Google Scholar]
- 21.Parekh AK, Goodman RA, Koh HK. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460–471. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinetti M, Basu J. Research on multiple chronic conditions: where we are and where we need to go. Med Care. 2014;52:S3–S6. doi: 10.1097/MLR.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed May 2, 2014]; http://www.fightchronicdisease.org/sites/fightchronicdisease.org/files/docs/Recommended-Topics-for-PCORI-Research-on-Multiple-Chronic-Conditions. [Google Scholar]
- 24.Warshaw G. Introduction: Advances and challenges in care of older people with chronic illness. Generation. 2006;30:5–10. [Google Scholar]
- 25.Arora NK. Letter: A complex patient or complex illnesses? Health Affairs. 2012;31(10):2354. doi: 10.1377/hlthaff.2012.0937. [DOI] [PubMed] [Google Scholar]
- 26.Akker Mvd, Buntinx F, Knotterus A. Comorbidity or multimorbidity: What’s in a name? Eur J Gen Pract. 1996;2:65–70. [Google Scholar]
- 27.Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: A systematic review. J Clin Epidem. 2014;67:254–266. doi: 10.1016/j.jclinepi.2013.09.021. 2014. [DOI] [PubMed] [Google Scholar]
- 28.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005 Mar;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 29.Friedman HS. Long-term relations of personality and health: Dynamism, mechanisms, tropisms. J Person. 2000;68:1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- 30.Swingle J, Braspenning J, Schellevis F, Stirbu-Wagner I, Westert G, Korevaar J. The prevalence of disease clusters in older adults with multiple chronic diseases—a systematic literature review. PLoS One. 2013;8(11):e79641. doi: 10.1371/journal.pone.0079641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and Medicare claims for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 32.Engel G. The need for a new medical model: A challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 33.Suls J, Rothman A. Evolution of the biopsychosocial model: Prospects and challenges. Health Psychol. 2004;23:119–125. doi: 10.1037/0278-6133.23.2.119. [DOI] [PubMed] [Google Scholar]
- 34.Page IH. The mosaic theory of hypertension. In: Bock KD, Cottier PT, editors. Essential Hypertension: An International Symposium. Berlin: Springer-Verlag; pp. 1–29. [Google Scholar]
- 35.Laurberg P, Knudsen N, Andersen S, Carle A, Pedersen IG, Karmisholt J. Thyroid function and obesity. Eur Thyroid J. 2012;1:159–167. doi: 10.1159/000342994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Turner BJ, Hollenbeak CS, Weiner M, Have TT, Tang SSK. Effect of unrelated comorbid conditions on hypertension management. Ann Int Med. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 38.Goh K-I, Cusick ME, Valle D, Childs B, Vidal M, Barabasi A-L. The human disease network. PNAS. 2007;104(21):8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn C, van Broeckhoven C, Gribbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. The Lancet. 1997;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 40.Lee D-S, Park KA, Christakis NA, Oltvai ZN, Barabasi A-L. The implications of human metabolic network topology for disease comorbidity. PNAS. 2008;105(29):9880–9885. doi: 10.1073/pnas.0802208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: a consensus on complexity. Lancet. 2010;375:2201–2202. doi: 10.1016/S0140-6736(10)60706-4. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 43.Sandhu MS, Dunger DB, Giovannucci E. Insulin, insulin-like growth factor I (IGF-1), IGF binding proteins, their biologic interactions, and colorectal cancer. JNCI. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 44.Gandhi S, Fleet JL, Bailey DG, McArthur E, Wald R, Rehman F, Garg AX. Calcium-channel blocker–clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310(23):2544–2553. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 45.Shelburne N, Adhikari B, Brell J, Davis M, Desvigne-Nickens P, Freedman A, Minasian L, Force T, Remick SC. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014 Sep 10;106(9) doi: 10.1093/jnci/dju232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments:what the cardiologist needs to know. Nat Rev Cardio. 2010;7(10):564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 47.Asnis GM, De La Garza R. Interferon-induced depression in chronic hepatitis C: A review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40(4):322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 48.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depression symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19:397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- 50.Morris T, Moore M, Morris F. Stress and chronic illness: The case of diabetes. J Adult Dev. 2011;18:70–80. [Google Scholar]
- 51.Selye H. The Stress of Life (rev. ed.) New York: McGraw-Hill; 1984. (original work published in 1956). [Google Scholar]
- 52.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- 53.Krantz DS, Manuck SB. Acute physiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psych Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 54.Kaplan JR, Manuck SB. Status, stress and atherosclerosis: The role of environment and individual behavior. Annals NY Acad Sciences. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 55.McCabe PM, Gonzales J, Zaias J, Szeto A, Kumar M, Herron A, Schneiderman N. Social environment influences the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Circulation. 2002;105:354–359. doi: 10.1161/hc0302.102144. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K. A practical and evidence-based approach to common symptoms. Ann Intern Med. 2014;161:579–586. doi: 10.7326/M14-0461. [DOI] [PubMed] [Google Scholar]
- 57.Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. In: Rachman S, editor. Contributions to Medical Psychology. Vol. 2. NY: Pergamon; 1980. pp. 7–30. [Google Scholar]
- 58.Pennebaker JW. The Psychology of Physical Symptoms. NY: Springer; 1982. [Google Scholar]
- 59.Skelton JA, Croyle RT, editors. Mental Representations in Health and Illness. NY: Springer; 1991. [Google Scholar]
- 60.Shin J-Y, Martin R, Suls J. Meta-analytic evaluation of gender differences and symptom measurement strategies in acute coronary syndromes. Heart & Lung. 2010;39:283–292. doi: 10.1016/j.hrtlng.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Prohaska TR, Keller ML, Leventhal EA, Leventhal H. Impact of symptoms and aging attribution on emotions and coping. Health Psychol. 1987;6:495–514. doi: 10.1037//0278-6133.6.6.495. [DOI] [PubMed] [Google Scholar]
- 62.Cameron L, Leventhal EA, Leventhal H. Seeking medical care in response to symptoms and life stress. Psychosom Med. 1995;57:37–47. doi: 10.1097/00006842-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Lugtenberg M, Burgers J, Clancey C, Westert G, Schneider E. Current guidelines have limited applicability to patients with comorbid conditions: A systematic analysis of evidence-based guidelines. Plos One. 2011;6:e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One. 2012;7(8):e41601. doi: 10.1371/journal.pone.0041601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 66.Weel C, Schellevis FG. Comorbidity and guidelines: Conflicting interests. Lancet. 2006;367:550–551. doi: 10.1016/S0140-6736(06)68198-1. [DOI] [PubMed] [Google Scholar]
- 67.Rooij M, van der Leeden M, Avezaat E, Hakkinen A, Klaver R, Maas T, Peter WF, Roorda LD, Lems WF, Dekker J. Development of co-morbidity-adapted exercise protocols for patients with knee osteoarthritis. Clinical Interv in Aging. 2014;9:829–842. doi: 10.2147/CIA.S55705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, Moller AC, Epstein LH, DeMott A, Hedeker D, Siddique J, Lloyd-Jones DM. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172(10):789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Annals of Family Medicine. 2012;10:134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linn BS, Linn MW, Gurel L. Cumulative Illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 71.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 72.Ferruci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277:728–734. [PubMed] [Google Scholar]
- 73.Akushevich I, Kravchenko J, Ukraintsea S, Arbeev K. Mortality risks among older adults with pre-existing age-related diseases. Experimental Gerontology. 2013;48:1395–1401. doi: 10.1016/j.exger.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiener JM, Tully J. Population aging in the United States of America: Implications for public programs. Int J Epidemiology. 2002;31:776–781. doi: 10.1093/ije/31.4.776. [DOI] [PubMed] [Google Scholar]