Abstract
By altering or eliminating delicate ecological relationships, non-indigenous species are considered a major threat to biodiversity, as well as a driver of environmental change. Global climate change affects ecosystems and ecological communities, leading to changes in the phenology, geographic ranges, or population abundance of several species. Thus, predicting the impacts of global climate change on the current and future distribution of invasive species is an important subject in macroecological studies. The African clawed frog (Xenopus laevis), native to South Africa, possesses a strong invasion potential and populations have become established in numerous countries across four continents. The global invasion potential of X. laevis was assessed using correlative species distribution models (SDMs). SDMs were computed based on a comprehensive set of occurrence records covering South Africa, North America, South America and Europe and a set of nine environmental predictors. Models were built using both a maximum entropy model and an ensemble approach integrating eight algorithms. The future occurrence probabilities for X. laevis were subsequently computed using bioclimatic variables for 2070 following four different IPCC scenarios. Despite minor differences between the statistical approaches, both SDMs predict the future potential distribution of X. laevis, on a global scale, to decrease across all climate change scenarios. On a continental scale, both SDMs predict decreasing potential distributions in the species’ native range in South Africa, as well as in the invaded areas in North and South America, and in Australia where the species has not been introduced. In contrast, both SDMs predict the potential range size to expand in Europe. Our results suggest that all probability classes will be equally affected by climate change. New regional conditions may promote new invasions or the spread of established invasive populations, especially in France and Great Britain.
Introduction
Plenty of evidence exists for impacts of climate change on ecosystems and ecological communities [1–8] Climate change modifies climatic factors such as ambient temperatures, precipitation, and the frequency of extreme weather events, which profoundly affects species’ geographical distributions [9]. Recent research reveals coherent patterns of ecological change across systems [10] concerning phenological changes, geographic range shifts and modifications in species abundance [11]. Rising ambient temperatures might promote range expansions beyond the northern range limits or favour altitudinal range shifts, while increasing temperatures enhance winter survival [9].
Non-indigenous species are expanding worldwide [12], and have been identified as a major driver of global biodiversity loss and environmental change [13–16]. Invasive species alter productivity, hydrology and nutrient cycles and thus, influence survival of native species and disrupt natural competition in host ecosystems [17].
Human-mediated transport for tourism or trade provides introduction pathways and therefore contributes to the rising number of introductions of alien species [18, 19]. Increases of biological invasions were found to coincide with the industrial revolution [18, 20, 21] and unprecedented acceleration of merchandise trade within the last 50 years led to progressive increases in the introduction of alien species [18]. Biological invasions are assumed to increase in the future in response to globalisation and climate change [18, 20–23]. Climate change is widely considered to exacerbate the impact of invasive species by making additional space suitable, enhancing survival and reproduction success, and by improving the competitive capacity of non-indigenous species [9, 24–26].
Depending on the physiological sensitivity to climatic conditions, the impact of climate change will vary among organisms (e.g. [27–29]) with poikilothermic taxa, such as amphibians, being particularly affected [11, 28, 30]. Due to behavioural traits, physiological processes and breeding phenology that closely depend on temperature and moisture, and a limited dispersal capacity, amphibians are especially sensitive and will be heavily affected by climate change [11, 31, 32]. However, climate change might also create opportunities for niche differentiation and evolution, by altering the composition of the resident biota, creating empty niches [33]. Further, impacts of climate change will likely vary geographically (e.g. [5, 11, 34, 35]). Although, considerable interest exists in predicting the spread and success of non-indigenous species, research linking climate change and biological invasions remain scarce [9, 36]. While the invasive potential of numerous species such as the invasive cane toad (Bufo marinus) in Australia [37, 38] was predicted to increase (e.g. [9, 39–41]) other studies suggest an opposite pattern e.g. for the American Bullfrog (Lithobates catesbeianus) in South America [42].
The African clawed frog, Xenopus laevis (Daudin, 1802), is one of the world's most widely distributed amphibians with populations originating from the Cape region in South Africa having become established on four continents (North America, South America, Asia and Europe) due to both accidental escape and voluntary release of laboratory animals [43–51]. While the establishment of introduced populations was most successful in areas with a Mediterranean climate, which closely resembles the environmental conditions of the Western Cape region, the persistence of several populations in cooler environments for decades suggests a capacity for long-term adaptation [52]. Recent research indicates that the global invasion potential of X. laevis has been severely underestimated with vast areas being potentially susceptible to invasion [50]. In addition, it has been claimed that climate change is likely to enhance this species’ invasion potential, favouring range expansion and population growth [53] particularly in Mediterranean climate regions.
Macroecological approaches represent a popular analytical tool to assess and predict the impacts of climate change (e.g. [54–56]) as well as to assess spatial patterns of biological invasions in order to prioritize regions for the early detection of invasion outbreaks (e.g. [42, 57, 58]). The utility of Species Distribution Models (SDMs) to predict future spread of non-indigenous species has been demonstrated repeatedly (e.g. [57, 59, 60]). For general assumptions and methods see Elith and Lethwick [61]. Previous research targeting X. laevis showed large areas in Asia, southern Australia, south-western Europe, North and South America to be particularly vulnerable to colonization [52]. Owing to its ability to settle on various continents and its expected impact on local wetland communities [46, 49, 62–65], X. laevis is recognized as a major invasive amphibian species worldwide [50]. For such species it is crucial to develop accurate models of potential colonization and range shifts accounting for short-term climate changes. Such models may help to identify zones that could be colonized, refine risk assessments, and target prevention measures. Aiming to contribute to the future management of X. laevis, this study investigates the present and forecasts the future potential invasion range of the species on a global scale, based on an updated and extended occurrence data set and a similar set of bioclimatic variables as those used by Measey et al. [50].
We hypothesize that rising ambient temperatures associated with climate change will likely increase occurrence probabilities in currently cooler environments e.g. along the present day northern range limits of the species. Thus, climate change will promote the expansion of existing populations and increase the probability of additional invasions in the northern hemisphere, while occurrence probabilities for populations from the southernmost range limits are likely to decrease.
Materials and Methods
In order to assess the present and future invasion potential of Xenopus laevis on a global scale georeferenced occurrence records, covering the species’ native distributional range in South Africa, as well as all known invasive populations in Europe, were obtained from recent literature [50] and supplemented by 286 new records collected during own field research (J.C., J.S., R.R., J.M., F.L., A.dV.). Until recently X. laevis was considered a species complex with a number of genetically distinct lineages [66]. Measey et al. [50] noted that all invasive populations were from the South African ‘Cape’ clade [67, 68] an area including the winter rainfall region and southern coast of South Africa. Since that time, De Busschere et al. [69] determined that the French invasion incorporated lineages from throughout the range of X. laevis. In addition, recent phylogeographic research supports the recognition of X. petersii, X. victorianus and X. poweri as separate species and therefore confines the native range of X. laevis sensu stricto to southern Africa (South Africa, Lesotho, Swaziland, Namibia, Botswana, Zimbabwe, Mozambique and Malawi) [70]. The occurrence data set for southern Africa used in this study was adjusted accordingly. It is important to note that this is a novel interpretation of the taxonomy and native range of X. laevis in comparison to that used by Measey et al. [50].
To prevent over‐fit and false inflation of model performance through spatially auto‐correlated species records [71–73], the comprehensive dataset of 1382 records was filtered, and clustered locality records were reduced to a single point within a specified Euclidian distance in environmental space using the spatially rarefy occurrence data tool for the ArcGIS SDM toolbox [74]. The final dataset used to build SDMs contained 826 records for South Africa, 37 for South America, 24 for North America, and 38 for Europe. SDMs built exclusively on occurrence data from the native range of invasive species tend to underestimate the potential invasive distribution, particularly when projecting onto climate change scenarios [75]. Hence, native and invasive occurrence records were pooled [50, 76] for the computation of SDMs. In this way a maximum amount of information on the species’ realized bioclimatic niche was integrated.
As environmental predictors, 19 bioclimatic variables available through the WorldClim-database ([77] http://www.worldclim.org/bioclim) were used. They represent minima, maxima and average values of monthly, quarterly, and annual ambient temperature as well as precipitation recorded between 1950 and 2000. All predictors had a spatial resolution of 2.5 arc min (approx. ~5 km resolution at the equator). Out of the total set of variables a set of nine predictors with pairwise Spearman rank correlation coefficients R2 < 0.75 were selected to minimize predictor correlation.
Subsequently, SDMs were computed using the machine learning algorithm Maxent version 3.3.3k [78, 79]. Maxent is supposed to exhibit a higher predictive performance than more conventional techniques [61] and has successfully been used to model the potential distribution of invasive species and to assess impacts of climate change [78]. Only linear, quadratic and product features were allowed in order to restrict model complexity, while extrapolation was not allowed to reduce uncertainties due to projections onto non-analogous climates [80, 81]. The Maxent model was trained by randomly splitting the species records into 70% used for model training and 30% for model testing applying a bootstrap approach. The Area Under the receiver operating characteristic Curve (AUC) [82] was used to evaluate the discrimination ability of the resulting SDM. Averages across 100 replicates were used for further processing. As the selection of an appropriate background is known to affect model performance [83], a circular buffer of 250 km around each locality record was selected as training area following Measey et al. [50].
Ensemble SDMs were computed using the biomod2 package version 3.2.2 [84] for Cran R [85] including the following eight algorithms: Generalized Linear Models (GLM), Generalized Boosting Models (GBM), Generalized Additive Models (GAM), Classification Tree Analysis (CTA), Artificial Neural Networks (ANN), Factorial Discriminant Analysis (FDA), Maxent, and Multivariate Adaptive Regression Splines (MARS) applying the same training background as for the Maxent analyses. We applied a bootstrapping approach with 100 replicates randomly subdividing the locality dataset in 70% for model training, whereas the remaining 30% were used for model evaluation using the AUC [82], True Skill Statistic (TSS) and Cohen’s Kappa [86]. The average projection across all replicates was used for further processing. The “Minimum training presence” (mtp) referring to the lowest generated probability estimate of the training data [87], was applied as presence-absence threshold. While threshold selection is a potential source for biases, the mtp has been shown to represent a confident method performing well for presence only SDMs [88] particularly for modelling potential distributions of invasive species [88, 89].
Areas requiring extrapolation beyond the training range of the variables were quantified using a multivariate environmental similarity surface (MESS) analysis [90] for Maxent and conceptually equivalent clamping masks for biomod2.
To predict the future potential distribution of X. laevis on a global scale, 11 general circulation models (GCMs: BCC-CSM1-1, CCSM4, GISS-E2-R, HadGEM2-AO, hadGEM2-ES, IPSL-CM5A-LR, MIROC-ESM-CHEM, MIROC-ESM, MIROC5, MRI-CGCM3 and NorESM1-M) representing simulations for four representative concentration pathways (RCP2.6, RCP4.5, RCP6, RCP8.5) for 2070 were obtained from the fifth assessment of the Intergovernmental Panel for Climate Change (IPCC AR5 WG1 2013; http://www.ipcc.ch, [91]). The selected RCPs represent four possible greenhouse gas emission trajectories ranging from low (RCP2.6) to high (RCP8.5) corresponding to increases in global radiative forcing, from pre-industrial times to 2100. These climate projections were statistically downscaled to match the bioclim variables using the delta method [77], (http://www.worldclim.org/downscaling) for details also see [92,93]. As differences between the selected GCMs might cause uncertainty in SDM projections [59], average values across all GCMs were calculated for each RCP respectively. Finally, SDMs were projected onto the derived future climate change scenarios.
In order to quantify impacts of different RCP scenarios onto the global invasion potential of X. laevis, the following predicted areas were determined: a) the entire SDM area using the ‘minimum training presence’ as threshold, b) the respective MESS area, and c) the SDM area–MESS area. A probability cut-off of 25%, 50%, and 75% onto the ‘SDM area–MESS area’ was applied to assess impacts for different probability classes. Further, the full model was partitioned into estimates for each continent. Subsequently, the invasion potential for X. laevis was determined on a continental scale as described above. Shift maps were generated to illustrate predicted gains, losses and stability of environmentally suitable space for all climate change scenarios following Bertelsmeier et al. [94]. Comparisons between the results obtained by our Maxent analyses, the results presented by Measey et al. [50] and the results obtained via biomod2 were performed by rescaling the probability output and subtracting the potential distribution grids from each other. Rescaling involved subtracting the minimum training presence threshold from each model and computation of percentages per grid cell relative to the maximum probability. The resulting maps quantitatively indicate for each area which SDM shows a higher probability.
Results
Model performance was 0.841 (AUCtest) and 0.846 (AUCtraining) for the maximum entropy model while weighted means of 0.631 for Cohen’s Kappa, 0.892 for AUC, and 0.685 for TSS were obtained for the ensemble model, demonstrating that both SDMs discriminate moderately well between suitable versus unsuitable space [79]. For the maximum entropy model the contribution of eight predictors exceeded 5%, while for the ensemble approach seven predictors had a contribution exceeding 5% (Table 1, S1 Table). Predictor contribution for the maximum entropy model was particularly high for ‘precipitation of the driest quarter’ (27.65%), ‘mean temperature of the wettest quarter’ (16.82%), ‘mean temperature of the coldest quarter’ (14.52%), and ‘precipitation of the warmest quarter’ (11.38%) (Table 1). Variables with high contribution in the ensemble model were ‘mean temperature of the coldest quarter’ (19.05%), ‘precipitation of the warmest quarter’ (16.57%), ‘mean temperature of the warmest quarter’ (13.92%), ‘precipitation of the driest quarter’ (12.56%) (Table 1). For the respective response curves see S1 Fig & S2 Fig.
Table 1. Variable contribution for the maximum entropy and the ensemble SDM.
| Variable Contribution (%) | |||
|---|---|---|---|
| ID | Bioclimatic Variable | Maxent SDM | Ensemble |
| Bio 17 | precipitation of driest quarter | 27.65 | 12.56 |
| Bio 8 | mean temperature of the wettest quarter | 16.82 | 7.61 |
| Bio 11 | mean temperature of coldest quarter | 14.52 | 19.05 |
| Bio 18 | precipitation of warmest quarter | 11.38 | 16.57 |
| Bio 19 | precipitation of coldest quarter | 8.25 | 8.25 |
| Bio 7 | temperature annual range | 6.99 | 4.96 |
| Bio 9 | mean temperature of driest quarter | 6.24 | 8.33 |
| Bio 16 | precipitation of wettest quarter | 6.21 | 7.95 |
| Bio 10 | mean temperature of warmest quarter | 1.93 | 13.92 |
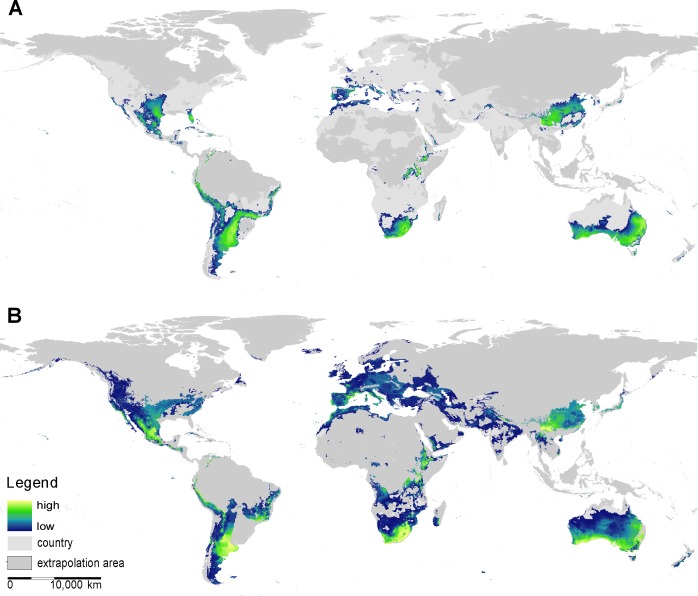
Both modelling approaches yielded similar global patterns for the present potential distribution of X. laevis. However, the more complex ensemble SDM predicted larger areas with slightly higher probabilities than the maximum entropy SDM (Fig 1 & S3 Fig). While the ensemble SDM predicted 28% of the world’s surface to be presently environmentally suitable for X. laevis, the maximum entropy SDM predicted only 12% (Table 2). As expected, high occurrence probability was predominantly predicted for areas resembling climatic characteristics of the South African Cape region with high annual variation in ambient temperatures, comparatively warm and dry summers, and mild and wet winters (S1 Fig & S2 Fig). More precisely, probability was correlated with mild winter temperatures (10–20°C), low precipitation during the wettest (<350 mm), and the coldest quarter (<500 mm) and high precipitation during the warmest quarter (400–600 mm) (S1 Fig & S2 Fig).
Fig 1.
Global projection of the potential distribution of X. laevis A) derived from the maximum entropy SDM; B) derived from the ensemble SDM. Probability ranging from moderate (dark blue) to highly suitable (yellow).
Table 2. Environmentally suitable space given as percent of the world’s surface area for current climatic conditions and projections onto climate change scenarios.
Percentages refer to the SDM-MESS area; values increasing with climate change scenarios are displayed in bold.
| Maximum entropy SDM | |||||
| Continent | % current | % RCP 2.5 | % RCP 4.5 | % RCP 6 | % RCP 8.5 |
| Africa | 9 | 6 | 5 | 5 | 3 |
| Europe | 4 | 8 | 9 | 10 | 11 |
| North America | 10 | 8 | 7 | 7 | 7 |
| South America | 26 | 23 | 21 | 21 | 20 |
| Australia | 40 | 33 | 31 | 30 | 28 |
| Asia | 8 | 9 | 10 | 10 | 11 |
| global | 12 | 12 | 11 | 11 | 11 |
| Ensemble SDM | |||||
| % current | % RCP 2.5 | % RCP 4.5 | % RCP 6 | % RCP 8.5 | |
| Africa | 24 | 16 | 12 | 12 | 9 |
| Europe | 21 | 30 | 33 | 35 | 38 |
| North America | 24 | 29 | 31 | 30 | 30 |
| South America | 30 | 31 | 28 | 28 | 26 |
| Australia | 74 | 72 | 66 | 66 | 56 |
| Asia | 24 | 20 | 19 | 19 | 18 |
| global | 28 | 27 | 26 | 26 | 25 |
According to both models, there are regions exhibiting high occurrence probabilities under current climatic conditions located on all continents (Fig 1), but percentages of environmentally suitable space varied greatly (Table 2). Both SDMs predict only moderate coverage with environmentally suitable space for X. laevis to presently exist in the northern hemisphere, while high coverage was predicted for Australia and South America (Table 2). Potentially highly suitable areas cover the south central United States (Texas, Kansas), western (stretching from Peru through Colombia and into Venezuela), eastern (eastern Brazil, stretching along the Paraná River), and southern South America (north-eastern Argentina). In Europe, particularly high probabilities are predicted in Portugal, eastern Spain, southern France, and Italy. In accordance with Measey et al. [50], both SDM approaches predict only moderate occurrence probability in Great Britain. In addition to the native distribution of X. laevis covering vast areas in southern Africa, there is a high occurrence probability in Morocco and the eastern Afromontane region (where no invasions by X. laevis have been reported so far). Moreover, both SDMs predict high suitability in China (Nanzhao plateau, Sichuan, Chinese plain), Japan, and southern Australia. The maximum entropy SDM highlights additional regions with high probabilities in Florida and south-eastern China (Zhejiang Province) (Fig 1, S3 Fig).
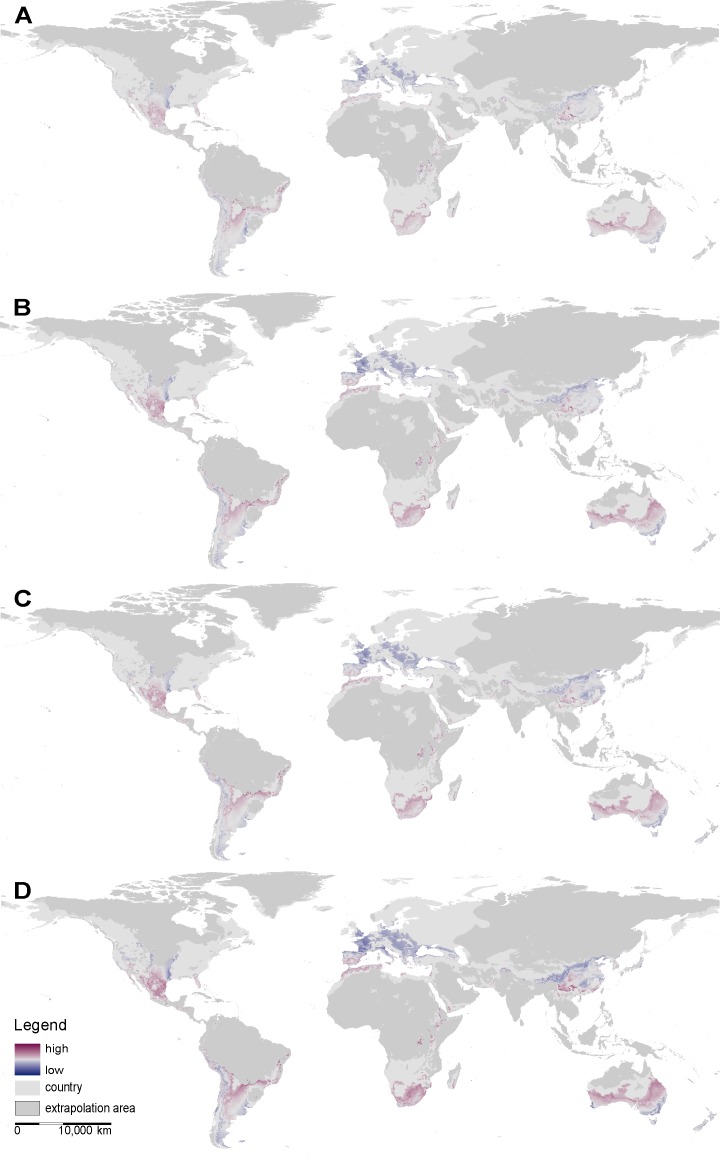
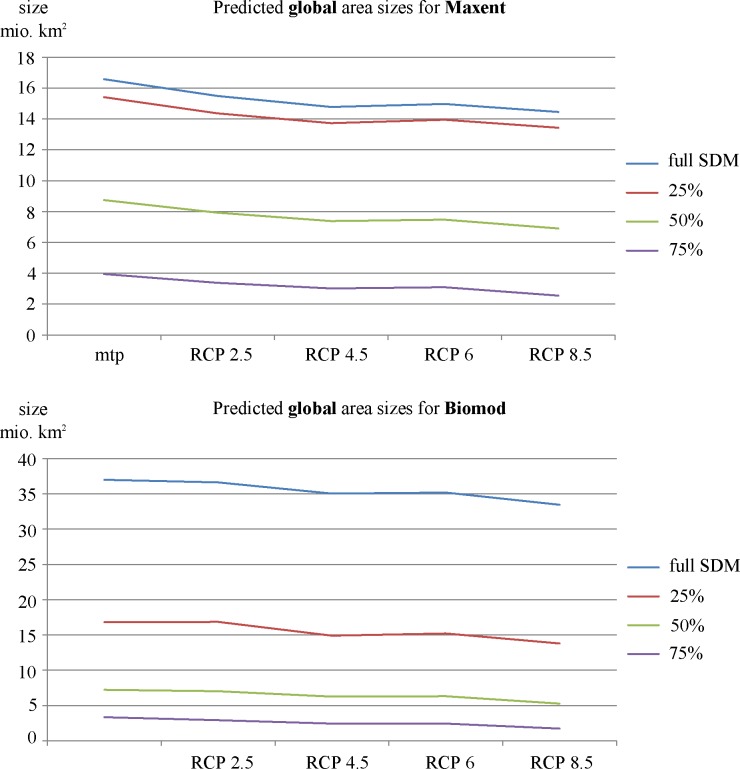
On a global scale, both SDM approaches predict suitable range sizes for X. laevis to decrease across all four RCP scenarios (Table 2, Fig 2 & Fig 3). However, the magnitude of decrease varies between RCP scenarios and between SDM approaches. For the maximum entropy SDM, the potentially suitable range size is predicted to decrease by 7 to 13% from RCP 2.5 to RCP 8.5, respectively. This corresponds to a maximum decrease of 1% in relation to the world’s surface area (Table 2). The ensemble SDM predicts a rather moderate decrease of 1 to 10%. Since the areas predicted by the ensemble SDM are generally larger, the portion of the world’s surface area predicted to be suitable shrinks by 3% (Table 2). A comparison between the full model and the respective probability cut-offs does not reveal significant differences between RCP scenarios (Fig 4), suggesting that probability classes will be equally affected by climate change.
Fig 2.
Global shift maps derived from Maxent illustrating predicted gains (dark violet) and losses (dark blue) of environmentally suitable space for different climate change scenarios; A) IPCC RCP2.6, B) IPCC RCP4.5, C) IPCC RCP6, D) IPCC RCP8.5.
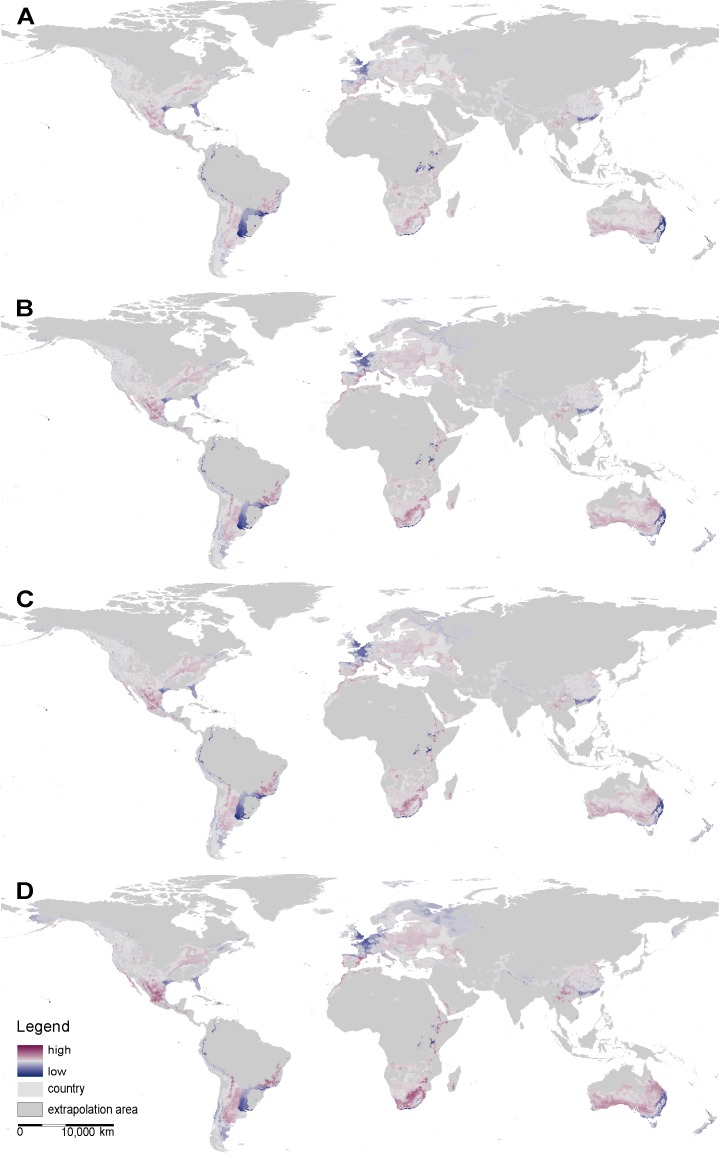
Fig 3.
Global shift maps derived from the ensemble SDM illustrating predicted gains (dark violet) and losses (dark blue) of environmentally suitable space for different climate change scenarios; A) IPCC RCP2.6, B) IPCC RCP4.5, C) IPCC RCP6, D) IPCC RCP8.5.
Fig 4.
Predicted development of area sizes suitable for X. laevis on a global scale; a) for the Maximum entropy SDM and; b) for the ensemble SDM. Mtp = minimum training presence, all areas sizes refer to SDM area–MESS area.
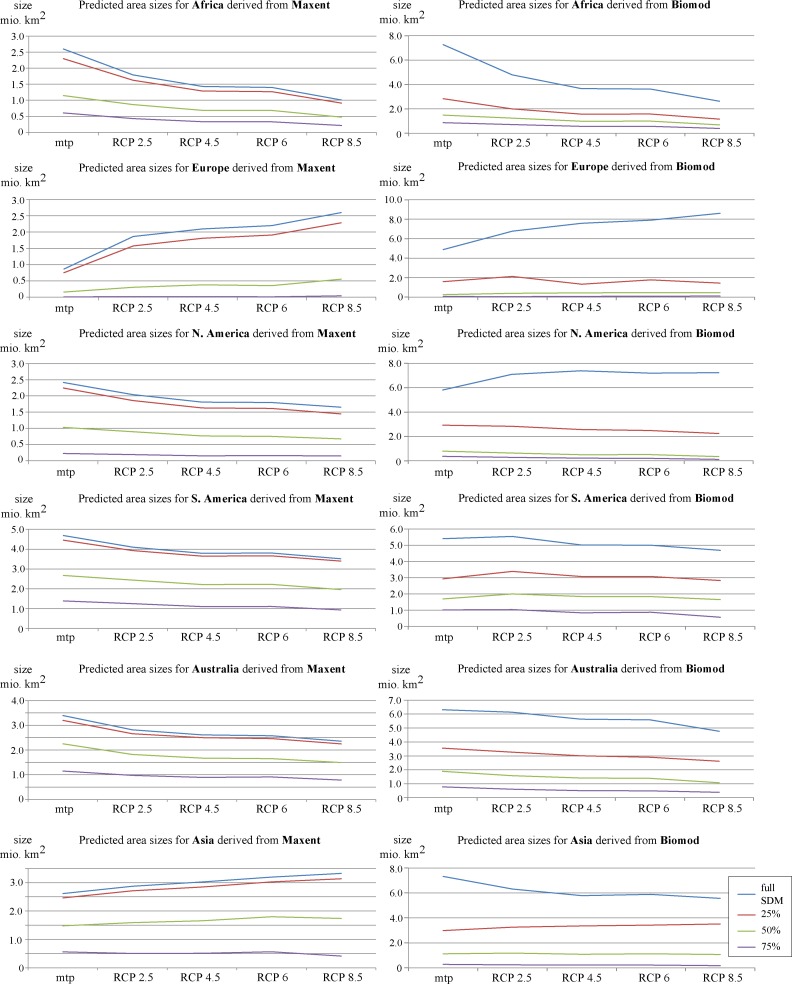
On a continental scale, both SDM approaches suggest range sizes to decrease for the species’ native range in South Africa, as well as for the invaded areas in South America and Australia, where the species has not been introduced (Fig 5, Table 2, S2 Table & S3 Table). In contrast, the potential range in Europe will expand in response to climate change (Table 2). Concordantly, shift maps highlight different magnitudes of expected gains in Europe (Fig 2 & Fig 3).
Fig 5.
Predicted development of area sizes suitable for X. laevis on a continental scale; left) for the Maximum entropy SDM, right) for the ensemble SDM. Mtp = minimum training presence, all areas sizes refer to SDM area–MESS area.
However, there are minor differences between the results of both approaches: while the maximum entropy SDM predicts range sizes to decrease for all probability classes, the ensemble SDM suggests range sizes to increase for North America when applying the mtp threshold (Fig 5). Furthermore, the ensemble SDM predicts increasing range sizes for the >25% probability class in Asia (Fig 5), while the maximum entropy model predicts increases for the mtp threshold, as well as for the probability classes >25% and >50% (Fig 5). Finally, the ensemble SDM predicts increasing range sizes only for the mtp threshold, while the maximum entropy SDM suggests an area expansion across all probability classes in Europe (Fig 5, S2 Table & S3 Table).
Discussion
Our predictions of the present potential distribution of Xenopus laevis generally agree with the findings presented by Measey et al. [50] highlighting analogous spatial extents and geographic locations. Both SDMs reveal large areas that can potentially be colonized on several continents. However, the overlap comparison of our SDMs with the prediction by Measey et al. [50] yields higher probability values on all continents for both our ensemble and our maximum entropy SDM (S3 Fig). These differences might be attributed to the different occurrence data sets used. While Measey et al. [50] applied a restricted definition within X. laevis using only records from a single clade occurring in the winter rainfall zone and southern coast of South Africa [66], the data used in this study was adjusted according to the findings of Furman et al. [70] resulting in a larger coverage for the species native range. Thus, the environmental space covered by the occurrence dataset used was larger than in Measey et al. [50].
Under current climatic conditions, both the Maxent and the ensemble model identify regions with suitable climatic conditions favouring invasion in Portugal, France, Sicily, California, Chile, and Japan, where invasive populations already exist. Both SDMs predict only moderate probability for Great Britain, where populations from Wales and Lincolnshire have recently been extirpated [53]. Furthermore, our results highlight areas in Spain (including the Balearic Islands), mainland Italy (including Sardinia), and southern France (including Corsica) to be highly vulnerable to potential invasions, as these regions exhibit suitable climatic conditions for X. laevis and are adjacent to established invasive populations. Globally, the same applies to Baja California and central Mexico.
Future projections of both SDM approaches identify regions that will likely become vulnerable to colonization in response to climate change. With an expected decrease of 1–3% (given as percentage of the worlds’ surface area) the overall magnitude of expected changes appears to be moderate, while the predicted global area suitable for X. laevis remains stable or slightly decreases with increasing RCP scenarios. However, predictions for Europe are the major exception to this general trend with particularly good prospects for the invasive populations in north-western Europe (Figs 2 & 3). Xenopus laevis is capable of enduring extreme conditions ([50] and references therein). However, reproduction seems to be triggered by rainfall and increasing temperatures [95, 96]. These physiological restrictions are well reflected in the variable contributions of the models. These were highest in the precipitation of driest and warmest quarter and in the temperature of the wettest, coldest and warmest quarters affecting reproduction cycles. While reproduction occurs throughout the year in California [97] the lower lethal limit of temperature tolerance in embryos was reported to be 10°C [95]. Harsh conditions only permitted infrequent reproduction in Great Britain [53] and rising temperatures will likely improve physiological performances, fecundity, breeding success, and increase the rate of larval development.
Thus, regional patterns may facilitate new invasions or promote a spread of the established invasive populations, especially in France and Great Britain, where populations persisted for decades [52]. While invasive populations are already spreading in France, British populations are presently considered extirpated [53]. Due to an increased environmental suitability caused by climate change along the northernmost boundaries of the species’ range, chances of successful establishment in Great Britain in case of re-introductions will increase in the future. Climate change is widely considered to exacerbate the impact of invasive species [9, 25] enhancing the invasive potential of some species (e.g. [9, 39–41]), including the invasive cane toad (Rhinella marina) in Australia [37, 38]. However, some studies suggest an opposite pattern e.g. for the global invasion potential of an assemblage of ant species [98], or the American Bullfrog (Lithobates catesbeianus) in South America [42].
For X. laevis, it has been claimed that climate change will likely favour range expansion and population growth [53] particularly in Mediterranean climate regions. While on a global scale our predictions reveal the species’ potential range to decrease in response to climate change, populations from the northern hemisphere are predicted to expand.
As X. laevis is kept as a model organism in laboratories all across the world and is still traded intensively [51, 98] Measey et al. [51] emphasize the importance of biosecurity at breeding facilities to prevent further escape and voluntary release of frogs and tadpoles [99]. While by now scientists working with X. laevis are likely to be aware of the species’ invasion potential [100], this frog is also readily available via the pet trade [98]. Once introduced, the species rapidly disperses using irrigation canals, ponds, and rivers as migration corridors, but also performs terrestrial migrations [101] even without rainfall [102]. Estimated annual spread of feral populations varied between 1 km [101] in France and 5.4 km [62] in Chile. Recent research found the maximum overland dispersal in native populations to be 2.3 km (Euclidean distance) within 6 weeks [102].
Although invasive X. laevis have demonstrably negative impacts on resident amphibian and fish communities [49, 63, 64, 103], attempts to eliminate invasive populations are limited. Recent studies stressed the urgent need for rigorous and comprehensive invasive species risk assessments to contribute to the development of management strategies [104, 105]. Prevention is generally considered more effective and cheaper than control and eradication of established populations [8, 25]. SDMs represent a quick and cost efficient tool to evaluate the current and future invasion potential of non-indigenous species. In addition, SDMs facilitate the identification of areas with high susceptibility to invasion and help to prioritize management actions. According to our results preventive measures should predominantly focus on the species’ northern range limits, particularly north-western Europe to prevent further spread and establishment of new populations as well as a re-introduction in Great Britain.
Even though considered particularly difficult in Mediterranean areas, eradication of invasive populations of X. laevis has been proposed [101] and an eradication program was established by the Portuguese Governmental Nature Conservation Institute in Oeiras, western Portugal in 2010 [50]. In addition, eradication was successfully executed in Scunthorpe, Humberside area, Great Britain [50]. According to our results eradication of established populations of X. laevis should focus on areas where populations are still small and scattered, but likely to expand in response to climate change e.g. Portugal and Sicily.
Supporting Information
Model contribution was assessed by building the model using a single corresponding predictor variable. The logistic output (probability of presence) is displayed on the Y axis. Red curves refer to mean responses of 100 replicate Maxent runs while the mean +/- one standard deviation is displayed in blue.
(PDF)
The logistic output (probability of occurrence) is displayed on the Y axis.
(PDF)
A) Overlap analysis of the ensemble SDM and the maximum entropy SDM, with red highlighting areas where the ensemble SDM yields higher probabilities and blue depicting areas where the maximum entropy SDM yields higher probabilities; B) Overlap analysis of the ensemble SDM and the SDM by Measey et al. (2012), with red indicating higher probability of the ensemble SDM and blue showing higher values for the SDM by Measey et al. (2012); C) Overlap analysis of the maximum entropy SDM and the SDM by Measey et al. (2012), with red highlighting regions with higher probability of the ensemble SDM and blue showing higher probability values for the SDM by Measey et al. (2012). Colour saturation increases with deviation of the models. Areas where both SDMs yield similar probability values are displayed in white.
(PDF)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by biodiversa (http://www.biodiversa.org/, project title: “Invasive biology of Xenopus laevis in Europe: ecology, impact and predictive models,” grant recipients: AH, RR, TB, DR) and the Deutsche Forschungsgemeinschaft (http://www.dfg.de/, DFG RO 4520/3-1, grant recipient: DR). The publication of this article was funded by the Open Access fund of the Leibniz Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res. 1999;50: 839–866. [Google Scholar]
- 2.Smith RC, Ainley D, Baker K, Domack E, Emslie S, et al. Marine Ecosystem Sensitivity to Climate Change Historical observations and paleoecological records reveal ecological transitions in the Antarctic Peninsula region. BioScience. 1999;49: 393–404. [Google Scholar]
- 3.Parmesan C, Root TL, Willig M. Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc. 2000;81: 443–450. [Google Scholar]
- 4.Peñuelas J, Flella I. Responses to a warming world. Science. 2001;294: 793–795. [DOI] [PubMed] [Google Scholar]
- 5.Pounds JA. Climate and amphibian declines. Nature. 2001;410: 639–664. [DOI] [PubMed] [Google Scholar]
- 6.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh- Gulberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416: 389–395. [DOI] [PubMed] [Google Scholar]
- 7.Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, et al. Extinction risk from climate change. Nature. 2004;427: 145–148. [DOI] [PubMed] [Google Scholar]
- 8.Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, et al. Impacts of biological invasions—what’s what and the way forward. Trends Ecol Evol. 2013;28: 58–66. 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Dukes JS, Mooney HA. Does global change increase the success of biological invaders?. Trends Ecol Evol. 1999;14: 135–139. [DOI] [PubMed] [Google Scholar]
- 10.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh- Gulberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416: 389–395. [DOI] [PubMed] [Google Scholar]
- 11.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421: 37–42. [DOI] [PubMed] [Google Scholar]
- 12.Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, et al. Biodiversity scenarios for the year 2100. Science. 2000;287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- 13.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10: 689–710. [Google Scholar]
- 14.Lodge DM. Responses of lake biodiversity to global changes In: Future scenarios of global biodiversity. Chapin FS III, Sala OE, Huber-Sannwald E, editors. Springer: New York; 2001. pp. 277–312. [Google Scholar]
- 15.Clavero M, García-Berthou E. Homogenization dynamics and introduction routes of invasive freshwater fish in the Iberian Peninsula. Ecol Appl. 2006;16: 2313–2324. [DOI] [PubMed] [Google Scholar]
- 16.Bálint M, Domisch S, Engelhardt CHM, Haase P, Lehrian S, Sauer J, Theissinger K, Pauls SU, Nowak C. Cryptic biodiversity loss linked to global climate change. Nature Clim Chang. 2011;1: 313–318. [Google Scholar]
- 17.Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V, Cox PM, Fisher V, Foley JA, Friend AD. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Chang Biol. 2001;7: 357–373. [Google Scholar]
- 18.Hulme PE. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol. 2009;46: 10–18. [Google Scholar]
- 19.Butchart S, Walpole M, Collen B, van Strien A, Scharlemann JPW, et al. Global biodiversity: indicators of recent declines. Science. 2010;328: 1164–1168. 10.1126/science.1187512 [DOI] [PubMed] [Google Scholar]
- 20.Perrings C, Dehnen-Schmutz K, Touza J, Williamson M. How to manage biological invasions under globalization. Trends Ecol Evol. 2005;20: 212–215. [DOI] [PubMed] [Google Scholar]
- 21.Meyerson LA, Mooney HA. Invasive alien species in an era of globalization. Front Ecol Environ. 2007;5: 199–208. [Google Scholar]
- 22.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20: 223–228. [DOI] [PubMed] [Google Scholar]
- 23.McNeely JA. As the world gets smaller, the chances of invasion grow. Euphytica. 2006;148: 5–15. [Google Scholar]
- 24.Corn PS. Climate change and amphibians. Anim Biodiv Conserv. 2005;28: 59–67. [Google Scholar]
- 25.Pyke CR, Thomas R, Porter RD, Hellmann JJ, Dukes JS, Lodge DM, Chavarria G. Current practices and future opportunities for policy on climate change and invasive species. Conserv Biol. 2008;22: 585–592. 10.1111/j.1523-1739.2008.00956.x [DOI] [PubMed] [Google Scholar]
- 26.Walther GR, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H. Alien species in a warmer world: risks and opportunities. Trends Ecol Evol. 2009;24: 686–693. 10.1016/j.tree.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 27.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105: 6668–6672. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinervo B, Mendez-De-La-Cruz F, Miles DB, Heulin B, Bastiaans E, et al. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328: 894–899. 10.1126/science.1184695 [DOI] [PubMed] [Google Scholar]
- 29.Rowland EL, Davison JE, Graumlich LJ. Approaches to evaluating climate change impacts on species: a guide to initiating the adaptation planning process. Environ. Manage. 2011;47: 322–337. 10.1007/s00267-010-9608-x [DOI] [PubMed] [Google Scholar]
- 30.Miller D, Summers J, Silber S. 2004. Environmental versus genetic sex determination: a possible factor in dinosaur extinction? Fertil Steril. 2004;81: 954–964. [DOI] [PubMed] [Google Scholar]
- 31.Blaustein AR, Belden LK, Olson DH, Green DM, Root TL, Kiesecker JM. Amphibian breeding and climate change. Conserv Biol. 2001;15: 1804–1809. [Google Scholar]
- 32.Beebee TJ. Amphibian breeding and climate. Nature. 2009;374: 219–220. [Google Scholar]
- 33.Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci. 2003;164: 165–184. [Google Scholar]
- 34.Beaumont LJ, Pitman A, Perkins S, Zimmermann NE, Yoccoz NG, Thuiller W. Impacts of climate change on the world’s most exceptional ecoregions. Proc Natl Acad Sci USA. 2011;108: 2306–2311. 10.1073/pnas.1007217108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pounds JA, Fogden, MP, Campbell JH. 1999. Biological response to climate change on a tropical mountain. Nature. 1999;398: 611–615. [Google Scholar]
- 36.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc Natl Acad Sci USA. 2002;99: 15497–15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban MC, Phillips BL, Skelly DK, Shine R. The cane toad's (Chaunus (Bufo) marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proc R Soc B. 2007;274: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherst RW, Floyd RB, Maywald GF. The potential geographical distribution of the cane toad, Bufo marinus L. in Australia. Conserv Biol. 1995;9: 294–299. [Google Scholar]
- 39.Kriticos DJ, Sutherst RW, Brown JR, Adkins SW, Maywald GF. Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp. indica in Australia. J Appl Ecol. 2003;40: 111–124. [Google Scholar]
- 40.Thuiller W, Richardson DM, Midgley GF. Will climate change promote alien plant invasions? In: Biological invasions. Springer, Berlin Heidelberg; 2007. Pp. 197–211. [Google Scholar]
- 41.Vilà M, Corbin JD, Dukes JS, Pino J, Smith SD. Linking plant invasions to global environmental change In: Terrestrial ecosystems in a changing world. Springer, Berlin Heidelberg; 2007. Pp. 93–102. [Google Scholar]
- 42.Nori J, Urbina-Cardona JN, Loyola RD, Lescano JN, Leynaud GC. Climate change and American Bullfrog invasion: what could we expect in South America. PloS ONE 2011;6: e25718 DOId: 10.1371/journal.pone.0025718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCoid MJ, Fritts TH. Female reproductive potential and winter growth of African Clawed frogs (Pipidae: Xenopus laevis) in California. California Fish and Game 1995;81: 39–42. [Google Scholar]
- 44.Tinsley RC, McCoid MJ. Feral populations of Xenopus outside Africa In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford University Press, Oxford; 1996. Pp. 81–94. [Google Scholar]
- 45.Fouquet A. 2001. Des clandestins aquatiques. Zamenis. 2001;6: 10–11. [Google Scholar]
- 46.Lobos G, Measey GJ. Invasive populations of Xenopus laevis (Daudin) in Chile. Herpetol J. 2002;12: 163–168. [Google Scholar]
- 47.Lillo F, Marrone F, Sicilia A, Castelli G. An invasive population of Xenopus laevis (Daudin, 1802) in Italy. Herpetozoa. 2005;18: 63–64. [Google Scholar]
- 48.Faraone FP, Lillo F, Giacalone G, Valvo ML. The large invasive population of Xenopus laevis in Sicily, Italy. Amphibia-Reptilia. 2008;29: 405–412. [Google Scholar]
- 49.Rebelo R, Amaral P, Bernardes M, Oliveira J, Pinheiro P, Leitão D. Xenopus laevis (Daudin, 1802), a new exotic amphibian in Portugal. Biol Invasions. 2010;12: 3383–3387. [Google Scholar]
- 50.Measey GJ, Rödder D, Green SL, Kobayashi R, Lillo F, Lobos G, Rebelo R, Thirion J- M. Ongoing invasions of the African clawed frog, Xenopus laevis: a global review. Biol Invasions. 2012;14: 2255–2270. [Google Scholar]
- 51.van Sittart L, Measey GJ. Historical perspectives on global exports and research of African clawed frogs (Xenopus laevis). T Roy Soc S Afr. 2016; 10.1080/0035919X.2016.1158747 [DOI] [Google Scholar]
- 52.Measey GJ, Tinsley RC. Feral Xenopus laevis in South Wales. Herpetol J. 1998; 8: 23–27. [Google Scholar]
- 53.Tinsley RC, Stott LC, Viney ME, Mable BK, Tinsley MC. Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biol Invasions. 2015;17: 3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison PA, Berry PM, Butt N, New M. Modelling climate change impacts on species’ distributions at the European scale: implications for conservation policy. Environ Sci Policy. 2006;9: 116–128. [Google Scholar]
- 55.Randin CF, Engler R, Normand S, Zappa M, Zimmermann NE, Pearman PB, Vittoz P, Thuiller W, Guisan A. Climate change and plant distribution: local models predict high‐elevation persistence. Glob Chang Biol. 2009;15: 1557–1569. [Google Scholar]
- 56.Ihlow F, Dambach J, Engler JO, Flecks M, Hartmann T, et al. On the brink of extinction? How climate change may affect global chelonian species richness and distribution. Glob Chang Biol. 2012;18: 1520–1530. [Google Scholar]
- 57.Ficetola GF, Maiorano L, Falcucci A, Dendoncker N, Boitani L, et al. Knowing the past to predict the future: land‐use change and the distribution of invasive bullfrogs. Glob Chang Biol. 2010;16: 528–537. [Google Scholar]
- 58.Kolbe JJ, Kearney M, Shine R. Modeling the consequences of thermal trait variation for the cane toad invasion of Australia. Ecol Appl. 2010;20: 2273–2285. [DOI] [PubMed] [Google Scholar]
- 59.Beaumont LJ, Hughes L, Pitman AJ. Why is the choice of future climate scenarios for species distribution modelling important? Ecol Lett. 2008;11: 1135–146. 10.1111/j.1461-0248.2008.01231.x [DOI] [PubMed] [Google Scholar]
- 60.Broennimann O, Guisan A. Predicting current and future biological invasions: both native and invaded ranges matter. Ecol Lett. 2008;4: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Syst. 2009;40: 677–697. [Google Scholar]
- 62.Lobos G, Jaksic FM. The ongoing invasion of African clawed frogs (Xenopus laevis) in Chile: causes of concern. Biodivers Conserv. 2005;14: 429–439. [Google Scholar]
- 63.Lillo F, Faraone FP, Lo Valvo M. Can the introduction of Xenopus laevis affect native amphibian populations? Reduction of reproductive occurrence in presence of the invasive species. Biol Invasions. 2011;13: 1533–1541. [Google Scholar]
- 64.Amaral P, Rebelo R. Diet of invasive clawed frog Xenopus laevis at Lage stream (Oeiras, W Portugal). Herpetol J, 2012;22: 187–190. [Google Scholar]
- 65.Lobos G, Cattan P, Estades C, Jaksic MF. Invasive African clawed frog Xenopus laevis in southern South America: key factors and predictions. Stud Neotrop Fauna Environ. 2013;48: 1–12. [Google Scholar]
- 66.Measey GJ, Channing A. Phylogeography of the genus Xenopus in southern Africa. Amphibia-Reptilia. 2003;24: 321–330. [Google Scholar]
- 67.Lillo F, Dufresnes C, Faraone FP, Lo Valvo M, Stöck M. Identification and potential origin of invasive clawed frogs Xenopus (Anura: Pipidae) in Sicily based on mitochondrial and nuclear DNA. Ital J Zool. 2013;80: 566–573. [Google Scholar]
- 68.Lobos G, Mendez MA, Cattan P, Jaksic F. Low genetic diversity of the successful invasive African clawed frog Xenopus laevis (Pipidae) in Chile. Stud Neotrop Fauna Environ. 2014;49: 50–60. [Google Scholar]
- 69.De Busschere C, Courant J, Herrel A, Rebelo R, Rödder D, Measey GJ, Backeljau T. Unequal contribution of native South African phylogeographic lineages to the invasion of the African clawed frog, Xenopus laevis, in Europe. PeerJ. 2016;4:e1659; 10.7717/peerj.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furman BL, Bewick AJ, Harrison TL, Greenbaum E, Gvoždík V, Kusamba C, Evans BJ. Pan‐African phylogeography of a model organism, the African clawed frog ‘Xenopus laevis’. Mol Ecol. 2015;24: 909–925. 10.1111/mec.13076 [DOI] [PubMed] [Google Scholar]
- 71.Veloz SD. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence‐only niche models. J Biogeogr. 2009;36: 2290–2299. [Google Scholar]
- 72.Hijmans RJ. Cross‐validation of species distribution models: removing spatial sorting bias and calibration with a null model. Ecology. 2012;93: 679–688. [DOI] [PubMed] [Google Scholar]
- 73.Boria RA, Olson LE, Goodman SM, Anderson RA. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol Model. 2014;275: 73–77. [Google Scholar]
- 74.Brown JL. SDMtoolbox: a python‐based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol Evol. 2014;5: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beaumont LJ, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib. 2009;15: 409–420. [Google Scholar]
- 76.Broennimann O, Guisan A. Predicting current and future biological invasions: both native and invaded ranges matter. Biol Lett. 2008;4: 585–589. 10.1098/rsbl.2008.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25: 1965–1978. [Google Scholar]
- 78.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259.Pounds JA. 2001. Climate and amphibian declines. Nature. 2006;410: 639–640. [DOI] [PubMed] [Google Scholar]
- 79.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31: 161–175. [Google Scholar]
- 80.Fitzpatrick MC, Hargrove WW. The projection of species distribution models and the problem of non-analog climate. Biodivers Conserv. 2009;18: 2251–2261. [Google Scholar]
- 81.Rocchini D, Hortal J, Lengyel S, Lengyel S, Jiménez-Valverde A, Ricotta C, Bacaro G, Chiarucci A. Accounting for uncertainty when mapping species distributions: the need for maps of ignorance. Prog Phys Geog. 2011;35: 211–226. [Google Scholar]
- 82.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988; 240: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 83.VanDerWal J, Shoo LP, Graham C, Williams SE. Selecting pseudo-absence data for presence-only distribution modeling: how far should you stray from what you know? Ecol Model. 2009;220: 589–594. [Google Scholar]
- 84.Thuiller W, Münkemüller T, Lavergne S, Mouillot D, Mouquet N, Schiffers K, Gravel D. A road map for integrating eco‐evolutionary processes into biodiversity models. Ecol Lett. 2013;16: 94–105. 10.1111/ele.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.R Development Core Team. R: A language and environment for statistical computing. Available: http://www.Rproject.org (15 February 2013).
- 86.Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol. 2006;43: 1223–1232. [Google Scholar]
- 87.Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr. 2007;34: 102–117. [Google Scholar]
- 88.Liu C, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography. 2005;28: 385–393. [Google Scholar]
- 89.Webber BL, Yates CJ, Le Maitre DC, Scott JK, Kriticos DJ, Ota N, Mc Neil A, Le Roux JK, Midgley GF. Modelling horses for novel climate courses: insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Divers Distrib. 2011;17: 978–1000. [Google Scholar]
- 90.Elith J, Kearney M, Phillips S. The art of modelling range‐shifting species. Meth Ecol Evol. 2010;1: 330–342. [Google Scholar]
- 91.Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463: 747–756. 10.1038/nature08823 [DOI] [PubMed] [Google Scholar]
- 92.Peterson AT, Nyari AS. Ecological niche conservatism and Pleistocene refugia in the Thrush-like Mourner, Schiffornis sp., in the Neotropics. Evolution. 2008;62: 173–183. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez-Villegas J, Jarvis A. Downscaling global circulation model outputs: the delta method decision and policy analysis Working Paper No. 1. J Pol Anal Manag. 2010;1: 1–18. [Google Scholar]
- 94.Bertelsmeier C, Luque GM, Hoffmann BD, Courchamp F. Worldwide ant invasions under climate change. Biodivers Conserv. 2014;24: 117–128. [Google Scholar]
- 95.Balinsky B. I. (1969). The reproductive ecology of amphibians of the Transvaal highveld. Zoologica africana, 4(1), 37–93. [Google Scholar]
- 96.McCoid MJ. An observation of reproductive-behaviour in a wild population of African clawed frogs, Xenopus laevis, in California, Calif fish game. 1985;71: 245–246. [Google Scholar]
- 97.McCoid MJ, Fritts TH. Observations of feral populations of Xenopus laevis (Pipidae) in southern California. Bull—South Calif Acad Sci. 1980;79: 82–86. [Google Scholar]
- 98.Herrel A, van der Meijden A. An analysis of the live reptile and amphibian trade in the USA compared to the global trade in endangered species. Herpetol J. 2014;24: 103–110. [Google Scholar]
- 99.Meyerson LA, Reaser JK. Biosecurity: moving toward a comprehensive approach. Bioscience. 2002;52: 593–600. [Google Scholar]
- 100.Vogel G. Proposed frog ban makes a splash. Science. 2008;319: 1472–1472. [DOI] [PubMed] [Google Scholar]
- 101.Fouquet A, Measey GJ. Plotting the course of an African clawed frog invasion in Western France. Anim Biol. 2006;56: 95–102. [Google Scholar]
- 102.De Villiers, FA. The dispersal ability, performance and population dynamics of Cape Xenopus frogs. MSc Thesis, Stellenbosch University, 2016.
- 103.Crayon JJ. Species account: Xenopus laevis In: Lannoo MJ, editor. Amphibian declines: the conservation status of United States species. University of California Press, Berkeley; 2005. Pp. 522–25. [Google Scholar]
- 104.McNeely JA, Mooney HA, Neville LE, Schei P, Waage JK, editors. Global strategy on invasive alien species. Gland, Switzerland: IUCN on behalf of the Global Invasive Species Programme; 2001. [Google Scholar]
- 105.National Invasive Species Council. Meeting the invasive species challenge: national invasive species management plan Washington, DC: National Invasive Species Council; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model contribution was assessed by building the model using a single corresponding predictor variable. The logistic output (probability of presence) is displayed on the Y axis. Red curves refer to mean responses of 100 replicate Maxent runs while the mean +/- one standard deviation is displayed in blue.
(PDF)
The logistic output (probability of occurrence) is displayed on the Y axis.
(PDF)
A) Overlap analysis of the ensemble SDM and the maximum entropy SDM, with red highlighting areas where the ensemble SDM yields higher probabilities and blue depicting areas where the maximum entropy SDM yields higher probabilities; B) Overlap analysis of the ensemble SDM and the SDM by Measey et al. (2012), with red indicating higher probability of the ensemble SDM and blue showing higher values for the SDM by Measey et al. (2012); C) Overlap analysis of the maximum entropy SDM and the SDM by Measey et al. (2012), with red highlighting regions with higher probability of the ensemble SDM and blue showing higher probability values for the SDM by Measey et al. (2012). Colour saturation increases with deviation of the models. Areas where both SDMs yield similar probability values are displayed in white.
(PDF)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.