Abstract
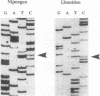
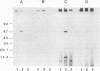
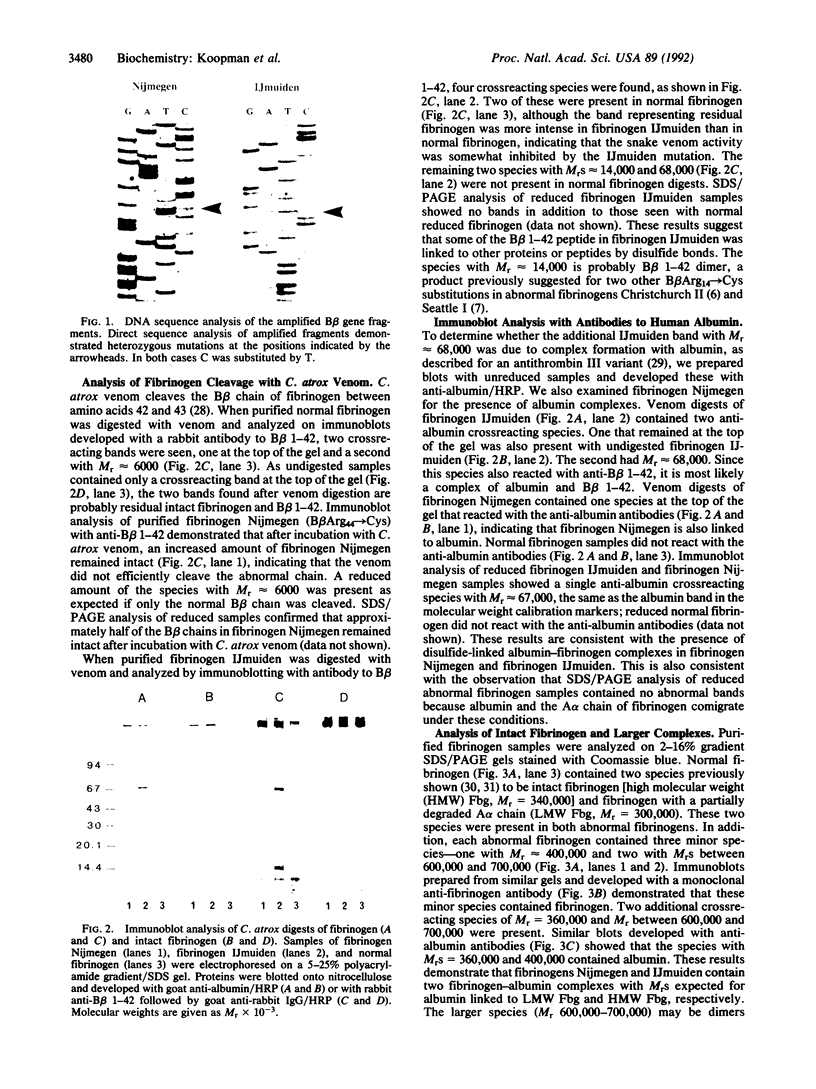
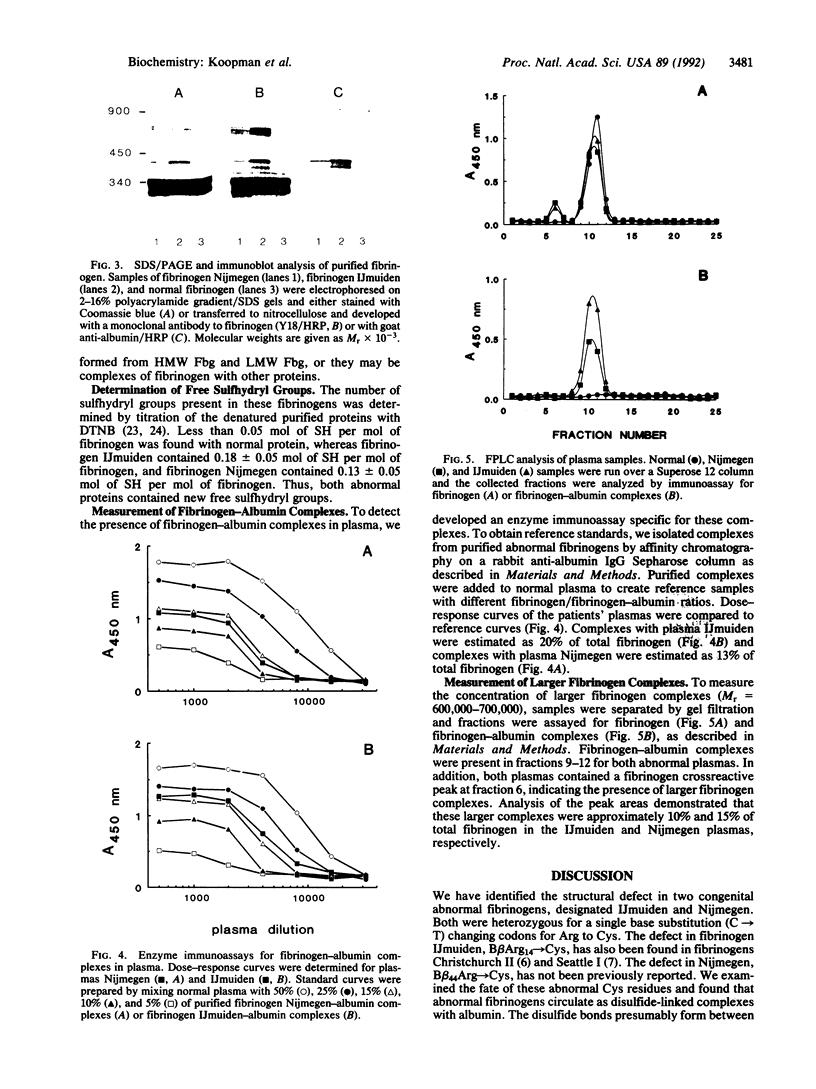
The molecular defects in two congenital abnormal fibrinogens, IJmuiden and Nijmegen, were determined by sequence analysis of genomic DNA amplified by the polymerase chain reaction. Both fibrinogens were heterozygous, IJmuiden having a B beta Arg14----Cys substitution and Nijmegen having a B beta Arg44----Cys substitution. Clotting induced by thrombin or Reptilase was impaired in both fibrinogens, indicating defective fibrin polymerization. Immunoblot analysis of both purified fibrinogens demonstrated that some of the abnormal molecules were linked by disulfide bonds to albumin. In addition, abnormal high molecular weight fibrinogen complexes with Mrs between 600,000 and 700,000 were present. Fibrinogen-albumin and high molecular weight complexes were also detected in the patients' plasmas. Quantitative analysis demonstrated that of the total plasma fibrinogen in the IJmuiden patient, 20% was linked to albumin and 10% was present as high molecular weight complexes. In plasma Nijmegen, 13% was linked to albumin and 15% was present as high molecular weight complexes. These results demonstrate that the additional abnormal cysteine in fibrinogens IJmuiden and Nijmegen resulted in the formation of disulfide-linked complexes with other proteins, predominantly albumin. We also found that a significant fraction of the abnormal fibrinogen molecules contained free sulfhydryl groups. These findings complicate interpretation of functional studies of these altered fibrinogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos E. S., van der Doelen A. A., van Rooy N., Schuurs A. H. 3,3',5,5' - Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoassay. 1981;2(3-4):187–204. doi: 10.1080/15321818108056977. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Que B. G., Rixon M. W., Mace M., Jr, Davie E. W. Characterization of complementary deoxyribonucleic acid and genomic deoxyribonucleic acid for the beta chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3244–3250. doi: 10.1021/bi00282a032. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Law S. W., Dennison O. E. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):71–75. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Engesser L., Koopman J., de Munk G., Haverkate F., Nováková I., Verheijen J. H., Briët E., Brommer E. J. Fibrinogen Nijmegen: congenital dysfibrinogenemia associated with impaired t-PA mediated plasminogen activation and decreased binding of t-PA. Thromb Haemost. 1988 Aug 30;60(1):113–120. [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Ireland H., Panico M., Di Marzo V., Blench I., Morris H. R. Formation of a covalent disulfide-linked antithrombin-albumin complex by an antithrombin variant, antithrombin "Northwick Park". J Biol Chem. 1987 Oct 5;262(28):13381–13384. [PubMed] [Google Scholar]
- Galanakis D. K., Henschen A., Peerschke E. I., Kehl M. Fibrinogen Stony Brook, a heterozygous A alpha 16Arg----Cys dysfibrinogenemia. Evaluation of diminished platelet aggregation support and of enhanced inhibition of fibrin assembly. J Clin Invest. 1989 Jul;84(1):295–304. doi: 10.1172/JCI114154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Haverkate F., Koopman J., Kluft C., D'Angelo A., Cattaneo M., Mannucci P. M. Fibrinogen Milano II: a congenital dysfibrinogenaemia associated with juvenile arterial and venous thrombosis. Thromb Haemost. 1986 Feb 28;55(1):131–135. [PubMed] [Google Scholar]
- Hong C. S., Stadler B. M., Wälti M., De Weck A. L. Dot-immunobinding assay with monoclonal anti-IgE antibodies for the detection and quantitation of human IgE. J Immunol Methods. 1986 Dec 24;95(2):195–202. doi: 10.1016/0022-1759(86)90406-0. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman J., Haverkate F., Briët E., Lord S. T. A congenitally abnormal fibrinogen (Vlissingen) with a 6-base deletion in the gamma-chain gene, causing defective calcium binding and impaired fibrin polymerization. J Biol Chem. 1991 Jul 15;266(20):13456–13461. [PubMed] [Google Scholar]
- Koopman J., Haverkate F., Koppert P., Nieuwenhuizen W., Brommer E. J., Van der Werf W. G. New enzyme immunoassay of fibrin-fibrinogen degradation products in plasma using a monoclonal antibody. J Lab Clin Med. 1987 Jan;109(1):75–84. [PubMed] [Google Scholar]
- Koppert P. W., Huijsmans C. M., Nieuwenhuizen W. A monoclonal antibody, specific for human fibrinogen, fibrinopeptide A-containing fragments and not reacting with free fibrinopeptide A. Blood. 1985 Sep;66(3):503–507. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipinska I., Lipinski B., Gurewich V. Fibrinogen heterogeneity in human plasma. Electrophoretic demonstration and characterization of two major fibrinogen components. J Lab Clin Med. 1974 Oct;84(4):509–516. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Miyata T., Terukina S., Matsuda M., Kasamatsu A., Takeda Y., Murakami T., Iwanaga S. Fibrinogens Kawaguchi and Osaka: an amino acid substitution of A alpha arginine-16 to cysteine which forms an extra interchain disulfide bridge between the two A alpha chains. J Biochem. 1987 Jul;102(1):93–101. doi: 10.1093/oxfordjournals.jbchem.a122046. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., DiOrio J. P., Müller M. F., Shainoff J. R., Siebenlist K. R., Amrani D. L., Homandberg G. A., Soria J., Soria C., Samama M. Studies on the ultrastructure of fibrin lacking fibrinopeptide B (beta-fibrin). Blood. 1987 Apr;69(4):1073–1081. [PubMed] [Google Scholar]
- Mosesson M. W., Galanakis D. K., Finlayson J. S. Comparison of human plasma fibrinogen subfractions and early plasmic fibrinogen derivatives. J Biol Chem. 1974 Jul 25;249(14):4656–4664. [PubMed] [Google Scholar]
- Pandya B. V., Cierniewski C. S., Budzynski A. Z. Conservation of human fibrinogen conformation after cleavage of the B beta chain NH2 terminus. J Biol Chem. 1985 Mar 10;260(5):2994–3000. [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hirose M. Determination of sulfhydryl groups and disulfide bonds in a protein by polyacrylamide gel electrophoresis. Anal Biochem. 1990 Aug 1;188(2):359–365. doi: 10.1016/0003-2697(90)90621-f. [DOI] [PubMed] [Google Scholar]
- Terukina S., Matsuda M., Hirata H., Takeda Y., Miyata T., Takao T., Shimonishi Y. Substitution of gamma Arg-275 by Cys in an abnormal fibrinogen, "fibrinogen Osaka II". Evidence for a unique solitary cystine structure at the mutation site. J Biol Chem. 1988 Sep 25;263(27):13579–13587. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ruijven-Vermeer I. A., Nieuwenhuizen W. Purification of rat fibrinogen and its constituent chains. Biochem J. 1978 Mar 1;169(3):653–658. doi: 10.1042/bj1690653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Dissociation of fibronectin from gelatin-agarose by amino compounds. Biochem J. 1978 Oct 1;175(1):333–336. doi: 10.1042/bj1750333. [DOI] [PMC free article] [PubMed] [Google Scholar]