Abstract
Purpose
To determine the cytokine levels in aqueous humor (AH) of Colombian patients with active ocular toxoplasmosis (OT), and to correlate them with their clinical characteristics.
Methods
27 Cytokines/chemokines were assayed in 15 AH samples (nine patients with diagnosis of OT biologically-confirmed and six controls that underwent cataract surgery). Correlations were assessed between cytokine/chemokine levels, type of inflammatory response (Th1, Th2, Th17, Treg), and clinical characteristics.
Results
Th2 predominant response was related to more severe clinical features. The presence of VEGF and IL-5 was related to higher number of recurrences. Growth factors (VEGF, FGF, PDGF-β), were related to higher number of lesions. Patients infected by type-I/III strains had a particular intraocular cytokine-pattern.
Conclusions
Th2 response was related to more severe clinical characteristics in patients infected by Type I/III strains. IL-5 and VEGF were associated with recurrences. We correlate for the first time, specific cytokine-patterns with clinical characteristics and with the infecting Toxoplasma strain.
Keywords: Toxoplasma gondii, Uveitis, Th2, Colombia, Intraocular cytokines
1. Introduction
Ocular toxoplasmosis (OT) is the most common cause of posterior uveitis and, in some countries, it is one of the most important causes of visual impairment [1]. The severity of disease varies greatly between patients [1]. OT is characterized by necrotizing retinopathy, triggered by the reactivation of dormant parasites within the retina [2]. Cytokine profiles in aqueous humor has been linked to clinical features for various causes of infectious uveitis [3–5] but the precise role of cytokines in toxoplasmic uveitis remains to be determined [6,7]. Cytokines can be pro- or anti-inflammatory, synergistic, antagonistic, pleiotropic, redundant, and interactive, depending on the local immunological environment [3–5]. Therefore, an ocular cytokine mapping or cytokinome will contribute to a better understanding of the physiopathology of specific forms of uveitis and of different outcomes, as occurs in OT, providing guidance for new targeted treatment [4,6,7]. A study on the ocular cytokinome in 27 immunocompetent French OT patients found no correlation with age, sex or region of origin of the patient; neither with time from symptom onset to sampling; degree of uveal inflammation; or the etiology of the infection (primary acquired or congenital). However, a specific local cytokine-profile for ocular toxoplasmosis was observed, distinct from other causes of uveitis [6]. Particularly high levels of IFN-γ, IL-6, and MIP-1β were frequently detected in samples from patients with ocular toxoplasmosis, as well as viral uveitis, whereas IL-17 was frequently detected in samples from patients with toxoplasmic but not viral uveitis [6]. Another prospective study using aqueous humor (AH) samples from French patients revealed enhanced Th1 (IL-2, IFN-γ) and Th2 (IL-13) cytokines, as well as inflammatory (IL-6, IL-17, MCP-1) and down-regulating (IL-10) immune mediators. In contrast, TNF-α was not up-regulated [7]. However, these results are representative for European (and North American) patients, where Type II strains predominate [8,9]. We recently found that cytokine patterns were strikingly different between French and Colombian patients with ocular toxoplasmosis [10]. Intraocular IFN-γ and IL-17 expression was lower, while higher levels of IL-13 and IL-6 were found in aqueous humor of Colombian patients [10]. These results are consistent with the hypothesis that South American strains may cause more severe OT due to an inhibition of the protective effect of IFN-γ [10]. Thus, our present work aimed to study the local cytokine profiles in Colombian patients with active OT, and to correlate them with the individual clinical manifestations, as well as with the type of infecting strain determined by serotyping.
2. Materials and methods
2.1. Patients and controls
We prospectively collected all consecutive patients who consulted the Quindío University Health-Center (Armenia, Colombia) between August 2008 and August 2010. This consultation is a tertiary-level center able to perform anterior chamber paracentesis. A complete ocular examination was conducted, including best-corrected Snellen visual acuity, slit-lamp biomicroscopy, tonometry, and indirect ophthalmoscopy. The clinical diagnosis of active OT was confirmed by biological tests on AH samples as previously described [11,12]. Screened patients with clinically suspected OT and positive for anti-Toxoplasma immunoglobulin G (IgG) antibodies in serum were subsequently diagnosed as confirmed OT when positive for Toxoplasma DNA by polymerase chain reaction (PCR) or for presence of specific local antibodies against Toxoplasma gondii by immunoblot in aqueous humor compared with immunoblot patterns in serum [12]. Six aqueous humor samples were used as controls from patients that underwent cataract surgery, in which OT was discarded by serological and molecular tests in AH, as described previously [11,12]. The study followed the tenets of the Declaration of Helsinki. All participants and controls were asked to participate voluntarily in the study. If they accepted then they signed an informed consent according to the Colombian legislation for research with humans (resolution 008430 of 1993 by the ministry of health). The University of Quindio Institutional Review Board approved the study (act number 14, 23 June 2008). Immunocompromised patients were excluded. Patients with a first OT episode had not received any anti-Toxoplasma treatment, patients with recurrences had not received treatment for at least 6 months before sampling. All known recurrences were noted, even if the episode was not observed by us, from the referring physician and clinical chart annotations as we described previously [13]. An assessment of inflammation level and anatomic classification of uveitis were carried out according to the criteria proposed by the International Uveitis Study Group (IUSG) [14]. The size of the retinochoroidal lesions was measured in disk-diameters (dd). Inflammation was defined according to the number of cells in vitreous examination. In correlation analysis, the number of cells and the levels of particular cytokines were evaluated. For qualitative analysis purposes, higher inflammation was defined if there were ≥3+ cells in vitreous examination, and moderate or lower inflammation if there were 2+ or fewer cells, considering the number of vitreous cells visualized in 3 mm × 1 mm slit beam, according to the Standardization of Uveitis Nomenclature (SUN) grading system [14].
2.2. Cytokine measurement in aqueous humor
In order to prevent changes in cytokine levels due to multiple freeze–thawing cycles, samples were immediately stored and maintained at −80 °C until analysis.
The Bio-Plex Pro Human 27-plex Panel assay (Bio-Rad) was used to measure cytokine and chemokine levels in 50 µl of the supernatants of aqueous humor of infected and control patients, according to the manufacturer’s recommendations. All measurements were done in duplicate. Concentrations were calculated using standard curves of known concentrations and levels of cytokines expressed in pg/ml for each cytokine. Data were analyzed with Bio-Plex Manager TM software V1.1.
2.3. Serotyping of Toxoplasma infections
Serotyping of Toxoplasma infections was performed using polymorphic synthetic peptides derived from the T. gondii dense granule proteins (GRA), GRA6 and GRA7 that detect the presence of strain specific antibodies raised against Type I/III or non-Type I/III GRA6/7 alleles in patients infected with Type I/III or non-Type I/III (Type II, atypical or non-determined) parasites respectively, as previously described [10,15].
2.4. Statistical analysis
Differences in proportions among groups were compared by the Fisher’s exact test and for non-parametric data, differences of means between two groups were analyzed by a Kruskal Wallis test, with the Epi-Info™ software, version 3.5.1 (CDC, Atlanta, USA). The statistical significance of the relationship between clinical features and cytokine profiles was studied by Spearman’s non-parametric correlation-test. Correlation between cytokine levels and serotyping results was analyzed by Kruskal Wallis tests, using the statistical package software SPSS, version 14 (SPSS Inc., Chicago, USA).
3. Results
3.1. Clinical and laboratory characteristics of Colombian patients with ocular toxoplasmosis
During the study period, 42 patients with clinical symptoms of OT underwent biological analysis: 20 cases (47.6%) were confirmed as OT, 13 (30.9%) were unequivocally discarded as non-toxoplasmic uveitis, and 9 (21.4%) had an inconclusive diagnosis. Toxoplasma DNA was detected by PCR in 11 out of 19 aqueous humor samples (57.8%), while local antibody synthesis was proved by immunoblot in 10 of 11 patients (34.8%). One patient was positive simultaneously for PCR and immunoblot assays. Median number of inflammatory cells in aqueous humor from OT patients was 2.5 (range 0–4), of 1.5 (range 0–4) in non-OT patients and of 2.5 (range 0–4) in patients with inconclusive diagnosis. Median number of recurrences was of 1.0 (range 0–9) in OT patients, of 1.5 (range 0–6) in non-OT and of 1.5 (range 0–4) in patients with inconclusive diagnosis. Mean number of lesions (active and non-active) was of 2 (range 1–6) in OT patients, of 2 (range 1–6) in non-OT patients and of 1 (range 1–5) in patients with inconclusive diagnosis.
3.2. Cytokine profiles in OT patients vs. controls
Only 9 of 20 cases (45%) with confirmed OT could be analyzed for cytokines in aqueous humor due to low amount of sample that remained after laboratory diagnosis. Non-statistically significant differences existed between patients where the measurement of cytokines in AH could be made and those where it was not possible, in age (median age: 25 years, range 20–82 vs. 42 years, range 20–86; p = 0.07) or gender distribution (% males 72 vs. 44; p = 0.36). Also not statistically significant differences were found for clinical characteristics (Table 1). All controls were women with indication for cataract surgery. The median age of control patients was 71 (range 60–82).
Table 1.
Comparison of clinical characteristics in patients with ocular toxoplasmosis where cytokine analysis was performed vs. those were it was not possible due to low amount of aqueous humor (AH) sample.
| Median (range) or percent (n/N) in patients where cytokine analysis was not possible |
Median (range) or percent (n/N) in patients with cytokine analysis |
P- value |
|
|---|---|---|---|
| Age of first clinical episode | 38.3 (16–85) | 20 (16–52) | 0.09 |
| Number of scars | 1 (0–4) | 2.5 (0–4) | 0.3 |
| Number of inflammatory cells in vitreous humor |
2.5 (1–4) | 2 (0.5–4) | 0.71 |
| Mean size of scars in disk diameters | 0.12 (0–1) | 0.5 (0–2.5) | 0.12 |
| Number of lesions | 2.5 (1–3) | 2.0 (1–6) | 0.9 |
| Number of recurrences episodes | 1 (0–9) | 1.5 (0–3) | 0.71 |
| Bilateral involvement | 11.1% (1/9) | 22.2% (2/9) | 1.0 |
| IgM anti-Toxoplasma positive test | 9.0% (1/11) | 11.1% (1/9) | 1.0 |
| Positive PCR in AH for Toxoplasma DNA | 60% (6/10) | 55% (5/9) | 1.0 |
| IgG anti-Toxoplasma (UI/ml) | 201 (90–421) | 194 (97–301) | 0.56 |
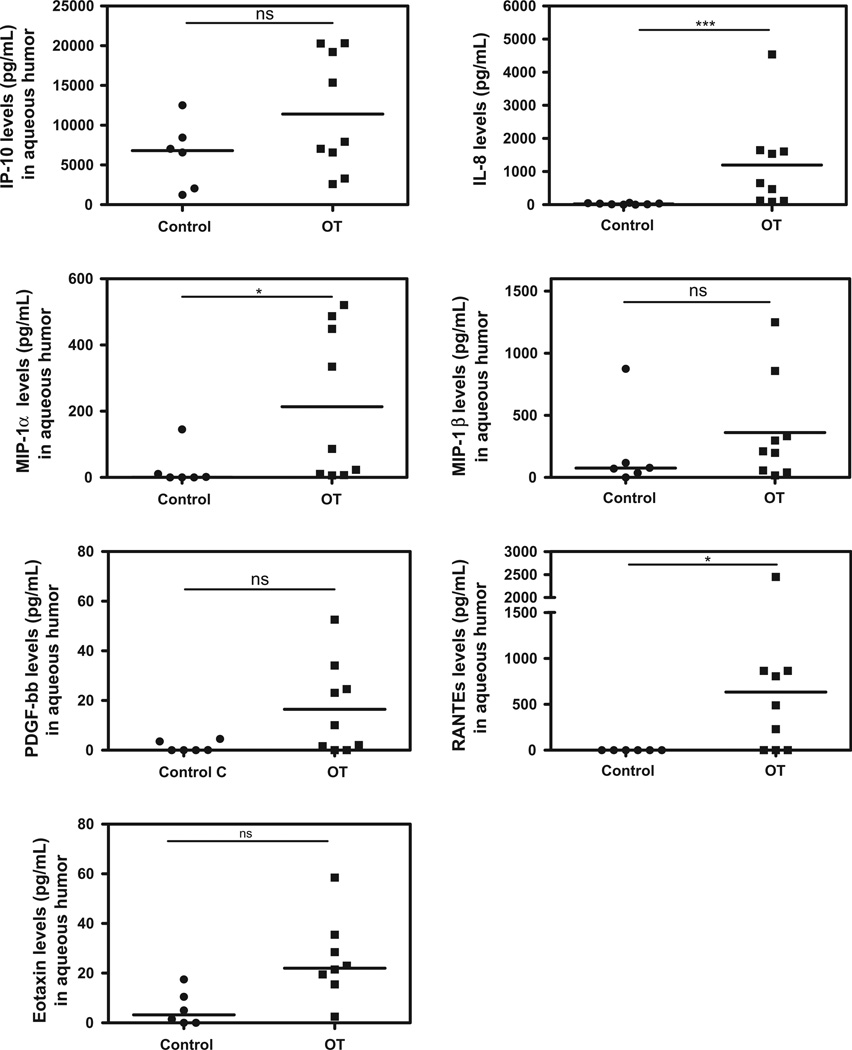
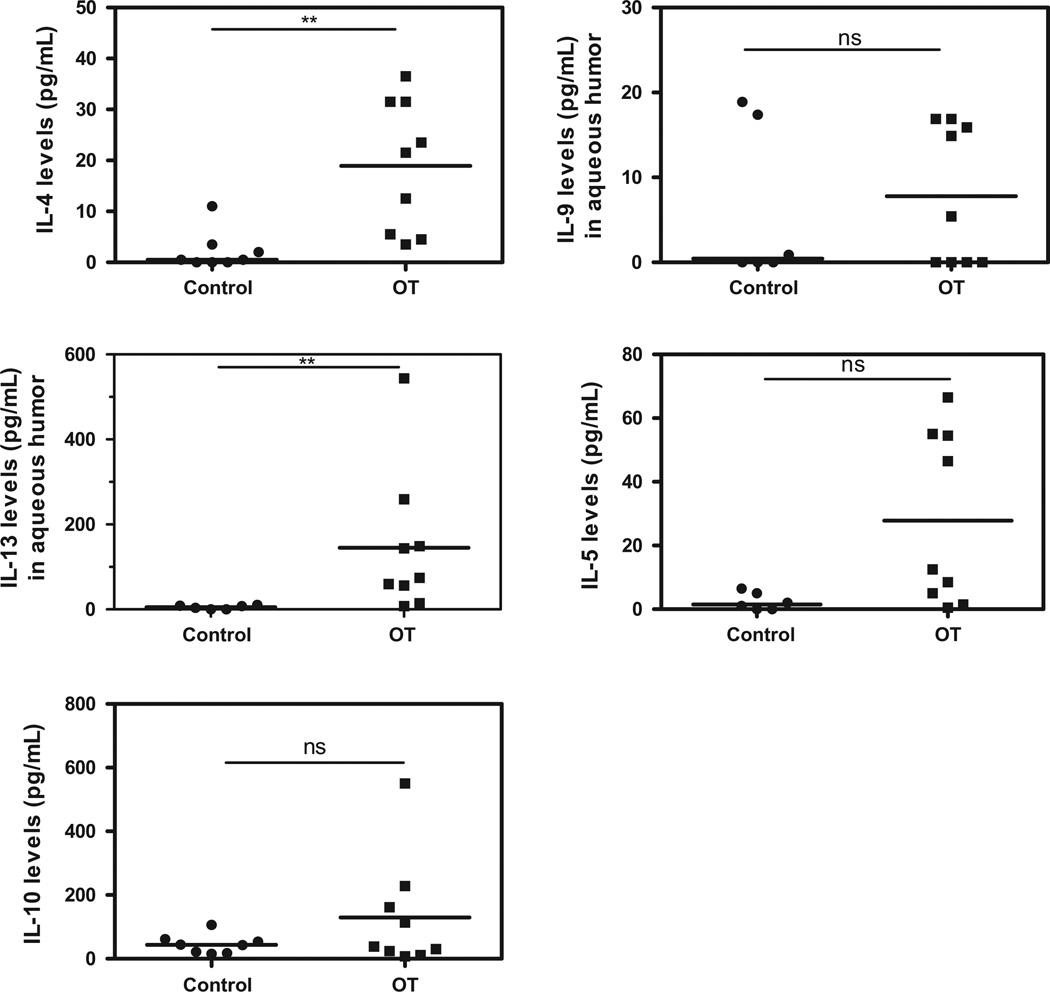
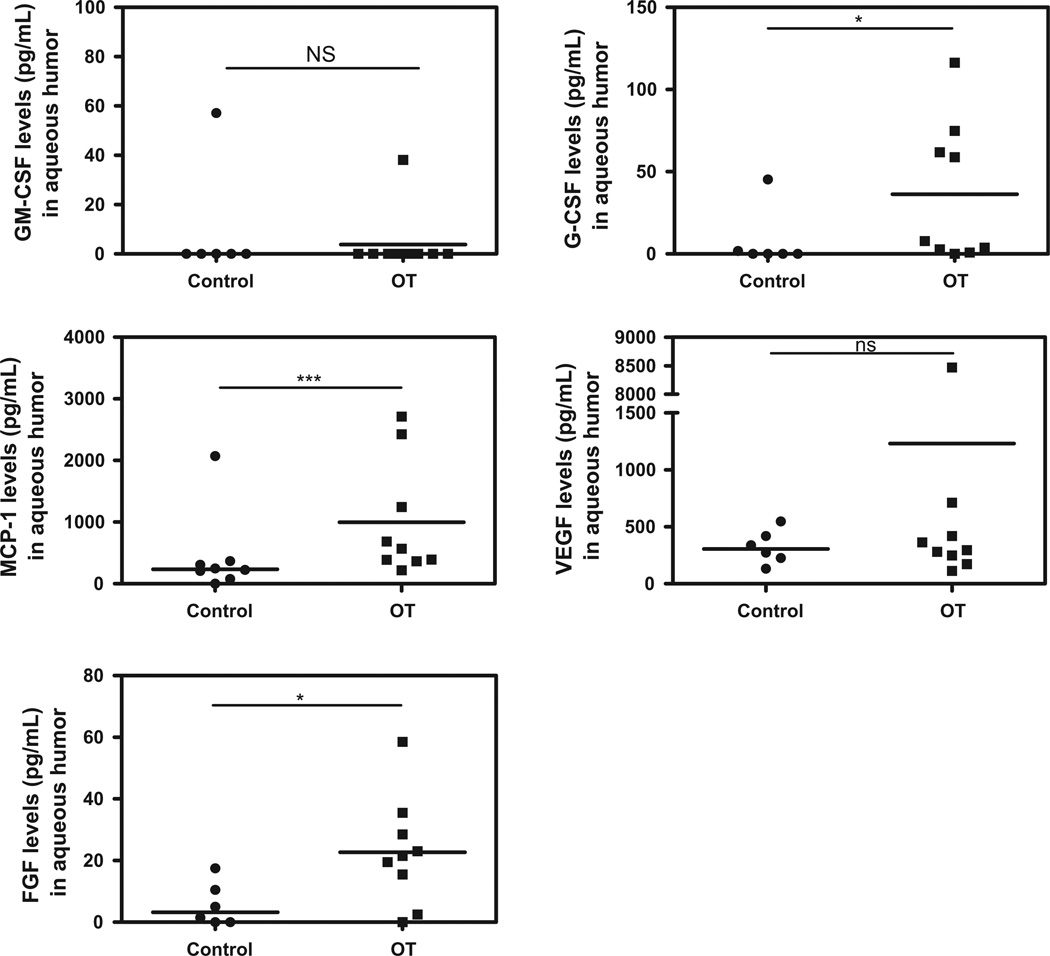
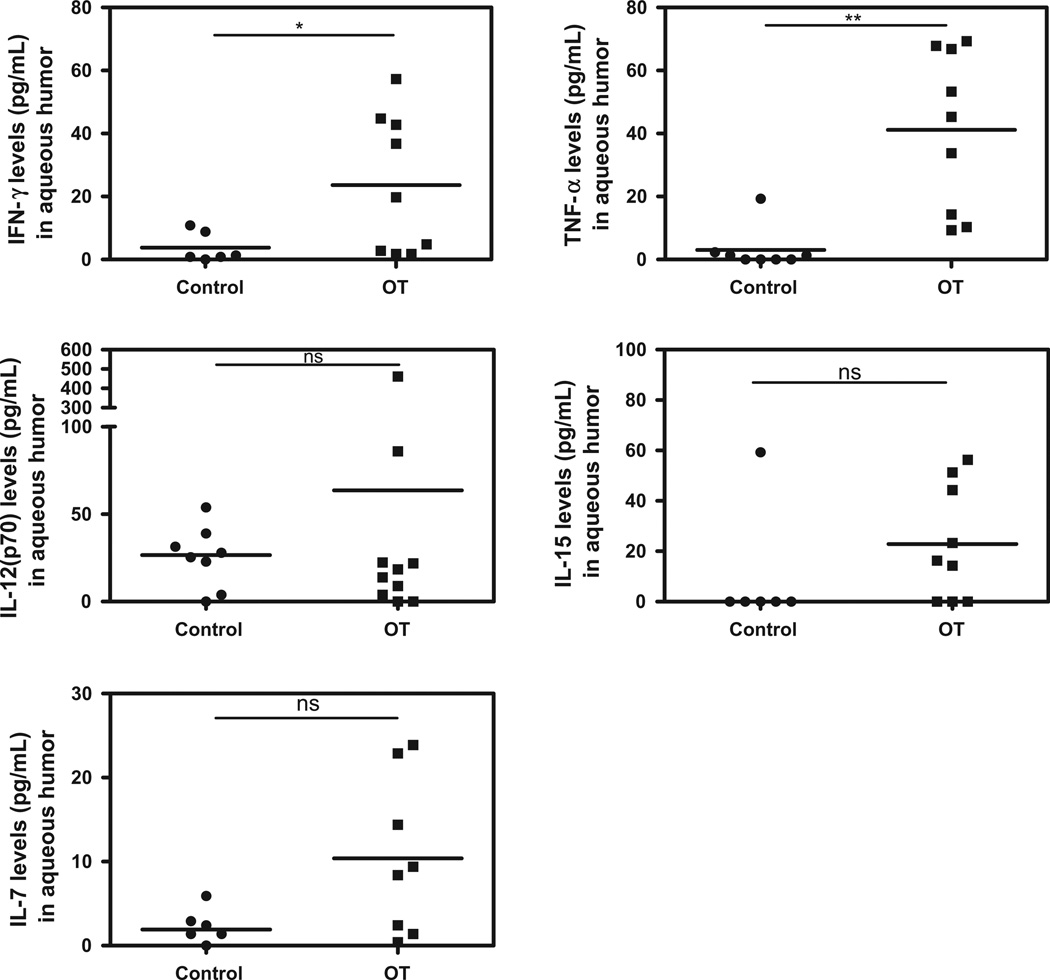
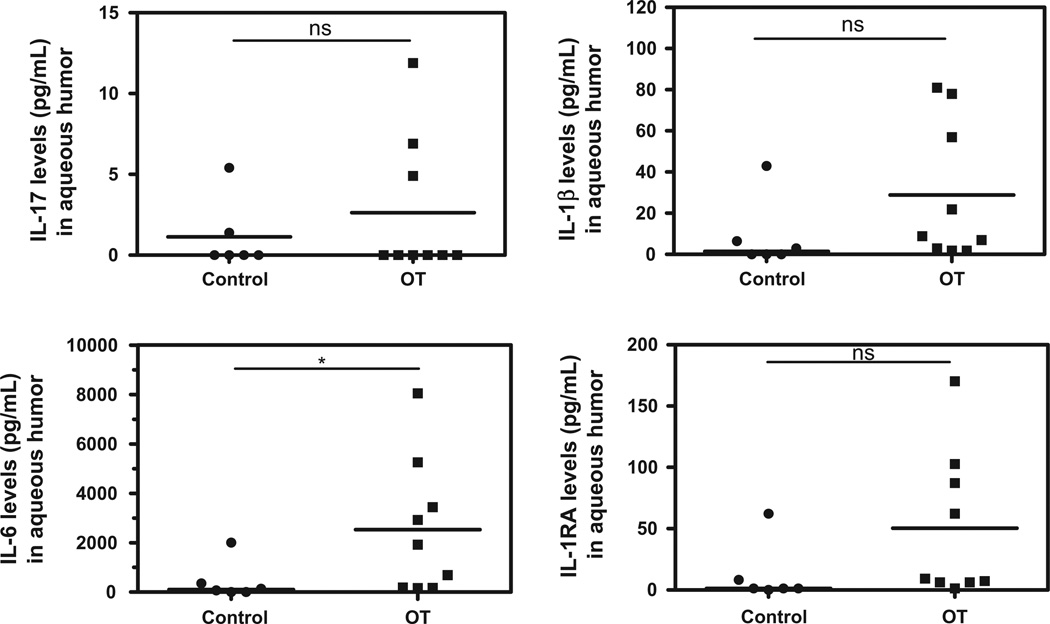
The expression pattern of intraocular immune modulators was heterogeneous in OT patients with high inter-individual variations compared to cataract patients (Figs. 1–5). However, levels of the pro-inflammatory cytokines/chemokines IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-bb, and RANTES (Fig. 1), as well as the growth factors, GM-CSF, G-CSF, FGF, (Fig. 2) were significantly higher in OT patients than in cataract controls. Some Th1 cytokines were also present at higher levels, such as IFN-γ followed by TNF-α, and IL-7 (Fig. 3), but not IL-17. However, in active OT patients, we found higher levels of the Th17 activators IL-1β and IL-6 and of the Th17 inhibitor IL-1RA, than in cataract controls (Fig. 4). Interestingly, the Th2 response was elevated in OT patients, mainly characterized by higher levels of IL-4, and IL-13, as well as the suppressive cytokine IL-10 (Fig. 5). IL10 was predominant over IFN-γ as determined by the IFN-γ/IL10 ratio of each patient (mean ± SD ratio for the OT patients: 0.28 ± 0.17).
Fig. 1.
Chemokines in AH of Colombian patients (n = 9) with OT vs. cataract controls (n = 6). Important expression of intraocular chemokines in active OT patients. Level of significance: *1 − α = 0.9 (90%). **1 − α = 0.95 (95%). ***1 − α = 0.99 (99%).
Fig. 5.
Th2 and Treg cytokine profile in AH of Colombian patients (n = 9) with OT vs. cataract controls (n = 6). Prominent Th2 response in active OT patients. Level of significance: *1 − α = 0.9 (90%). **1 − α = 0.95 (95%). ***1 − α = 0.99 (99%).
Fig. 2.
Proinflammatory growth factors, angiogenesis and wound healing factors in AH of Colombian patients (n = 9) with OT vs. cataract controls (n = 6). Higher levels of the pro-inflammatory growth factors in active OT patients compared to cataract controls. Level of significance: *1 − α = 0.9 (90%). **1 − α = 0.95 (95%). ***1 − α = 0.99 (99%).
Fig. 3.
Th1 cytokine profile in AH of Colombian patients (n = 9) with OT vs. cataract controls (n = 6). Higher levels of Th1 cytokines in active OT patients compared to controls. Level of significance: *1 − α = 0.9 (90%). **1 − α = 0.95 (95%). ***1 − α = 0.99 (99%).
Fig. 4.
Th17 cytokine profile in AH of Colombian patients (n = 9) with OT vs. cataract controls (n = 6). Counter-balance of Th17 activators (IL-1β and IL-6), and Th17 inhibitor (IL-1RA) in active OT patients, compared to controls in which the expression of these factors is low or there are not expression. Level of significance: *1 − α = 0.9 (90%). **1 − α = 0.95 (95%). ***1 − α = 0.99 (99%).
3.3. Clinical data correlate with cytokine profiles
Table 2 summarizes the statistically significant correlations between clinical characteristics and cytokine levels. Age was positively correlated with IL-12, TNF-α, IL-7, IL-4, IL-17, IL-1b and IL-1RA levels. The number of active lesions was positively correlated with VEGF, FGF, PDGF-bb, IL-12, and IL-13 levels. The size of active lesions was positively correlated with IFN-γ, TNF-α, IL-7, IL-4, IL-13, IP-10, IL-1b, IL1RA, MIP-1a, MIP-1β, RANTES and FGF. The size of inactive lesions was negatively correlated with FGF. The number of inactive lesions was positively correlated with VEGF. Vitreous inflammation was positively correlated with TNF-α and IFN-γ levels. The total number of recurrences was positively correlated with IL-5 and VEGF. Finally, the number of scars was positively correlated with VEGF levels.
Table 2.
Spearman’s correlation of clinical characteristics and levels of intraocular cytokines (pg/ml) in patients with active OT.
| Cytokine | Age | Size active lesions DD |
Size inactive lesions DD |
Number of inactive lesions |
High vitreous Inflammation |
Number of recurrences |
Number of scars |
|---|---|---|---|---|---|---|---|
| IL-12 | .727(*) | NS | NS | NS | NS | NS | NS |
| IFN-g | NS | .676(*) | NS | NS | .709(*) | NS | NS |
| TNF-a | .803(**) | .725(*) | NS | NS | .688(*) | NS | NS |
| IL2 | NS | NS | NS | NS | NS | NS | NS |
| IL-7 | .668(*) | .780(*) | NS | NS | NS | NS | NS |
| IL-15 | NS | NS | NS | NS | NS | NS | NS |
| IL-4 | .840(**) | .728(*) | NS | NS | NS | NS | NS |
| IL-10 | NS | NS | NS | NS | NS | NS | NS |
| IL-13 | NS | .759(*) | NS | NS | NS | NS | NS |
| IL-5 | NS | NS | NS | NS | NS | .685(*) | NS |
| IL-9 | NS | NS | NS | NS | NS | NS | NS |
| IP-10 | NS | .690(*) | NS | NS | NS | NS | NS |
| EOTAXIN | NS | NS | NS | NS | NS | NS | NS |
| IL-17 | .785(*) | NS | NS | NS | NS | NS | NS |
| IL-1b | .762(*) | .811(**) | NS | NS | NS | NS | NS |
| IL1-ra | .679(*) | .809(**) | NS | NS | NS | NS | NS |
| IL-6 | NS | NS | NS | NS | NS | NS | NS |
| IL-8 | NS | NS | NS | NS | NS | NS | NS |
| MCP-1 | NS | NS | NS | NS | NS | NS | NS |
| MIP-1a | NS | .845(**) | NS | NS | NS | NS | NS |
| MIP-1b | NS | .725(*) | NS | NS | NS | NS | NS |
| G-CSF | NS | NS | NS | NS | NS | NS | NS |
| GM-CSF | NS | NS | NS | NS | NS | NS | NS |
| PDGF-bb | NS | NS | NS | NS | NS | NS | NS |
| VEGF | NS | NS | NS | .720(*) | NS | .747(*) | .720(*) |
| RANTES | NS | .772(*) | NS | NS | NS | NS | NS |
| FGF | NS | .690(*) | −.707(*) | NS | NS | NS | NS |
NS: non-significant correlation.
Level of significance (two tailed).
1 − α = 0.9 (90%).
1 − α = 0.95 (95%).
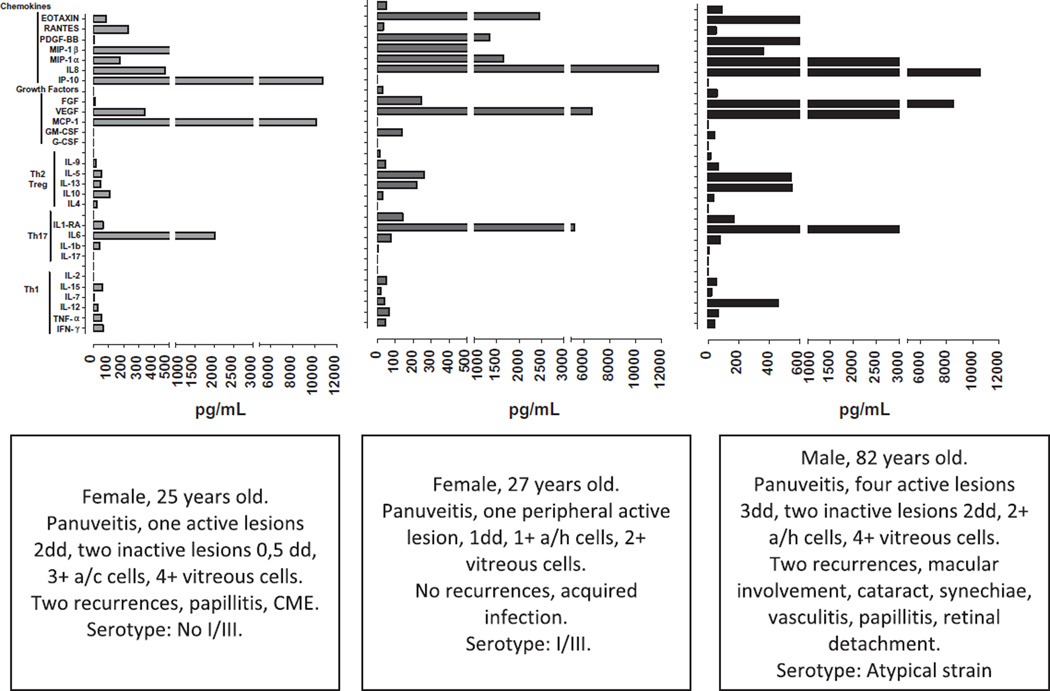
We draw a cytokine profile for each OT patient (see examples in Fig. 6). Despite obvious inter-individual variations, some patterns could be observed: chemokines and growth factors were predominant in all patients; MCP-1, IP-10 and IL-6 had high levels; IL-17 and IFN-α had extremely low levels and expression of IL-12 was related with higher inflammation level.
Fig. 6.
Examples of individual typical cytokine-profiles patterns in AH of patients with active OT.
3.4. Cytokine profile and Toxoplasma serotyping
Four out of nine OT patients showed Type I/III serotypes, while five patients exhibited non-Type I/III serotypes. We compared median cytokine levels between these two groups. Several cytokines were indeed present in higher concentration in OT patients with a I/III serotype: IL-12, IL-13, IL-17, IL-1β, IL-5, IL-7, IL-1ra, IL-4, G-CSF, PDGF-bb and TNF-α (Table 3). A trend towards higher inflammation scores was observed in I/III vs. non-I/III patients (median scores 2.5 vs. 1, p = 0.3). No significant correlation was found between serotype and other clinical characteristics (bilateral lesions, papillitis, number of recurrences, number of lesions, vasculitis, or synechiae).
Table 3.
Cytokines with significant different levels according to infecting T. gondii strain in Colombian patients with active OT.
| Cytokine group | Cytokine | Serotype | P-value (Kruskal–Wallis) | |
|---|---|---|---|---|
| I/III Cytokine median (range) pg/ml N: 4 |
No I/III Cytokine median (range) pg/ml N: 5 |
|||
| Th1 | TNF-a | 67.3 | 14.3 | 0.05 |
| (33.8–69.3) | (9.3–53.3) | |||
| IL-7 | 21.4 | 3.4 | 0.03 | |
| (9.4–23.9) | (2.4–14.4) | |||
| IL-12 | 63.4 | 8.9 | 0.05 | |
| (13.9–460.9) | (0–31.4) | |||
| Th17 | IL-17 | 5.9 | 0 | 0.03 |
| (0–11.9) | (0–0) | |||
| Th17 activators | IL-1b | 76.9 | 14.9 | 0.05 |
| (21.9–80.9) | (6.4–56.9) | |||
| Th17 inhibitors | IL1-ra | 122.55 | 8.3 | 0.04 |
| (62.3–170.3) | (7.3–87.3) | |||
| Th2 | IL-4 | 31.5 | 5.5 | 0.05 |
| (12.5–36.5) | (3.5–23.5) | |||
| IL-5 | 50.5 | 6.5 | 0.05 | |
| (8.5–66.5) | (0.5–50.5) | |||
| IL-13 | 166.5 | 48.5 | 0.05 | |
| (59.5–543.5) | (7.5–143.5) | |||
| Growth factors | G-CSF | 95.55 | 29.8 | 0.05 |
| (45.3–140.8) | (0–61.8) | |||
| Chemokines | PDGF-bb | 29.35 | 4.6 | 0.01 |
| (23–52.6) | (0–10.1) | |||
4. Discussion
The eye presents an immune privileged environment, which leads to tolerization rather than activation of antigen-specific T-cells [16]. Retinal cells, like the Retinal Pigmented Epithelial (RPE) cells, constitutively express immunosuppressive mediators and receptors [17]. This particular ocular situation is also observable with antibody production in the eye, but not in serum during an OT episode [11]. It has to be kept in mind, however, that immune reactions do take place in the eye, especially in case of local injury or infection. However, they are regulated in a different manner than in peripheral tissues. In the brain, microglial cells were found to react to inflammation with the production of IL-13 and cell death, thus protecting neuronal survival [18]. This and other protective reactions, particular to the CNS, are probably also at play in the retina, but very few data are available yet. Because of the special environment, we studied the cytokine patterns directly in the eye, rather than in serum. We and others previously identified cytokines that play essential roles in the inflamed eye [3–7]. Elevated aqueous humor concentrations of cytokines have been reported for different types of uveitis, and diverse cytokine profiles were shown to be characteristic of specific diseases [4–6]. In consequence, cytokine patterns may serve as diagnostic and prognostic monitoring tools for the clinician [19], but are also useful to understand the immunopathogenic mechanisms of infectious uveitis [4].
Here, we looked for the local immune response associated with different clinical appearance. We determined cytokine levels in aqueous humor from nine Colombian patients with ocular toxoplasmosis, by using the same recruitment criteria and the same methodology used in our previous work [6,7,10]. Importantly, we only included biologically confirmed OT cases, rather than all patients with presumed OT, which may limit the impact of other studies [20]. While the ocular signs of toxoplasmic retinochoroiditis are highly suggestive of this disease, they may be mimicked by other infections [11] and symptoms may be atypical in some cases [21]. In our patient series, 30% of suspicious retinochoroiditis cases were found not to be due to toxoplasmosis.
Most of previous human studies actually dealt with Type II strain infection, predominant in Europe and North America. Retinal lesions seem, in these cases, be induced by a non-appropriate Th17 type response and controlled by production of protective IFN-γ [6,7]. In contrast, we recently reported more severe ocular infections in South America due to highly variable Toxoplasma strains and characterized by a completely different local immune response pattern and much higher ocular parasite loads [2,10]. Here, we confirm this specific South American cytokine pattern which suggests that the particular severity of ocular toxoplasmic infection is due to a predominant Th2 response thus preventing effective parasite control. Accordingly, IL13 and IL4 levels were correlated with size and number of lesions. Although IFN-γ and TNF-α levels were elevated in OT compared to cataract controls, they stayed far below the levels reported in French patients [10]. Additionally, the low IFN γ/IL10 ratio indicated a predominance of immunosuppressive over efficient anti-parasitic responses. The lower Th1 response in Colombian patients can be explained by a specific modulation of the immune response by South American strains. In vitro studies demonstrated that strains of the types I and III inhibit the NFκB pathway resulting in reduced IFN-γ production, whereas Type II strains induce it [22]. In support of this, we observed significantly different local cytokine profiles in patients with I/III strain serotypes, compared to patients with non-I/III strain serotypes. The heterogeneous clinical and immunological aspect observed in our Colombian patients is most likely a consequence of a very heterogeneous parasite population in Colombia [10,23] compared to a very homogeneous Type II infection in France [24]. More refined serotyping methods will give a more precise picture in future studies. Genotyping of the Toxoplasma virulence factor ROP18 identified an association between infection by a parasite with the virulent allele of ROP18 and a more inflammatory ocular reaction, whereas current serotyping techniques could not [25].
High levels of IL-6, IL-12p70 and MCP-1, but also IL-10 were found to be associated with more inflammation. Particularly IL-6 is a major proinflammatory cytokine in uveitis and elevated intraocular levels were found in AH of patients with uveitis of diverse origins, including ocular toxoplasmosis, viral uveitis, Fuchs heterochromic uveitis syndrome [32–38]. IL-6 can enhance the progression of the parasite by activation of STAT-3, which is an inhibitor of the protective cytokine IL-12 [39].
Interferon gamma-induced protein 10 (IP-10) was positively correlated with size of active lesions. IP-10 is secreted by monocytes, endothelial cells and fibroblast and is a powerful chemoattractant for various immune cells [30], therefore inducing a highly inflammatory reaction within the eye, leading to larger lesions. The positive correlation that we found between size of active lesions and other cytokines (IFN-γ, TNF-α, IL-7, IL-1b, IL-1 ra) and the chemokine RANTES can be explained by their pro-inflammatory effect. Additionally, the number of active lesions was correlated with IL-12 levels. This inflammatory reaction is, however, largely counterbalanced by a predominant IL-4 and IL10 response in our Colombian patients. IL-10 is an immunomodulatory cytokine produced by various cells [40], often concomitant with IFN-γ or IL-17 [41], as a negative feedback mechanism. This also explains the counterintuitive positive correlation of IL-10 with some inflammatory processes and cytokines. Regarding all these results, IL-10 seems to be central to the induction of the permissive state seen in the eyes of South American OT patients.
The number of recurrences was correlated with IL-5 expression. This cytokine has an important role in the induction of a Th2 response and antibody production by enhancing specific IgA production [31], which has been described as predictor of recurrences in ocular toxoplasmosis [32]. This makes IL-5 a good candidate for a prognostic marker. Equally, VEGF levels were positively correlated with numbers of recurrences and inactive lesions. This could contribute to the formation of choroidal neovascular-membranes [29], frequently observed in Colombian OT patients [1].
We finally found a positive correlation between age and levels of IL-4 and TNF-α. Aging in humans is related with progressive decline in T cell numbers and increased production of TNF-α [26], as well as increased secretion of IL-4, reflecting an age-dependent accumulation of memory T cells [27,28].
In conclusion, for the first time it was found that there are significant correlates of specific cytokine patterns with clinical characteristics in OT, such as inflammation, recurrences and the infecting T. gondii strain. These results will help to build new working hypothesis about the differences in therapeutic response and prognosis in OT and to test immunomodulatory options for the treatment of this important ocular infection.
Acknowledgments
We would like to thank the Universidad del Quindío, Hôpitaux Universitaires de Strasbourg for the PHRC Grant 2007-3964, and Université de Strasbourg for additional financial support.
Funding
This work was supported by Colciencias [Grant 111345921861], the Ecos Nord Program, Fondation pour la Recherche Médicale, and the Intramural Research Program of the National Institutes of Health and NIAID.
References
- 1.de-la-Torre A, López-Castillo CA, Gómez-Marín JE. Incidence and clinical characteristics in a Colombian cohort of ocular toxoplasmosis. Eye (Lond) 2009;23:1090–1093. doi: 10.1038/eye.2008.219. [DOI] [PubMed] [Google Scholar]
- 2.Pfaff A, de-la-Torre A, Rochet E, Brunet J, Sabou M, Sauer A, et al. New clinical and experimental insights into Old World and neotropical ocular toxoplasmosis. Int J Parasitol. 2014;44:99–107. doi: 10.1016/j.ijpara.2013.09.007. http://dx.doi.org/10.1016/j.ijpara.2013.09.00. [DOI] [PubMed] [Google Scholar]
- 3.Lacomba MS, Martin CM, Chamond C, Galena JMG, Omar M, Esterez C. Aqueous and serum interferon gamma, interleukin (IL-2, IL-4 and IL-10) in patients with uveitis. Arch Ophthalmol. 2000;118:768–772. doi: 10.1001/archopht.118.6.768. [DOI] [PubMed] [Google Scholar]
- 4.Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin Med Res. 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang M, Cheung G, Vania M, Chen J, Yang H, Li J, et al. Aqueous cytokine and chemokine analysis in uveitis associated with tuberculosis. Mol Vis. 2012;18:565–573. [PMC free article] [PubMed] [Google Scholar]
- 6.Lahmar I, Abou-Bacar A, Abdelrahman T, Guinard M, Babba H, Ben Yahia S, et al. Cytokine profiles in toxoplasmic and viral uveitis. J Infect Dis. 2009;199:1239–1249. doi: 10.1086/597478. [DOI] [PubMed] [Google Scholar]
- 7.Sauer A, Pfaff AW, Villard O, et al. Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J Infect Dis. 2012;206:1319–1329. doi: 10.1093/infdis/jis486. [DOI] [PubMed] [Google Scholar]
- 8.Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LM, Tan HK, Wallon M, et al. European Multicentre Study on Congenital Toxoplasmosis (EMSCOT). Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis. 2008;2(8):e277. doi: 10.1371/journal.pntd.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de-la-Torre A, Sauer A, Bourcier T, Speeg-Schatz C, Ballonzoli L, Ajzenberg D, et al. Severe South American ocular toxoplasmosis is associated with decreased IFN-γ/IL-17A and increased IL-6/IL-13 intraocular levels. PLoS Negl Trop Dis. 2013;7(11):e2541. doi: 10.1371/journal.pntd.0002541. http://dx.doi.org/10.1371/journal.pntd.000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garweg JG, de Groot-Mijnes JD, Montoya JG. Diagnostic approach to ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:255–261. doi: 10.3109/09273948.2011.595872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villard O, Cimon B, Franck J, Fricker-Hidalgo H, Godineau N, Houze S, et al. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Network from the French National Reference Center for Toxoplasmosis. Diagn Microbiol Infect Dis. 2012;73:231–235. doi: 10.1016/j.diagmicrobio.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 13.de-la-Torre A, Rios-Cadavid AC, Cardozo-García CM, Gomez-Marín JE. Frequency and factors associated with recurrences of ocular toxoplasmosis in a referral centre in Colombia. Br J Ophthalmol. 2009;93(8):1001–1004. doi: 10.1136/bjo.2008.155861. [DOI] [PubMed] [Google Scholar]
- 14.Deschenes J, Murray PI, Rao NA, Nussenblatt RB. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16:1–2. doi: 10.1080/09273940801899822. [DOI] [PubMed] [Google Scholar]
- 15.McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, et al. Toxoplasmosis study group. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009) Clin Infect Dis. 2012;54:1595–1605. doi: 10.1093/cid/cis258. http://dx.doi.org/10.1093/cid/cis25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein-Streilein J. Immune regulation and the eye. Trends Immunol. 2008;29:548–554. doi: 10.1016/j.it.2008.08.002. http://dx.doi.org/10.1016/j.it.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102–180. doi: 10.1016/j.preteyeres.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin WH, Lee DY, Park KW, Kim SU, Yang MS, Joe EH, et al. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia. 2004;15:142–152. doi: 10.1002/glia.10357. [DOI] [PubMed] [Google Scholar]
- 19.Fisson S, Ouakrim H, Touitou V, Baudet S, Ben Abdelwahed r, Donnou S, et al. Cytokine profile in human eyes: contribution of a new cytokine combination for differential diagnosis between intraocular lymphoma or uveitis. PLoS One. 2013;8(2):e52385. doi: 10.1371/journal.pone.0052385. http://dx.doi.org/10.1371/journal.pone.0052385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutra MS, Béla SR, Peixoto-Rangel AL, Fakiola M, Cruz AG, Gazzinelli A, et al. Association of a NOD2 gene polymorphism and T-helper 17 cells with presumed ocular toxoplasmosis. J Infect Dis. 2013;207:152–163. doi: 10.1093/infdis/jis640. http://dx.doi.org/10.1093/infdis/jis640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds EM, Holland GN, Stanford MR, Yu F, Siu WO, Shah KH, et al. Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol. 2008;146:856–865. doi: 10.1016/j.ajo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallego C, Saavedra C, Gómez Marín JE. Direct genotyping of animal and human isolates of Toxoplasma gondii from Colombia (South America) Acta Tropica. 2006;97:161–167. doi: 10.1016/j.actatropica.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Fekkar A, Ajzenberg D, Bodaghi B, Touafek F, Le Hoang P, Delmas J, et al. Direct genotyping of Toxoplasma gondii in ocular fluid samples from 20 patients with ocular toxoplasmosis: predominance of type II in France. J Clin Microbiol. 2011;49:1513–1537. doi: 10.1128/JCM.02196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez V, de-la-Torre A, Gómez Marín JE. Characterization of ROP18 alleles in human toxoplasmosis. Parasitol Int. 2014;63:463–469. doi: 10.1016/j.parint.2013.10.012. http://dx.doi.org/10.1016/j.parint.2013.10.01. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Chiplunkar S, Kim C, Yel L, Gollapudi S. Effect of age on molecular signaling of TNF-alpha-induced apoptosis in human lymphocytes. Mech Ageing Dev. 2003;124:503–509. doi: 10.1016/s0047-6374(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 27.Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, et al. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- 28.Li SP, Miller RA. Age-associated decline in IL-4 production by murine T lymphocytes in extended culture. Cell Immunol. 1993;151:187–195. doi: 10.1006/cimm.1993.1230. [DOI] [PubMed] [Google Scholar]
- 29.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 30.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 31.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191–236. doi: 10.1016/S0065-2776(08)01006-7. http://dx.doi.org/10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]
- 32.Gómez Marín JE, Montoya MT, Castaño JC, Alvarado F, Duque AM, Chemla C, et al. Frequency of specific anti-Toxoplasma gondii IgM, IgA and IgE in Colombian patients with acute and chronic ocular toxoplasmosis. Mem Inst Oswaldo Cruz. 2000;95:89–94. doi: 10.1590/s0074-02762000000100014. [DOI] [PubMed] [Google Scholar]
- 33.Iyer JV, Connolly J, Agrawal R, Yeo TK, Lee B, Au B, et al. Cytokine analysis of aqueous humor in HIV patients with cytomegalovirus retinitis. Cytokine. 2013;64:541–547. doi: 10.1016/j.cyto.2013.08.006. http://dx.doi.org/10.1016/j.cyto.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Takase H, Futagami Y, Yoshida T, Mochizuki M, Kamoi K, Sugita S, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 35.van Kooij B, Rothova A, Rijkers GT, deGroot Mijnes J. Distinct cytokine and chemokine profiles in the aqueous humor of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006;142:192–194. doi: 10.1016/j.ajo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura T, Sonoda KH, Ohguro N, et al. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology. 2009;48:347–355. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Asrar AMA, Struyfb S, Kangavea D, Al-Obeidana SS, Opdenakkerb G, Geboesch K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139:177–184. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Perez VL, Papaliodis GN, Chu D, Anzaar F, Christen W, Foster CS. Elevated levels of interleukin 6 in the vitreous fluid of patients with pars planitis and posterior uveitis: the Massachusetts eye & ear experience and review of previous studies. Ocul Immunol Inflamm. 2004;12:193–201. doi: 10.1080/092739490500282. [DOI] [PubMed] [Google Scholar]
- 39.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturallyoccurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]