Abstract
In chronic infections and cancer, T cells are exposed to persistent antigen and/or inflammatory signals. This scenario is often associated with the deterioration of T cell function: a state called ‘exhaustion’. Exhausted T cells lose robust effector functions, express multiple inhibitory receptors and are defined by an altered transcriptional programme. T cell exhaustion is often associated with inefficient control of persisting infections and tumours, but revitalization of exhausted T cells can reinvigorate immunity. Here, we review recent advances that provide a clearer molecular understanding of T cell exhaustion and reveal new therapeutic targets for persisting infections and cancer.
During acute infections or vaccinations, naive T cells are activated and differentiate into effector T cells over the course of 1–2 weeks1,2. This differentiation is accompanied by robust proliferation, transcriptional, epigenetic and metabolic reprogramming, and the acquisition of cardinal features of effector T cells such as effector function, altered tissue homing and dramatic numerical expansion1,2. Following the peak of effector expansion, the resolution of inflammation and the clearance of antigen, most activated T cells die, but a subset persists and transitions into the memory T cell pool. These memory T cells downregulate much of the activation programme of effector T cells, yet they maintain the ability to rapidly reactivate effector functions upon restimulation2. In addition, memory T cells develop a key memory property of antigen-independent self-renewal, which is a type of stem cell-like, slow division that is driven by interleukin-7 (IL-7) and IL-15. There is considerable diversity and complexity of memory T cell subsets and differentiation following acute infections or vaccinations (for example, effector memory T cells versus central memory T cells)2. However, a key aspect of the development of functional, persisting memory T cells is that after the effector phase, memory development occurs in the absence of ongoing antigen stimulation and high levels of persisting inflammation.
By contrast, during chronic infections and cancer — which involve persistent antigen exposure and/or inflammation — this programme of memory T cell differentiation is markedly altered3. An altered differentiation state, termed T cell exhaustion, usually manifests with several characteristic features, such as progressive and hierarchical loss of effector functions, sustained upregulation and co-expression of multiple inhibitory receptors, altered expression and use of key transcription factors, metabolic derangements, and a failure to transition to quiescence and acquire antigen-independent memory T cell homeostatic responsiveness3–5 (FIG. 1). Although T cell exhaustion was first described in chronic viral infection in mice6,7, it has also been observed in humans during infections such as HIV and hepatitis C virus (HCV), as well as in cancer3,5. Importantly, while T cell exhaustion prevents optimal control of infections and tumours, modulating pathways overexpressed in exhaustion — for example, by targeting programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte antigen 4 (CTLA4) — can reverse this dysfunctional state and reinvigorate immune responses3,5,8,9. Whereas T cell exhaustion and the reversal of this state of dysfunction have considerable relevance for tumours, an in-depth discussion of T cell exhaustion in cancer is beyond the scope of this Review and has been covered elsewhere recently10,11.
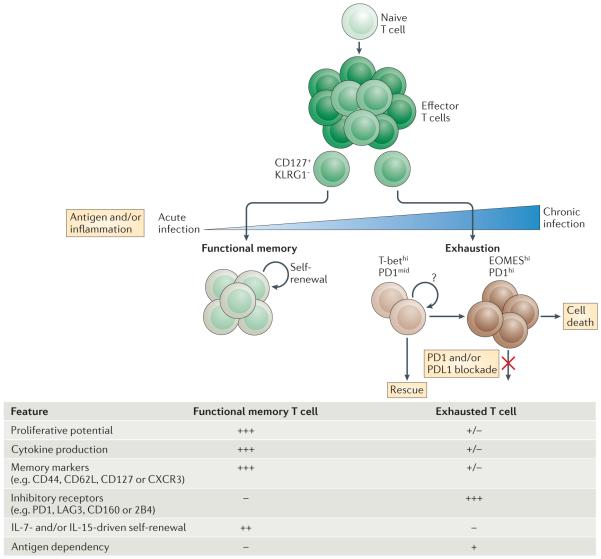
Figure 1. Progressive development of T cell exhaustion.
Upon infection, naive T cells are activated by antigen, co-stimulation and inflammation, and they exponentially proliferate to form effector populations1,2. Whereas the majority of effector CD8+ T cells that express killer cell lectin-like receptor subfamily G member 1 (KLRG1) die during the contraction phase, a population of effector CD8+ T cells that retains CD127 expression can give rise to memory or exhausted CD8+ T cells. In the setting of acute infection, where antigen and/or inflammation is cleared, effector CD8+ T cells further differentiate into functional memory CD8+ T cells that can produce multiple cytokines (such as interferon-γ (IFNγ), tumour necrosis factor (TNF) and interleukin-2 (IL-2)) and mount robust recall responses upon secondary infection1,2. These memory T cells are also maintained efficiently long term without antigen via IL-7- and IL-15-driven homeostatic self-renewal102. By contrast, during chronic infection, antigen and inflammation persist after the effector phase. As infection progresses and T cell stimulation continues, T cells lose effector functions in a hierarchical manner and become exhausted3. Typically, functions such as IL-2 production and cytokine polyfunctionality, as well as high proliferative capacity, are lost early; this is followed by defects in the production of IFNγ, TNF and chemokines, as well as in degranulation. T cell exhaustion is also accompanied by a progressive increase in the amount and diversity of inhibitory receptors that are expressed, including programmed cell death protein 1 (PD1), lymphocyte activation gene 3 protein (LAG3), 2B4, CD160 and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT). Ultimately, if the severity or duration of the infection is high or prolonged, virus-specific T cells can be lost (‘deletion’). Variables — such as the level and number of inhibitory receptors expressed, strength of antigen stimulation, availability of CD4+ T cell help and the duration of infection — can all influence the severity of exhaustion. Exhausted T cell populations are heterogeneous, and subsets of T-bethi PD1mid and EOMEShi PD1hi CD8+ exhausted T cells exist12. Only the T-bethi PD1mid subset is responsive to reinvigoration by blockade of the PD1 pathway42. CXCR3, CXC-chemokine receptor 3; PDL1, PD1 ligand 1.
Of note, exhausted T cells are not inert (BOX 1). These cells retain suboptimal but crucial functions that limit ongoing pathogen replication or tumour progression. Despite this host–pathogen ‘stalemate’ mediated by exhausted T cells, these cells are not effective in eradicating pathogens or tumours, and there has been considerable interest in avoiding or reversing exhaustion. The demonstration that T cell exhaustion is reversible (at least at the population level) rather than a terminal or irreversible fate provides a substantial clinical opportunity to use immunotherapy to improve immunity9. Although the immunological effects of these human treatments remain to be fully defined, emerging results support the notion that reversal of T cell exhaustion in humans is a causative mechanism for the marked antitumour effect that is seen in many patients receiving agents that block the PD1 pathway.
Box 1. Evolutionary perspective on T cell exhaustion.
What is the biological significance of exhausted T cells to the host? First, it is important to point out that exhausted T cells are not inert. In nearly all cases, exhausted T cells have some level of residual function, and this residual function (or other as yet unappreciated properties of exhausted T cells) may be important in vivo. For example, exhausted T cells can drive epitope mutation during chronic infections109, and depletion of CD8+ T cells during the chronic phase of simian immunodeficiency virus (SIV) infection results in rapid and marked exacerbation of viraemia and progression to AIDS137,138. This result indicates that although exhausted CD8+ T cells are poorly functional, they are not functionally inert and have a crucial role in maintaining a virus–host stalemate by restraining viral replication. Moreover, whereas epitope escape in SIV, HIV and hepatitis C virus (HCV) infection often occurs early, escape in the chronic phase of infection — and also probably in the setting of tumour immune editing — may be driven by exhausted T cells. Finally, when the ability to sustain exhausted CD8+ T cell populations is compromised, all containment of chronic infection can be lost12. In settings of chronic infection where immunopathology can occur, T cell exhaustion may serve as a mechanism to protect against tissue damage139. In this context, one might envision that for viruses that cannot be fully eradicated, at least containing these pathogens to low levels might be the evolutionary driver of T cell exhaustion. For herpesviruses, for example, continually driving a virus back into viral latency may require local effector function, but a robust proliferative burst such as might occur for central memory T cells (or even effector memory T cells) might prove pathogenic. Evidence for such a scenario may exist for herpes simplex virus (HSV)140,141, although whether this applies for other herpesviruses remains a subject of some debate. Many persisting pathogens have evolved strategies to establish a host–pathogen balance, and there are many examples of viruses that cause chronic infections by evading immunity through the expression of immune evasion genes and through other strategies. However, in many if not most cases, uncontrolled pathogen replication does not occur, and a balance between pathogen replication and immune control is usually achieved. Overall, despite decreased functionality, the raison d’être for exhausted T cells may be to establish a host–pathogen stalemate for some persisting infections. Other persisting pathogens seem to have exploited this immunological ‘niche’ to continue to replicate, spread and cause disease.
In this Review, we outline the characteristic features of altered functionality that are hallmarks of T cell exhaustion and focus on major advances in three key areas: first, inhibitory receptors and negative regulatory pathways; second, lack of canonical memory T cell properties and maintenance; and third, altered transcriptional control including recently defined progenitor and terminal progeny subsets of exhausted T cells controlled by T-bet and eomesodermin (EOMES)12. Where relevant, we also discuss similarities and differences between CD4+ and CD8+ exhausted T cells.
Developmental pathways of exhaustion
Persistent antigen exposure
A central question in this field has been: what causes T cell exhaustion? The development of CD8+ T cell exhaustion probably integrates information from altered inflammatory and tissue microenvironments, other lymphocyte populations such as CD4+ T cells, B cells and regulatory T (TReg) cells, and also inhibitory signals from cytokines and cell surface inhibitory and co-stimulatory receptors (FIG. 2). However, one key feature seems to be the chronic and probably continuous exposure to antigen rather than acutely terminated or intermittent exposure. Additional factors, including lack of CD4+ T cell help13 and perhaps instructive signals directly from inhibitory receptors14, probably also contribute to T cell exhaustion. Early studies in the chronic lymphocytic choriomeningitis virus (LCMV) model demonstrated that the severity of exhaustion (and ultimately the deletion of antigen-specific T cells) correlated with the level of antigen stimulation15. The importance of the level of antigen persistence in exhaustion was also confirmed in other mouse models and in human HIV-1 infection16–18. Indeed, if CD8+ T cells are primed during infection with a chronic strain of LCMV but removed from persisting antigen stimulation early (approximately 1 week after infection), these cells can differentiate into fully functional CD8+ memory T cells19,20. However, if these T cells are instead exposed to a persisting antigen for 2–4 weeks, T cell exhaustion becomes established, and these cells do not recover normal memory differentiation simply by removal from antigen exposure19,20. These observations are consistent with the notion that early antiviral treatment of HIV infection, and possibly HCV infection, in humans can preserve functional T cell responses17,21. In addition, specific T cell receptor (TCR)-dependent pathways, including those mediated by nuclear factor of activated T cells (NFAT) and sprouty homologue 2 (SPRY2), have been implicated in T cell exhaustion, consistent with a role for ongoing TCR stimulation22–24. Furthermore, chronic antigen stimulation also leads to sustained expression of PD1 through NFAT cytoplasmic 1 (NFATc1)25. It is likely that PD1 further modulates the level of TCR signalling and provides an important feedback loop or rheostat to temper chronic antigen stimulation26,27 (FIG. 3). Thus, the level and duration of chronic antigen stimulation and infection seem to be key factors that lead to T cell exhaustion and correlate with the severity of dysfunction during chronic infection.
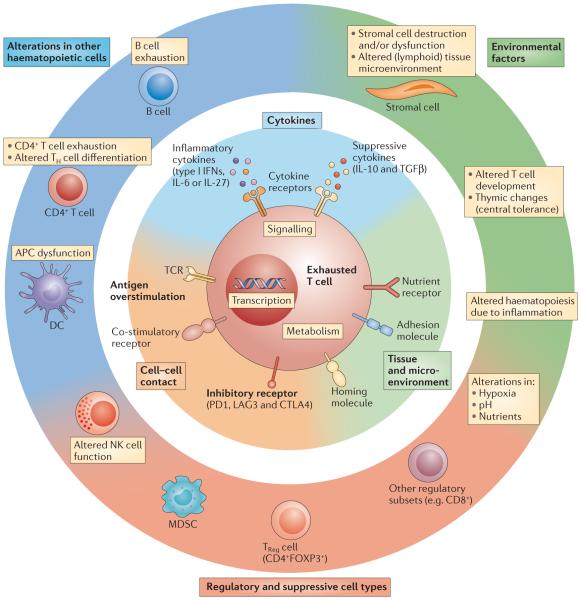
Figure 2. Overview of mechanisms of T cell exhaustion.
Pathways implicated in regulating T cell exhaustion can be classified into three general categories (centre and inner circle): cell-to-cell signals including prolonged T cell receptor (TCR) engagement (signal 1) and co-stimulatory and/or co-inhibitory signals (signal 2); soluble factors such as excessive levels of inflammatory cytokines (for example, type I interferons (IFNs)) and suppressive cytokines including interleukin-10 (IL-10) and transforming growth factor-β (TGFβ); and tissue and microenvironmental influences driven by changes in the expression levels of chemokine receptors, adhesion molecules and nutrient receptors. This last class of influences may include altered tissue distribution and/or migratory patterns and lead to changes in pathways sensing oxygen tension (the von Hippel–Lindau tumour suppressor (VHL) and/or hypoxia-inducible factor (HIF) pathways), pH and nutrient levels. Tissue destruction and altered lymphoid organization may have a major role. Other immune cell types and stromal cells could be the source of many of these changes (outer circle). Cell types such as antigen-presenting cells (APCs), CD4+ T cells, natural killer (NK) cells, B cells and regulatory cells (for example, myeloid-derived suppressor cells (MDSCs) and regulatory T (TReg) cells) have been implicated in CD8+ T cell exhaustion. Overall, during chronic infections, cell-intrinsic and cell-extrinsic signals are probably integrated and thereby negatively influence T cell differentiation and promote exhaustion. The precise balance of these signals may determine the severity and/or qualitative aspects of T cell exhaustion in different disease settings. CTLA4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; FOXP3, forkhead box P3; LAG3, lymphocyte activation gene 3 protein; PD1, programmed cell death protein 1; TH cell, T helper cell.
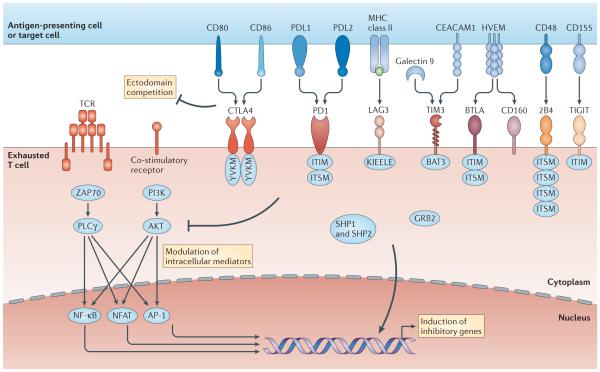
Figure 3. Molecular pathways of inhibitory receptors associated with T cell exhaustion.
Ligand and receptor pairs for inhibitory pathways are depicted, showing the intracellular domains of receptors that contribute to T cell exhaustion. Many inhibitory receptors have immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and/or immunoreceptor tyrosine-based switch motifs (ITSMs) in their intracellular domains; however, some receptors have specific motifs, such as YVKM for cytotoxic T lymphocyte antigen 4 (CTLA4) and KIEELE for lymphocyte activation gene 3 protein (LAG3). The molecular mechanisms of inhibitory receptor signalling are also illustrated and can be classified as: ectodomain competition (inhibitory receptors sequester target receptors or ligands); modulation of intracellular mediators (local and transient intracellular attenuation of positive signals from activating receptors such as T cell receptors and co-stimulatory receptors); and induction of inhibitory genes. Multiple inhibitory receptors are responsible for these three mechanisms. AP-1, activator protein 1; BAT3, HLA-B-associated transcript 3 (also known as BAG6); BTLA, B and T lymphocyte attenuator; CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1; GRB2, growth factor receptor-bound protein 2; HVEM, herpes virus entry mediator (also known as TNFRSF14); NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; PD1, programmed cell death protein 1; PDL1, PD1 ligand 1; PI3K, phosphoinositide 3-kinase; PLCγ, phospholipase Cγ; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains; TIM3, T cell immunoglobulin and mucin domain-containing protein 3.
Inhibitory receptors
Inhibitory receptors are crucial negative regulatory pathways that control autoreactivity and immunopathology28. Although inhibitory receptors are transiently expressed in functional effector T cells during activation, higher and sustained expression of inhibitory receptors is a hallmark of exhausted T cells. The inhibitory signalling pathway mediated by PD1 in response to binding of PD1 ligand 1 (PDL1) and/or PDL2 offers an illustrative example27,29,30. Whereas our understanding of the molecular mechanisms by which the inhibitory receptor PD1 controls T cell exhaustion remains incomplete, there are several mechanisms by which inhibitory receptors such as PD1 might regulate T cell function5,29 (FIG. 3): first, by ectodomain competition, which refers to inhibitory receptors sequestering target receptors or ligands and/or preventing the optimal formation of microclusters and lipid rafts (for example, CTLA4 (REF. 31)); second, through modulation of intracellular mediators, which can cause local and transient intracellular attenuation of positive signals from activating receptors such as the TCR and co-stimulatory receptors32–34; and third, through the induction of inhibitory genes14.
Whereas there is some knowledge about PD1, our understanding of the intracellular mechanisms of action of inhibitory receptors — including those of PD1 — is incomplete. The intracellular domain of PD1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM)35. In vitro studies suggest a role for the ITSM in recruiting the tyrosine-protein phosphatase SHP1 (also known as PTPN6) and/or SHP2 (also known as PTPN11)33,36. The role of the ITIM in PD1 function remains poorly understood. Other evidence implicates a role for PD1 signalling in modulating the phosphoinositide 3-kinase (PI3K), AKT and RAS pathways32,37, and also links PD1 to cell cycle control38. Notably, much of our information about how PD1 controls T cell signalling is derived from in vitro studies of acutely activated T cells. In vivo studies of the role of PD1 during acute T cell activation and expansion suggest a possible role for PD1 signalling in either increasing mobility paralysis39 or decreasing migratory arrest26, depending on the context. Finally, signalling downstream of PD1 may in fact induce the expression of genes that could negatively regulate the expression of effector genes, such as BATF, which encodes the activator protein 1 (AP-1) family member basic leucine zipper transcription factor ATF-like14. Despite this elegant work, it is unclear how these observations relate to exhausted T cells exposed to chronic infection in vivo.
PD1 expression is rapidly upregulated upon T cell activation30, and it may persist at moderate levels in healthy humans40,41, indicating that PD1 expression alone is not a unique feature of exhausted T cells. However, during chronic infections PD1 expression can be substantially higher than observed on functional effector or memory CD8+ T cells42,43. During chronic infection, sustained upregulation of PD1 is usually dependent on continued epitope recognition44, although examples exist of residual PD1 expression even after removal of persisting antigen signalling20,45.
In addition to PD1, exhausted T cells express a range of other cell surface inhibitory molecules (FIG. 3). Exhausted T cells can co-express PD1 together with lymphocyte activation gene 3 protein (LAG3), 2B4 (also known as CD244), CD160, T cell immunoglobulin domain and mucin domain-containing protein 3 (TIM3; also known as HAVCR2), CTLA4 and many other inhibitory receptors18. Typically, the higher the number of inhibitory receptors co-expressed by exhausted T cells, the more severe the exhaustion. Indeed, although individual expression of PD1 or other inhibitory receptors is not indicative of exhaustion, co-expression of multiple inhibitory receptors is a cardinal feature. These co-expression patterns are mechanistically relevant, as simultaneous blockade of multiple inhibitory receptors results in synergistic reversal of T cell exhaustion. This concept was demonstrated for PD1 and LAG3 (REF. 18) in chronic LCMV infection, and for PD1 and CTLA4 in HIV infection46, other infections47 and cancer48,49. Many other combinations of inhibitory receptors such as PD1 and TIM3 (REFS 50–53) can also co-regulate exhausted T cells. PD1 and CTLA4 blockade in patients with melanoma demonstrated impressive tumour control54, and clinical trials of other combinations of agents blocking inhibitory receptors are underway (for example, ClinicalTrials.gov identifiers NCT01968109, NCT02210117 and NCT02408861, which are among >120 other trials involving the PD1 pathway). Overall, these data on the role of inhibitory receptors in co-regulation of T cell exhaustion suggest that these pathways are non-redundant. These molecules come from diverse structural families, bind ligands with distinct expression patterns and have distinct intracellular signalling domains (FIG. 3). Thus, there is the potential to tailor or tune the type and magnitude of exhausted T cell reinvigoration.
In addition to inhibitory receptors, it has become clear that co-stimulatory receptors are involved in T cell exhaustion29. For example, desensitization of co-stimulatory pathway signalling through the loss of adaptor molecules can serve as a mechanism of T cell dysfunction during chronic infection. The signalling adaptor tumour necrosis factor receptor (TNFR)-associated factor 1 (TRAF1) is downregulated in dysfunctional T cells in HIV progressors, as well as in chronic LCMV infection55. Adoptive transfer of CD8+ T cells expressing TRAF1 enhanced control of chronic LCMV infection compared with transfer of TRAF1-deficient CD8+ T cells, which indicates a crucial role for TRAF1-dependent co-stimulatory pathways in this setting55. It has also been possible to exploit the potential beneficial role of co-stimulation to reverse exhaustion by combining agonistic antibodies to positive co-stimulatory pathways with blockade of inhibitory pathways. 4-1BB (also known as CD137 and TNFRSF9) is a TNFR family member and positive co-stimulatory molecule that is expressed on activated T cells. Combining PD1 blockade and treatment with an agonistic antibody to 4-1BB dramatically improved exhausted T cell function and viral control56. Although a simple model of positive versus negative co-stimulation during T cell exhaustion probably has mechanistic validity, the diversity of pathways and much of the experimental data suggest that specific qualitative signals may be imparted by distinct co-stimulatory and co-inhibitory pathways.
Soluble mediators
Soluble molecules are a second class of signals that regulate T cell exhaustion (FIGS 2,3); these include immunosuppressive cytokines such as IL-10 and transforming growth factor-β (TGFβ) and inflammatory cytokines such as type I interferons (IFNs) and IL-6. For example, the IL-10–IL-10 receptor (IL-10R) pathway has received considerable attention for its role in T cell exhaustion. Blockade of IL-10 restores T cell function and improves viral control during chronic viral infections, demonstrating that IL-10 promotes or fosters T cell exhaustion57,58. Studies of LCMV infection in mice and HIV in humans demonstrated that many cell types can be the source of IL-10 during chronic infection including dendritic cells (DCs), monocytes and/or CD4+ T cells59–61. The most relevant source of this cytokine remains a matter of debate, although recent studies suggest that TH1 cells expressing B lymphocyte-induced maturation protein 1 (BLIMP1; also known as PRDM1) are important IL-10 producers in chronic LCMV infection62. Simultaneous blockade of IL-10 and the PD1 pathway in mice synergistically reverses CD8+ T cell exhaustion and enhances viral control, which indicates a role for IL-10 in controlling CD8+ T cell exhaustion63. Some evidence suggests a potential connection between the PD1 pathway and IL-10 production through induction of IL-10 by monocytes following ligation of PD1 by PDL1 (REF. 60). Despite the clear evidence that blocking IL-10 reverses exhaustion, the molecular events downstream of IL-10 signalling (presumably via signal transducer and activator of transcription 3 (STAT3)), which shape T cell exhaustion, remain to be more precisely defined.
TGFβ has also been implicated in T cell exhaustion. Phosphorylation of SMAD2 in CD8+ T cells was increased during chronic LCMV infection compared with acute infection, which suggests a role for TGFβ signalling. In vivo inhibition of TGFβ signalling in CD8+ T cells through expression of a dominant-negative receptor improved the function of exhausted T cells64. However, recent studies in mice using systemic administration of a TGFβ inhibitor or a blocking antibody found little benefit of these treatments65,66. Although it is difficult to directly compare the genetic approach to antibody- and inhibitor-based strategies, these observations may warrant further evaluation of the role of this immunoregulatory pathway in driving CD8+ T cell exhaustion.
The type I IFNs IFNα and IFNβ (hereafter referred to as IFNα/β) are crucial inflammatory cytokines that have essential antiviral functions early during infection. In most cases, acute viral control is dramatically compromised in the absence of IFNα/β signalling; this was recently confirmed in simian immunodeficiency virus (SIV) infection of macaques67. Moreover, IFNα/β signalling can provide a critical ‘signal 3’ for proper activation and differentiation of CD8+ T cells following priming68. In addition to these crucial innate antiviral effects, however, recent studies have demonstrated a surprising role for chronic type I IFN signalling in facilitating viral persistence by promoting immune suppression and lymphoid tissue destruction during chronic infection. Despite the direct antiviral activity of IFNα/β, blocking this pathway during LCMV clone 13 infection paradoxically enhanced control of chronic viral replication and reversed and/or prevented T cell exhaustion69,70. Interestingly, applying this IFNα/β blockade early (within the first 1–2 days after infection) prevented later development of T cell exhaustion, suggesting a possible early role for IFNα/β signalling in ‘programming’ exhaustion. The IFNα/β effect seemed to operate via CD4+ T cells, though the precise mechanism remains to be defined. For example, IFNα/β can induce several immunoregulatory molecules including IL-10, PDL1 and indoleamine 2,3-dioxygenase (IDO) in antigen-presenting cells (APCs) and other cell types71. Thus, whereas IFNα/β signals enhance viral control early during infection directly and indirectly (that is, by promoting effector CD8+ T cell differentiation), chronic exposure to IFNα/β can be detrimental and/or enhance T cell dysfunction during chronic infections. Indeed, exposure to chronic inflammation alone (that is, in the absence of TCR signalling) can impair optimal CD8+ memory T cell differentiation72. By contrast, other inflammatory pathways — including those induced by IL-6 and IL-27 — may be required for controlling chronic viral infection and avoiding CD8+ T cell exhaustion73,74. Although there is no clear evidence that IL-6 and IL-27 have direct intrinsic effects on CD8+ T cell exhaustion, these cytokines regulate CD4+ T cell differentiation and/or exhaustion during chronic viral infection. Thus, among inflammatory cytokines, there are some such as IL-6 and IL-27 that may have positive effects and skew T cells away from exhaustion, whereas others such as IFNα/β have more complex roles including positive and negative effects depending on the context. Effects of these cytokines on CD4+ T cell responses, such as enhanced IL-21 production, may have down-stream effects on preventing CD8+ T cell exhaustion (see below).
Regulatory cells
It is well known that forkhead box P3-expressing (FOXP3+) CD4+ TReg cells influence immune responses during many infections75,76 (FIG. 2). TReg cells can suppress T cell responses in acute infection and in the acute phase of chronic infection. The frequency of TReg cells can be increased in human chronic HIV and HCV infections, in which TReg cells may limit the responses of antiviral effector T cells in vitro76. However, it is still unclear whether TReg cells can directly cause CD8+ T cell and FOXP3−CD4+ T cell exhaustion. As TReg cells are a source of IL-10, TGFβ and perhaps other suppressive cytokines (for example, IL-35)76, such a scenario might exist. However, precisely how TReg cells affect the development of T cell exhaustion remains incompletely defined. Recent work in the chronic LCMV model system demonstrated an interaction between TRegcells and the PD1 pathway in regulating exhausted CD8+ T cells because simultaneous depletion of TReg cells and blockade of PD1 signalling had a highly synergistic effect on viral control and reversal of exhaustion77. These data suggest a role for TReg cells in CD8+ T cell exhaustion. In addition to FOXP3+CD4+ TReg cells, other regulatory cell types — such as immunoregulatory APCs, myeloid-derived suppressor cells (MDSCs)78,79, natural killer (NK) cells and even CD8+ regulatory populations80–82 — may influence viral persistence during chronic infections and directly or indirectly promote T cell exhaustion. Professional APCs can be direct targets of viruses, particularly during persisting infections, and dysfunction of DC cytokine production and cross-presentation has been reported in some chronic infections78,83. Indeed, early dysfunction of DCs may have a major role in facilitating viral persistence in some contexts83. Furthermore, destruction of tissue architecture and fibrosis of secondary lymphoid organs have been reported in both mice and human chronic infection84–86. Changes in lymphoid tissue architecture may contribute to poor T cell responses, but precisely how these anatomical features influence T cell exhaustion remains to be fully defined.
In addition to a possible role for TReg cells and other regulatory populations, positive signals from other cells are crucial in avoiding T cell exhaustion. It has long been known that loss of CD4+ T cell help during pathogen persistence can underlie or contribute to defective CD8+ T cell responses. Depletion of CD4+ T cells in the chronic LCMV model results in failed containment of chronic infection and more severe CD8+ T cell exhaustion13. In HIV infection, loss of CD4+ T cells is associated with increased exhaustion of CD8+ T cells and with disease progression. Although CD4+ T cells can clearly become exhausted (BOX 2), the impact of changes in the CD4+ T cell response on CD8+ T cell exhaustion is highly relevant. One key factor produced by CD4+ T cells during chronic infections is IL-21. In the absence of IL-21 signalling, CD8+ T cell exhaustion is substantially worse during chronic LCMV infection87–89, with similar observations in HIV infection90,91. IL-21 and CD4+ T cell help are also essential for optimal antibody responses, and recent work highlights the importance of antibody, including non-neutralizing antibody, during ongoing chronic viral infections92–95. It is interesting in this respect that antiviral CD4+ T cells generated during chronic infection demonstrate a skewing towards the T follicular helper (TFH) cell phenotype95,96. Further investigation is required to determine whether the key role of IL-21 in limiting CD8+ T cell exhaustion is only due to a direct effect on CD8+ T cells or whether it also includes a component of antibody-mediated reduction in antigen load.
Box 2. CD4+ T cell exhaustion.
Virus-specific CD4+ T cells also lose effector function during chronic viral infections19,96,142,143. In fact, dysregulation of CD4+ T cell responses may be a crucial event in failed immune control in human chronic infections144,145. Exhausted CD4+ T cells, similar to exhausted CD8+ T cells, display reduced production of effector cytokines (such as tumour necrosis factor and interferon-γ (IFNγ)) and express high levels of programmed cell death protein 1 (PD1)96. In addition, exhausted CD4+ T cells have characteristics that are distinct from exhausted CD8+ T cells. For instance, poor effector function is often apparent earlier for virus-specific CD4+ T cells than for CD8+ T cells during chronic infection19,96,143, and exhausted CD4+ T cells often produce interleukin-10 (IL-10) and/or IL-21 (REFS 57,95,96). Moreover, there is evidence of a skewing towards T follicular helper (TFH) cells during chronic viral infections95,96 that may be dependent on chronic IFNα/β signalling146. These observations are intriguing and may provide insights that are relevant to cryoglobulinaemia and to altered antibody production during some human chronic infections147–149, as well as for the importance of IL-21 signalling in sustaining exhausted CD8+ T cells87–89. Thus, patterns of differentiation for exhausted CD4+ T cells suggest perhaps more complex regulation than is required for the differentiation of exhausted CD8+ T cells.
Overall, although various negative and positive regulatory pathways have been implicated in exhaustion, the role of these pathways in actually causing CD8+ T cell exhaustion has been less clear, in part because we are only just beginning to understand the developmental origin of exhausted CD8+ T cells.
Origin, homeostasis and durability
Questions have arisen about the origin of exhausted CD8+ T cells. Are these cells simply persisting effector T cells? How are exhausted CD8+ T cells, different from other dysfunctional T cells, such as senescent T cells or anergic T cells (TABLE 1)? Whereas there are some similarities between exhausted and CD8+ T cells, the transcriptional profiles of these two subsets are distinct4,97. Phenotypic markers can be identified that distinguish effector T cells from exhausted T cells, including markers that are highly expressed on effector CD8+ T cells — such as CD44, LY6C and killer cell lectin-like receptor subfamily G member 1 (KLRG1) — but are expressed at low or intermediate levels on exhausted T cells, as well as markers such as inhibitory receptors that are expressed at substantially higher levels by exhausted CD8+ T cells (TABLE 1). In addition, whereas CD8+ effector T cells co-express the transcription factors T-bet and EOMES98, exhausted T cell subsets express either T-bet or EOMES in a somewhat mutually exclusive pattern12 (see below).
Table 1.
A comparison of dysfunctional T cell responses
| Feature | Exhaustion | Anergy | Senescence | Refs |
|---|---|---|---|---|
| Main cause | Excessive and continuous stimulation |
Suboptimal stimulation | Repetitive stimulation | 3,5,100 |
| Nature and time frame | Persistent overstimulation of signal 1 and signal 3 leads to progressive loss of effector and memory function (typically within a few weeks) |
When signal 2 and/or signal 3 are either absent or weak at priming, T cell dysfunction occurs at an earlier time point (typically within a few days) |
Intermittent and multiple stimulations after the primary response lead to a limit in cell replication at later time point (typically within months to years) |
3,5,15, 20,100,101 |
| Proliferative capacity | Low | Low | Low | 3,100 |
| Expression of inhibitory markers |
High (and multiple markers are expressed) |
? | Low |
11,18,41, 99,100 |
| Expression of NK cell receptor markers |
None or low | None | High |
11,18,41, 99,100 |
| Expression of CD57 (human) and KLRG1 (mouse) |
None or low | ? | High |
11,18,41, 99,100 |
| Effector function | Low to moderate | None or low | High | 3,100,101 |
| Telomerase activity | Low | ? | Low | 3,100 |
| Primed properly | Yes | No | Yes | 3,5 |
Question mark depicts unknown effects. KLRG1, killer cell lectin-like receptor subfamily G member 1; NK, natural killer.
Recent studies have directly compared the transcription programmes of exhausted CD4+ and CD8+ T cells in the lymphocytic choriomeningitis virus (LCMV) model system96. These studies confirmed a core transcriptional signature of T cell exhaustion common to both CD4+ and CD8+ T cells that included some transcription factors, inhibitory receptors, IFNα/β signature genes, and dysregulated pathways such as proliferation. However, despite this similar core programme, exhausted CD4+ T cells were distinct from exhausted CD8+ T cells. For example, whereas PD1 expression was common to both lineages, the expression pattern of other inhibitory receptors was distinct for each subset46,96. Of note, the expression pattern of transcription factors differed extensively; exhausted CD4+ T cells had altered expression of, for example, GATA-binding protein 3, B cell lymphoma 6 (BCL-6) and Helios, which was not observed for exhausted CD8+ T cells. Together with altered expression of IL-10 and IL-21 and the redirection of CD4+ T cell responses from T helper 1 cells to TFH-like cells95,96,146, these data suggest an altered and perhaps more complex differentiation programme for exhausted CD4+ T cells than previously appreciated. Indeed, transcription factor co-expression patterns in exhausted CD4+ T cells reveal substantial heterogeneity in this population96. Thus, although our understanding of exhaustion in CD4+ T cells still lags behind that in CD8+ T cells, the existing data suggest a distinct pattern of differentiation that may include enhanced skewing towards TFH cell-like cells, altered immunoregulatory pathways and a lineage-specific network of transcription factor control96. Future studies are needed to explore this heterogeneity in differentiation in more detail and to define the pathways and mechanisms that might be used to reverse dysfunction and reinvigorate exhausted CD4+ T cells.
Some of these observations also suggest a distinction between phenotypically defined senescence and exhaustion (TABLE 1). Whereas senescent CD8+ T cells are often defined by high expression of markers such as KLRG1 and/or CD57 (REFS 41,99,100), exhausted CD8+ T cells tend to have low expression of these markers97. Furthermore, exhausted CD8+ T cells have high expression of PD1, whereas senescent cells do not101. Although both exhausted and senescent CD8+ T cells have low proliferative capacity, this difference in phenotype suggests a potential distinction between these two cell states100. Analysis of the lineage origin of exhausted CD8+ T cells in the chronic LCMV model has demonstrated that exhausted T cells do not derive from the ‘senescent’ KLRG1+ subset of effector CD8+ T cells but rather from the CD127+ subset of memory precursors that are present in the effector phase20 (FIG. 1). These studies reinforce the notion that exhaustion and senescence are distinct and that exhausted CD8+ T cells arise from a skewed pattern of memory T cell differentiation rather than from a residual population of effector CD8+ T cells.
Exhausted CD8+ T cells have maintenance characteristics that are distinct from memory T cells that are generated after acute infection. Following acute infection, CD8+ memory T cells can persist long term in the absence of antigen via IL-7- and IL-15-driven slow, steady homeostatic turnover (see below). This self-renewal capacity develops after the effector phase as CD8+ memory T cells acquire high co-expression of both CD122 (also known as IL-2Rβ) and CD127, which are the key receptor subunits for responsiveness to IL-7 and IL-15 (REFS 1,3,102). By contrast, exhausted CD8+ T cells have lower expression of CD122 and CD127 during chronic infection and are unable to respond efficiently to IL-7 or IL-15. As a result, exhausted T cells are maintained poorly when adoptively transferred to infection (antigen)-free recipients, although in some settings small numbers of these cells may persist45,103–107 (TABLE 1). Virus-specific CD8+ T cells become ‘addicted’ to their cognate antigen, and continuous TCR stimulation is needed for the maintenance of exhausted T cells103,104. A similar scenario is also likely to occur in humans based on apparent loss of virus-specific CD8+ T cells after antiviral treatment or epitope escape21,108,109. This antigen-dependent maintenance is accompanied by extensive ongoing division of a subset of exhausted CD8+ T cells; for example, a subset of exhausted T cells gives rise to cells that divide at least 5–7 times per week for a prolonged period of time in chronic LCMV infection12,104. This maintenance pattern is in stark contrast to the slow homeostatic turnover of memory CD8+ T cells, for which there are only one or two divisions in a month. Similar maintenance phenotypes have also been reported for T cells responding to persisting herpesviruses110,111.
A key unanswered question, therefore, is: what happens to exhausted CD8+ T cells in clinical settings in which chronic infections are cured? Will these exhausted CD8+ T cells disappear? Will they differentiate into functional T cells? As we rapidly move towards a drug-mediated cure of HCV infection — a setting that often shows marked T cell exhaustion — understanding the fate of exhausted T cells after cure, and determining whether these cells can provide protection if re-exposed to HCV, will become critical. Similar scenarios may also exist in cancer immunotherapy. Better defined cellular and molecular mechanisms that underlie the persistence of exhausted CD8+ T cells will be important in answering these questions.
Homeostatic cytokines
In contrast to the immunosuppressive cytokines described above, common γ-chain cytokines can positively affect T cell responses during chronic viral infections. IL-2 treatment during chronic LCMV infection resulted in an increase in the number of antigen-specific CD8+ T cells and increased viral control, consistent with reversal of T cell exhaustion112. Combining IL-2 treatment with blockade of the PD1-mediated inhibitory pathway had striking synergistic effects for re-invigorating exhausted CD8+ T cells and decreasing viral load113. IL-7 treatment early during chronic LCMV infection augmented T cell responses and enhanced viral clearance, effects that were in part dependent on inhibition of suppressor of cytokine signalling 3 (SOCS3) and on endogenous IL-6 (REFS 114,115). This early IL-7 treatment probably occurred at time points before severe loss of IL-7 responsiveness during chronic LCMV infection. As discussed above, IL-21 also has an important role during chronic infection. IL-21, produced primarily by antigen-specific CD4+ T cells, is required to sustain antiviral CD8+ T cell responses and prevent exhaustion during chronic LCMV infection87–89,91. However, IL-21 may also restrict virus-driven TReg cell populations116. Despite many important insights about the role of common γ-chain cytokines in chronic infection, it remains unclear where and when these signals are important in vivo during chronic infections, what regulates their availability and how signalling via IL-21R, for example, alters the cellular and molecular mechanisms that maintain exhausted CD8+ T cells.
Maintaining exhausted T cell populations
The identification of subsets of exhausted CD8+ T cells defined by expression of PD1, CD44, other phenotypic markers and the transcription factors T-bet and EOMES has enabled the lineage dynamics of these populations to be defined12,42 (FIG. 1). Specifically, T-bet and EOMES delineate key subsets of exhausted CD8+ T cells and a proliferative hierarchy that maintains exhausted T cell populations12. Exhausted CD8+ T cells are under considerable strain to maintain ongoing antigen-driven activation and proliferation without depleting the ‘clone’ of antigen-specific CD8+ T cells. T-bet and EOMES cooperate to sustain the overall exhausted CD8+ T cell pool. Specifically, two distinct subpopulations exist: a small T-bethiPD1mid progenitor pool with residual proliferative potential and a numerically larger population of EOMEShiPD1hi terminal progeny with higher co-expression of other inhibitory receptors and limited proliferative capacity12 (FIG. 1). This role for T-bet is interesting because whereas high levels of T-bet expression are closely associated with terminal differentiation in acute infection, high T-bet expression in chronic infection partially represses the expression of PD1 and other inhibitory receptors and identifies a subset of exhausted CD8+ T cells that retains some (albeit weakened) proliferative capacity during chronic infection12,42,117. Persistent antigen causes T-bethiPD1mid to lose T-bet expression, undergo proliferation and convert to EOMEShiPD1hi cells12. The resulting terminal progeny have weaker cytokine production but enhanced killing ability. They also accumulate in peripheral tissues and have a total population size that is ~20-fold greater than the T-bethi progenitor pool. Importantly, PD1 pathway blockade seems to only reinvigorate the T-bethi subset, while having little to no impact on the EOMEShi cells42, indicating an important aspect of population dynamics in checkpoint blockade-mediated reversal of T cell exhaustion. Importantly, genetic deletion of either transcription factor leads to loss of the respective subset (that expresses high levels of the transcription factor), failed long-term maintenance of the exhausted CD8+ T cell pool and loss of the ability to establish a host–pathogen stalemate, resulting in high viral loads. Although these lineage relationships were defined in the chronic LCMV model, central aspects of this model — including the existence of exhausted CD8+ T cells subsets defined by T-bet and EOMES, and a bias towards the EOMEShi terminally differentiated subset with more severe infection — have been confirmed in human HCV and HIV infection12,118. Persistently high levels of antigen might be anticipated to cause continuous proliferation and depletion of the progenitor pool. Although there is some evidence for erosion of the T-bethi progenitor pool in prolonged viraemia12, whether this population self-renews is unclear. It is also possible that replenishment could occur from new thymic emigrants primed on persisting antigen119,120. Although mice that have undergone thymectomy do not have major defects in maintaining antiviral CD8+ T cell responses during chronic infection121, and adoptively transferred TCR-transgenic cells become exhausted and are maintained long-term without thymic contribution117,122, new thymic emigrants could make quantitative or qualitative contributions to virus-specific CD8+ T cell responses in the long term. Thus, the existence of a proliferative hierarchy for the maintenance of exhausted CD8+ T cells provides a framework for understanding the durability of these cells over time, but many important questions remain about the signals involved and molecular mechanisms underlying this process. In addition, how these events change during infections with different levels and/or types of persistence remains to be explored.
Transcriptional control of exhaustion
Recent studies have applied genomic approaches to investigate the transcriptional circuitry underlying T cell exhaustion. Both CD4+ and CD8+ exhausted T cells have a transcriptional profile that is markedly different from effector and memory CD4+ or CD8+ T cells, including major changes in the expression of inhibitory and co-stimulatory receptors, transcription factors, signalling molecules, cytokine and chemokine receptors, and genes involved in metabolism4,96,97. Thus, in addition to phenotyping, fate-mapping and functional analysis, genomic studies also support the concept that exhausted T cells represent a unique state of T cell differentiation. These studies have also revealed key differences between the exhausted phenotypes of CD4+ and CD8+ T cells (BOX 2).
Although considerable progress has been made in defining centrally important transcription factors, a lineage-specific transcription factor for exhausted T cells has not been identified. Nonetheless, in addition to T-bet and EOMES discussed above, the transcription factors BLIMP1, NFAT, BATF, von Hippel–Lindau disease tumour suppressor (VHL), FOXO1 and FOXP1 have been implicated in CD8+ T cell exhaustion12,14,22,24,117,123–126, and it is likely that other transcription factors involved in T cell exhaustion remain to be identified. A key concept that has emerged, however, is that although these transcription factors can have roles in other T cell populations, the expression pattern, the genes controlled and the manner in which key transcription factors operate can be distinct in exhausted T cells compared with in other T cell subsets (FIG. 4). In other words, there are exhaustion-specific functions for key transcription factors. For example, whereas T-bet is expressed by, and has a functional role in the formation of, terminally differentiated CD8+ T cells in acute infection1,127, during chronic infection T-bet controls the population of non-terminal progenitor cells within the exhausted T cell pool12 (see above and FIG. 1). Similarly, EOMES is involved in central memory T cells following acute infection, when it is involved in controlling quiescence and homeostatic turnover128–130. However, during chronic infection EOMES controls the formation of a terminally differentiated (non-quiescent) subset of exhausted T cells that is highly enriched in peripheral tissues12. This distinct context-specific reuse of transcription factors was further interrogated using transcriptional network analysis, which demonstrated that the same transcription factors can have markedly different functions in memory CD8+ T cells versus exhausted CD8+ T cells4. The concepts of context-specific transcription factor function and combinatorial transcription factor control of cellular differentiation and/or lineage development have also been observed in other settings131,132. These different functions could occur for various reasons including the availability of distinct cofactors, concentration-dependent binding to different genomic sites and altered genomic accessibility due to epigenetic changes (FIG. 4).
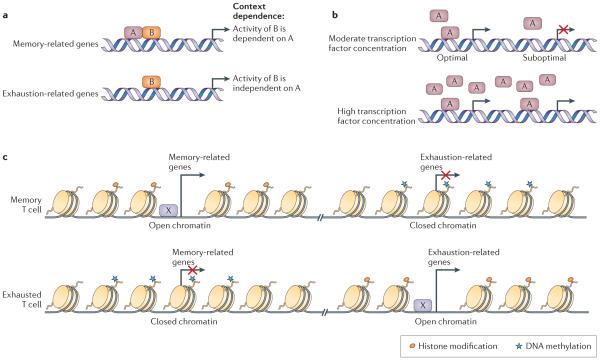
Figure 4. Transcriptional and epigenetic mechanisms of T cell exhaustion.
Altered usage of key transcription factors is associated with the altered transcriptional and developmental programme of T cell exhaustion. Several potential mechanisms exist. a | Different use of transcription factor binding partners is one potential mechanism for distinct context-dependent transcription factor activity. In the example shown, transcription factor B is closely associated with memory-related genes in the context of acute infection where transcription factor A is abundant (top panel). However, the same transcription factor B is linked with exhaustion-related genes in chronic infection (lower panel). Post-translational modifications of transcription factors and/or their subcellular localization may also be important in this setting. b | The dosage or concentration of a transcription factor could also provide a mechanism for context-dependent transcriptional function. Here, a transcription factor binds only at specific high-affinity binding sites if the amount of the factor is low (top panel). By contrast, at high transcription factor concentrations binding can occur more broadly (that is, occurring also at lower affinity sites), which leads to different transcriptional activity (lower panel). c | DNA methylation, histone modifications and the ‘chromatin landscape’ resulting from overall epigenetic regulation could provide a mechanism for context-specific transcription factor function in exhausted T cells (transcription factor X in this example). The enhancer landscape of a cell may determine how different transcription factors function.
The epigenome provides the context in which transcription factors function. Although global epigenetic landscape information does not yet exist for exhausted T cells, studies of the Pdcd1 locus (which encodes PD1) have been informative (FIG. 4b). Analysis of the Pdcd1 promoter region in acutely resolved LCMV infection demonstrated that these regions were largely demethylated in the effector phase and then became remethylated as infection resolved and CD8+ T cell memory formed122. By contrast, the Pdcd1 locus became completely demethylated in chronic LCMV infection and no remethylation was observed, even when viral titres and PD1 protein expression by exhausted CD8+ T cells decreased122. Similar data were obtained in studies examining well-controlled HIV infection133. These observations and other studies134 suggest not only an important layer of epigenetic regulation on gene expression in CD8+ T cell exhaustion but also provide evidence for a durable imprint of exhaustion in the epigenome. Future studies are clearly warranted to define global epigenetic changes in more detail, to determine how such an imprint will affect the success of immunotherapies and the development of memory following cure of chronic infections.
Open questions and future directions
Although advances over the past decade have shed considerable light on the mechanisms of T cell exhaustion and have revealed novel therapeutic opportunities, there are still many important unanswered questions. The recognition of potent and diverse negative regulatory pathways, such as the PD1 pathway, that can be targeted experimentally and clinically has been a major step forward with the potential to revolutionize immunotherapy of chronic infections and cancer. Despite this promise, we still understand surprisingly little about the molecular mechanisms by which these pathways operate, and where, when and how these negative pathways signal and initiate or execute a programme of exhaustion. Moreover, we still lack a true molecular understanding of the intriguing observations of synergy when targeting multiple pathways simultaneously for reversal of T cell exhaustion. In addition to inhibitory receptors, one of the emerging defining features of T cell exhaustion is the altered use of key transcription factors (FIG. 4). In general, we are only just beginning to understand the transcriptional coordination of T cell exhaustion, how these transcriptional networks change over time and what happens to these events upon reversal of exhaustion.
In addition to understanding the molecular mechanisms of inhibitory receptor and transcription factor function in T cell exhaustion, several other key areas are likely to reveal substantial new insights into fundamental aspects of T cell exhaustion. First, we lack an understanding of the epigenetic landscape of T cell exhaustion (FIG. 4). A second related issue is to define the nature of terminal differentiation in the exhausted T cell pool. Cellular reprogramming studies have demonstrated that, if taken back to an embryonic stem cell, exhausted CD8+ T cells can give rise to new naive T cells135. Although this is important in demonstrating the concept of reprogramming, such an approach may be challenging to implement clinically. Thus, it will be important to define the nature of terminal differentiation versus reprogramming that can occur for exhausted T cells at the epigenetic level. Given the clinical opportunities with PD1 blockade and also the ability to cure chronic infections such as HCV, it will be crucial to investigate these issues. Finally, numerous recent studies have addressed the critical importance of metabolism in regulating the T cell differentiation136. Although emerging studies have begun to investigate these issues for chronic infections124,125, a more comprehensive understanding of metabolic alterations in exhausted T cells will probably be highly informative.
In summary, T cell exhaustion represents a distinct state of T cell differentiation with considerable clinical relevance. Recent work defining the mechanisms of T cell exhaustion has provided many insights as well as clinical opportunities. Despite this progress, our understanding of T cell exhaustion and how to most effectively reverse this state remains incomplete. Future mechanistic and clinical studies are needed to develop the next generation of immune-based interventions for chronic infections and cancer.
Acknowledgements
The authors thank the members of Wherry laboratory for discussions. In particular, they would like to thank J. Kurachi for technical assistance and figure preparation, and E. Stelekati and K. Pauken for helpful comments. The authors’ work is supported by The Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Young Scientists (B) 22790453 to M.K.), the Uehara Memorial Foundation of Japan (M.K.) and the US National Institutes of Health (grants AI105343, AI112521, AI082630, AI095608 and HHSN266200500030C to E.J.W.).
Glossary
- Effector memory T cells
A subset of memory T cells that resides in peripheral tissues and retains high levels of effector function such as the production of interferon-γ and granzymes.
- Central memory T cells
A subset of memory T cells that expresses CD62L and CC-chemokine receptor 7 and resides mainly in the lymph nodes and spleen. These cells have high proliferative potential upon antigen re-encounter.
- Lymphocytic choriomeningitis virus (LCMV)
A virus that induces a strong T cell response and therefore provides good mouse model of infection. Although they only differ by two nucleotides, the Armstrong strain of LCMV causes acute infection and the clone 13 strain causes chronic infection. As major antigenic epitopes are conserved between these two strains, the fate of antigen-specific T cells can be easily compared (that is, memory after infection with the Armstrong strain versus exhaustion after infection with the clone 13 strain).
- Antigen-presenting cells (APCs)
T cells can be activated when antigen is displayed by major histocompatibility complexes (MHC restriction). This antigen presentation is largely executed by professional APCs, particularly dendritic cells. APCs also influence T cell differentiation by producing cytokines such as type I interferons and interleukin-12.
- Senescent T cells
Cells that enter a terminal differentiation state owing to excessive cell replication. This state is associated with irreversible cell cycle arrest and telomere shortening.
- Anergic T cells
An unresponsive state that is induced by suboptimal stimulation (that is, signal 1 without signal 2) at the time of priming by antigen.
- Epigenetic landscape
Historically, this is proposed to refer to embryonic development and how genes might interact with their surroundings to lead to the expression of a certain phenotype. The current view of the epigenetic landscape is that overall epigenetic changes such as (but not limited to) DNA methylation and histone modifications, are accumulated genome wide and have strong impact on cellular differentiation.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
References
- 1.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;131:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Doering TA, et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. This study shows that memory and exhausted CD8+ T cells have partially non-overlapping modules and centrally connected genes that are thought to be the hubs or foci of biological processes. This reference also indicates that transcription factors have distinct connections in exhausted CD8+ T cells compared with memory CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. References 6 and 7 describe T cell exhaustion as the dysfunction and subsequent physical deletion of antigen-specific T cells during chronic viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. This study shows that exhausted CD8+ T cells can be reinvigorated by blocking PD1 signalling during chronic viral infection in mice. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nat. Rev. Immunol. 2014;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. This study shows the lineage relationships, hierarchy and cooperative maintenance of subpopulations of exhausted CD8+ T cells during chronic infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. This study shows that CD4+ T cell help is also required to sustain CD8+ T cell responses during chronic viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J. Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streeck H, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. This study shows that inhibitory receptors are co-expressed by exhausted T cells, that their expression is correlated with the severity of T cell exhaustion, and that co-blockade of multiple inhibitory receptors synergistically reinvigorates exhausted T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasprowicz V, et al. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J. Virol. 2010;84:1656–1663. doi: 10.1128/JVI.01499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnellini P, et al. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl Acad. Sci. USA. 2007;104:4565–4570. doi: 10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu YL, et al. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J. Clin. Invest. 2014;124:198–208. doi: 10.1172/JCI70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez GJ, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. This study shows that AP-1-independent NFAT activity promotes T cell anergy and exhaustion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda T, et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40:235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 28.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 29.Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J. Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki K, Youngblood B, Ahmed R. Programmed cell death 1-directed immunotherapy for enhancing T-cell function. Cold Spring Harb. Symp. Quant. Biol. 2013;78:239–247. doi: 10.1101/sqb.2013.78.019869. [DOI] [PubMed] [Google Scholar]
- 31.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison J. Bsource-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Msourcel. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton KL, et al. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J. Immunol. 2014;192:782–791. doi: 10.4049/jimmunol.1302663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley JL. Immunol. Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 37.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11:4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinselmeyer BH, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duraiswamy J, et al. Phenotype, function, and gene expression profiles of programmed death-1hi CD8 T cells in healthy human adults. J. Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolfi DV, et al. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J. Leukoc. Biol. 2013;93:825–836. doi: 10.1189/jlb.0912438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by anti-PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. This study identifies subsets of exhausted CD8+ T cells and shows that exhausted CD8+ T cells with intermediate expression of PD1 can be reinvigorated by PD1 blockade, whereas a PD1hi subset cannot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 44.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utzschneider DT, et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 2013;14:603–610. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann DE, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 47.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat. Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grosso JF, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J. Immunol. 2009;182:6659–6669. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kassu A, et al. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J. Immunol. 2010;185:3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. This study shows that co-blockade of PD1 and CTLA4 in humans leads to impressive tumour regression compared with monotherapy in patients with melanoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J. Exp. Med. 2012;209:77–91. doi: 10.1084/jem.20110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vezys V, et al. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J. Immunol. 2011;187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. References 57 and 58 show that the immunoregulatory cytokine IL-10 has an important role in promoting or sustaining T cell exhaustion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng CT, Oldstone MB. Infected CD8α– dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc. Natl Acad. Sci. USA. 2012;109:14116–14121. doi: 10.1073/pnas.1211910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richter K, et al. Macrophage and T cell produced IL-10 promotes viral chronicity. PLoS Pathog. 2013;9:e1003735. doi: 10.1371/journal.ppat.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parish IA, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J. Clin. Invest. 2014;124:3455–3468. doi: 10.1172/JCI66108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brooks DG, et al. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl Acad. Sci. USA. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garidou L, Heydari S, Gossa S, McGavern DB. Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J. Virol. 2012;86:7060–7071. doi: 10.1128/JVI.00164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boettler T, Cheng Y, Ehrhardt K, von Herrath M. TGF-β blockade does not improve control of an established persistent viral infection. Viral Immunol. 2012;25:232–238. doi: 10.1089/vim.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. References 69 and 70 show that while acutely antiviral, sustained type I IFN activity has detrimental effects on antiviral T cell immunity and paradoxically fosters viral persistance and T cell exhaustion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stelekati E, et al. Bystander chronic infection negatively impacts development of CD8+ T cell memory. Immunity. 2014;40:801–813. doi: 10.1016/j.immuni.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harker JA, Dolgoter A, Zuniga EI. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4+ T cell responses and viral control during chronic infection. Immunity. 2013;39:548–559. doi: 10.1016/j.immuni.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 76.Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol. Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penaloza-MacMaster P, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013;13:652–664. doi: 10.1016/j.chom.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 2013;255:210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holderried TA, Lang PA, Kim HJ, Cantor H. Genetic disruption of CD8+ T activity enhances the immune response to viral infection. Proc. Natl Acad. Sci. USA. 2013;110:21089–21094. doi: 10.1073/pnas.1320999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joosten SA, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc. Natl Acad. Sci. USA. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MBA. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 2004;113:737–745. doi: 10.1172/JCI20243. This study shows that DCs can be infected by LCMV and that altered DC development and/or function is associated with reduced antigen presentation and with T cell dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mueller SN, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl Acad. Sci. USA. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schacker T. The role of secondary lymphatic tissue in immune deficiency of HIV infection. AIDS. 2008;22(Suppl. 3):13–18. doi: 10.1097/01.aids.0000327511.76126.b5. [DOI] [PubMed] [Google Scholar]
- 86.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 89.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. References 87–89 show that IL-21 produced by antigen-specific CD4+ T cells during chronic LCMV infection is needed to sustain antiviral CD8+ T cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams LD, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chevalier MF, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Straub T, et al. Nucleoprotein-specific nonneutralizing antibodies speed up LCMV elimination independently of complement and FcγR. Eur. J. Immunol. 2013;43:2338–2348. doi: 10.1002/eji.201343565. [DOI] [PubMed] [Google Scholar]
- 94.Richter K, Oxenius A. Non-neutralizing antibodies protect from chronic LCMV infection independently of activating FcγR or complement. Eur. J. Immunol. 2013;43:2349–2360. doi: 10.1002/eji.201343566. [DOI] [PubMed] [Google Scholar]
- 95.Fahey LM, et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crawford A, et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 99.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 100.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 101.Wirth TC, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 103.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia F, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS. doi: 10.1097/00002030-200106150-00002. [DOI] [PubMed] [Google Scholar]
- 106.Ortiz GM, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl Acad. Sci. USA. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Altfeld M, et al. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Invest. 2002;109:837–843. doi: 10.1172/JCI14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alter G, et al. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 2003;171:477–488. doi: 10.4049/jimmunol.171.1.477. [DOI] [PubMed] [Google Scholar]
- 109.Jamieson BD, et al. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J. Immunol. 2003;171:5372–5379. doi: 10.4049/jimmunol.171.10.5372. [DOI] [PubMed] [Google Scholar]
- 110.Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 111.Snyder CM, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 113.West EE, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pellegrini M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 115.Nanjappa SG, Kim EH, Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117:5123–5132. doi: 10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmitz I, et al. IL-21 restricts virus-driven TReg cell expansion in chronic LCMV infection. PLoS Pathog. 2013;9:e1003362. doi: 10.1371/journal.ppat.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kao C, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. This study shows that the transcription factor T-bet is differentially used in memory T cells compared with exhausted T cells and that T-bet directly represses expression of the gene encoding PD1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buggert M, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10:e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vezys V, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson JJ, et al. CD8 T cells recruited early in mouse polyomavirus infection undergo exhaustion. J. Immunol. 2012;188:4340–4348. doi: 10.4049/jimmunol.1103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller NE, Bonczyk JR, Nakayama Y, Suresh M. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J. Virol. 2005;79:9419–9429. doi: 10.1128/JVI.79.15.9419-9429.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Youngblood B, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. This study provides initial insights into the epigenetic changes that underlie T cell exhaustion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doedens AL, et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staron MM, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stephen TL, et al. Transforming growth factor β-mediated suppression of antitumour T cells requires FoxP1 transcription factor expression. Immunity. 2014;41:427–439. doi: 10.1016/j.immuni.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paley MA, et al. Technical advance: fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J. Leukoc. Biol. 2013;93:307–315. doi: 10.1189/jlb.0812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Banerjee A, et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou X, et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mullen AC, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trompouki E, et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Youngblood B, et al. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J. Immunol. 2013;191:540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang F, et al. Epigenetic manipulation restores functions of defective CD8+ T cells from chronic viral infection. Mol. Ther. 2014;22:1698–1706. doi: 10.1038/mt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nishimura T, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 136.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 138.Jin X, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cornberg M, et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front. Immunol. 2013;4:475. doi: 10.3389/fimmu.2013.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khanna KM, Lepisto AJ, Decman V, Hendricks RL. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 2004;16:463–469. doi: 10.1016/j.coi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 141.Frank GM, et al. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 143.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morou A, Palmer BE, Kaufmann DE. Distinctive features of CD4+ T cell dysfunction in chronic viral infections. Curr. Opin. HIV AIDS. 2014;9:446–451. doi: 10.1097/COH.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schulze Zur Wiesch J, et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 2012;209:61–75. doi: 10.1084/jem.20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Osokine I, et al. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc. Natl Acad. Sci. USA. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76:818–824. doi: 10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haas A, Zimmermann K, Oxenius A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J. Virol. 2011;85:12102–12113. doi: 10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hunziker L, et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]