Differentiating multiple system atrophy (MSA) from Parkinson disease with orthostatic hypotension (PD + OH) can be very difficult clinically. All patients with PD + OH have neuroimaging evidence of cardiac sympathetic denervation, whereas most MSA patients have evidence of intact innervation [1]. Occasionally, patients with autopsy-proven MSA have been found to have loss of cardiac sympathetic innervation, by in vivo neuroimaging [2] or by post-mortem decreased tyrosine hydroxylase immunoreactivity in epicardial nerves [3]. In PD, deposits of immunoreactive alpha-synuclein (SYN-ir) occur intraneuronally in Lewy bodies and Lewy neurites, whereas in MSA deposits of SYN-ir occur in glial cytoplasmic inclusions (GCIs). This raises the possibility of a “hybrid disease,” in which alpha-synuclein would be deposited in both neurons and glial cells.
Here we report the case of a patient with MSA who had in vivo neuroimaging evidence of cardiac sympathetic denervation that was confirmed by post-mortem catecholamine neurochemistry, without alpha-synuclein deposition in central or sympathetic neurons. Moreover, the pattern of abnormalities in tissue levels of catechols in the putamen and myocardium in this case fit with a particular mechanism of catecholamine neuron death, independent of neuronal synucleinopathy.
Our patient, a Greek man, noted erectile dysfunction at about 62 years old. At 65 he developed urinary retention with incontinence, followed soon afterward by orthostatic lightheadedness, occasional fainting, decreased sweating, and constipation. At a medical evaluation at the age of 68 he had stridor. His family reported the patient also had dream enactment behavior, slow movement, and decreased facial expression. All these symptoms and signs persisted, without cognitive changes. His movement disorder did not improve with levodopa/carbidopa treatment, but this did seem to improve his orthostatic tolerance. The past medical history included retroperitoneal fibrosis at the age of 56, which caused unilateral renal failure and necessitated ureteral bypass surgery. He also had adult-onset hypothyroidism. There were no neurologic diseases in his family. After giving informed consent to participate in an IRB-approved protocol, the patient was evaluated at the NIH Clinical Center at the age of 71. He had a postural tremor of both upper extremities, without resting tremor. There were asymmetric rigidity of all limbs, hypomimia, bradykinesia, and hypokinesia. The gait was unstable with short, shuffling steps.
Pertinent autonomic and neurological test results included the following: In response to the Valsalva maneuver there was a progressive decrease in beat-to-beat blood pressure during Phase II and no phase IV pressure overshoot, indicating baroreflex-sympathoneural failure. Baroreflex-cardiovagal gain, based on the Phase II hemodynamic data, was low at 0.17 ms/mmHg (normal >2 ms/mmHg). There was an attenuated orthostatic increase in plasma norepinephrine (NE) (Table 1), which was also consistent with baroreflex-sympathoneural failure. Analysis of cerebrospinal fluid (obtained more than 72 h after discontinuing levodopa/carbidopa) revealed decreased levels of NE, dihydroxyphenylglycol (DHPG), and dihydroxyphenylacetic acid (DOPAC), consistent with central noradrenergic and dopaminergic deficits [4]. There was severely decreased olfaction by the University of Pennsylvania Smell Identification Test, whereas results of the Quantitative Sudomotor Axon Reflex Test were normal. Positron emission tomographic imaging showed decreased putamen 18F-DOPA-derived radioactivity and low left ventricular myocardial 18F-dopamine-derived radioactivity (Fig. 1).
Table 1.
Plasma and tissue concentrations (fmol/mg wet weight) of catechols in control subjects (CON, means ± SEM) and the patient with multiple system atrophy (MSA). Numbers in parentheses are numbers of subjects.
| Plasma | CON | MSA Patient | % Of CON |
| NE Supine | 1.64 ± 0.09 (62) | 1.02 | 62% |
| NE Upright | 3.39 ± 0.27 (26) | 1.20 | 35% |
| CSF NE | 0.90 ± 0.07 (32) | 0.28 | 31% |
| CSF DOPAC | 2.37 ± 0.22 (32) | 1.26 | 53% |
| CSF DHPG | 11.02 ± 0.57 (32) | 9.78 | 89% |
| Putamen | CON | MSA Patient | % Of CON |
| DOPA | 1597 ± 383 (25) | 471 | 29% |
| DA | 16,848 ± 2019 (25) | 500 | 8.9% |
| DOPAL | 339 ± 24 (24) | 36 | 3.4% |
| DOPAC | 2616 ± 460 (24) | 54 | 1.6% |
| NE | 198 ± 26 (25) | 17.9 | 31% |
| DHPG | 14.6 ± 2.3 (24) | 0.9 | 5.6% |
| Myocardium | |||
| DOPA | 219 ± 28 (23) | 275 | 126% |
| DA | 72.5 ±15.6 (23) | 7.9 | 10.9% |
| DOPAL | Not detected | Not detected | |
| DOPAC | 35.6 ± 7.1 (23) | 3.6 | 10.1% |
| NE | 1540 ± 247 (23) | 3.3 | 0.2% |
| DHPG | 90.5 ± 19.5 (23) | 0.6 | 0.7% |
Fig. 1.
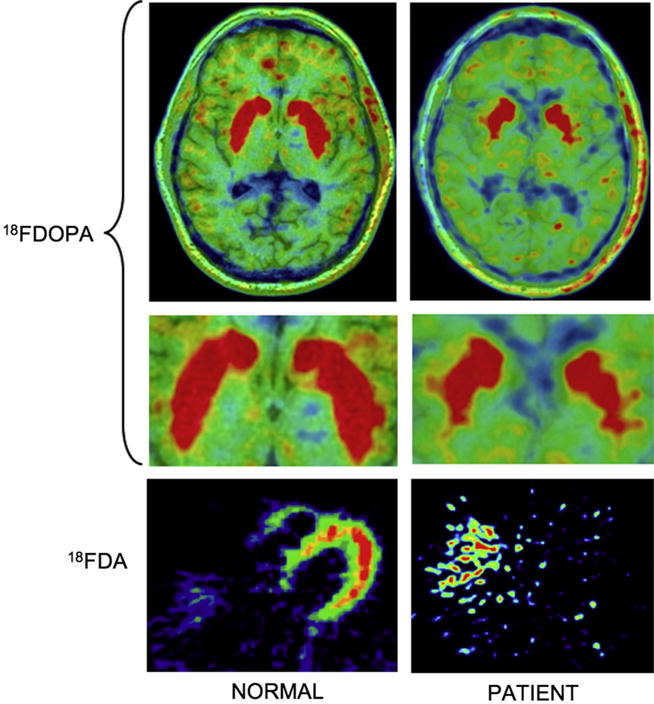
18F-DOPA (top and blow-up in middle) and 18F-dopamine (bottom) PET scans in control subjects (left) and in the current patient with MSA (right). There is neuroimaging evidence for decreased, distorted putamen 18F-DOPA-derived radioactivity and markedly decreased cardiac sympathetic innervation.
About a year later, at the age of 72, the patient died from progressive respiratory failure. He was autopsied, and the brain and extra-cranial tissues were harvested, with the post-mortem interval (time from death to tissue freezing) about 8 h. Gross examination of the brain revealed putamen atrophy, and neurohistopathology demonstrated SYN-ir deposits in glia but not in neurons or sympathetic ganglia. Putamen tissue DA and DOPAC were drastically decreased—by 92% and 98%—while tissue DOPA and NE were decreased to lesser extents—by 71% and 69%. Tissue 3,4-dihydroxyphenylacetaldehyde (DOPAL) was built up with respect to DA (7.2% vs. 2.0% in controls), indicating a shift from vesicular sequestration to oxidative deamination of cytosolic DA [5]. The ratio of DOPAC:DOPAL was low (1.5 vs. 7.7 in controls), consistent with concurrently decreased aldehyde dehydrogenase activity [5]. In apical myocardial tissue, NE was markedly decreased (by 99.8% from control), with an elevated DHPG:NE ratio (0.18 vs. 0.059 in controls), which fit with a shift from vesicular sequestration to oxidative deamination of cytosolic NE in sympathetic nerves.
This case illustrates that in MSA, cardiac sympathetic denervation can occur even without Lewy bodies or alpha-synuclein deposition in neurons. To our knowledge, this is the first report of cardiac sympathetic denervation without alpha-synuclein deposition in neurons. While the ergot-derived dopamine agonists carry a risk of retroperitoneal fibrosis, in this case the retroperitoneal fibrosis pre-dated any motor or sensory symptoms and the use of any dopaminergic medications. We do not know of any reported connection between retroperitoneal fibrosis and the subsequent development of multiple system atrophy or other synucleinopathy. The abnormal patterns of tissue levels of catechols in the putamen and myocardium suggest a shift from vesicular uptake to oxidative deamination of cytosolic catecholamines, catecholaldehyde buildup, and decreased catecholaldehyde metabolism, as has been reported in sporadic PD [5]. Since DOPAL is cytotoxic, it is possible that this triad is part of a death process in catecholaminergic neurons.
Acknowledgments
The research reported here was supported by the Division of Intramural Research, NINDS.
References
- 1.Goldstein DS, Orimo S. Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clin Auton Res. 2009;19:133–6. doi: 10.1007/s10286-009-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raffel DM, Koeppe RA, Little R, Wang CN, Liu S, Junck L, et al. PET measurement of cardiac and nigrostriatal denervation in parkinsonian syndromes. J Nucl Med. 2006;47:1769–77. [PubMed] [Google Scholar]
- 3.Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, et al. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol Berl. 2007;113:81–6. doi: 10.1007/s00401-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Holmes C, Sharabi Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain. 2012;135:1900–13. doi: 10.1093/brain/aws055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, et al. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]


