Abstract
Objective
Diagnosis of amyotrophic lateral sclerosis (ALS) and primary lateral sclerosis (PLS) is often difficult due to absence of disease biomarkers. Our aim was to investigate quantitative susceptibility mapping (QSM) of the motor cortex as a potential quantitative biomarker for the diagnosis of ALS and PLS.
Materials and Methods
Utilizing an institutional review board approved retrospective database, QSM images for 16 patients with upper motor neuron disease (12 with ALS and 4 with PLS; mean age 56.3; 56% male) and 23 control patients (mean age 56.6; 57% male) were reviewed. Two neuroradiologists, blinded to diagnosis, qualitatively assessed QSM, T2, T2*, and T2 FLAIR-weighted images. Relative motor cortex susceptibility (RMCS) was quantitatively calculated by subtracting adjacent white matter/CSF signal intensity from mean motor cortex susceptibility on the axial image most representative of the hand lobule, and receiver operating characteristic (ROC) analysis was performed. The Fisher’s exact and Student’s t tests were used to evaluate for statistical differences between the groups.
Results
Qualitatively, QSM had higher diagnostic accuracy than T2, T2*, or T2 FLAIR for the diagnosis of ALS/PLS. Quantitatively, RMCS was found to be significantly higher in patients with motor neuron disease than in control patients (46.0 and 35.0, respectively; p<0.001). ROC analysis demonstrated an area-under-the-curve of 0.88 (p<0.0001) and an optimal cutoff value of 40.5 ppb for distinguishing between control and ALS/PLS patients (sensitivity, 87.5%; specificity, 87.0%).
Conclusions
QSM is a sensitive and specific quantitative biomarker of iron deposition in the motor cortex in ALS and PLS.
Introduction
Amyotrophic lateral sclerosis (ALS) is an idiopathic, progressive neurodegenerative disease affecting upper and lower motor neurons that invariably results in death, with mean survival ranging from 3 to 5 years. The majority of ALS cases are sporadic, with multifactorial pathophysiological mechanisms leading to disease (1). Diagnosis relies on documented progressive upper and lower motor neuron signs and symptoms involving the brain and multiple spinal cord regions of innervation (1). Primary lateral sclerosis (PLS) is a less common, more slowly progressive neurodegenerative disease that only affects upper motor neurons. There remains debate as to whether ALS and PLS are two distinct disorders or whether they represent a continuum of the same disease process (2).
In addition to serial clinical assessment, the diagnosis of ALS and exclusion of its mimics is supported by laboratory tests including blood and CSF analysis, nerve conduction studies, electromyography, and muscle biopsy. The current role of imaging lies mainly in excluding structural lesions such as syringohydromyelia or multiple sclerosis. Diagnosis is commonly delayed, with a median time from symptom onset to diagnosis of 14 months in ALS (1), and a minimum four year symptom duration in PLS (3). In addition, published false positive ALS diagnosis rates range from 8 to 44% (1). Due to the clinical importance of establishing early diagnosis, avoiding false positives, and predicting the rate of progression, there has been great interest in potential imaging biomarkers.
Conventional imaging findings in ALS include motor cortex T2 hypointensity (4–6) and corticospinal tract T2 hyperintensity (7,8). Conventional imaging findings in PLS are similar and include corticospinal tract T2 hyperintensity, motor and premotor cortical atrophy, and T2 hypointensity within the precentral gyrus (8,9). Postmortem studies have shown that motor cortex hypointensity on 7T T2*-weighted gradient recall echo (GRE) images corresponds to accumulation of iron within microglia in middle and deep layers of the motor cortex (5), providing evidence that the paramagnetic effect of iron causes motor cortex susceptibility in ALS. Qualitative motor cortex scoring of hypointensity on T2*-weighted images in patients has been shown to correlate with disease severity (using ALS-FRS-R) in ALS patients, with the majority of patients demonstrating increased qualitative motor cortex susceptibility 6 months after baseline imaging, suggesting correlation with disease progression (6). Despite some understanding of the imaging findings in ALS/PLS, diagnosis remains a challenge.
Quantitative susceptibility mapping (QSM) is a novel imaging technique that allows quantitative assessment of tissue magnetic susceptibility. QSM measures tissues’ magnetic susceptibilities, which quantitatively reflects the degree of magnetization in response to an applied magnetic field. The voxel value in QSM is determined by its concentration of paramagnetic and diamagnetic species, which exhibit positive and negative susceptibility values, respectively. While T2*-weighted images qualitatively show hypointensity for both paramagnetic and diamagnetic species, QSM is able to distinguish between paramagnetic species (such as iron), which appear hyperintense, and diamagnetic species (such as calcium), which appear hypointense. A study correlating QSM with iron concentration as determined by postmortem mass spectroscopy found a strong linear correlation between tissue magnetic susceptibility and iron concentration in gray matter structures (10).
The aim of this case-control study is to investigate the diagnostic accuracy of QSM and its potential to serve as a quantitative biomarker for the diagnosis of ALS and PLS.
Methods
ADS and TL had control of the data.
Data acquisition
This study was approved by our institutional review board. All consecutive patients referred from a peripheral nerve and muscle disease clinic for clinically indicated MR imaging of the brain to exclude structural causes of their symptoms were retrospectively included in this study. Among this group, chart review was performed by two experienced neurologists; any patients who did not have either possible, probable, or definite ALS per the El Escorial criteria (11), or clinically confirmed PLS, were excluded. For patients meeting these criteria, the following clinical data was collected: (1) date of disease onset; (2) type of motor neuron disease (MND), separated into possible, probable, or definite amyotrophic lateral sclerosis (ALS) and primary lateral sclerosis (PLS); (3) percent predicted forced vital capacity (FVC); (4) ALS-FRS-R (revised ALS Functional Rating Scale) (12); and (5) Medical Research Council (MRC) upper extremity strength scores, if available. FVC, ALS-FRS-R, and MRC measurements were all performed within two months of imaging. Twenty-three control patients, matched for age and sex with the cases, were selected from the same IRB-approved retrospective database. This control group included patients with anatomically normal MR examinations for age; any patients with upper/lower motor neuron disease, stroke, hemorrhage, or other structural abnormality were excluded. All patients were imaged on a 3.0T scanner (GE Excite HD). T2-weighted, T2*-weighted, T2 FLAIR, and QSM images were all acquired in oblique axial planes parallel to the anterior-posterior commissure line. T2-weighted images were acquired using the following parameters: TR=6s, TE=81ms, FA=90°, NEX=2, ETL=23, and voxel size=0.47×0.94×3mm3. T2 FLAIR images were acquired using the following parameters: TR=6s, TE=130ms, TI=1.85s, FA=90°, NEX=1, ETL=140, and voxel size=1.2×1.2×1.2mm3. The T2* and QSM images were acquired using the same 3D multi gradient echo sequence (TE/ΔTE/#TE = 5ms/5ms/11, TR/FA/BW=59ms/20°/±62.50 kHz, voxel size=0.57×0.75×2mm3). The T2* images are composite images obtained by taking the weighted sum of all of the magnitude images at different echoes. In order to obtain QSM images, real and imaginary images were saved, from which the magnetic field inhomogeneity was estimated. The estimated field inhomogeneity was further deconvolved by a magnetic dipole field to obtain the QSM image. To overcome noise amplifications in the deconvolution, the morphology enabled dipole inversion (MEDI) method was used for QSM reconstruction (13–15).
Qualitative analysis
Two neuroradiologists (AJT and AG, with 10 and 3 years of experience, respectively) who were blinded to the presence/absence of MND performed qualitative scoring of the following four imaging features: (1) motor cortex T2 hypointensity, (2) motor cortex T2* hypointensity, (3) motor cortex QSM hyperintensity, and (4) corticospinal tract T2 hyperintensity. Each neuroradiologist assigned scores for motor cortex T2/T2* hypointensity and corticospinal tract T2 hyperintensity as follows: 0, absent; 1, present, mild; or 2, present, marked. In our experience, in patients without motor cortex pathology, the motor cortex is slightly more hyperintense than the rest of the supratentorial cortex (including the sensory cortex) on the QSM images. Therefore, scores for motor cortex QSM hyperintensity were assigned by each neuroradiologist, as follows: 0, slightly more hyperintense than sensory cortex; 1, moderately more hyperintense than sensory cortex; or 2, markedly more hyperintense than sensory cortex. Example images of each of the qualitative scores are provided in the supplemental figures (1S–4S).
Quantitative analysis
Guided by a board-certified neuroradiologist with a certificate of added qualification (10 years experience), the central sulcus and image most representative of the hand lobule were selected for each side, and the motor cortex of each subject was drawn in a standardized fashion. A semi-automated program based on OsiriX v.5.8.1 (Pixmeo Sarl, Geneva, Switzerland) was used to refine the segmentation between motor cortices and adjacent white matter and CSF by applying a simple threshold. The threshold was heuristically adjusted to ensure continuity and completeness of the cortex, and was typically in the range of 10 to 20 ppb (parts per billion). Relative susceptibility (in ppb) was calculated by subtracting adjacent susceptibility from motor cortex susceptibility.
Statistical analysis
For the qualitative analysis, Fisher’s exact test was used to evaluate for differences between the MND and control groups and the kappa statistic was calculated to evaluate inter-observer agreement between the two neuroradiologists who made qualitative assessments of the T2, T2*, QSM, and T2 FLAIR images. For the quantitative analysis, the mean relative motor cortex susceptibility (RMCS) of the right and left hand lobules was calculated for each patient. XLSTAT version 2013 6.02 (Addinsoft, France) was used to perform the statistical analyses. Linear regression was performed between these mean values and the clinical scores (ALS-FRS-R, percent predicted FVC, and MRC upper extremity strength score). The two-tailed Student’s t-test was used to evaluate for statistical differences between MND cases and controls, with p values less than 0.05 considered statistically significant. Receiver operating characteristic (ROC) analysis was performed utilizing the quantitative RMCS values for both the control and MND patients. For all analyses, a p value of less than 0.05 was considered statistically significant. An optimal cutoff value distinguishing cases and controls was established, and diagnostic test characteristics, including sensitivity and specificity were calculated.
Results
Among the 25 patients referred from the peripheral nerve and muscle disease clinic for MRI including QSM between 12/1/2011 and 11/30/2013, 16 met the El Escorial criteria for ALS (12) or had clinically confirmed PLS and were included in the study. Of the 9 excluded patients, 5 had unspecified motor neuropathy, 2 had possible atypical PLS, 1 had Parkinson disease, and 1 had the superoxide dismutase 1 mutation (which has been linked to familial ALS) but was normal functionally. Representative T2*-weighted images and QSM are shown in Figure 1. Demographic and clinical characteristics of the 16 patients included in the study are summarized in Table 1.
Figure 1.
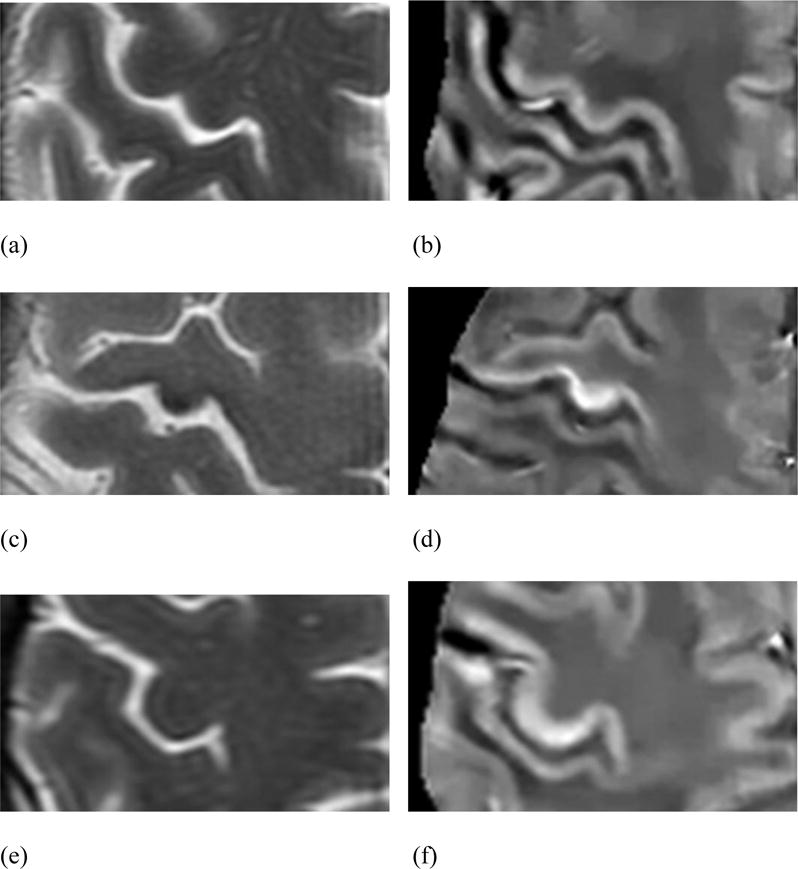
Axial T2-weighted and QSM images in a 50-year-old male control patient (a and b); a 42-year-old man with definite ALS (c and d), with an MRC upper extremity strength score of 57/100 and an ALS-FRS-R of 30; and a 49-year-old woman with probable ALS (e and f), with an MRC upper extremity strength score of 82/100 and an ALS-FRS-R of 43. Of note, the patient with definite ALS in Figs. 1c and 1d presented with profound left upper extremity weakness, and the RMCS scores for his right and left hand lobules were 89.3 ppb and 74.2 ppb, respectively.
Table 1.
Demographic data, clinical data, and RMCS for the 16 patients with MND.
| Pt # | Age at time of MRI | Sex | Duration of symptoms at time of MRI (mo) | Diagnosis; El Escorial Category | Other Comments | ALS-FRS-R at time of MRI (/48) | FVC at time of MRI (percent predicted) | MRC upper extremity strength score (/100) | Mean RMCS (ppb) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | M | 37 | ALS; definite | 30 | 49 | 57 | 81.7 | |
| 2 | 61 | M | 25 | ALS; possible | LMN predominant; became probable ALS on follow-up | 42 | 121 | 94 | 43.9 |
| 3 | 49 | F | 38 | ALS; probable | 43 | 151 | 82 | 47.9 | |
| 4 | 57 | M | 93 | PLS | 47 | 102 | 98 | 43.6 | |
| 5 | 60 | M | 11 | ALS; possible | With bulbar involvement | 44 | N/A* | 100 | 44.2 |
| 6 | 66 | F | 15 | ALS; definite | C9orf 72 mutation; concurrent FTLD | 38 | 73 | 96 | 45.7 |
| 7 | 73 | M | 25 | PLS | 41 | N/A* | 90 | 51.7 | |
| 8 | 68 | M | 65 | PLS | 33 | N/A* | 80 | 45.9 | |
| 9 | 41 | M | 22 | ALS; probable | Concurrent FTLD | 38 | N/A* | 91 | 39.1 |
| 10 | 67 | F | 20 | ALS; definite | 38 | 100 | 72 | 41.2 | |
| 11 | 60 | F | 7 | ALS; definite | Hemiplegic variant | 33 | 49 | 95 | 47.8 |
| 12 | 61 | M | 11 | ALS; probable | Also has cognitive dysfunction | 40 | 85 | 88 | 47.0 |
| 13 | 63 | F | 28 | ALS; definite | 37 | 61 | 86 | 45.8 | |
| 14 | 46 | M | 47 | PLS | 45 | N/A* | 100 | 49.1 | |
| 15 | 19 | F | 8 | ALS; definite | 47 | 105 | 100 | 17.1 | |
| 16 | 67 | F | 18 | ALS; probable | 38 | 61 | 91 | 43.8 |
Note: ALS indicates amyotrophic lateral sclerosis; PLS, primary lateral sclerosis; LMN, lower motor neuron; FTLD, frontotemporal lobe dementia; ALS-FRS-R, ALS revised functional rating scale; FVC, forced vital capacity; MRC, Medical Research Council;
N/A*, no FVC available at time of MRI; RMCS, relative motor cortex susceptibility; ppb, parts per billion.
The average age for the 16 MND patients and control patients was 56.3 and 56.6, respectively. The percent of the UMND and control groups that were male were 56.3 and 56.5%, respectively.
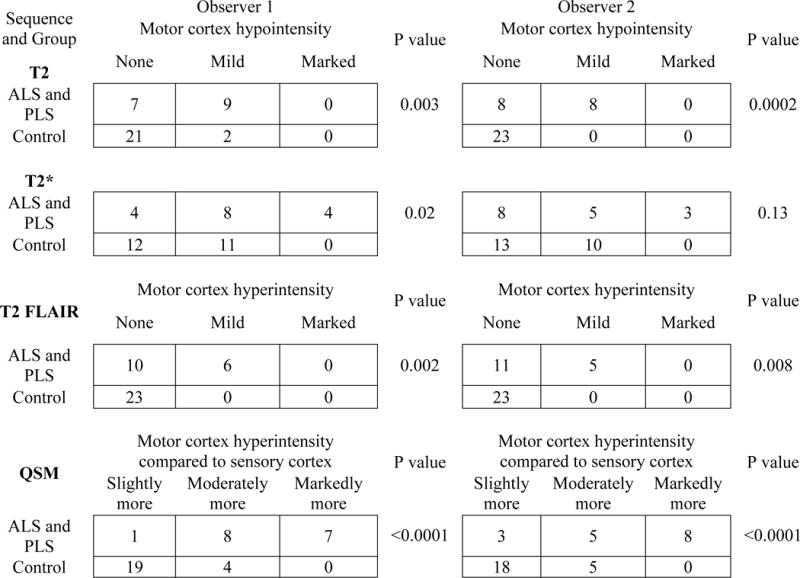
Table 2 shows how each neuroradiologist qualitatively assessed subjects and controls using T2, T2*, T2 FLAIR, and QSM sequences. Statistically significant differences between MND and control patients were observed for all sequences except for the T2* images by observer 2.
Table 2.
Two neuroradiologists’ (observers 1 and 2) qualitative analysis of signal intensity in subjects and controls on T2, T2*, T2 FLAIR, and QSM sequences. P values were generated using Fisher’s exact test.
|
Table 3 shows mean sensitivity, specificity, and diagnostic accuracy for the detection of MND, as well as the interobserver variability as measured by the weighted kappa statistic. Highest diagnostic accuracy of 83% was achieved when the two observers used QSM and counted either moderately or markedly more hyperintense motor cortex compared to sensory cortex as positive for MND. QSM showing markedly more hyperintensity in the motor than the sensory cortex, and T2 FLAIR showing presence of corticospinal tract T2 hyperintensity, each had the highest specificity (100% for both observers). Interobserver agreement was best for QSM and T2 FLAIR sequences.
Table 3.
Two observers’ mean sensitivity, specificity, and diagnostic accuracy for the diagnosis of MND using qualitative analysis. A test was considered positive if there was mild/marked hypointensity on T2 or T2*, mild or marked hyperintensity on T2 FLAIR, or moderately/markedly more hyperintensity on QSM. The last row shows the weighted kappa between both observers for each sequence.
| T2 | T2* | T2 FLAIR | QSM | |
|---|---|---|---|---|
| Sensitivity | 53% | 69% | 34% | 88% |
| Specificity | 96% | 54% | 100% | 80% |
| Accuracy | 78% | 60% | 73% | 83% |
| Kappa | 0.66 | 0.80 | 0.89 | 0.89 |
Representative T2-weighted and QSM images for two cases and one control are shown in Figure 1.
In the quantitative analysis, patients with MND (n=16) had significantly higher RMCS than control patients (n=23), 46.0 and 35.0 ppb with standard deviations of 12.3 and 6.5 ppb, respectively (p<0.001). ALS patients (n=12) also had significantly higher RMCS than control patients (n=23), 45.4 and 35.0 ppb, respectively (p=0.005). PLS patients (n=4) also had significantly higher RMCS than control patients (n=23), 47.6 and 35.0 ppb, respectively (p<0.001). There was a trend towards higher motor cortex susceptibility in patients with definite ALS compared to those with probable/possible ALS (46.6 and 44.3 ppb, respectively), which was not statistically significant (p=0.80). Figure 2 shows that PLS patients had slightly higher RMCS than ALS patients (47.6 and 45.4 ppb, respectively), but this difference also was not statistically significant (p=0.77) and PLS patients had significantly longer mean duration of symptoms at the time of imaging (57.5 and 20.0 months, respectively; p=0.001).
Figure 2.
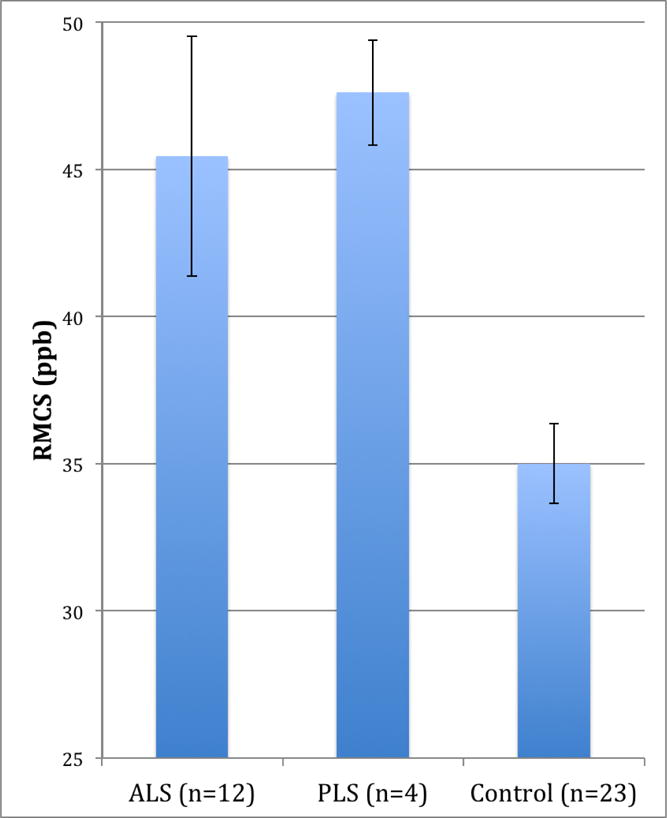
Relative motor cortex susceptibility by diagnosis stratified into ALS, PLS, and control groups, with bars indicating standard error for each group. Note: RMCS, relative motor cortex susceptibility.
Figure 3 shows the receiver operating characteristic (ROC) curve including all 39 patients in the study (16 MND patients and 23 control patients). The area under the ROC curve was 0.88 (p < 0.0001; 95% confidence interval, 0.752 to 0.998). The optimal cutoff value for RMCS was found to be 40.5 ppb, which yielded a sensitivity of 87.5% (95% CI, 62.5 to 97.5%) and a specificity of 87.0% (95% CI, 66.8 to 96.1%).
Figure 3.
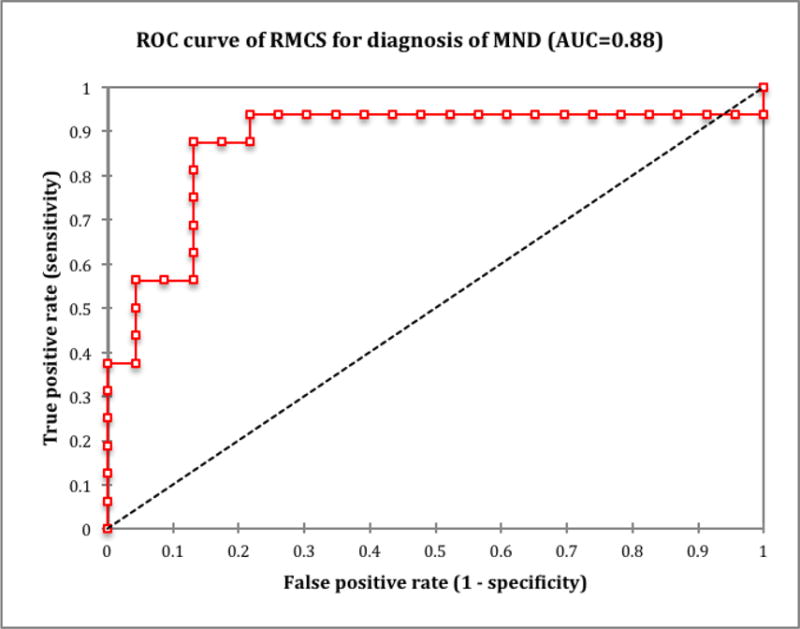
Receiver operating characteristic (ROC) curve for relative motor cortex susceptibility value’s ability to predict the presence or absence of MND (ALS or PLS). Note: RMCS, relative motor cortex susceptibility; AUC, area under the curve.
Supplemental figures 5Sa–c show correlations between mean RMCS and clinical measures of disease severity; modest inverse correlation with ALS-FRS-R, weak inverse correlation with FVC (among the 11 of 16 patients where FVC data were available at the time of MRI), and modest inverse correlation with MRC upper extremity strength score.
Discussion
The diagnosis of ALS and PLS is made difficult by the absence of a quantitative biomarker of disease. Imaging is currently utilized primarily to exclude structural lesions. Conventional and advanced imaging techniques have been investigated to aid in diagnosis. Conventional MRI studies have shown motor cortex T2 hypointensity (4–6) and corticospinal tract T2 hyperintensity (8,9) in ALS and PLS patients. Qualitative scoring of T2* hypointensity has been correlated with disease progression (6). T2/T2* hypointensity in the motor cortex in ALS has been related to iron accumulation, specifically as ferritin within microglial cells within the middle and deep layers of the motor cortex (5). It is currently unknown whether the increased iron deposition is due to primary iron metabolism dysregulation or is secondary to iron scavenging by glial cells following neuronal loss (16–18). Regardless of the mechanism of its deposition, the presence of increased iron within the motor cortex provides a potential biomarker of disease.
In this study, we performed both qualitative and quantitative analysis using QSM to investigate its diagnostic utility in ALS and PLS, and confirmed that both qualitative and quantitative methods were able to diagnose ALS and PLS with high diagnostic accuracy. Qualitatively, QSM demonstrating either moderately or markedly more motor than sensory cortex hyperintensity had the highest diagnostic accuracy (83%) among the four sequences analyzed (QSM, T2, T2*, and T2 FLAIR). Corticospinal tract T2 hyperintensity was very specific for the presence of MND (100% for both observers), consistent with previously published data (7). However, the sensitivity of corticospinal tract T2 hyperintensity was poor (38 and 31% for observers 1 and 2, respectively). Marked motor cortex hypointensity on QSM was also very specific for the presence of ALS or PLS (also 100% for both observers), with a higher sensitivity than T2 FLAIR (50 and 44% for observers 1 and 2, respectively). Quantitatively, patients with ALS and PLS had statistically significantly higher RMCS than control patients, likely on the basis of iron deposition as demonstrated in previous pathological examination of the motor cortices of ALS patients (5). While PLS patients had slightly higher RMCS than ALS patients, this difference was not statistically significant. Furthermore, the PLS patients had a significantly longer mean duration of disease than the ALS patients (57.5 and 20.0 months, respectively), likely due to the more indolent course of PLS.
There were modest inverse correlations between mean RMCS and ALS-FRS-R and MRC upper extremity strength score, and a weak inverse correlation with percent predicted FVC. It is possible that statistically significant correlations were not observed due to the relatively small sample size, particularly for percent predicted FVC, where data at the time of the MRI was available for only 11 of the 16 patients. Other than the patient with the highest motor cortex susceptibility of 81.7 ppb (patient #1, who had rapidly progressive, profound weakness, and hence the greatest functional impairment) and the patient with the lowest susceptibility of 17.1 ppb (patient #15, who had a nearly normal ALS-FRS-R of 47), the patients with upper motor neuron disease were predominantly clustered between RMCS values of 41 and 48 ppb. While RMCS values in this range were higher than those in the control group, it may not provide an adequately large range to discriminate between graded measures of disease severity.
It is important to note several limitations of this study. First, we employed a relatively small, retrospective case-control study with known disease cases and normal controls without subjects having intermediate pre-test probability of upper motor neuron disease. We believe that such an approach is justifiable in the initial assessment of QSM technology in ALS and PLS as our study confirms the ability of QSM to discriminate patients with disease and normal patients. However, we acknowledge that future investigations will require studying diagnostic accuracy in a prospective cohort of patients not yet diagnosed with motor neuron disease but in whom there is clinical suspicion of motor neuron disease (19). Second, because QSM is an emerging technology, certain assumptions were made including that the susceptibility of water is defined as zero; however, this has yet to be explicitly verified. Therefore, at this time it is necessary to use an internal control (the subcortical white matter and CSF adjacent to the segmented motor cortex) to calculate relative susceptibility. With continued improvement of the QSM technique and validation of values of absolute tissue susceptibility, a cutoff for absolute motor cortex susceptibility independent of scanning hardware/software could be determined. Third, patients with advanced disease could not be included in this study for logistical reasons related to their disability. This could bias our case group towards more mild/moderate disease severity, with two possible consequences; a) a possible limit on our ability to gain more than moderate correlations with ALS-FRS-R and MRC upper extremity strength score, and b) possible lessening of the observed difference in RMCS between cases and controls. Finally, cortical iron deposition has been reported in other pathological states. Patients with cerebrovascular disease (multiple small infarctions in the white matter and/or basal ganglia but no large vessel infarctions) have been shown to have qualitative T2 shortening in the perirolandic cortex (motor to a greater degree than sensory cortex) (20) and the visual cortex (21), probably due to iron deposition. In Alzheimer disease, iron deposition has been demonstrated in deep gray matter and in the parietal cortex (22). Patients with beta-thalassemia major have demonstrated iron accumulation in the motor cortex, temporal cortex, and deep gray structures (23). Iron deposition in the motor cortex has also been well documented to occur in the process of normal aging (20,24–26), which was addressed in this study by age-matching control and disease patients. Nonetheless, these are potential confounding factors that should be considered when future studies attempt to establish a RMCS cutoff for the diagnosis of ALS/PLS.
The findings from our case-control study require further validation in a larger-scale prospective cohort study to determine whether QSM of the motor cortex can aid in the early diagnosis of motor neuron disease and can serve as imaging biomarker for disease severity. Earlier and more confident diagnosis of ALS would allow earlier treatment with riluzole, an inhibitor of glutamate release that has been shown to improve survival in two large randomized controlled trials (27–29). Earlier physical therapy, occupational therapy, and rehabilitation services may improve quality of life (30,31). Furthermore, earlier diagnosis may be beneficial if any of the promising investigational therapies in clinical trials (32) prove effective. Future clinical trials could use QSM as a surrogate endpoint given its feasibility, accuracy, and reproducibility.
Conclusion
Our aim was to investigate QSM of the motor cortex as a potential quantitative biomarker for the diagnosis of ALS and PLS. In this case-control study, patients with ALS and PLS were found to have significantly higher relative motor cortex susceptibility than control patients, likely due to accelerated iron deposition. QSM was found to be accurate for both qualitative and quantitative detection of this increased tissue susceptibility. QSM thus has the potential to aid in earlier, more confident diagnosis of MND and could serve as a quantitative biomarker.
Supplementary Material
Acknowledgments
The authors would like to thank Sumit Niogi, MD, PhD for his comments on the manuscript.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- QSM
quantitative susceptibility mapping
- PLS
primary lateral sclerosis
- MND
motor neuron disease
- RMCS
relative motor cortex susceptibility
- FVC
forced vital capacity
- ALS-FRS-R
revised ALS functional rating scale
- MRC
Medical Research Council
- MEDI
morphology enabled dipole inversion
References
- 1.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia MC, Rowe A, Findlater K, Orange JB, Grace G, Strong MJ. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007;64(2):232–6. doi: 10.1001/archneur.64.2.232. [DOI] [PubMed] [Google Scholar]
- 3.Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve. 2007;35(3):291–302. doi: 10.1002/mus.20728. [DOI] [PubMed] [Google Scholar]
- 4.Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology. 1993;189(3):843–6. doi: 10.1148/radiology.189.3.8234713. [DOI] [PubMed] [Google Scholar]
- 5.Kwan JY, Jeong SY, Van Gelderen P, et al. Iron Accumulation in Deep Cortical Layers Accounts for MRI Signal Abnormalities in ALS: Correlating 7 Tesla MRI and Pathology. PLoS One. 2012;7(4):e35241. doi: 10.1371/journal.pone.0035241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignjatović A, Stević Z, Lavrnić S, Daković M, Bačić G. Brain iron MRI: A biomarker for amyotrophic lateral sclerosis. J Magn Reson Imaging. 2013;38(6):1472–9. doi: 10.1002/jmri.24121. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Ulug AM, Zimmerman RD, Lin MT, Rubin M, Beal MF. The diagnostic utility of FLAIR imaging in clinically verified amyotrophic lateral sclerosis. J Magn Reson Imaging. 2003;17(5):521–7. doi: 10.1002/jmri.10293. [DOI] [PubMed] [Google Scholar]
- 8.Cheung G, Gawel MJ, Cooper PW, Farb RI, Ang LC, Gawal MJ. Amyotrophic lateral sclerosis: correlation of clinical and MR imaging findings. Radiology. 1995;194(1):249–56. doi: 10.1148/radiology.194.1.7997565. [DOI] [PubMed] [Google Scholar]
- 9.Riad SM, Hathout H, Huang JC. High T2 signal in primary lateral sclerosis supports the topographic distribution of fibers in the corpus callosum: assessing disease in the primary motor segment. AJNR Am J Neuroradiol. 2011;32(4):E61–4. doi: 10.3174/ajnr.A2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62(3):1593–9. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 12.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Liu J, De Rochefort L, et al. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: Comparison with COSMOS in human brain imaging. Magn Reson Med. 2011;66(3):777–83. doi: 10.1002/mrm.22816. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Liu T, De Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012;59(3):2560–8. doi: 10.1016/j.neuroimage.2011.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2013;69(2):467–76. doi: 10.1002/mrm.24272. [DOI] [PubMed] [Google Scholar]
- 16.Goodall EF, Haque MS, Morrison KE. Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. J Neurol. 2008;255(11):1652–6. doi: 10.1007/s00415-008-0945-0. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell RM, Simmons Z, Beard JL, Stephens HE, Connor JR. Plasma biomarkers associated with ALS and their relationship to iron homeostasis. Muscle Nerve. 2010;42(1):95–103. doi: 10.1002/mus.21625. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi M, Brown RH, Rogers JT, Cudkowicz ME. Serum ferritin and metal levels as risk factors for amyotrophic lateral sclerosis. Open Neurol J. 2008;2:51–4. doi: 10.2174/1874205X00802010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutjes AWS, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PMM. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005;51(8):1335–41. doi: 10.1373/clinchem.2005.048595. [DOI] [PubMed] [Google Scholar]
- 20.Hirai T, Korogi Y, Sakamoto Y, Hamatake S, Ikushima I, Takahashi M. T2 shortening in the motor cortex: effect of aging and cerebrovascular diseases. Radiology. 1996;199(3):799–803. doi: 10.1148/radiology.199.3.8638008. [DOI] [PubMed] [Google Scholar]
- 21.Korogi Y, Hirai T, Komohara Y, Okuda T, Ikushima I, Kitajima M, Shigematu Y, Sugahara T, Takahashi M. T2 shortening in the visual cortex: effect of aging and cerebrovascular disease. AJNR Am J Neuroradiol. 1997;18(4):711–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology. 2009;253(2):497–504. doi: 10.1148/radiol.2532082324. [DOI] [PubMed] [Google Scholar]
- 23.Metafratzi Z, Argyropoulou MI, Kiortsis DN, Tsampoulas C, Chaliassos N, Efremidis SC. T2 relaxation rate of basal ganglia and cortex in patients with beta-thalassaemia major. Br J Radiol. 2001;74(881):407–10. doi: 10.1259/bjr.74.881.740407. [DOI] [PubMed] [Google Scholar]
- 24.Ngai S, Tang YM, Du L, Stuckey S. Hyperintensity of the precentral gyral subcortical white matter and hypointensity of the precentral gyrus on fluid-attenuated inversion recovery: variation with age and implications for the diagnosis of amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2007;28(2):250–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Karaarslan E, Arslan A. Perirolandic cortex of the normal brain: low signal intensity on turbo FLAIR MR images. Radiology. 2003;227(2):538–41. doi: 10.1148/radiol.2272020311. [DOI] [PubMed] [Google Scholar]
- 26.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 27.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 28.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425–31. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 29.Miller R, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;(3) doi: 10.1002/14651858.CD001447.pub3. Art. No.: CD001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller RG, Brooks BR, Swain-Eng RJ, et al. Quality improvement in neurology: amyotrophic lateral sclerosis quality measures: report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology. 2013;81(24):2136–40. doi: 10.1212/01.wnl.0000437305.37850.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbesman M, Sheard K. Systematic Review of the Effectiveness of Occupational Therapy–Related Interventions for People With Amyotrophic Lateral Sclerosis. J Occup Ther. 2014;68(1):20–6. doi: 10.5014/ajot.2014.008649. [DOI] [PubMed] [Google Scholar]
- 32.Gordon PH. Amyotrophic Lateral Sclerosis: An update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013;4(5):295–310. doi: 10.14336/AD.2013.0400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


