Abstract
Adherence of an embryo to the uterus represents the most critical step of the reproductive process. Implantation is a synchronized event between the blastocyst and uterine luminal epithelium leading to structural and functional changes for further embryonic growth and development. The milieu comprising the complex process of implantation is mediated by estrogen through diverse but interdependent signaling pathways. Mouse models have demonstrated the relevance of the expression of estrogen modulated paracrine factors to uterine receptivity and implantation window. More importantly, some factors seem to serve as molecular links between different estrogen pathways promoting cell growth, acting as molecular chaperones or amplifying estrogenic effects. Abnormal expression of these factors can lead to implantation failure and infertility. This review provides an overview of several well characterized signaling pathways that elucidates molecular cross-talk involved in the uterus during early pregnancy.
Keywords: Estrogen, implantation, uterus, pregnancy, signaling
Introduction
Reproduction is a fundamental aspect of life. The World Health Organization (WHO) has recognized infertility, or the inability to reproduce, a worldwide health concern with a lifetime prevalence ranging from 6.6% to 26.4% (Boivin, et al. 2007). Although much advancement has been made using assisted reproductive technologies (ART) to achieve higher pregnancy rates by improving the selection of high-quality embryos, the implantation process is still very illusive.
The development of the preimplantation embryo and the differentiation of the uterus are distinct processes occurring simultaneously in early gestation and must be synchronized in order for successful implantation (Paria, et al. 1993; Psychoyos 1973a). It has been shown that in a mouse, implantation occurs when the developed blastocyst attaches to the luminal epithelium of the uterine endometrium on the evening of day 4 of pregnancy (Das, et al. 1994; Enders and Schlafke 1969). This attachment of the embryo to the epithelial lining promotes the disappearance of epithelium via a mechanism through entosis (cell-eat-cell) by the trophoblast cells followed by apoptosis at the site of implantation (Li, et al. 2015; Parr, et al. 1987) and subsequent stimulation of stromal cell proliferation and differentiation into secretory decidual cells, therefore forming decidualization at the blastocyst site (Huet-Hudson, et al. 1989).
These structural and functional changes occurring in the uterus promote receptivity to the invading blastocyst. This receptivity phase of the uterus is short-lived and primarily mediated by estrogen and progesterone (Paria et al. 1993; Psychoyos 1973a). The estrogenic effects in the mouse uterus are biphasic: early (phase I) responses occur within 6 hours and are characterized by water inhibition, macromolecular uptake and alteration in genes involved in vascular permeability. Late (Phase II) responses occur between 18–30 hours and are characterized by increased epithelial cell proliferation (Huet-Hudson et al. 1989). The presence of progesterone (P4) is inhibitory to estrogen-mediated epithelial proliferation, which can be detected on day 4 (D4) of gestation (Das and Martin 1973; Li, et al. 2011; Martin, et al. 1973; Pan, et al. 2006). Ovariectomized mice on the morning of D4 before preimplantation estrogen secretion exhibit delayed implantation due to blastocyst dormancy (Yoshinaga and Adams 1966). When the uterus in ovariectomized mice is exposed to progesterone alone it renders it a neutral or pre-receptive endometrium; however, receptivity for implantation is observed when exposed to estrogen (Paria et al. 1993). This demonstrates the crucial role of estrogen in the process of implantation.
The mechanisms by which estrogen transforms a progesterone-primed uterus to the receptive state, activates blastocysts and initiates implantation are not clearly delineated. The classical estrogen-signaling pathway is through nuclear estrogen receptors ERα (ESR1) and ERβ (ESR2), which act as ligand-inducible transcription factors (Beato, et al. 1995; Tsai and O’Malley 1994). However, there is increasing evidence that gene activation and cell function modulation are initiated by estrogen through a nuclear ER-independent manner. Studies with ERα null mice and also in those with wild-type mice in which both ERα and ERβ antagonist ICI 182,780 was utilized to silenced ligand-dependent ER functions, have demonstrated estrogen mediated gene expression suggesting an alternate signaling pathway (Das, et al. 2000; Das, et al. 1997; Hou, et al. 2004).
Implantation failure and infertility is associated with aberrations in molecular pathways. The knowledge attained with the development of knockout mouse models and conditional gene deletions has advanced uterine biology immensely. This is a review of the knowledge gained from previous studies in mice attempting to delineate the mechanisms of estrogen signaling. Understanding the estrogen pathways and its mediated events during early pregnancy is critical to further advancement in ART protocols that will improve treatment of this worldwide health condition.
Role of estrogen receptors during early pregnancy
Estrogen plays a pivotal role in the observed changes of the uterus during early pregnancy. In mice, during the first two days of gestation, pre-ovulatory estrogen stimulates proliferation of the luminal and glandular epithelial cells (phase I estrogen secretion). Once the corpora lutea is formed on day 3 of gestation, progesterone secretion stimulates stromal cell proliferation, which becomes further potentiated by pre-implantation estrogen (phase II estrogen secretion) on day 4, the day of implantation (Huet-Hudson et al. 1989). This second wave of estrogen prior to implantation ceases epithelial cell proliferation and allows for differentiation to occur (Tan, et al. 1999). During the remodeling of the uterine epithelium, the epithelial cells lose polarity through down-regulation of the cell-to-cell adhesion molecules E-cadherin (Daikoku, et al. 2011; Li et al. 2015). Epithelial cells also acquire inhibition of the glycoprotein mucin 1 (MUC1) and develop protrusions along the apical surface (DeSouza, et al. 1998; Surveyor, et al. 1995). Increased endometrial capillary permeability at the location of the blastocyst is also exhibited lending to implantation and subsequent decidualization of stromal cells (Matsumoto, et al. 2002a; Psychoyos 1973b).
The classic physiologic actions of estrogen on its target organ are mediated by its binding to ER activates the receptor by promoting dimerization and then translocation to the nucleus to bind its responsive element in the DNA (Kumar and Chambon 1988). The distribution and expression of ER subtypes varies due to their tissue-specific physiologic functions in various organ systems. ERα, for example, is mainly present in mammary gland tissue, uterus, thecal cells of the ovary, bone, liver, adipose tissue, testes and epididymis of the male reproductive organs and the stroma of the prostate. ERβ is mainly found in the epithelium of the prostate, bladder, granulosa cells of the ovary, colon adipose tissue and the immune system (Dahlman-Wright, et al. 2006; Heldring, et al. 2007). Although ERα is the predominant isoform in certain tissues, both receptors have high affinity to estradiol-17β (E2) in the same estrogen response element (ERE) and they share approximately 95% and 55% homology in the DNA-binding domain and the hormone-binding domain, respectively (Kuiper, et al. 1997; Tremblay, et al. 1997). However, it has been demonstrated that the biological disruption of ERα gene causes infertility through defects in the reproductive tract and gonads of female mice while disruption of the ERβ gene by the insertion of neo-cassette into exon 3 is associated with only disruption of ovulation (Couse, et al. 2005; Eddy, et al. 1996; Krege, et al. 1998; Lubahn, et al. 1993).
The innovation of genetically induced mice has allowed for further knowledge of estrogen signaling. Studies using ERα and ERβ knockout (KO) mice have demonstrated that ERα is essential for endometrial receptivity (Buchanan, et al. 1999; Cooke, et al. 1997; Lubahn et al. 1993). Similarly, studies utilizing PR null mouse strains have demonstrated that uterine stromal cells are the mediators of progesterone inhibitory effects on estrogen induced proliferative response of the uterine epithelium (Kurita, et al. 1998). Simultaneously, Tan et al demonstrated that there is compartmentalization of uterine ERα, but extremely low to undetectable expression of ERβ, is associated with early periimplantation days of gestation (Tan et al. 1999). Early gestation (days 1 and 2) ERα mRNA is primarily localized in the luminal and glandular epithelium while localization is additionally seen in the stroma on days 3 and 4; however, by day 8 of gestation ERα exhibits down regulation in decidual cells immediately surrounding the embryo. Collectively, these studies suggest that specific regulation of ER gene expression seems to define the implantation window.
Additionally, the analysis of the implantation window has demonstrated that the estrogen effects on the endometrium are tightly regulated. Ma et al demonstrated that lower estrogen levels tend to sustain the receptivity of the uterus; however, higher concentrations shut down this time window, although the exact mechanism is not well understood (Ma, et al. 2003). NCOA6 is a coactivator for multiple nuclear receptors and has been demonstrated that Ncoa6 KO mice fail to develop due to defects noted in the placenta and other tissues (Kuang, et al. 2002; Mahajan and Samuels 2005). Kawagoe et al demonstrated that NCOA6 regulates estrogen sensitivity and signaling affecting the uterine receptivity status (Kawagoe, et al. 2012). Using a conditional KO of Ncoa6 in mice, Kawagoe was able to demonstrate that loss of NCOA6 results in ERα accumulation in stromal cells and accumulation of steroid receptor coactivator 3 (SRC3), a potent ERα coactivator (Kawagoe et al. 2012). Therefore, the loss of NCOA6 leads to the inability to attenuate estrogen sensitivity via an accumulation of ERα and SRC3 at the implantation site rendering the uterus non-receptive with pregnancy failure.
These observations suggest a localized site of coordinated effects of estrogen on its target tissue. Since both stroma and epithelium express ERα, one would assume that estrogen-induced epithelial proliferation is controlled directly through the interaction with the specific nuclear steroid receptor. However, studies have demonstrated that target tissue estrogen-induced response is not necessarily related to its affinity or occupancy to the receptor (Das et al. 1997), since an estrogen receptor antagonist, ICI-182,780 failed to inhibit uterine estrogen responsive lactoferrin (Ltf) gene expression and water imbibition induced by certain estrogens in ERKO mice, however, this ICI-182,780 indeed suppressed the uterine Ltf expression in wild-type mice induced after E2, which indicated an estrogen signaling independent of both ERα and ERβ.
Distinct estrogen signaling pathways
Specific functions of AF-1 and AF2 domains of ERα
Binding of ER at genomic sites regulates gene expression. Different physiologic responses are initiated by estrogen binding to ER leading to receptor conformational changes that are required for transcriptional activity. Two transactivation function domains mediate transcriptional activation: activation fuction-1 (AF-1) in the N-terminal domain and activation function-2 (AF-2) in the C-terminal ligand-binding domain (LBD) (Kushner, et al. 2000; Tremblay, et al. 1999). Both AFs have unique differential gene activation through cell type-specific co-activators (Hsia, et al. 2010; Xu, et al. 1998). Previous studies demonstrated that the significance of these specific domains with regards to the functionality of ER depends on AF-1 (Merot, et al. 2004).
However, although reproduction is affected in ERα null mice (Lubahn et al. 1993), several estrogen effects still persist, such as early responses to uterine edema and gene expression (Das et al. 2000; Das et al. 1997) and vascular injury response (Iafrati, et al. 1997). In the uterus of this null mouse, through alternative splicing, a chimeric small ERα protein (~55 kDa), in which 64 amino acid residues mainly for the B region can be partially deleted from the N-terminal A/B regions of ERα (Couse, et al. 1995). In addition, studies also reported uterine detection of a short form of ERα transcript, representing the deletion of a portion of exon 2 followed by insertion of a frame shift and at least two stop codons at the 5’-end of exon 3 (Couse et al. 1995), but the significance of this remains unknown. The truncated small ERα variant lacks the AF-1 domain, which Pendaries et al. demonstrated could be partially dispensable to mediate the estrogenic effects in the uterus since the variant possesses a residual estrogen-dependent transcriptional activity with an intact AF-2 region (Couse et al. 1995; Pendaries, et al. 2002). Further studies revealed the crucial role of AF-2 for estrogen mediated endometrial epithelial proliferation using antagonists and selective ER modulators (SERMs) (Arao, et al. 2011). However, further studies also revealed that AF-1 activation function is required for the E2-induced uterine epithelial proliferation, whereas it is partially dispensable for the induction of uterine edema by chronic estrogen stimulation (Abot, et al. 2013). Additionally, Kurita et al. revealed differences in the estrogen induced proliferative responses between human and mouse epithelial cells, which seems to be species specific with regards to the utilization of the AF domains within the ERα (Kurita, et al. 2005). Therefore, further investigations into these domains to evaluate the specific physiologic roles of AF-1 and AF-2 are still needed.
Interdependent regulation by uterine epithelial and stromal cells
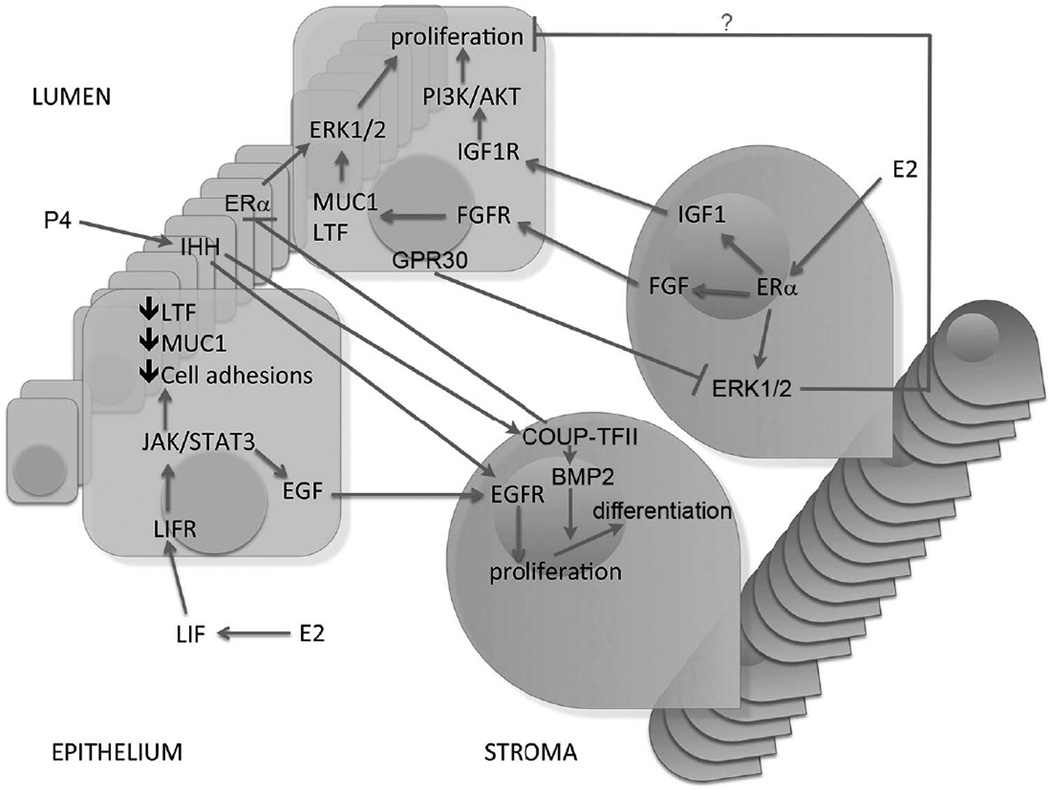
Deletion of ERα in uterine epithelial cells leads to infertility, however, this receptor loss does not prevent estrogen induced epithelial cell proliferation (Winuthayanon, et al. 2010). In this regard, tissue recombination studies have also shown that ERα action in stromal cells mediates the estrogenic proliferation events in the epithelium via a paracrine manner (Cooke et al. 1997; Cunha, et al. 2004). In addition, Pawar et al also showed that epithelial ERα controls uterine decidualization via a paracrine mechanism through epithelial-stromal cross-talk during the early implantation (Pawar, et al. 2015). Similarly down regulation of the progesterone receptor in the uterine epithelium is depended on stromal ERα (Kurita, et al. 2000). The theory of interdependency between the endometrial epithelium and stroma proposes an intercellular cross talk through different signaling pathways (Figure 1), which can mimic the effects of the traditional ligand-receptor pathway.
Figure 1.
Leukemia inhibitory factor (LIF) signaling
LIF is a well-characterized paracrine factor produced by the glandular epithelium under estrogen stimulation that regulates implantation (Stewart, et al. 1992). LIF executes its biological function by activating its own receptor (LIFR) followed by the recruitment of glycoprotein 130 (GP130) (Taga and Kishimoto 1997). Yang et al demonstrated the expression patterns of LIFR and gp130 in the luminal epithelium on day 4 of pregnancy in mice (Yang et al 1995). LIF acts on the luminal epithelium to activate Janus kinase (JAK), a non-receptor tyrosine kinase, which mediates the phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3) (Heinrich, et al. 1998; Tomida, et al. 1999). Lif null mice demonstrate normal ER and PR expression but absent expression of EGF-life growth factors such as heparin binding epidermal growth factor (Hbegf), amphiregulin (Areg), and epiregulin (Ereg) near the blastocyst on day 4 of gestation (Song, et al. 2000). Although the exact function of EGF factors is unknown, the EGF receptors are expressed on stromal cells during pregnancy suggesting a role as paracrine mediators driving stromal proliferation (Figure 1) (Song et al. 2000; Xie, et al. 2007). Furthermore, Stat3 null mice demonstrate increased epithelial expression of estrogen regulated genes Ltf and Muc1, which heighten estrogen signaling allowing for persistent proliferation in the luminal epithelium and a lack of proliferation in the stromal layer (Sun, et al. 2013), indicating an undifferentiated uterine state. Collectively these findings demonstrate that the loss of the LIF-STAT3 signaling pathway culminates in undifferentiated uterine epithelium and therefore non-receptive to the embryo implantation.
Indian hedgehog (IHH) signaling
IHH, a member of the hedgehog gene family, is a progesterone regulated factor produced in the epithelium and controls stromal function via paracrine mechanisms (Figure 1) (Matsumoto, et al. 2002b; Takamoto, et al. 2002). Using a conditional knockout mouse, Ihhd/d, studies have demonstrated that in the absence of Ihh a uterine non-receptive state is achieved secondary to failure of stromal cell proliferation and vascularization along with increased estrogen signaling during the periimplantation phase (Franco, et al. 2010; Lee, et al. 2006). The lack of stromal cell proliferation is in part due to Ihh’s regulation of the EGFR in the stromal compartment, which allows the stroma to be activated by the EGF factors produced by the epithelium secondary to estrogen stimulation (Franco et al. 2010). These observations suggest that the hedgehog signaling cascade plays a crucial role in the events occurring just prior to decidualization.
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) signaling
Previously studies have shown that epithelial IHH stimulates COUP-TFII [also known as nuclear receptor subfamily 2, group F, member 2 (Nr2f2)], a stromal factor that mediates decidualization (Lee, et al. 2010; Lee et al. 2006; Takamoto et al. 2002). Using PR-Cre to cause conditional ablation of endometrial COUP-TFII in mice demonstrate a defect linked to decreased expression of bone morphogenic protein 2, factor produced by the stroma in response to progesterone stimulation (Kurihara, et al. 2007). This aberration results in failure to undergo structural changes involved in decidualization. Additionally, COUP-TFII -deficient mice show an increase in epithelial ERα expression and increased estrogen activity resulting in Ltf and Muc1 expression (Kurihara et al. 2007). Furthermore, studies have shown that the loss of epithelial ERα activity by COUP-TFII is critical for successful progression of embryo implantation and decidualization (Lee et al. 2010). Overall, these studies conclude that COUP-TFII plays a major role in epithelial remodeling and differentiation through controlling ERα activity to support the initiation of embryo implantation.
Fibroblastic growth factor (FGF)/Insulin-like growth factor (IGF) signaling
Stromal factors that regulate epithelial function have also been identified in the intercellular communication pathways, which play a critical role in the implantation window. Specifically, fibroblast growth factors (FGFs) and insulin-like growth factor-1 (IGF1) have been proposed for stromal epithelial communication in a variety of tissues. The FGF family is a group of stromal ERα-induced paracrine factors that act on the epithelium to activate ERK1/2 signaling cascades that stimulate epithelial proliferation (Figure 1) (Li et al. 2011). In this regard, based on uterine co-culture experiments, evidence suggest that estrogen mediated epithelial proliferation may involve stroma-derived factors FGF10 and BMP8a (Chung, et al. 2015). With the FGF10 receptor, FGFR2, primarily detected in the epithelial cells in both the co-culture system and the adult ovariectomized uteri, collectively these results suggest that FGF10/FGFR2 signaling may be specifically involved in the stroma-epithelial cross-talk during early pregnancy. However, Filant et al. demonstrated that conditional ablation of FGFR2 after birth results in abnormal basal cell appearance and stratification in the luminal epithelium, as well as, subfertility that progressed to infertility (Filant, et al. 2014). These results show the critical importance of FGFR2 in postnatal uterine development of LE and female fertility; however, further studies are needed to delineate the molecular mechanism resulting in the observed phenomenon in FGFR2 null mice that lead to complete infertility in multiparous FGFR2 mutant mice. Similarly IGF1, following estrogen stimulation, is abundantly detected in the uterus with IGF1R identified in the epithelium (Kapur, et al. 1992; Murphy and Ghahary 1990). A lack of IGF1 expression is observed in ERKO mice stimulated with estrogen, validating these previous findings (Hewitt, et al. 2010). The fact that IGF1R and IGF1 are abundantly expressed in the uterine epithelium, suggests that IGF1 may be a paracrine mediator involved in the epithelial proliferation during early pregnancy. It is hypothesized that IGF1 stimulates activation of PI3/AKT pathway in the epithelium, which phosphorylates and inactivates glycogen synthase kinase 3 beta (GSK3β), allowing for epithelial proliferation (Zhu and Pollard 2007). When analyzing the role IGF1 in IGF1 knockout mice, Sato et al. demonstrated that uterine growth is supported by systemic IGF1in the absence of local IGF1 production (Sato, et al. 2002). This suggests that local IGF1 is not a direct mediator to estrogen effects in the uterus but rather systemic IGF1 may be the key factor for growth.
Wnt signaling
The biological effect of estrogen can also be associated with Wnt signaling pathways. Wnt is a family of genes that encode a large group of glycoproteins that have a critical role in embryonic development and are also involved in tumorigenesis (Smalley and Dale 1999). The canonical Wnt signaling pathway, which involves regulation of β-catenin, has been the most widely studied. The activation of Wnt signaling stabilizes intracellular β-catenin by antagonizing kinase activity of GSK3β. In the absence of Wnt signaling, GSK3β forms a multimolecular complex with axin (a bridging molecule), adenomatous polyposis coli and β-catenin, leading to phosphorylation and then subsequent degradation via ubiquitination pathway of β-catenin. When activated, β-catenin translocates to the nucleus and forms a complex with downstream effectors such as lymphoid enhancer factor (Lef)/T cell factor (Tcf) family which stimulate transcription of Wnt Target genes. These target genes are involved in cellular organization during embryonic development, proliferation and differentiation as well as cell-to-cell communication and cell fate specification (Smalley and Dale 1999).
Previously, studies have shown that Wnt4 expression is upregulated at the site of embryo implantation during decidualization (Daikoku, et al. 2004). Further studies revealed that Wnt4 plays a key role in implantation and decidualization (Franco, et al. 2011) and this action is mediated downstream of progesterone via β-catenin signaling pathway in uterine stromal activity with proliferation and differentiation (Li, et al. 2013; Rider, et al. 2006).
We previously demonstrated the presence of an ER-independent pathway of estrogen stimulation via Wnt pathway (Hou et al. 2004). After exposing ERα KO (ERKO) mice with estrogen, prompt stabilization and localization of β-catenin in the nucleus of uterine epithelial cells was observed. This finding was confirmed with the injection of adenovirus-driven expression of SFRP2, a Wnt antagonist suppressed rapidly by estrogen during the early phase in the uterus in an ER-independent manner, since (Das et al. 2000), demonstrating down regulation of β-catenin and halting epithelial cell growth without affecting early estrogen effects (Hou et al. 2004). Similarly, studies have also shown that Wnt/β-catenin downstream effectors Lef1 and Tcf3 are upregulated in an estrogen independent manner (Ray, et al. 2008). Through immunofluorescence studies, Lef1/Tcf3 localization was confirmed in the epithelial cells after estrogen exposure and interestingly found to be interacting with ERα in a time-dependent manner (Ray et al. 2008). Furthermore, evidence was provided for an ERα and Tcf3/Lef1 complex occupying a certain DNA region of estrogen responsive gene promoters, suggesting a non-classical induction mechanism of the Wnt/β-catenin pathway that is necessary in the estrogen-dependent gene regulation.
GPR30 signaling
GPR30 (also known as GPER1), a G-protein coupled receptor, has been implicated in early non-genomic signaling mediated by E2. In mouse uterus, GPR30 localizes primarily in the uterine epithelial cells (Gao, et al. 2011). Studies from GPR30 knock-out mice appear to imply that GPR30’s role in uterine biology is minimal for estrogenic growth regulation (Martensson, et al. 2009; Otto, et al. 2009; Wang, et al. 2008). In contrast, utilizing selective activation of GPR30 by G-1, studies have shown that GPR30 is involved in regulating early signaling events, including the inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals in the uterine stromal compartment, suggesting a paracrine signaling is involved (Figure 1) (Gao et al. 2011). However, it should be noted that this study was unable to exclude the possibility through the off-target effects of G-1. Moreover, further studies should be considered to show that Gper1 null mice are insensitive to G-1 in the above uterine effects. Overall, studies show that GPR30 can act as a negative regulator of ERα-dependent uterine growth in response to E2.
Molecular links between the phase I and phase II estrogenic responses in the uterus
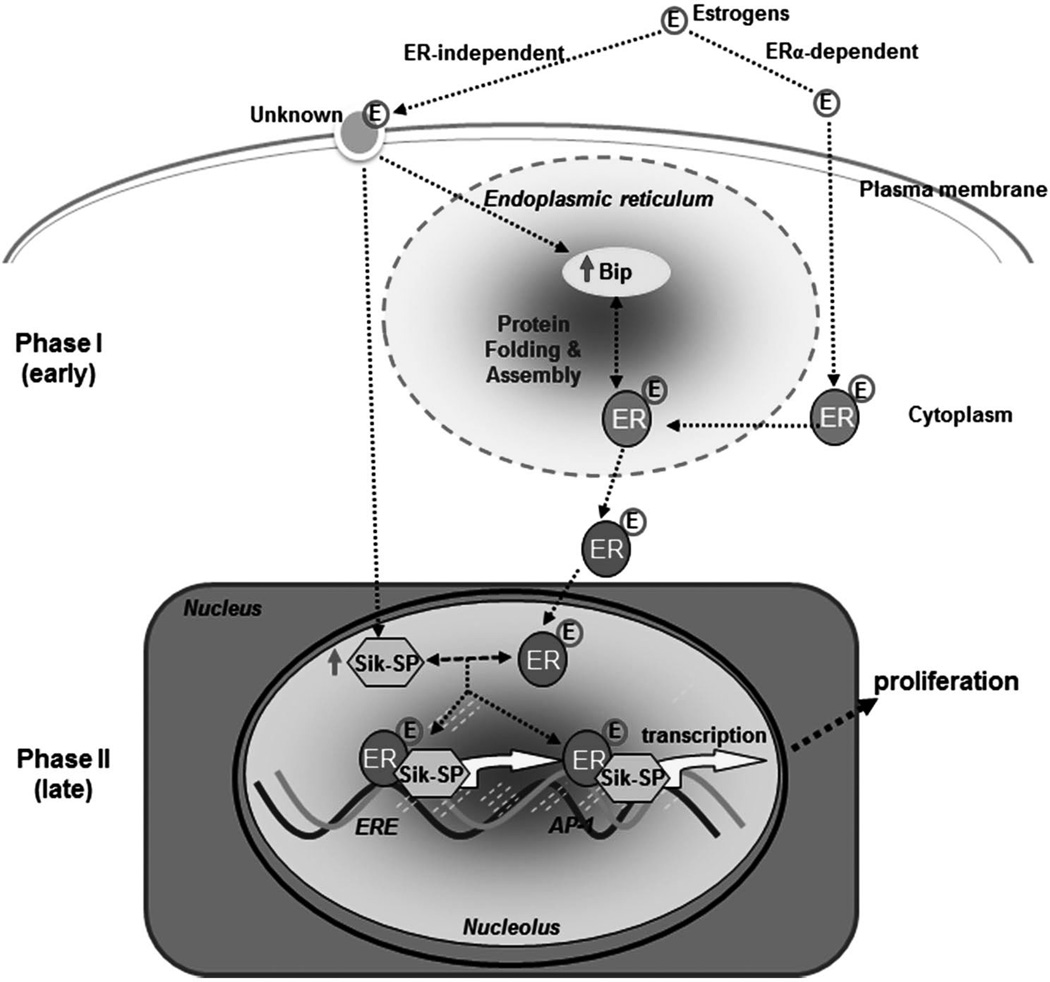
Early (phase I) and late (phase II) estrogenic responses in the uterus have been recognized for more than 70 years, yet mechanisms involved in their regulation remain controversial. One concept is that an early events(s), occurring within the first 6 h, prepares the uterus for later (18–30 h) increase in DNA synthesis, cell proliferation and protein synthesis. An alternate view is that the late growth phase is a result of the continuous presence of a stimulus. Discussion of either concept usually makes the assumption that all of the responses are dependent upon ligand interaction with one of the two estrogen receptor isoforms (ERα and ERβ). However, we and others have shown that ERα null mice (ERKO) or wild-type mice in which ER functions are silenced by ER antagonist ICI 182,780 manifest expression of several early genes in response to 4-hydroxyextradiol-17β or a xenoestrogen (kepone), as well as induction of early responses such as water imbibition and macromolecular uptake by 4-hydroxyextradiol-17β (Das, et al. 1998; Das et al. 2000; Das et al. 1997; Hewitt, et al. 2003; Hou et al. 2004; Ray, et al. 2006; Watanabe, et al. 2003). Furthermore, studies have also shown that ICI was able to suppress expression of Ltf, a well characterized estrogen-responsive uterine gene, in the wild-type mice after E2, indicating the effectiveness of ICI in this study (Das et al. 1998; Das et al. 1997). Utilizing the same effective dose of ICI (Das et al. 1998; Das et al. 1997), we have identified two such ER-independent uterine genes Bip and Sik-SP that are regulated by E2 in ERKO mice (Das et al. 2000). The bimodal nature of estrogen effects coupled with phase I ER independent estrogenic responses and phase II, mostly ER dependent responses has ignited interest in understanding the pathways linking these two phases.
Role of Bip
Bip, also known as grp78 encoded by Hspa5, is a member of the heat shock protein HSP70 chaperone family and it is induced by estrogen in an ER independent manner as a phase I response (Das et al. 2000; Ray et al. 2006). It is a protein that resides in the endoplasmic reticulum (Figure 2), where assembly of newly synthesized peptides occurs, and is abundantly present during cell proliferation and differentiation particularly at the site of embryo implantation during decidualization (Simmons and Kennedy 2000). As a chaperone molecule, the role of BIP is for functional maturation of steroid hormone receptors. In the mouse uterus it mediates estrogen dependent responses through molecular association with ERα (Ray et al. 2006). Studies have demonstrated through an in vivo and in vitro mouse model that suppression of Bip antagonizes ERα mediated gene transcription and compromises estrogen-dependent phase II growth response (uterine epithelial cell proliferation) with sustained phase I responses (water accumulation and macromolecular uptake). Most interesting, is the lack of growth response in the presence of ERKO state even if Bip is upregulated (Ray, et al. 2007). Although this study analyzed xenoestrogen and Bip, it demonstrates the close relationship between Bip and ERα in regulation of uterine growth. Together, studies suggest that the functional activation of ERα via Bip plays a role in coordinating phase I responses with those of phase II for regulated growth and differentiation via estrogen signaling in the mouse uterus.
Figure 2.
Some organochlorine compounds, such as polychlorinated biphenyls, are highly persistent organic pollutants in many industrial nations. These compounds have gained attention recently secondary to their potential for adverse effects on health and reproduction. The reproductive toxicity is thought to be due to their estrogen-like properties, hence they are categorized as xenoestrogens. The ability to bind to ERα, allows for mimicking effect on target organ function, although the mechanisms are not well defined (Das et al. 1997). There are however, significant differences in coactivator recruitment and transcriptional activation in tissues exposed to xenoestrogens corresponding to distinct biological effects causing endocrine disruption. Furthermore, these compounds are effective at very low doses comparable to their level of exposure making them very potent estrogens (Ray et al. 2007).
Knowing the critical role that Bip plays in regulation of estrogen-dependent ERα mediated gene transcription and growth, the xenoestrogen mediated effects with regards to upregulation of Bip under certain conditions could be potentially harmful in respect to enhanced uterine estrogenicity. Specifically, the xenoestrogen kepone can induce sustainable levels of uterine Bip without involving ER, which in turn regulates the kepone-dependent ERα mediated gene expression (Ray et al. 2007). Furthermore, with the notion that stress can regulate Bip expression and the ability of uterine growth via stress induced estrogen response in mice, studies have demonstrated endogenous Bip via stress-related signals contributes to uterine estrogenicity for kepone (Ray et al. 2007). Thus the combination of a variety of signals in the body, such as stress, and xenoestrogens can act as plausible risk factor enhancing estrogenicity and therefore major health concerns.
Role of Sik-SP
The nucleolus is the nuclear subdomain that primarily carries out assembly of ribosomal subunits in eukaryotic cells. A recent study has uncovered an unexpected role for uterine estrogen signaling which involves a nucleolar protein Sik-similar protein (Sik-SP, also known as Nop58/Nop5/Nol5) (Chung, et al. 2012). Studies have shown that the expression of uterine Sik-SP is tightly regulated by E2 in an ER-independent manner, but is still required for the control of ERα-mediated late uterine functions (Figure 2) (Chung et al. 2012; Das et al. 2000). Specifically, using both the in vivo and in vitro co-culture approaches, studies have shown that E2-induced Sik-SP directly interacts with ERα to mediate ERα-dependent gene regulation and is necessary to coordinate the biphasic responses in the uterus for its appropriate growth under the direction of E2. Overall, this finding of ERα-independent early Sik-SP contributing to ERα-regulated events adds new insights to our understanding of nucleolar involvement in uterine estrogen signaling.
Taken together, these studies provide evidence of non-classical pathways that mediate estrogen actions in a time dependent fashion, possibly shedding a light on how the biphasic: phase I and phase II estrogenic responses are molecularly linked to mediate uterine cell proliferation.
ER-independent genes associate with embryo implantation
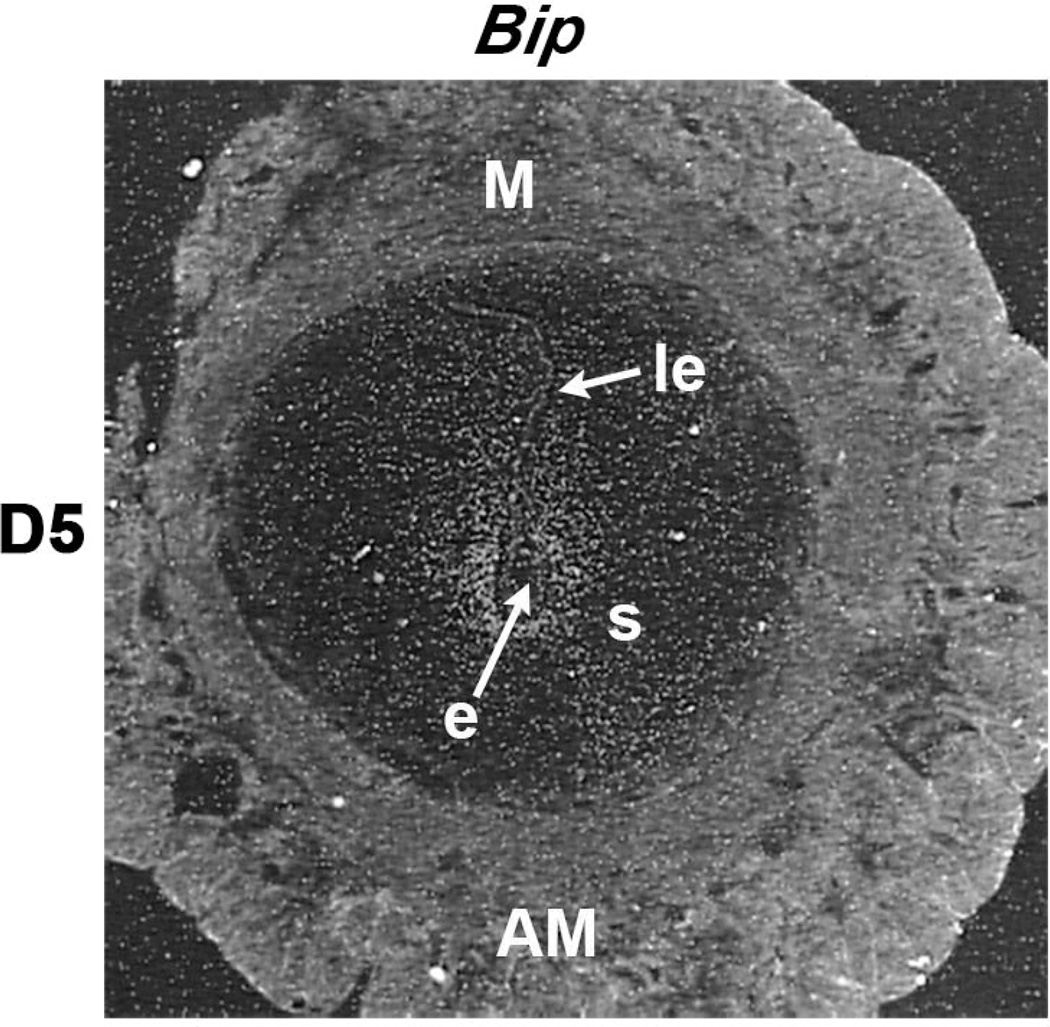
To understand the functional significance of estrogen-induced ER-independent early uterine genes, studies were undertaken to determine whether E2-administration in the delayed implantation model in mice enhances the expression of Bip and Sik-SP at the site of implantation. Indeed, results demonstrated that these genes are specifically up-regulated in the sub-luminal stromal cells at the site of the implanting embryo following activation with E2; however, the delayed stage of the uterus does not show any expression at the site of embryo (Chung et al. 2012; Reese, et al. 2001). Furthermore, this induced expression is consistent with the status of expression in normal implantation sites on D5 for Bip (Figure 3) and Sik-SP (Chung et al. 2012). Taken together, the studies have shown that these ER-independent genes are physiologically important during the onset of embryo implantation under the direction of E2.
Figure 3.
Conclusions
This review has served as an update of the literature describing the molecules involved in estrogen signaling in the mouse uterus during early pregnancy. We have discussed the signaling pathways that are ER dependent and ER independent as well as, the molecular links that shed light into the complexity of the bimodal estrogen actions occurring in early pregnancy. Dysregulation of the cross-talk between these pathways can lead to implantation failure through the inability to obtain a receptive uterine epithelium. Environmental toxins can mimic estrogen pathways, however the mediated effects differ from normal through the enhanced estrogenicity of the uterus creating a non-receptive uterine epithelium. Continued research into the mechanisms involved in estrogen signaling will expand our understanding of this delicate and time sensitive event. Understanding the molecular interactions will provide the knowledge needed to improve current treatments of infertility through the exploration of new ideas, techniques and technology.
Acknowledgments
Funding
This work was supported in part by grants from National Institute of Health (NIH) (ES07814 and HD56044 to SKD) and March of Dimes (#22-FY13-543).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, et al. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci U S A. 2011;108:14986–14991. doi: 10.1073/pnas.1109180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR, Cooke PS. Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology. 1999;140:484–491. doi: 10.1210/endo.140.1.6448. [DOI] [PubMed] [Google Scholar]
- Chung D, Gao F, Jegga AG, Das SK. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol Cell Endocrinol. 2015;400:48–60. doi: 10.1016/j.mce.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Gao F, Ostmann A, Hou X, Das SK. Nucleolar Sik-similar protein (Sik-SP) is required for the maintenance of uterine estrogen signaling mechanism via ERalpha. Mol Endocrinol. 2012;26:385–398. doi: 10.1210/me.2011-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, Demayo F, Maxson R, et al. Conditional Deletion of MSX Homeobox Genes in the Uterus Inhibits Blastocyst Implantation by Altering Uterine Receptivity. Dev Cell. 2011 doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- Das RM, Martin L. Progesterone inhibition of mouse uterine epithelial proliferation. J Endocrinol. 1973;59:205–206. doi: 10.1677/joe.0.0590205. [DOI] [PubMed] [Google Scholar]
- Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. Estrogen targets genes involved in protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-alpha and -beta. J Biol Chem. 2000;275:28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci U S A. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- DeSouza MM, Mani SK, Julian J, Carson DD. Reduction of mucin-1 expression during the receptive phase in the rat uterus. Biol Reprod. 1998;58:1503–1507. doi: 10.1095/biolreprod58.6.1503. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Enders AC, Schlafke S. Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat. 1969;125:1–29. doi: 10.1002/aja.1001250102. [DOI] [PubMed] [Google Scholar]
- Filant J, DeMayo FJ, Pru JK, Lydon JP, Spencer TE. Fibroblast growth factor receptor two (FGFR2) regulates uterine epithelial integrity and fertility in mice. Biol Reprod. 2014;90:7. doi: 10.1095/biolreprod.113.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW, DeMayo FJ. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod. 2010;82:783–790. doi: 10.1095/biolreprod.109.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Ma X, Ostmann AB, Das SK. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERalpha) phosphorylation signals. Endocrinology. 2011;152:1434–1447. doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW. Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev. 2010;62:1227–1237. doi: 10.1016/j.addr.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr, Lubahn DB, O’Donnell TF, Jr, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- Kapur S, Tamada H, Dey SK, Andrews GK. Expression of insulin-like growth factor-I (IGF-I) and its receptor in the peri-implantation mouse uterus, and cell-specific regulation of IGF-I gene expression by estradiol and progesterone. Biol Reprod. 1992;46:208–219. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- Kawagoe J, Li Q, Mussi P, Liao L, Lydon JP, DeMayo FJ, Xu J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell. 2012;23:858–865. doi: 10.1016/j.devcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Zhang H, Pereira FA, Yuan Y, DeMayo FJ, Ko L, Xu J. Deletion of the cancer-amplified coactivator AIB3 results in defective placentation and embryonic lethality. J Biol Chem. 2002;277:45356–45360. doi: 10.1074/jbc.C200509200. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988;55:145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation. 2005;73:313–322. doi: 10.1111/j.1432-0436.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11:358–365. doi: 10.1016/j.celrep.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH. Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev. 2005;26:583–597. doi: 10.1210/er.2004-0012. [DOI] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002a;277:29260–29267. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002b;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- Merot Y, Metivier R, Penot G, Manu D, Saligaut C, Gannon F, Pakdel F, Kah O, Flouriot G. The relative contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha transcriptional activity depends upon the differentiation stage of the cell. J Biol Chem. 2004;279:26184–26191. doi: 10.1074/jbc.M402148200. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36:211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine epithelial estrogen receptor-alpha controls decidualization via a paracrine mechanism. Mol Endocrinol. 2015;29:1362–1374. doi: 10.1210/me.2015-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci U S A. 2002;99:2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos A. Endocrine Control of Egg Implantation. Washington, D.C: American Physiology Society; 1973a. [Google Scholar]
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973b;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Ray S, Hou X, Zhou HE, Wang H, Das SK. Bip is a molecular link between the phase I and phase II estrogenic responses in uterus. Mol Endocrinol. 2006;20:1825–1837. doi: 10.1210/me.2006-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Xu F, Li P, Sanchez NS, Wang H, Das SK. Increased level of cellular Bip critically determines estrogenic potency for a xenoestrogen kepone in the mouse uterus. Endocrinology. 2007;148:4774–4785. doi: 10.1210/en.2007-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Xu F, Wang H, Das SK. Cooperative control via lymphoid enhancer factor 1/T cell factor 3 and estrogen receptor-alpha for uterine gene regulation by estrogen. Mol Endocrinol. 2008;22:1125–1140. doi: 10.1210/me.2007-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- Rider V, Isuzugawa K, Twarog M, Jones S, Cameron B, Imakawa K, Fang J. Progesterone initiates Wnt-beta-catenin signaling but estradiol is required for nuclear activation and synchronous proliferation of rat uterine stromal cells. J Endocrinol. 2006;191:537–548. doi: 10.1677/joe.1.07030. [DOI] [PubMed] [Google Scholar]
- Sato T, Wang G, Hardy MP, Kurita T, Cunha GR, Cooke PS. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology. 2002;143:2673–2679. doi: 10.1210/endo.143.7.8878. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Kennedy TG. Induction of glucose-regulated protein 78 in rat uterine glandular epithelium during uterine sensitization for the decidual cell reaction. Biol Reprod. 2000;62:1168–1176. doi: 10.1095/biolreprod62.5.1168. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–230. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of gp130 or stat3 shows implantation failure with increased estrogenic responses. Mol Endocrinol. 2013;27:1492–1501. doi: 10.1210/me.2013-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–3647. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida M, Heike T, Yokota T. Cytoplasmic domains of the leukemia inhibitory factor receptor required for STAT3 activation, differentiation, and growth arrest of myeloid leukemic cells. Blood. 1999;93:1934–1941. [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Labrie F, Giguere V. Dominant activity of activation function 1 (AF-1) and differential stoichiometric requirements for AF-1 and −2 in the estrogen receptor alpha-beta heterodimeric complex. Mol Cell Biol. 1999;19:1919–1927. doi: 10.1128/mcb.19.3.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H, Iguchi T. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol. 2003;30:347–358. doi: 10.1677/jme.0.0300347. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A. 2007;104:15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]