Summary
Methicillin resistance creates a major obstacle for treatment of Staphylococcus aureus infections. The resistance gene, mecA, is carried on a large (20kb to >60kb) genomic island, staphylococcal cassette chromosome mec (SCCmec), that excises from and inserts site-specifically into the staphylococcal chromosome. However, although SCCmec has been designated a mobile genetic element, a mechanism for its transfer has not been defined. Here we demonstrate the capture and conjugative transfer of excised SCCmec. SCCmec was captured on pGO400, a mupirocin-resistant derivative of the pGO1/pSK41 staphylococcal conjugative plasmid lineage, and pGO400::SCCmec (pRM27) was transferred by filter-mating into both homologous and heterologous S. aureus recipients representing a range of clonal complexes as well as S. epidermidis. The DNA sequence of pRM27 showed that SCCmec had been transferred in its entirety and that its capture had occurred by recombination between IS257/431 elements present on all SCCmec types and pGO1/pSK41 conjugative plasmids. The captured SCCmec excised from the plasmid and inserted site-specifically into the chromosomal att site of both an isogenic S. aureus and a S. epidermidis recipient. These studies describe a means by which methicillin resistance can be environmentally disseminated and a novel mechanism, IS-mediated recombination, for the capture and conjugative transfer of genomic islands.
Keywords: staphylococci, methicillin resistance, conjugation, gene transfer, SCCmec, genomic island
Abbreviated Summary to Accompany Graphical Abstract
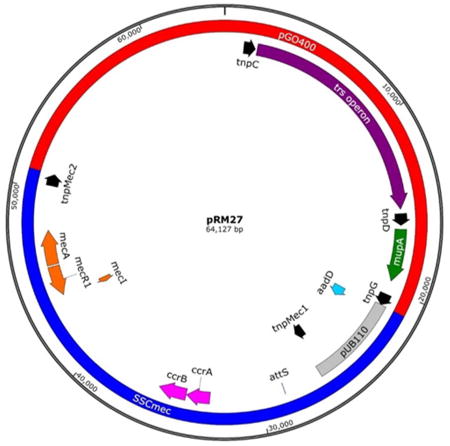
SCCmec, the genomic island containing the gene that encodes methicillin resistance, was captured on a conjugative plasmid by recombination at IS elements and transferred by conjugation between different Staphylococcus aureus sequence types and between S. aureus and Staphylococcus epidermidis. In some of the recipients SCCmec excised from the plasmid and inserted site-specifically into the recipient chromosome. This is the first demonstration of a mechanism for the interspecies dissemination of methicillin resistance among staphylococci.
Introduction
Staphylococcus aureus, a component of the normal human microflora, is an opportunistic pathogen that is a leading cause of both hospital and community-acquired infections in the United States (Kluytmans et al, 1997, Anderson et al, 2014, Dukie et al, 2013, Dantes et al 2011). Its prevalence as a cause of infections is largely due to the development of resistance to antimicrobial agents, most importantly beta-lactam antibiotics (Dantes et al 2011). These bacteria are collectively called methicillin-resistant S. aureus or MRSA. Not only does methicillin resistance preclude treatment of infections with the most effective group of antibiotics, it is usually associated with resistance to additional antibacterial agents, producing the multiresistant phenotype and further compromising therapy (Chambers and Deleo, 2009).
Methicillin resistance is mediated by the acquisition of the gene mecA, encoding a new drug-resistant target (PBP2a), which is always carried on a genomic island referred to as staphylococcal cassette chromosome mec (SCCmec) (Katayama et al, 2000). These elements vary in size from approximately 20 kb to > 60 kb and are divided into types, currently types I to XII, based on the sequences of mecA regulators (mecR1 and mecI) and serine recombinases (ccrAB and ccrC) that mediate the excision, circularization, and chromosomal insertion of SCCmec (Katayama et al, 2000, Wang and Archer, 2010, IWG-SCC, 2009). All of these elements also contain from one to four copies of the insertion sequence, IS257/431. The variable content of SCCmec includes integrated plasmids and transposons that often contain additional antibiotic resistance genes (IWG-SCC, 2009). The Ccr-mediated excision and insertion of SCCmec has been used as evidence that the element is mobile. Further evidence for SCCmec as a mobile genetic element is provided by the presence of identical SCCmec elements in different staphylococcal species (Shore and Coleman, 2013, Wisplinghoff et al, 2003) and different SCCmec types in S. aureus strains with the same genetic background (Enright et al, 2002). The appearance of SCCmec type IV in S. epidermidis, years before the same element became widespread in S. aureus has led to the speculation that coagulase-negative staphylococci (CoNS) serve as the reservoir for SCCmec (Wisplinghoff et al, 2003).
Conjugative plasmids of the pGO1/pSK41 lineage, carrying identical transfer genes and oriT sequences, are widespread among MRSA and CoNS, and can transfer between multiple different staphylococcal species. These plasmids carry multiple antibiotic resistance genes that integrate and excise from the plasmid by means of IS431/257-mediated recombination or transposition (Caryl and O’Neill, 2009, Berg et al, 1998, Leelaporn et al, 1996). The presence of multiple identical 790 kb IS elements in all SCCmec types and on all conjugative plasmids led us to hypothesize that these IS elements may serve as recombination sites for the capture and integration of SCCmec into a model conjugative plasmid with its subsequent transfer into appropriate recipients. The following report details the successful confirmation of this hypothesis.
Results
Construction of donor strain and determination of SCCmec excision kinetics
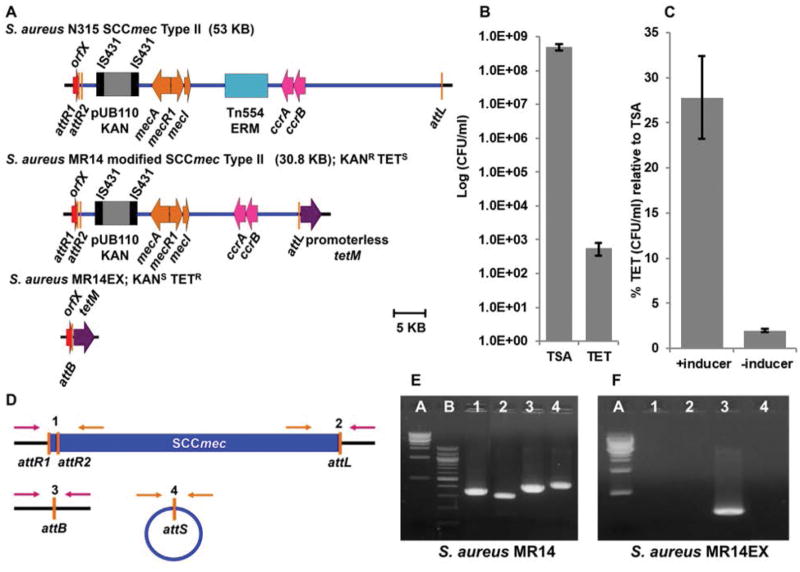
Following CcrAB-mediated excision of SCCmec from the chromosome, the element is circularized by the joining of the terminal att sites, attR and attL, to form attS. The circularized element has not been shown to replicate. It was felt that the first step in SCCmec transfer would be the plasmid rescue of the excised circular element and it was therefore necessary to characterize the SCCmec excision kinetics from the donor MRSA strain, N315. In order to measure the excision frequency of SCCmec, a positive selection strain for excision (S. aureus MR14) was created by modifying S. aureus N315 such that excision would generate tetracycline resistance (Fig. 1A). The promoterless tetracycline-resistance gene, tetM, was inserted just downstream of attL so that when SCCmec excised the gene would be driven by the orfX promoter at the attR end of the element. orfX is the gene containing the attB site into which SCCmec always inserts. We found, however, that there were promoter sequences in SCCmec proximal to attL that produced tetracycline resistance in the absence of excision, so we progressively deleted DNA until there was no baseline tetracycline resistance. The deletion removed 15 kb between ccrAB and attL. None of the genes contained in this region have been shown to have a role in either SCCmec excision or the expression of methicillin resistance. Sixteen open reading frames were deleted, three transposase fragments, five genes encoding a two component system duplicated elsewhere in the core genome and eight hypothetical genes of unknown function. In addition, the transposon Tn554 was deleted from the element to eliminate erythromycin resistance so that we could potentially use this resistance phenotype as a recipient selection marker in future transfer studies. The resulting donor strain derivative of N315 was designated MR14 and SCCmec was reduced to 30.8 kb, 22.2 kb smaller than the original, a size that was also felt to be easier to capture on a conjugative plasmid. The spontaneous excision frequency of SCCmec in MR14 was first determined by comparing the tetracycline resistance (excised) population to the total population of bacterial cells (Fig. 1B) and was found to be 10−6. This low spontaneous excision frequency is a reflection of the stability of chromosomally integrated SCCmec and is supported by previous observations that integration is favored over excision (Wang et al, 2015). In order to improve the opportunity for plasmid capture of the excised circular element it was necessary to increase the SCCmec excision frequency. Since it has previously been shown that overproduction of CcrAB drives excision and prevents reinsertion (Wang and Archer, 2010), we induced ccrAB expression by incorporating the genes on a plasmid, pWA46, under the control of a cadmium-inducible promoter resulting in an SCCmec excision frequency of 28% (Fig. 1C). The excision frequency in the absence of cadmium induction was higher than the spontaneous excision rate both because ccrAB was on a high-copy number plasmid and the cadmium-inducible promoter has baseline activity in the absence of inducer. It was also important to determine if pWA46 could completely drive excision, with no subsequent reinsertion, producing an MR14 population with no remaining chromosomal SCCmec. We used high-level kanamycin resistance (100μg/ml in agar for selection), encoded within SCCmec on the integrated plasmid pUB110, as a SCCmec selection marker rather than methicillin resistance because the latter can have variable expression and is not reliable for primary selection. After six days of passage of MR14 in the presence of cadmium we plated the strain on kanamycin and saw no colonies indicating the complete loss of the excised circular element without its reinsertion.
Fig. 1.
(A) S. aureus N315, MR14, MR14EX. S. aureus strain N315 contains a 53 kb SCCmec type II element. The gene mecA encodes methicillin resistance; mecR1 and mecI encode mecA regulators; and ccrAB encode recombinases for SCCmec excision and integration. N315 was modified to construct MR14 for positive selection for excision. A promoterless tetracycline resistance gene, tetM, was inserted immediately downstream of attL, and Tn554 and 15.4 kb of DNA upstream of tetM were deleted. MR14EX is the excised tetracycline-resistant derivative of MR14 with tetM expression driven from the orfX promoter.
(B) S. aureus MR14 spontaneous excision. Serial dilutions of an overnight MR14 culture were plated on TSA and TSA with tetracycline (TET). The frequency of spontaneous excision was the excised population (TET) divided by the total population (TSA). Bars indicate the standard deviation of three independent experiments.
(C) S. aureus MR14 pWA46 inducible excision monitored by positive selection on tetracycline in the presence or absence of inducer (5μM cadmium chloride). Excision percentages were determined by plating overnight cultures on TSA and TSA with tetracycline and comparing growth on tetracycline to total growth without antibiotic selection. Bars indicate the standard deviation of three independent experiments.
(D) Diagram of the PCR approach used to determine integrated, excised, and circular SCCmec. Primer sets 1 and 2 amplify attR and attL integrated junctions, respectively. Primer set 3 amplifies excised chromosomal junction (attB). Primer set 4 amplifies excised circular SCCmec junction (attS).
(E) PCR analysis of strain MR14. Lane A, 1 kb molecular size marker; lanes 1 and 2, integrated attR and attL amplicons, respectively; lane 3, excised chromosomal junction (attB); lane 4, circular SCCmec junction (attS).
(F) PCR analysis of strain MR14EX. Same lanes as in Fig. 1E. Lane 3 shows only the excised chromosomal junction (attB) is present. Lane 4 shows the circular SCCmec intermediate (attS), following excision, has been lost.
A polymerase chain reaction (PCR)-based approach was also designed to monitor excision and confirm the presence or loss of the excised circular SCCmec element (Fig. 1D). Using this approach, we demonstrated that MR14 undergoing spontaneous excision is composed of a mixed population of integrated, excised, and circular SCCmec products (Fig. 1E). However, with passage in the absence of SCCmec selection, as described above, the non-replicating circular intermediate was lost confirming the production of a completely excised population. (S. aureus MR14EX; Fig. 1F). A donor strain was thus produced that could be used to select for SCCmec plasmid rescue: any colonies growing on tetracycline and kanamycin after passage in cadmium would have completely excised SCCmec (tetracycline resistance) and a circular intermediate rescued on a replicating plasmid (kanamycin resistance).
SCCmec capture on a conjugative plasmid
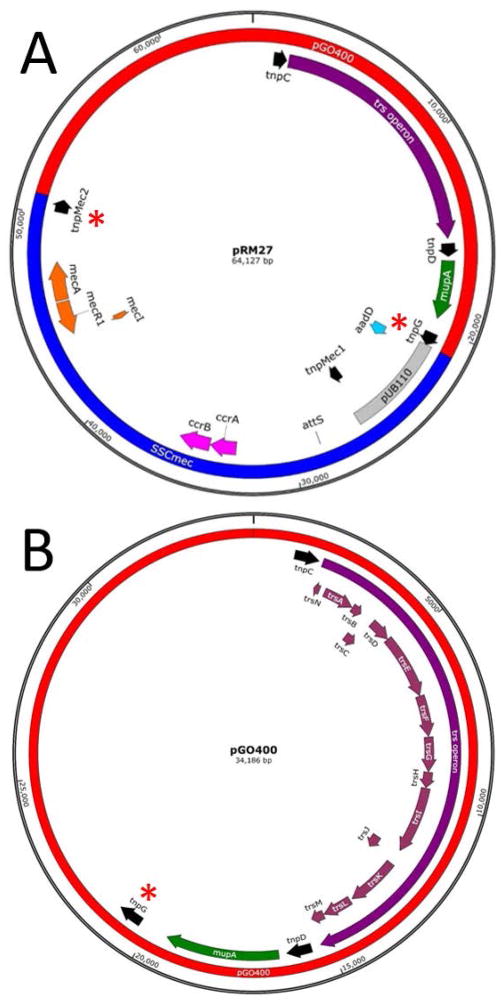
The plasmid that was used to rescue excised SCCmec was pGO400, a 34 kb mupirocin-resistant member of the pGO1/pSK41 family of staphylococcal conjugative plasmids (Morton et al, 1995; Fig. 5). The initial hypothesis was that rescue would be accomplished by homologous recombination between copies of IS431/257. Two of these elements are present in MR14 within SCCmec, flanking integrated pUB110, and three are present on pGO400. Following introduction of pGO400 into MR14 the strain was passaged for nine days in the presence of cadmium to drive CcrAB-induced excision, tetracycline to select the excised population, chloramphenicol to maintain pWA46 and mupirocin to maintain pGO400. Cells were then plated on kanamycin to identify colonies with plasmid-captured SCCmec. From a total population of 8.7 × 108 CFU/ml, there was an average of 7.5 × 101 CFU/ml that grew in the presence of kanamycin, tetracycline, mupirocin, and chloramphenicol.
Fig. 5.
(A) pRM27 is a 64,127 bp plasmid consisting of the conjugative plasmid pGO400 (red) and captured, circularized SCCmec (blue). Loci of interest include the trs operon (purple) responsible for the conjugative transfer of the plasmid between strains and species; mupirocin resistance gene, mupA (green); IS elements (black), three from pGO400 (tnpC, tnpD, and tnpG) and two from SCCmec (tnpMec1 and tnpMec2). The insertion of SCCmec into tnpG and its duplication to form tnpMec2 are marked with red asterisks. The latter two flank pUB110 (grey) which also contains the gene for kanamycin resistance (aadD, light blue). Also shown are the mec operon (orange) and the ccr operon (pink) encoding CcrA and CcrB, the proteins responsible for site-specific recombination between attS (marked) and attB in the S. aureus genome. Accession number KT780705.
(B) Chromosomal map of the conjugative plasmid pGO400 showing the 3 IS431/IS257 elements in black, tnpG, the IS element into which SCCmec inserted is marked with an asterisk, mupirocin resistance gene mupA in green and the genes of the transferase (trs) operon in maroon. Accession number KT780704.
Conjugative transfer of captured SCCmec
SCCmec capture in MR14 could have either been on pWA46 due to ccrAB homology or pGO400 due to IS-mediated homology. In order to isolate pGO400::SCCmec plasmids, filter mating was performed into N315EX, an isogenic recipient with no chromosomal SCCmec, as determined by PCR using primers that were positive for attB and negative for attR and attL as shown in figure 1D. N315ex was made resistant to novobiocin and rifampin by serial passage in these antibiotics (Fig 2). Colonies growing on novobiocin, rifampin, mupirocin and kanamycin but susceptible to chloramphenicol and tetracycline (donor markers) were further analyzed. The recipients were all heterotypically resistant to oxacillin. PCR analysis confirmed the presence of pGO400 by amplification of mupA and SCCmec by amplification of mecA (Fig. 3A). There was also an amplicon for the circular junction, attS, which could only be present if excised circular SCCmec had integrated into another replicon by recombining at a site other than an att site. The presumed pGO400::SCCmec plasmid was designated pRM27. In addition, amplicons were present for both attR and attL, the left and right integration junctions, confirming the integration of SCCmec into the chromosome. A mating was next performed using the N315EX pRM27 transconjugant as donor and a heterologous strain, RN4220, as recipient. N315 is a member of clonal complex (CC) 5, sequence type (ST) 5, and RN4220 is of the genetically distantly-related CC8(ST8). CC5 and CC8 are two of the most common MRSA clonal complexes (Dantes et al 2011, Wisplinghoff et al, 2003). RN4220 was made tetracycline resistant by the insertion of pCN36, which served as the recipient selection marker (Fig. 2). Transfer was confirmed by growth in the presence of recipient and pRM27 antibiotic selection and the absence of donor markers; by PCR showing the presence of RN4220 and the absence of N315 chromosomal markers; and by the presence of plasmid markers mecA, mupA and attS (Fig 3B). It is of interest that there was no PCR evidence for SCCmec integration into the RN4220 chromosome (PCR positive for attB with no product for attR or attL) as there was for N315EX (Fig 1E and F; Fig. 3A and 3B). If selection pressure on pRM27 was maintained with kanamycin and mupirocin, pRM27 was stable in RN4220 and RN4220 pCN36 pRM27 was used as a donor in additional matings. However, in the absence of kanamycin selection, SCCmec was lost from pRM27 on subsequent matings at a rate of approximately 2% per mating, as determined by the number of kanamycin susceptible transconjugants.
Fig. 2.
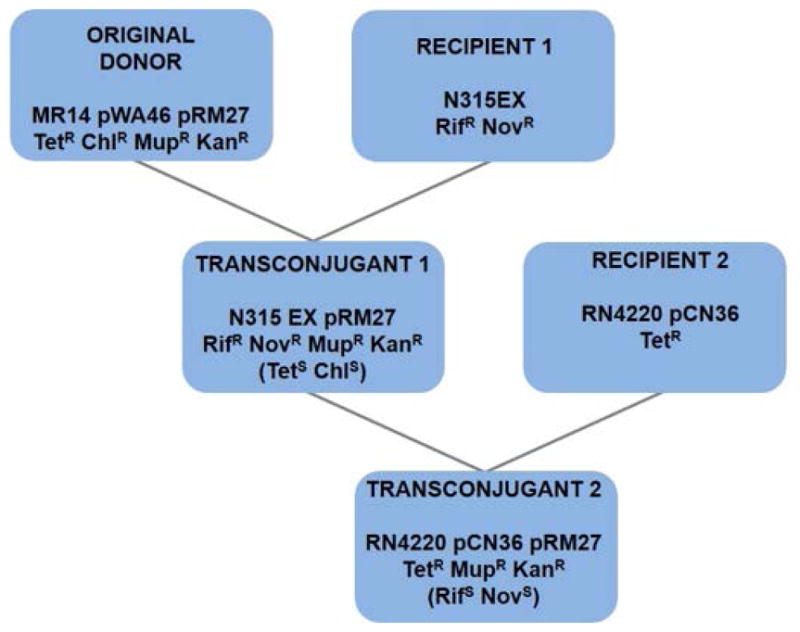
Outline of filter matings for pRM27 transfer. First mating, original donor (MR14 pWA46 pRM27, apparent captured pGO400::SCCmec) with recipient 1 (N315EX, isogenic recipient with excised SCCmec). Resulting transconjugants 1 (N315EX pRM27) became the donor for a second mating with recipient 2 (RN4220 pCN36, heterologous recipient). The presence of different antibiotic resistance markers in the donor and recipient cells allowed for transconjugant selection. Tet, tetracycline; Chl, chloramphenicol; Mup, mupirocin; Kan, kanamycin; Nov, novobiocin; Rif, rifampin.
Fig. 3.
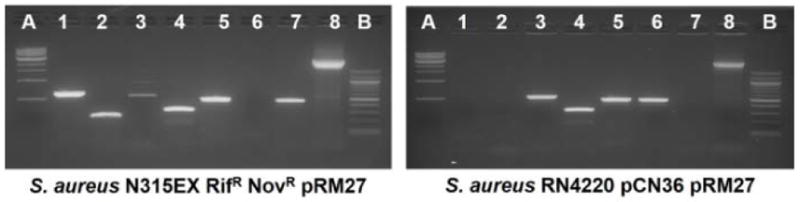
(A) PCR analysis to characterize transconjugant N315EX pRM27. Lane A, 1 kb molecular size marker; lanes 1 and 2, integrated attR and attL amplicons, respectively; lane 3, excised chromosomal junction (attB); lane 4, circular SCCmec junction (attS); lane 5, mecA; lane 6, orf0042, specific to RN4220; lane 7, ermA, specific to N315; lane 8, mupA; lane B, 500 bp molecular size marker
(B) PCR analysis to characterize transconjugant RN4220 pCN36 pRM27. The lanes are the same as in Fig. 3A.
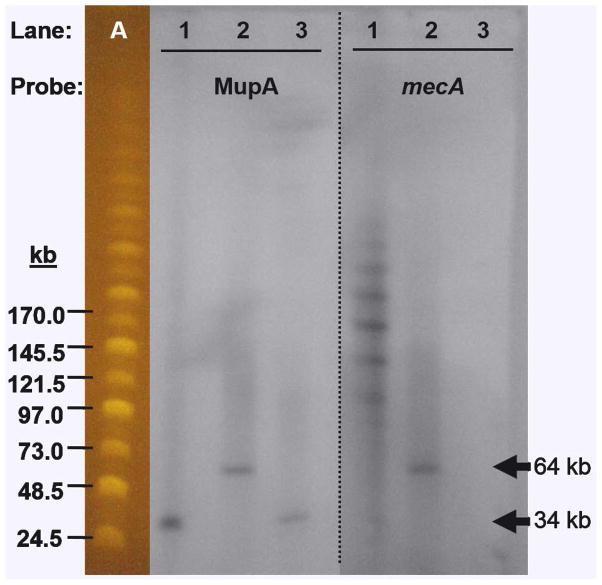
In order to further confirm the capture and transfer of SCCmec, Southern blotting was performed as shown in Fig. 4. RN4220 pCN36 pRM27 was digested with NarI, an enzyme that did not cut in SCCmec but cut once in pGO400. In RN4220, both mecA and mupA probes hybridized to fragments of 64 kb, the predicted size of pGO400 (34 kb) plus SCCmec from MR14 (30.8 kb; Fig. 4, lanes 3). However, the mupA probe for N315EX pRM27 (Fig. 4, lane 1) hybridized only to a fragment the size of pGO400 while the mecA probe showed a ladder of hybridization with each band approximately 31 kb larger than the one below. This was interpreted to mean that there almost complete excision of SCCmec from pRM27 with its integration into the chromosome. The ladder of mecA hybridization to N315EX pRM27 was felt to indicate that the colony had representatives of from one to six end-to-end, concatameric chromosomal insertions (Fig. 4, lane 2). We confirmed the concatameric nature of insertions by digesting the DNA from this strain with SacI, an enzyme that cuts only once in SCCmec. A subsequent Southern blot showed the ladder collapsed into a single 30kb band (SI Appendix. Fig S1). Although pRM27 was not seen in the Southern blot of the N315EX transconjugant, it was confirmed to be present by its transfer from N315EX pRM27 into RN4220 on a subsequent mating.
Fig. 4.
Southern hybridization analysis of NarI restriction-digested DNA separated by pulsed field gel electrophoresis. There is a single NarI cleavage site within pGO400 and no site within SCCmec. Lane A, MidRangeII PFG marker; lanes 1 N315EX pRM27, lanes 2, RN4220 pCN36 pRM27; lanes 3, RN4220 pGO400. The left panel was hybridized with a probe for mupA and the right panel for mecA.
The capture of SCCmec on pGO400 was conclusively demonstrated by determining the DNA sequence of pRM27. This was accomplished by determining the whole genome sequence of RN4220 pCN36 pRM27. The map of pRM27 is shown in Fig. 5 and demonstrates that the juncture of pGO400 and SCCmec occurs at IS elements, confirming the integration by IS-mediated recombination.
Transfer characteristics of pRM27
We sought to explore the recipient range of pRM27 by filter matings between RN4220 pCN36 pRM27 and a variety of S. aureus and S. epidermidis recipients. We chose methicillin-susceptible S. aureus (MSSA) recipients of different clonal complexes (CCs) as well as methicillin-susceptible S. epidermidis (MSSE). Conjugative pRM27 transfer was successful into two different MSSA strains of CC1 (both ST1 but with different accessory elements) and a clinical MSSA isolate of CC5 different from strain N315 (ST5 for N315 and ST105 for 18-1-2). The two S. aureus strains into which pRM27 did not transfer had low conjugation frequencies for pGO400 (SI Appendix, Table S3). Most importantly, we were able to transfer pRM27 into S. epidermidis, and PCR analysis of S. epidermidis transconjugants revealed SCCmec chromosomal integration (Table 1, Supporting Information, Fig. S2) at the unique orfX attB site that is present in all sequenced staphylococcal species. This is the only site into which SCCmec has ever been shown to integrate. Furthermore, pRM27 from S. epidermidis was able to transfer back into S. aureus RN4220 and MR14EX (Table 1)
Table 1.
Filter matings and conjugation frequencies of pRM27. Conjugation frequencies are expressed as the number of transconjugants divided by the number of donors. Donor RN4220 pCN36 pRM27 was resistant to kanamycin, mupirocin, and tetracycline. Recipients were resistant to rifampin and novobiocin except for S. epidermidis VCU129 which was resistant to erythromycin. Donor S. epidermidis VCU129 pRM27 was resistant to kanamycin, mupirocin, and erythromycin. Sa, S. aureus; Se, S. epidermidis; CC, clonal complex; NC, not classified; ST, sequence type.
| Donor Strain | Recipient Strain | Recipient Species | CC(ST) | Donors CFU/ml | Recipients CFU/ml | Recipient + pRM27 transconjugants CFU/ml | Conjugation Frequency |
|---|---|---|---|---|---|---|---|
| RN4220 pCN36 pRM27 | Cowan I | Sa | 30(30) | 9.80E+09 | 8.40E+09 | 0 | 0 |
| RN4220 pCN36 pRM27 | 8325 | Sa | 8(8) | 1.74E+08 | 2.10E+08 | 2.00E+01 | 1.15E-07 |
| RN4220 pCN36 pRM27 | RN4220 | Sa | 8(8) | 1.13E+09 | 1.34E+09 | 3.80E+02 | 3.36E-07 |
| RN4220 pCN36 pRM27 | MR14EX | Sa | 5(5) | 1.04E+09 | 3.50E+09 | 1.10E+02 | 1.06E-07 |
| RN4220 pCN36 pRM27 | NRS242 | Sa | 121(121) | 1.23E+09 | 7.40E+08 | 0 | 0 |
| RN4220 pCN36 pRM27 | MW2EX | Sa | 1(1) | 1.02E+09 | 1.13E+09 | 6.00E+00 | 5.88E-09 |
| RN4220 pCN36 pRM27 | MSSA476 | Sa | 1(1) | 1.88E+09 | 1.02E+09 | 1.00E+00 | 5.32E-10 |
| RN4220 pCN36 pRM27 | 18-1-2 | Sa | 5(105) | 1.24E+09 | 1.18E+09 | 3.80E+01 | 3.06E-08 |
| RN4220 pCN36 pRM27 | VCU113 | Se | NC | 1.63E+08 | 7.40E+08 | 0 | 0 |
| RN4220 pCN36 pRM27 | VCU129 | Se | 193(193) | 1.03E+09 | 1.11E+09 | 8.40E+01 | 8.16E-08 |
| S. epidermidis VCU129 pRM27 | RN4220 | Sa | 8(8) | 2.30E+08 | 1.70E+08 | 4.50E+02 | 1.96E-06 |
| S. epidermidis VCU129 pRM27 | MR14EX | Sa | 5(5) | 2.10E+08 | 3.60E+08 | 6.00E+01 | 2.86E-07 |
Discussion
The transfer of SCCmec could take place by a number of mechanisms that are available in staphylococci. Virtually all staphylococci contain phage integrated in their genome and the transduction of small SCCmec types between compatible S. aureus strains has been demonstrated (Scharn et al, 2013). In addition, specialized genomic pathogenicity islands, called SaPIs, have been described that coopt the capsids of larger phage to transfer themselves between S. aureus strains at high frequency (Novick et al, 2010). However, several SCCmec characteristics argue against transduction or phage-mediated transfer as a predominant method for the dissemination of the element. First, any transfer postulates would have to explain transfer between different staphylococcal species. While it is possible that broad host-range staphylococcal transducing phage exist in commensal species the absence of homology flanking SCCmec between sequences in S. aureus and CoNS would make transduction an unlikely transfer mechanism. In addition, the nonreplicative nature of the circular intermediate provides no mechanism for phage encapsidation and recircularization in the recipient (Novick et al, 1986). In contrast, staphylococcal conjugative plasmids are prevalent (Archer and Scott, 1991), exist in multiple species (Archer and Scott, 1991), and readily transfer between species (Archer and Johnston, 1983, Forbes and Schaberg, 1983). A pGO1/pSK41-like plasmid served as the capture replicon for transposon Tn1546, carrying vancomycin resistance, which was initially resident in Enterococcus faecalis (Weigel et al, 2003). Second, most SCCmec types are > 40 kb (IWG-SCC, 2009) and known staphylococcal generalized transducing phages, such as 80α or ϕ11, are members of the Siphoviridae with genome sizes of 39 - 43 kb (Deghorain and Van Melderen, 2012, Christie et al, 2010). Since the sequences of large SCCmec elements in S. epidermidis are identical to those in S. aureus (Wisplinghoff et al, 2003, Gill et al, 2005), phage would have to exist that could package large genomes and move them between the two species. Those phage have not yet been identified and, therefore, if they exist they must be rare. However, conjugative plasmids exist in many bacterial species that are > 100 kb and can readily incorporate large quantities of additional DNA (Grohmann et al, 2003). In the current study we doubled the size of pGO400 with the addition of SCCmec to form pRM27.
Another potential mechanism for SCCmec transfer in staphylococci would be by conjugative mobilization where oriT sequences present in a chromosomal genomic island are nicked by conjugative plasmid enzymes and single-stranded DNA is mobilized to transfer as part of the conjugative process. Chromosomal elements containing an oriT are called integrating conjugative elements (ICEs) and are present in many gram-negative bacteria (Daccord et al, 2010). While it is possible that some plasmids that integrate into SCCmec by IS431/257-mediated recombination/nonresolved replicative transposition contain an oriT site that could allow the element to be mobilized, a search of closed S. aureus genomes for known oriT sequences found only a single example, and this oriT was not in an SCCmec. Thus, it is unlikely that most SCCmec types are examples of ICEs.
Finally, as reported recently by Morikawa et al (Morikawa et al, 2012), staphylococci can take up DNA by transformation when competence genes are activated by sigma factor H (SigH). The activation of SigH and transformation took place in a small fraction of the cell population. In this study the N315 SCCmec region was transformed at low frequency (10−8) into an isogenic N315ex recipient. Since no attempt was made to excise SCCmec in this study it is likely that the transforming DNA was the chromosomally integrated element. Thus, flanking homology would be required for integration as with phage-mediated DNA transfer making it unlikely that this transfer mechanism would occur with heterologous donor DNA and recipients, including DNA and recipients of different species. In addition, there has been no demonstration that transformation can occur by this mechanism in coagulase-negative staphylococci and, therefore, would be a mechanism for interspecies transfer. Nevertheless, as a rare event DNA including the circular element could conceivably be taken up by transformation, making this a plausible means for SCCmec horizontal gene transfer. The demonstration of spontaneous SCCmec transfer by filter mating without the manipulation of the donor strain was attempted on numerous occasions but was never successful. This is likely due to the occurrence of multiple rare events at frequencies that are too low in the aggregate to be detected at the limit of the number of bacteria that can be grown using standard laboratory culture techniques. As we have shown in this study, the frequency of spontaneous SCCmec excision is about 10−6 and, once excised, the element rapidly reintegrates into its att site (Wang et al, 2015). The best recombination frequency of two 790 bp areas of DNA homology provided by IS431/257 can be calculated at approximately 10−3 (Khasanov et al, 1992). Finally, the best conjugation frequency between homologous donor and recipient demonstrated in this study was approximately 10−7 (Table 1). Thus, the aggregate frequency would exceed 10−12 and it would require a population of donor bacteria at least this large to detect a single event. Therefore, to increase our opportunity to detect transfer we increased the donor SCCmec excision frequency to 20% by overproducing CcrAB; engineered a positive selection marker for complete excision; and used a separate antibiotic resistance marker to select for SCCmec maintenance by conjugative plasmid rescue. While these manipulations may not completely reflect the rare events that take place in nature to allow the spontaneous transfer of SCCmec they provide proof of principle. Furthermore, conditions may exist in nature that increase the expression of ccrAB and, therefore, SCCmec excision, improving the chances for conjugation-mediated transfer (Stojanov et al, 2013, Higgins et al, 2009).
In addition to SCCmec transfer, we demonstrated the spontaneous excision of the element from the conjugative plasmid and insertion into the recipient chromosome at the attB site within orfX. We hypothesize that SCCmec excises from pRM27 by IS-mediated recombination and is then directed to attB for chromosomal insertion by CcrAB. We have previously shown that integration is highly favored over excision (14) and observed, in the current study, that multiple copies of the transferred element inserted end-to-end as concatamers. The existence of end-to-end insertions of different SCC elements at the attL site has been seen in clinical isolates (Diep et al, 2006). It is also of interest that pRM27 transferred into a heterologous host, RN4220, but the SCCmec element remained associated with the plasmid in the presence of selection and did not insert into the chromosome. The absence of SCCmec integration in the chromosome of RN4220 may be explained by differences in the sequence surrounding the integration site (attB) between S. aureus strains N315EX and RN4220. Sequence differences one to two kb surrounding attB have been shown to be important for CcrAB-mediated integration and suggest that CcrAB types from different elements may have preferred integration site sequences (Wang et al, 2012).
We also found that pRM27 transferred into some S. aureus recipients but not others. While this may merely reflect low filter mating transfer frequencies it may also reflect preferential mating recipients. Those recipients into which pRM27 successfully transferred were of clonal complexes and sequence types CC5(ST5), CC8(ST8) and CC1(ST1). CC5 and CC8 are the most prevalent clonal complexes and sequence types among MRSA worldwide (Chambers and Deleo, 2009, Enright et al, 2002), and CC1 is the clonal complex representing the first community-acquired MRSA isolates (CDC, 1999, Baba et al, 2002). The reasons for a type preference are not clear but pRM27 could be a useful tool in the exploration of the epidemiology of the global spread of methicillin resistance.
In this study we have shown a plausible mechanism for the transfer of SCCmec that is consistent with observations from clinical isolates. We have transferred a 30.8 kb element that approximates the size of Types I and IV SCCmec; we have moved the element into recipients that represent the two most clinically prevalent and unrelated MRSA clonal complexes as well as into the CoNS species, S. epidermidis, that may serve as a reservoir for this element; we have shown that the element can excise from the plasmid and insert into the chromosome of a recipient with the appropriate insertion site sequence; and we have shown that, in the absence of specific selection, the element is lost from the plasmid, either inserting into the chromosome or excising and being lost on subsequent plasmid transfer. This may explain the failure to find SCCmec sequences on conjugative plasmids in clinical isolates. The prevalence of pGO1/pSK41-like conjugative plasmids that can provide up to eight individual 790 kb IS257/431 sites for homologous recombination (Caryl and O’Neill, 2009, Berg et al, 1998) supports not only the availability of a transport vehicle but the opportunity for IS-mediated recombination to occur. Thus, IS-mediated recombination and conjugation describe mechanisms for SCCmec capture and transfer that are plausible and prevalent in the important staphylococcal species responsible for clinical infections.
Experimental Procedures
Strains, media and bacterial identification
All of the strains used in the present study are listed in Table S1. S. aureus and S. epidermidis strains were cultured in tryptic soy broth (TSB) (Difco, Lawrence, KS), and Escherichia coli cells were cultured in Luria-Bertani (LB) (Difco). Escherichia coli Top10 (Invitrogen, Carlsbad, CA) was used for gene cloning. Antibiotics and concentrations, used when appropriate for selection, were as follows: ampicillin (100 μg/ml), chloramphenicol (10 μg/ml), tetracycline (10 μg/ml), erythromycin (10 μg/ml), kanamycin (100 μg/ml), mupirocin (10 μg/ml), rifampin (10 μg/ml), and novobiocin (5 μg/ml) (Sigma-Aldrich, St. Louis, MO). Bacteria in mating studies were identified as S. aureus or S. epidermidis by species-specific PCR amplicons, phenol red mannitol salt agar, and standard laboratory identification techniques.
Tn554 and 15.4 kb deletion mutant construction
Tn554 and 15.4 kb upstream of attL within SCCmec in N315 were deleted by allelic replacement mutagenesis, using vector pKOR1 (Bae and Schneewind, 2006). Two 1-kb fragments, one upstream and one downstream of the region to be deleted, were amplified by PCR, using primers shown in Table S2; digested with BamHI (NEB, Ipswich, MA) (Tn554) or EcoRI (NEB) (15.4 kb); and ligated overnight with T4 DNA ligase (NEB). The ligated product was mixed with the plasmid pKOR1 and BP clonase II (Invitrogen), incubated at room temperature for 5 h, and transformed into E. coli TOP10-competent cells. The resulting plasmids were recovered from E. coli, introduced into RN4220 by electroporation, transduced into S. aureus N315 using phage 80α, and selected on TSB agar plates containing chloramphenicol (10 μg/ml). A colony of N315 containing the plasmid was grown at 42°C to inhibit replication of the temperature-sensitive (TS) plasmid and colonies growing on chloramphenicol were again selected. Colonies that had undergone secondary recombination between the sequences flanking the region to be deleted were selected by inducing the culture with anhydrotetracycline (200 ng/ml). Chromosomal deletion was confirmed by PCR and DNA sequencing (Eurofins MWG Operon, Huntsville, AL).
tetM insertion mutant construction
The gene tetM from pCN36 was inserted downstream of SCCmec attL in N315 by allelic replacement mutagenesis, using vector pKOR1. The In-Fusion HD Cloning Kit (Clontech, Mountain View, CA) was used following the manufacturer’s instructions to create the insertion construct consisting of the promoterless tetM gene flanked by 1 kb of N315 DNA up and down stream of attL in a pUC19 vector backbone. The resulting construct was transformed into E. coli TOP10-competent cells. The resulting plasmid was recovered from E. coli and the construct was transferred into pKOR1 and strain N315 as described above.
Conjugative filter matings
Overnight TSB cultures of donor and recipient strains grown in the presence of appropriate antibiotic selection were mixed in a 1:1 ratio (2 ml of donor strain and 2 ml of recipient strain). The combined cells were centrifuged at 4,000 rpm for 15 min and washed once with 1X phosphate buffered saline (PBS). The combined cell pellet was resuspended in 2 ml 1X PBS and forced through a sterile syringe onto a 25 mm diameter, 0.45 μm pore- size nitrocellulose filter (EMD Millipore, Darmstadt, Germany). The filter was placed on TSA in the absence of antibiotic pressure, bacteria side up, and incubated at 37°C overnight. Cells were vortexed off of the filters in 1 ml of 1X PBS and were plated on TSA containing appropriate antibiotics, selecting for donors, recipients, and transconjugants.
PCR to confirm integration and excision of SCCmec
Primers used for PCR are listed in Table S2. Template genomic DNA was isolated using the QIAGEN DNeasy Blood and Tissue Handbook Kit according to manufacturer’s instructions (QIAGEN, Valencia, CA). PCR was performed using a TopTaq DNA polymerase kit (QIAGEN) and following manufacturer’s instructions (QIAGEN). PCR reaction conditions were as follows: one initial cycle at 95°C for 3 min followed by 30 cycles of [94°C 30 s to denature, (Primer melting temperature in °C -5)°C 30 s for hybridization, 70°C one min per kb for extension] followed by a final extension at 70°C for 10 min and then chilled to 12°C. PCR products were purified using the QIAGEN PCR purification kit following manufacturer’s instructions (QIAGEN) and confirmed by DNA sequencing (Eurofins MWG Operon).
Pulsed-field gel electrophoresis (PFGE)
Bacterial strains subjected to PFGE analysis were streaked on TSA plates supplemented with appropriate antibiotics and grown overnight at 37°C. Overnight cultures were then prepared from a single colony in TSB supplemented with appropriate antibiotics and grown at 37°C with shaking at 200 rpm. Agarose plugs containing bacterial cells in suspension were then prepared as previously described (Bannerman et al, 1995). DNA in 1% agarose gel plugs was digested by restriction enzymes NarI or SacI (NEB), where noted, in a 400 μl reaction volume with 120U restriction enzyme per plug. Plugs were loaded into the gel, along with a MidRange II PFG marker (NEB). The gel was placed in a CHEF-DR III Pulse Field Electrophoresis System (Bio-Rad, Hercules, CA) and the following settings were used for electrophoresis: 6 v/cm, 1 s initial switching time, 30 s final switching time, 22 h run time, 14°C, 120° included angle) (Bannerman et al, 1995).
mecA and mupA probe construction
Full-length mecA and mupA purified PCR products were randomly primed and labeled with Digoxigenin-11-2′-deoxy-uridine-5′-triphosphate (DIG-11-dUTP) using the DIG DNA Labeling and Detection Kit (Roche, Mannheim, Germany) following manufacturer’s protocol. Primers for probe amplification are listed in Table S2.
Southern blot hybridization and detection
Pulsed-field DNA gels were soaked for 10 min in 0.2 N HCl to depurinate the DNA and then rinsed in ddHO. Gels were then soaked in denaturation solution (1.5 M NaCl, 0.5 N NaOH) for 45 min, rinsed with distilled water, soaked in neutralization solution (1 M Tris-HCl pH 7.4, 1.5 M NaCl) for 30 min, rinsed in ddH2O and soaked in fresh neutralization solution for 15 min. DNA was transferred from agarose gels to a positively charged nylon membrane (Roche) by capillary transfer for 24 h as previously described (38). The DNA was then fixed to the membrane by UV irradiation in a UV Stratalinker 2400 (Stratagene, La Jolla, CA) at 120,000 μjoules/cm2. Nylon membranes were prehybridized in an appropriate volume (20 ml/100 cm2 membrane) of DIG Easy Hyb buffer (Roche) at 42°C Following prehybridization, 25 ng/ml DIG-labeled DNA probes were denatured at 95°C for 5 min and rapidly cooled on ice. The denatured probes were added to an appropriate volume (3.5 ml/100 cm2 membrane) of pre-heated DIG Easy Hyb buffer (Roche). The prehybridization solution was decanted from the membranes and the probe- Hyb buffer mixtures were added to the membranes and incubated overnight in tightly sealed containers with gentle agitation at 42°C. The membranes were then washed twice at room temperature in low stringency wash solution (2X SSC [3M NaCl, 0.3M NaCitrate, pH 7.0], 0.1% SDS) for 5 min each with shaking, and twice for 15 min at 42°C with shaking in high stringency wash solution (0.5X SSC, 0.1% SDS). Membranes were washed using supplied reagents in the DIG Wash and Block Buffer Set (Roche) according to the manufacturer’s instructions. Detection was performed using the DIG DNA Labeling and Detection Kit (Roche) following manufacturer’s protocol and using supplied reagents. DIG-labeled hybrids were detected with an anti-DIG- alkaline phosphatase conjugate and visualized with the substrates NBT (nitroblue tetrazolium salt) and BCIP (5-bromo-4-chloro-3-indolyl phosphate, toluidinium salt) (Sambrook et al, 1989).
Sequencing of pRM27
To isolate high-quality DNA from RN4220 pCN36 pRM27, the QIAamp DNA Blood Mini Kit was used following the manufacturer’s protocol for the QIAGEN Maxi tip 500/G (QIAGEN, Valencia, CA). Whole genome sequencing was performed at UNC Pacific Biosciences High Throughput Sequencing Facility (Chapel Hill, NC). The assembly was also performed at UNC and made using the HGAP2 algorithm provided in the PacBio’s SMRTportal package (version 2.3.0). Accession numbers for the DNA sequence of plasmids pGO400 and pRM27 are KT780704 and KT780705 respectively.
Supplementary Material
Acknowledgments
This work was supported in part by grant 5RO1AI035705-20 from the National Institute of Health, National Institute of Allergy and Infectious Diseases. Whole genome sequencing was performed at UNC Pacific Biosciences High Throughput Sequencing Facility (Chapel Hill, NC). We thank them for their advice and help with sequence assembly.
Footnotes
Author Contributions
M. D. Ray:
- Designed research
- Performed research
- Contributed new reagents or analytic tools
- Analyzed data
- Wrote the paper
S. Boundy:
- Designed research
- Performed research
- Contributed new reagents or analytic tools
- Analyzed data
- Wrote the paper
G. L. Archer:
- Designed research
- Analyzed data
- Wrote the paper
References
- Anderson DJ, Moehring RW, Sloane R, Schmader KE, Weber DJ, Fowler VG, Jr, Smathers E, Sexton DJ. Bloodstream infections in community hospitals in the 21st century: a multicenter cohort study. Plos One. 2014;9(3):e91713. doi: 10.1371/journal.pone.0091713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer GL, Johnston JL. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983;24:70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer GL, Scott J. Conjugative transfer genes in staphylococcal isolates from the United States. Antimicrob Agents Chemother. 1991;35:2500–2504. doi: 10.1128/aac.35.12.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TI, Takeuchi F, Kuroda M, Yuzawa H, Aoki K-I, et al. Genome and virulence determinants of high virulence community-acquired MRSA. The Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bannerman TL, Hancock GA, Tenover FC, Miller JM. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray RA. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caryl JA, O’Neill AJ. Complete nucleotide sequence of pGO1, the prototype conjugative plasmid from the Staphylococci. Plasmid. 2009;62:35–38. doi: 10.1016/j.plasmid.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus – Minnesota and North Dakota, 1997–1999. JAMA. 1999;282:1123–1125. [PubMed] [Google Scholar]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–461. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie GE, Matthews Am, King DG, Lane KD, Olivarez NP, Tallent SM, et al. The complete genomes of Staphylococcus aureus bacteriophages 80 and 80α –implications for the specificity of SaPI mobilization. Virology. 2010;407:381–390. doi: 10.1016/j.virol.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daccord A, Ceccarelli D, Burrus V. Integrating conjugative elements of the SCT/R391 family trigger the excision and drive the mobilization of a new class of Vibrio genomic islands. Molecular Microbiology. 2010;78:576–588. doi: 10.1111/j.1365-2958.2010.07364.x. [DOI] [PubMed] [Google Scholar]
- Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States. JAMA Intern Med. 2011;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deghorain M, Van Melderen L. The staphylococci phages family: An overview. Viruses. 2012;4:3316–3335. doi: 10.3390/v4123316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan THV, Chen JH, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. The Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Dukie VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PloS One. 2013;8:e52722. doi: 10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes BA, Schaberg DR. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983;153:627–934. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, et al. Insights on evolution of virulence and resistance from the a complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann E, Muth G, Espinosa M. Conjugative plasmid transfer in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:277–301. doi: 10.1128/MMBR.67.2.277-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins PG, Rosato AE, Seifert H, Archer GL, Wisplinghoff H. Differential Expression of ccrA in Methicillin-Resistant Staphylococcus aureus Strains Carrying Staphylococcal Cassette Chromosome mec Type II and IVa Elements. Antimicrob Agents Chemother. 2009;53:4556–4558. doi: 10.1128/AAC.00395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasanov FK, Zvingila DJ, Zainullin AA, Prozorov AA, Bashkirov VI. Homologous recombination between plasmid and chromosomal DNA in Bacillus subtilis requires approximately 70 bp of homology. Mol Gen Genet. 1992;234:494–497. doi: 10.1007/BF00538711. [DOI] [PubMed] [Google Scholar]
- Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelaporn L, Firth N, Paulsen IT, Skurray RA. IS257-mediated cointegration in the evolution of a family of staphylococcal trimethoprim resistance plasmids. J Bacteriol. 1996;178:6070–6073. doi: 10.1128/jb.178.20.6070-6073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K, Takemura AJ, Inose Y, Tsai M, Nguyen Thi LT, et al. Expression of a Cryptic Secondary Sigma Factor Gene Unveils Natural Competence for DNA Transformation in Staphylococcus aureus. PLoS Pathog. 2012;8:e1003003. doi: 10.1371/journal.ppat.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton TM, Johnston JL, Patterson J, Archer GL. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother. 1995;39:1272–1280. doi: 10.1128/aac.39.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Edelman I, Lofdahl S. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J Mol Bio. 1986;192:209–220. doi: 10.1016/0022-2836(86)90360-8. [DOI] [PubMed] [Google Scholar]
- Sambrook JE, Fritsch EF, Maniatis T, editors. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scharn CR, Tenover FR, Goering RV. Transduction of staphylococcal cassette chromosome mec elements between strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:5233–5238. doi: 10.1128/AAC.01058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore AC, Coleman DC. Staphylococcal cassette chromosome mec: recent advances and new insights. Int J Med Microbiol. 2013;303:350–359. doi: 10.1016/j.ijmm.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Stojanov M, Sakwinska O, Moreillon P. Expression of SCCmec cassette chromosome recombinases in methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 2013;68:749–757. doi: 10.1093/jac/dks494. [DOI] [PubMed] [Google Scholar]
- Wang L, Ahmed MH, Safo MK, Archer GL. A plasmid-borne system to assess the excision and integration of staphylococcal cassette chromosome mec mediated by CcrA and CcrB. J Bacteriol. 2015;197:2754–2761. doi: 10.1128/JB.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Archer GL. Roles of CcrA and CcrB in excision and integration of staphylococcal cassette chromosome mec, a Staphylococcus aureus genomic island. J Bacteriol. 2010;192:3204–3212. doi: 10.1128/JB.01520-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Safo M, Archer GL. Characterization of DNA sequences required for the CcrAB-mediated integration of staphylococcal cassette chromosome mec, a Staphylococcus aureus genomic island. J Bacteriol. 2012;194:486–498. doi: 10.1128/JB.05047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Rosato AE, Enright MC, Noto M, Craig W, Archer GL. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob Agents Chemother. 2003;47:3574–3579. doi: 10.1128/AAC.47.11.3574-3579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.