Summary
The epigenome is a key determinant of transcriptional output. Perturbations within the epigenome are thought to be a key feature of many, perhaps all cancers, and it is now clear that epigenetic changes are instrumental in cancer development. The inherent reversibility of these changes makes them attractive targets for therapeutic manipulation and a number of small molecules targeting chromatin-based mechanisms are currently in clinical trials. In this perspective we discuss how understanding the cancer epigenome is providing insights into disease pathogenesis and informing drug development. We also highlight additional opportunities to further unlock the therapeutic potential within the cancer epigenome.
Keywords: Cancer, epigenome, targeted therapy, chromatin, histone modification
Introduction
Epigenetics refers to heritable traits that are not attributable to changes in DNA sequence. In more specific terms, it can be used to describe how chromatin-associated proteins and reversible chemical modifications of DNA and histone proteins maintain transcriptional programs by regulating chromatin structure (Strahl and Allis, 2000). Since all cells in a multicellular organism contain essentially the same DNA, the development of specialized cell types requires a tightly regulated process that leads to the expression of genes critical for a specific cell type; and repression of genes required for alternative cell fates. One important process by which cells regulate cell type specific gene expression is the packaging of DNA into chromatin in a fashion that modulates transcriptional output in a given cell type. Therefore, epigenetic regulatory mechanisms are instrumental in dictating cell identities and have been implicated in fundamental processes such as development, stem cell self-renewal, differentiation, genome integrity and proliferation (Tessarz and Kouzarides, 2014). Epigenetic gene regulation is primarily achieved through the collaboration of multiple regulatory pathways; involving sequence specific DNA binding transcription factors, long non-coding RNAs (lncRNAs), ATP-dependent nucleosome remodeling, DNA methylation, introduction of histone variants, and post-translational modification (PTM) of histone proteins. Histone proteins can be modified at specific amino acid residues by diverse chemical moieties including methylation, acetylation, phosphorylation, ubiquitination and SUMOylation (Kouzarides, 2007). Identification of the chromatin regulatory proteins involved in mediating, removing and binding these modifications has expedited our understanding of the biology of many of these PTMs (Figure 1). Moreover, examining the genome-wide localization of histone PTMs, DNA methylation and chromatin regulatory proteins in a wide spectrum of biological contexts has allowed researchers to begin defining “epigenomic landscapes” and how these relate to cellular phenotypes (Bernstein et al., 2010). Combining these data with gene expression profiles has provided significant insights into the biological significance of many of these entities as they relate to gene expression (Brien and Bracken, 2009).
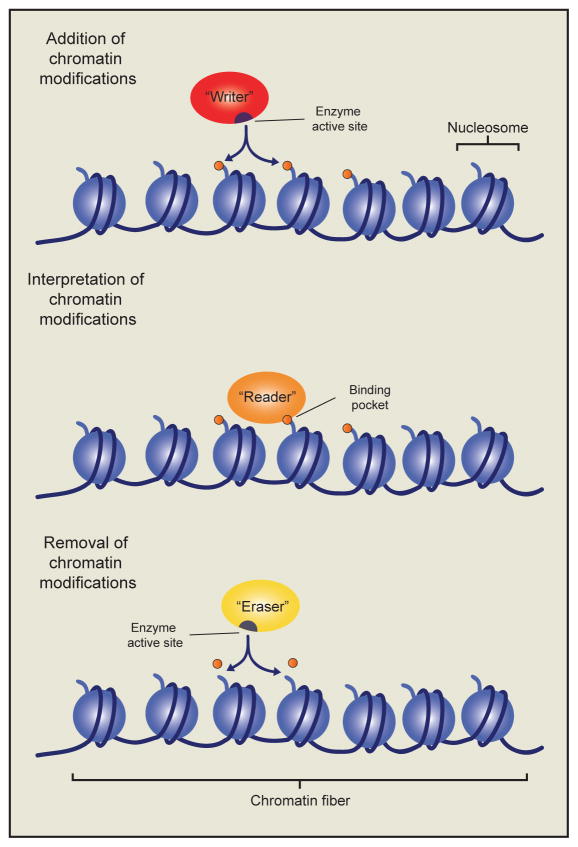
Figure 1. Chromatin writers, readers and erasers in epigenetic gene regulation.
The addition of histone post-translational modifications is catalyzed by a class of enzymes known as chromatin “writers”. The modifications established by these writers may affect gene transcription by altering electrostatic interactions within or between adjacent nucleosomes. Alternatively they may act as binding substrates for another class of chromatin regulators called chromatin “readers”. Chromatin readers employ characteristic binding domains, such as chromo-, bromo-, and PHD-finger domains to bind nucleosomes marked by specific modifications, or a combination of modifications. Chromatin readers may themselves possess additional chromatin modifying activities, or alternatively recruit additional proteins to modify the local chromatin environment. Finally, chromatin “erasers” catalyze the removal of histone modifications, thereby reversing their biochemical effects on the chromatin fiber.
It has been appreciated for some time that deregulation of the epigenomic landscape is a common feature of a number of diseases, including cancer. Some of the earliest observed epigenetic abnormalities in cancer cells were alterations in the patterns of DNA methylation and histone acetylation (Feinberg and Vogelstein, 1983; Fraga et al., 2005; Greger et al., 1989; Sakai et al., 1991). Localized hypermethylation of gene promoter regions leading to transcriptional repression of tumor suppressor genes such as CDKN2A and RB1 were subsequently found to be a common feature of many cancers (Baylin and Jones, 2011). Moreover, DNA hypermethylation events also occur over broad sub-chromosomal domains encompassing multiple tumor suppressor genes (Coolen et al., 2010; Frigola et al., 2006). The chemotherapeutic agents azacitidine (5-azacytidine) and decitabine (5-aza-2′-deoxycytidine); when used at low doses were shown to irreversibly inhibit the enzymatic activity of the DNA methyltransferase enzyme DNMT1 and induce global hypomethylation. These drugs represent the first targeted epigenetic therapies and were FDA approved in 2005 for the treatment of myelodysplastic syndrome (MDS) and are currently recommended as first line treatments for high-risk MDS patients (Issa and Kantarjian, 2009). Global loss of histone acetylation is also a common feature of many cancers (Fraga et al., 2005), and has been associated with unfavorable patient outcomes in certain cases (Seligson et al., 2005). Vorinostat (Suberoylanilide hydroxamic acid – SAHA) which inhibits the activity of histone deacetylase (HDAC) enzymes, leading to global increases in histone acetylation, was granted FDA approval for the treatment of advanced cutaneous T-cell lymphoma in 2006 (Mann et al., 2007; Wagner et al., 2010). Since this initial success, the HDAC inhibitors romidepsin and panobinostat have been approved for use in cutaneous T-cell lymphoma and multiple myeloma, respectively (Khan and La Thangue, 2012; Laubach et al., 2015). Recently, studies that combine DNA methylation and HDAC inhibitors have been initiated; with several clinical trials currently ongoing examining the utility of these combinations in hematopoietic malignancies and solid tumors (Falkenberg and Johnstone, 2014). Moreover, pre-clinical studies have indicated that combination therapies involving HDAC inhibitors and some of the emerging epigenetic therapies may prove clinically beneficial (Fiskus et al., 2014; Mazur et al., 2015).
Until recently it was unclear whether epigenetic changes play a causal role in cancer development or whether they merely are a result of the cancerous state. However, recent cancer genome sequencing studies have shown that genes encoding chromatin regulatory proteins are among the most commonly mutated gene sets in cancer (Garraway and Lander, 2013). In fact, 25–30% of the identified cancer driver mutations affect genes encoding chromatin regulatory proteins (Garraway and Lander, 2013; Vogelstein et al., 2013). These findings indicate that altered epigenetic states do not just correlate with cancer but likely drive disease pathogenesis. This has spurred a significant research effort to understand how altered epigenetic states drive cancer cell phenotypes and how to therapeutically exploit these phenomena. We have only begun to scratch the surface with regard to our mechanistic understanding of the cancer epigenome, but this limited knowledge has already delivered tangible successes with regard to our ability to therapeutically manipulate cancer promoting epigenetic states and cancer associated gene expression programs (Table 1) (Cai et al., 2015).
Table 1.
Selection of targeted epigenetic therapies in use or under clinical investigation in cancer patients
| Chromatin writers | |||||
|---|---|---|---|---|---|
|
| |||||
| Protein | Biological function | Cancer type | Small-molecule(s) | Clinical Trial | References |
| DOT1L | H3K79 methylation | AML, ALL | EPZ-5676 |
NCT01684150 NCT02141828 |
Daigle et al., 2013 |
| EZH2 | H3K27 methylation | Non-Hodgkin lymphoma, SNF5-deficient MRTs | GSK2816126, EPZ-6438, CPI-1205 |
NCT01897571 NCT02082977 NCT02395601 |
McCabe et al., 2012; Knutson et al., 2013 |
| DNMT1 | DNA Methylation (Hemi-methylated CpG sites) | MDS, CMML | Azacitidine, Decitabine | FDA approved | Issa and Kantarjian 2009 |
|
| |||||
| Chromatin Readers | |||||
|
| |||||
| BET-family | Acetyl-lysine binding | AML, MDS, lymphoma, glioblastoma, MNC, multiple myeloma, Breast cancer, NSCLC, Prostate cancer, Pancreatic cancer | OTX015, CPI-0610 |
NCT01713582 NCT01949883 NCT02157636 NCT02158858 NCT02259114 NCT02296476 NCT02303782 |
Filippakopoulos et al., 2010; Delmore et al., 2011; Zuber et al., 2011 |
|
| |||||
| Chromatin erasers | |||||
|
| |||||
| LSD1 | H3K4/K9 demethylation | AML, MDS, SCLC | GSK2879552, Tranylcypromine |
NCT02034123 NCT02177812 NCT02261779 NCT02273102 |
Harris et al., 2012; Schenk et al.; 2012 Mohammad et al., 2015 |
| HDAC1/2/3 | Lysine deacetylation | Cuteanous T cell lymphoma, multiple myeloma | Vorinstat, Romidepsin, Panobinostat | FDA approved | Mann et al., 2007; Khan and La Thangue 2012 |
AML - Acute myeloid leukemia, ALL - Acute lymphoid leukemia, MRT - Malignant rhabdoid tumor, MDS - Myelodysplastic syndrome, CMML - Chronic myeloproliferative leukemia, NMC - NUT midline carcinoma, NSCLC - Non-small cell lung cancer, SCLC - Small cell lung cancer
In this perspective we will discuss how our burgeoning understanding of the cancer epigenome and chromatin regulatory mechanisms are yielding significant insights into the mechanisms underlying disease development; and providing a rational means to identify drug targets for the treatment of certain cancers. This is a very exciting time in the field, with a number of targeted epigenetic therapies in ongoing clinical trials. However, the relatively small number of drug targets identified to date is still a limiting factor and we will also discuss how a more comprehensive approach to studying the cancer epigenome will be necessary in order to truly harness the therapeutic potential therein.
Exploiting oncogenic chromatin activities
Oncogenic chromatin writers
The histone methyltransferase (HMT) EZH2 is subject to recurrent gain-of-function mutations in B-cell lymphoma (Morin et al., 2011, 2010). EZH2 is a lysine methyltransferase and the catalytic subunit of the PRC2 complex; as part of this complex, EZH2 mediates the mono-, di- and tri-methylation of lysine 27 of histone H3 (H3K27me1/2/3) (Conway et al., 2015). EZH2 primarily functions as a transcriptional repressor through deposition of H3K27me3 at the promoter regions of PRC2 target genes (Margueron and Reinberg, 2011). In addition, EZH2 mediated H3K27me2 may also be important for transcriptional repression by maintaining the inactive state of intergenic enhancer elements (Ferrari et al., 2014; Lee et al., 2015). Heterozygous point mutations of EZH2 are found in 22% of diffuse large B-cell lymphoma cases and 10% of follicular lymphoma cases. These mutations affect key residues within the active site of the catalytic Su(var)3-9, enhancer of zeste, trithorax (SET) domain (McCabe et al., 2012; Morin et al., 2010). In vitro analysis has shown that the preferred enzymatic activity of wild type EZH2 is the conversion of H3K27me0-to-me1 and H3K27me1-to-me2, while the enzyme is relatively inefficient at the final conversion step to H3K27me3. However, owing to alterations in substrate binding modality, the lymphoma associated EZH2 SET domain mutants exhibit an enhanced ability to convert H3K27me2-to-me3 (Antonysamy et al., 2013; M. T. McCabe et al., 2012; Sneeringer et al., 2010; Wigle et al., 2011; Yap et al., 2011). This finding suggests that wild type and mutant EZH2 collaborate to push the kinetics of PRC2 activity towards increased H3K27me3 production. Indeed, lymphoma cell lines containing EZH2 gain-of-function mutations exhibit globally increased levels of H3K27me3, with a concomitant decrease in H3K27me2 (McCabe et al., 2012; Yap et al., 2011). Chromatin immunoprecipitation coupled with next generation sequencing (ChIP-seq) for H3K27me3 in an in vivo mouse model of Ezh2-mutant lymphoma demonstrated that mutant EZH2 drives aberrant repression of genes required for cell cycle exit and appropriate differentiation of Germinal Center B-cells via accumulation of H3K27me3 at gene promoter regions (Béguelin et al., 2013). Taken together, these data support the idea that mutant EZH2 directly promotes lymphoma development through alterations in H3K27 methylation levels, leading to a block in Germinal Center B-cell differentiation and enhanced proliferation (Figure 2).
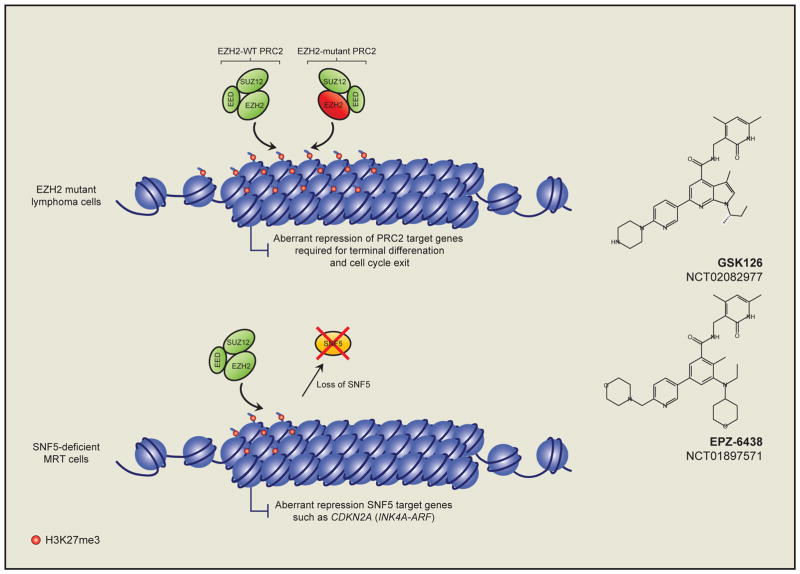
Figure 2. Targeting the oncogenic function of EZH2 in lymphoma and solid tumors.
Heterozygous point mutations of the EZH2 SET domain in non-Hodgkin lymphomas leads to an enhanced accumulation of H3K27me3 on the promoters of PRC2 target genes in Germinal Center B-cells (Upper panel). This causes aberrant silencing of these genes, many of which are required for terminal B-cell differentiation and cell cycle exit. Small-molecule inhibitors (right panels) of EZH2 enzymatic activity are currently in clinical trials for the treatment of lymphoma patients with activating EZH2 mutations. Moreover, these molecules are also in trials for the treatment of SNF5-deficient malignant rhabdoid tumors (MRTs) where SNF5-loss facilitates aberrant EZH2-mediated silencing of SNF5 target genes, such as the tumor suppressor CDKN2A (lower panel).
This observation has made EZH2 enzymatic activity an attractive target for treatment of EZH2-mutant lymphomas. Several highly selective small molecule EZH2 SET domain inhibitors have been developed (Knutson et al., 2012; McCabe et al., 2012; Qi et al., 2012). Interestingly, these molecules were shown to reduce H3K27me3 levels to a similar degree in cells, regardless of their EZH2-mutational status (McCabe et al., 2012; Qi et al., 2012). However, EZH2-mutant cells are acutely sensitive to EZH2 inhibition both in vitro and in vivo, where EZH2 inhibition leads to up-regulation of repressed H3K27me3 marked genes and apoptotic cell death (Béguelin et al., 2013; McCabe et al., 2012). These data lend further support to the notion that aberrant H3K27me3 accumulation is essential for the survival of EZH2-mutant lymphoma cells. Based on these pre-clinical successes, some of these compounds have now entered phase I clinical trials for the treatment of relapsed refractory B-cell lymphoma, and advanced solid tumors (Figure 2, and discussed further below) - NCT02395601, NCT01897571, and NCT02082977 at www.clinicaltrials.gov.
NSD2 (also known as MMSET or WHSC1) encodes an HMT, responsible for mediating di-methylation of H3K36 (H3K36me2). NSD2 primarily functions as a transcriptional activator by depositing intragenic H3K36me2 at active genes (Huang et al., 2013; Kuo et al., 2011), although it may also function in transcriptional repression in limited contexts (Marango et al., 2008). NSD2 was initially discovered because of its involvement in a recurrent chromosomal translocation t(4;14), occurring in 20% of multiple myeloma (MM) cases (Chesi et al., 1998). This translocation places NSD2, and its neighboring gene FGFR3, under the control of the IgH locus Eμ enhancer, leading to increased expression of both genes, though NSD2 appears to be the oncogene in this setting (Keats et al., 2003; Santra et al., 2003). NSD2 is also subject to recurrent gain-of-function mutations in lymphoid malignancies, with the highest prevalence (14%) in pediatric acute lymphoblastic leukemias (ALL) carrying an ETV6-RUNX1 fusion (Jaffe et al., 2013; Oyer et al., 2014). These mutations result in a glutamic acid to lysine switch at position 1099 (E1099K) within the SET domain of NSD2, leading to increased enzymatic activity in vitro. Consistent with this, global levels of H3K36me2 are increased in cells harboring either an E1099K activating NSD2 mutation or a t(4;14) translocation (Huang et al., 2013; Jaffe et al., 2013). Moreover, H3K36me2 ChIP-seq in t(4;14) rearranged MM cells demonstrates that H3K36me2 marked domains undergo a broad expansion into intergenic space as a result of increased NSD2 expression (Kuo et al., 2011; Popovic et al., 2014). Consistent with the fact that higher order H3K36 methylation directly inhibits the enzymatic activity of the PRC2 complex (Schmitges et al., 2011; Yuan et al., 2011), this expansion of H3K36me2 is accompanied by global losses of H3K27me2/3 (Jaffe et al., 2013). While the exact mechanism by which NSD2 drives malignancy remains unclear, the broad gain of H3K36me2 and loss of repressive H3K27 methylation may lead to an opening of the chromatin landscape, creating a more permissive or “stem cell-like” state which is more amenable to additional reprogramming by transcription factors (Nimura et al., 2009). Consistent with this, expression profiling of t(4;14) MM cells revealed that these cells have increased expression of genes associated with self-renewal, and reduced expression of genes associated with mature B-cell functions (Huang et al., 2013; Kuo et al., 2011; Popovic et al., 2014). Interestingly, despite the global loss of H3K27 methylation, H3K27me3 is focally increased at certain gene promoters, many of which correlate with aberrantly repressed B-cell differentiation genes (Popovic et al., 2014). Together these findings suggest that NSD2 activation drives a broad rewiring of the epigenome, which may in turn block terminal differentiation thereby contributing to oncogenesis.
Several studies have demonstrated that the activity of the NSD2 SET domain is essential for the observed chromatin/transcriptional changes and tumorigenicity in MM and lymphoma cells (Jaffe et al., 2013; Kuo et al., 2011). This supports the notion that the changes in H3K36me2 are instrumental for development of these cancers and suggests that targeting the NSD2 SET domain with a specific small-molecule would be an excellent therapeutic approach. However, to date no potent inhibitors of NSD2 have been published (Cain, 2013). In addition to targeting the NSD2 SET domain, a number of recent studies have demonstrated that certain chromatin reading domains in NSD2 are essential for the oncogenic function of the protein (Huang et al., 2013; Popovic et al., 2014). For example, NSD2 protein fragments lacking functionality in the second, third or fourth PHD-finger domain or the second PWWP-domain have reduced transforming activity. These domains potentially mediate the interaction of NSD2 with chromatin, or with ancillary protein cofactors that are important for NSD2 function. It will be important to further elucidate the function of these domains; since they too may be viable drug targets for the treatment of NSD2 activated cancers.
Oncogenic chromatin readers
NUT midline carcinoma (NMC) is an aggressive form of squamous cell cancer that occurs in all age groups and NMC patients have a dismal average overall survival of just 6.7 months (French, 2014). This disease is characterized by translocations of the NUT gene, which typically fuse NUT to one of two bromodomain and extra terminal (BET) family genes, BRD4 or BRD3. Together these translocations, BRD4-NUT t(15;19) and BRD3-NUT t(15;9), account for approximately 80% of all NMC cases. Alternatively NUT may also be indirectly linked to BRD4, through fusion to the BRD4 associated protein NSD3 - NSD3-NUT t(15;8) (French et al., 2014; Shen et al., 2015). This propensity for linkage of NUT to BET family proteins suggests that their collaborative function is important for disease pathogenesis. The function of wild type NUT is unclear, though it has been reported to interact with the lysine acetyltransferases p300/CBP and enhance their activity (Reynoird et al., 2010). BET family proteins are acetyl-lysine readers, that promote transcription by interacting with, and recruiting the positive transcription elongation factor (P-TEFb) and Mediator complexes to chromatin (Jang et al., 2005; Jiang et al., 1998; Lovén et al., 2013; Yang et al., 2005). BRD-NUT fusion proteins bind chromatin in distinctive foci associated with elevated levels of histone acetylation (French et al., 2008). Recent ChIP experiments demonstrate that these foci correspond to broad “megadomains” of BRD-NUT binding, ranging from 100KB–1.4MB in size, typically delimited by topological chromatin domains (Alekseyenko et al., 2015). Moreover, these megadomains are also associated with elevated binding of pTEFb and Mediator components (Wang and You, 2015). Several genes within these megadomains such as MYC (Grayson et al., 2014), TP63 and MED24 (Alekseyenko et al., 2015) exhibit increased expression in a BRD-NUT dependent manner and are essential for maintaining the undifferentiated and highly proliferative state of NMC cells. Together these data suggest that BRD-NUT megadomains promote the expression of genes that drive NMC pathogenesis.
Interestingly, the formation of these megadomains requires the function of both the BET bromodomains and the acetyltransferase activity of p300/CBP (Reynoird et al., 2010). This suggests a model wherein p300/CBP mediated histone acetylation generates a binding substrate for BET bromodomains, leading to spreading of BRD-NUT into the surrounding the chromatin fiber (Alekseyenko et al., 2015; French, 2014). The fact that BET bromodomains are critical for this process has been therapeutically exploited using the small-molecule BET inhibitor JQ1 (Filippakopoulos et al., 2010). Treatment of NMC cell lines with JQ1 induces a rapid differentiation and proliferation arrest. Moreover, JQ1 treatment improves survival in murine xenograft models. Based on these pre-clinical successes, several derivatives of JQ1 with improved pharmacodynamic properties are currently in clinical trials for the treatment of NMC and other cancers (discussed further below) - NCT01587703, NCT01713582, NCT01949883, NCT01987362, and NCT01943851 at www.clinicaltrials.gov.
Exploiting non-oncogene chromatin dependencies
Chromosomal rearrangements at 11q23 are associated with acute leukemia which led to the discovery of the MLL1 gene (Djabali et al., 1992; Gu et al., 1992; Tkachuk et al., 1992; Ziemin-van der Poel et al., 1991). MLL1 translocations are found in ~10% of patients with acute leukemia and are generally associated with an unfavorable prognosis (Krivtsov and Armstrong, 2007; Muntean and Hess, 2012). MLL1 rearrangements are more frequently present in infant and pediatric patients, with the highest frequency of 80% in infant ALL (Greaves, 1996). MLL1 is primarily thought to function as a transcriptional activator at least in part through tri-methylation of H3K4 (H3K4me3) at the promoters of active genes. In MLL-rearranged leukemia the N-terminus of MLL1 is fused to one of over 70 different fusion partners; however, MLL-AF4 t(4:11), MLL-AF9 t(9:11), MLL-ENL t(11;19), MLL-AF10 t(10;11) and MLL-ELL t(11;19) are the most common translocations, collectively accounting for the vast majority (>80%) of MLL-rearrangements seen in patients (Krivtsov and Armstrong, 2007). MLL-rearranged leukemias exhibit a strikingly low rate of somatic mutation (Andersson et al., 2015), supporting the notion that MLL-fusions are the primary drivers of these leukemias. Despite the loss of the C-terminal SET domain of MLL1 in MLL-fusions, genome-wide levels of H3K4me3 are not reduced in MLL-fusion leukemia cells; suggesting that alternative H3K4 methyltransferase(s) maintain H3K4 methylation in these cells (Bernt et al., 2011; Mishra et al., 2014). Indeed recent evidence suggests that maintaining H3K4 methylation levels in MLL-fusion leukemia stem cells may be important for the leukemic potential of these cells (Wong et al., 2015). This highlights the importance of identifying the H3K4 methyltransferase(s) involved in maintaining H3K4 methylation levels in MLL-fusion leukemia. Some of the first hints toward the mechanisms by which MLL-fusion proteins mediate leukemogenesis came from the realization that a number of the most common MLL-fusion partners exist in overlapping protein complexes whose functions are associated with transcriptional elongation (Deshpande et al., 2012). The HMT DOT1L, which mediates mono-, di- and tri-methylation of H3K79 (H3K79me1/2/3), is a component of several of these complexes and perturbations in normal H3K79me2 patterns are a feature of MLL-rearranged leukemia (Guenther et al., 2008; Krivtsov et al., 2008). DOT1L-mediated deposition of H3K79me2 downstream of active gene promoters is believed to be important for efficient transcriptional elongation and active gene expression (Nguyen and Zhang, 2011). Subsequent studies demonstrated that the aberrant levels of H3K79me2 in MLL-rearranged leukemia accumulate specifically at MLL-fusion target genes, indicating that this phenomenon is likely driven by MLL-fusion protein mediated recruitment of DOT1L to these sites (Bernt et al., 2011) (Figure 3). These genes include several key regulators of hematopoietic stem cell identity, such as HOXA cluster genes and MEIS1. It has recently been shown that a critical function of DOT1L mediated H3K79 methylation is to inhibit repressive mechanisms that would otherwise silence MLL-fusion driven gene expression (Chen et al., 2015). These data support the hypothesis that MLL-fusion proteins cause a block in hematopoietic cell differentiation by preventing repression of stem cell regulators.
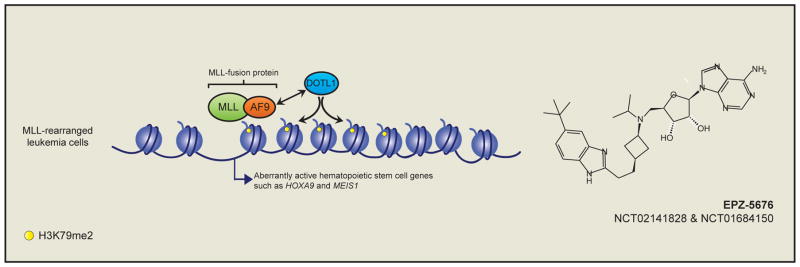
Figure 3. Targeting MLL-rearranged leukemia through co-opted DOT1L activity.
Studies have demonstrated that MLL-fusion proteins, such as MLL-AF9, recruit aberrant chromatin activity in the form of DOT1L mediated H3K79me2 to the promoter of MLL-fusion target genes in primitive hematopoietic cells. DOT1L activity is essential for the expression of MLL-fusion target genes such as HOXA9 and MEIS1, and this requirement has been exploited through the development of small-molecule inhibitors of DOT1L enzymatic activity (Right panel). DOT1L inhibitors are currently in clinical trials for the treatment of MLL-rearranged leukemia.
Genetic inactivation of Dotl1 in a mouse model of MLL-AF9 driven leukemia led to prolonged survival, demonstrating that DOT1L-mediated H3K79me2 is important for leukemogenesis (Bernt et al., 2011; Jo et al., 2011; Nguyen et al., 2011). Strikingly, Dot1l inactivation specifically results in downregulation of MLL-AF9 target genes, suggesting that inhibiting DOT1L may provide a specific means to target these leukemias. Parallel studies demonstrated that pharmacological inhibition of DOT1L enzymatic activity with a small-molecule inhibitor reduced H3K79me2 levels in vitro and in vivo, leading to reduced expression of MLL-AF9 target genes (Daigle et al., 2011) (Figure 3). These early successes lead to the development of an improved DOT1L inhibitor (Daigle et al., 2013), which is currently in ongoing clinical trials for the treatment of patients with MLL-rearranged leukemia - NCT02141828 and NCT01684150 at https://clinicaltrials.gov.
Until the discovery of the first histone demethylase LSD1 (also known as KDM1A) histone demethylation was thought to be a passive process, accomplished through dilution of methylated histones during cell division. LSD1 is a member of the FAD-dependent monooxidase family of enzymes and is primarily responsible for mediating the demethylation of the mono- and di-methylated forms of H3K4 and H3K9 (H3K4me1/me2 and H3K9me1/me2) (Metzger et al., 2005; Shi et al., 2004). Interest in LSD1 as a potential therapeutic target in cancer was spurred by observations of increased LSD1 expression in several cancers including neuroblastoma (Schulte et al., 2009), prostate cancer (Kahl et al., 2006), bladder cancer (Kauffman et al., 2011), colorectal and lung cancer (Hayami et al., 2011; Mohammad et al., 2015) and certain hematopoietic cancers (Harris et al., 2012). Significantly, recent studies using both genetic inactivation and pharmacologic inhibition of LSD1 in the context of AML have provided evidence for a functional link between LSD1 and leukemia (Harris et al., 2012; Schenk et al., 2012). Moreover, LSD1 inhibition can sensitize AML cells to all-trans-retinoic acid (ATRA) induced differentiation, and combining LSD1 inhibition with ATRA treatment reduces engraftment of primary AML patient samples in xenograft models (Schenk et al., 2012). Interestingly, LSD1 inhibition is not associated with global increases in either H3K4me1/2 or H3K9me1/2 levels across the genome; and the underlying epigenetic mechanism(s) of how LSD1 inhibition affects leukemia cell phenotypes is still unclear. This is confounded by the fact that focal decreases, as well as increases in H3K4me2, are apparent in LSD1 inhibitor treated leukemia cells (Harris et al., 2012; Schenk et al., 2012). LSD1 inhibition has also recently been shown to have clinical efficacy in small cell lung cancer (SCLC) (Mohammad et al., 2015). As with AML, LSD1 inhibition in the setting of SCLC does not have a global effect on H3K4me1/2 levels. Moreover, LSD1 inhibition results in generally non-overlapping transcriptional changes in sensitive cell lines. As such a clear mechanistic understanding of the role of LSD1 in sustaining leukemia and lung cancer cells is currently lacking. However, despite the lack of a clear mechanistic understanding of the role of LSD1 in leukemia and SCLC these pre-clinical successes have lead to LSD1 inhibitors entering clinical trials for the treatment of these diseases – NCT02273102, NCT02177812, NCT02261779 and NCT02034123 at https://clinicaltrials.gov.
Exploiting transcriptional addictions
A number of recent studies have demonstrated that several chromatin regulatory proteins, namely BRD4, CDK7 and CDK9, all of which play general roles in transcriptional activation, are specifically required for cancer cell survival in certain contexts (Chipumuro et al., 2014; Christensen et al., 2014; Dawson et al., 2011; Delmore et al., 2011; Kwiatkowski et al., 2014; Wang et al., 2015; Zuber et al., 2011). As mentioned above, BRD4 binds acetylated histones and modulates the localization and function of transcriptional complexes such as pTEFb and Mediator. CDK7 and CDK9 are cyclin-dependent kinases and components of the TFIIH and pTEFb complexes, respectively. Within these complexes CDK7 and CDK9 promote transcription by phosphorylating RNA polymerase II (RNAPII), thereby enabling active transcriptional initiation and elongation (Larochelle et al., 2012; Zhou et al., 2012). Given the general role of BRD4, CDK7 and CDK9 in facilitating transcription, the fact that cancer cells show a greater dependency on these proteins is somewhat surprising, but there is growing evidence that cancer cells may be significantly addicted to high rates of transcription (Lin et al., 2012). Interestingly, an overarching theme emerging from these studies is that inhibiting these proteins leads to preferential downregulation of super-enhancer associated genes (Chipumuro et al., 2014; Kwiatkowski et al., 2014; Lovén et al., 2013; Shi and Vakoc, 2014; Wang et al., 2015). Super-enhancers are clusters of enhancer elements that activate the expression of genes required for cell identity; and in cancer cells, super-enhancers also drive the expression of key oncogenes (Lovén et al., 2013). ChIP-seq studies have demonstrated that BRD4 and CDK7 are preferentially bound at super-enhancers (Chipumuro et al., 2014; Lovén et al., 2013), and recent work indicates that mutations in enhancer elements, or oncogenic transcription factors may drive the formation of super-enhancers in tumor cells (Mansour et al., 2014; Oldridge et al., 2015). Taken together, these findings suggest that inhibiting certain components of the general transcriptional machinery provides a means to target transcriptional addictions to oncogenic transcription factors such as MYC (Shi and Vakoc, 2014), MYCN (Chipumuro et al., 2014), TAL1 (Kwiatkowski et al., 2014) and others (Christensen et al., 2014). A critical next step will be to understand how these addictions develop and to determine which specific components of the transcriptional machinery are required in which cancer types.
Exploiting chromatin based mechanisms of acquired drug resistance
A significant clinical challenge in cancer treatment is the emergence of therapeutic resistance. Rewiring of signaling programs and epigenomic changes are increasingly being recognized as mechanisms of acquired drug resistance (Brown et al., 2014; Holohan et al., 2013; Sharma et al., 2010). For example, breast cancers with activated PI3K and ERBB2 signaling are typically treated with kinase inhibitors targeting these pathways. However, resistance often emerges owing to upregulation of alternative receptor tyrosine kinase (RTK) genes which compensate for the inhibited enzyme (Azuma et al., 2011; Britschgi et al., 2012; Chandarlapaty et al., 2011; Rexer et al., 2011). Combination therapies targeting multiple RTKs can in some instances circumvent resistance, but the multitude of upregulated RTKs limits the effectiveness of this approach. Recent work has demonstrated that combining PI3K or ERBB2 inhibitors with BET bromodomain inhibition re-sensitizes previously resistant breast cancer cells to kinase inhibition (Stratikopoulos et al., 2015; Stuhlmiller et al., 2015). The effectiveness of adding BET inhibitors in this context can be explained by increased BRD4 binding at the promoter/enhancer regions of transcriptionally activated RTKs in response to PI3K inhibition, suggesting that BRD4 facilitates the upregulation of alternative RTKs (Stratikopoulos et al., 2015). Moreover, BRD4 also appears to be important for transcriptional activation of the downstream target genes of these RTKs (Stuhlmiller et al., 2015), further explaining the utility of BET inhibitors in this setting. BRD4 inhibition may also have utility in prostate cancers resistant to androgen receptor (AR) inhibition. Sustained AR signaling is a primary driver of castration-resistant prostate cancer, and inhibition of AR signaling is used as a therapeutic approach in this subset of patients. Unfortunately, these patients often develop resistance to AR inhibitors and invariably succumb to disease (Watson et al., 2015). BRD4 directly interacts with the N-terminus of the AR in a bromodomain dependent manner, and AR signaling-positive prostate cancer cells are exquisitely sensitive to BET inhibition (Asangani et al., 2014). BRD4 and AR co-localize on chromatin and BET inhibition leads to a robust downregulation of AR target genes, suggesting that BET inhibition might be an approach to circumvent acquired resistance to AR inhibitors. Another example of resistance mediated by epigenetic changes concerns patients with T-cell acute lymphoblastic leukemia (T-ALL) driven by activating mutations in NOTCH1. Small molecule γ–secretase inhibitors (GSI) block NOTCH1 signaling in T-ALL lymphoblasts, but response to these inhibitors is only transient, suggesting that resistance limits their clinical efficacy (Palomero and Ferrando, 2009). GSI-resistant cells exhibit increased BRD4 binding on chromatin compared to GSI-naive cells, and maintain MYC expression via alternative enhancer usage at the MYC locus, independent of NOTCH1 (Knoechel et al., 2014; Yashiro-Ohtani et al., 2014). This renders GSI resistant cells markedly more sensitive to JQ1 treatment, and in vivo GSI-JQ1 combination therapy is significantly more effective against primary human TALL than treatment with either single agent (Knoechel et al., 2014). These findings demonstrate that acquired drug resistance may in fact be commonly driven by epigenetic changes, which may be addressed by incorporating epigenetic modulators in combination therapies.
A number of recent studies have identified mechanisms of acquired resistance to novel epigenetic therapies. For example, resistance to the BET bromodomain inhibitors JQ1 and I-BET in MLL-AF9 AML cells is mediated in part by alternative enhancer usage at the MYC locus; which maintains expression levels of MYC in resistant cell lines (Fong et al., 2015; Rathert et al., 2015). Interestingly, this appears to be downstream of the WNT signaling pathway and inhibition of WNT signaling may provide a means to circumvent resistance in this setting (Fong et al., 2015). Significantly, a distinct mechanism of BET inhibitor resistance has been described in triple-negative breast cancer cells (Shu et al., 2016). In this setting, resistant cells accumulate increased levels of phosphorylated BRD4, which appears to be crucial for the acquisition of resistance. Despite being bound by JQ1 phosphorylated BRD4 remains on chromatin indicating that the association of BRD4 with chromatin in this context is bromodomain independent. It will be important to further characterize the underlying mechanism(s) involved in this resistance phenotype; particularly in the context of the signaling pathway(s) driving BRD4 phosphorylation since modulation of these pathways may re-sensitize cells to BET inhibition. Taken together, these studies demonstrate that non-overlapping, and perhaps context dependent mechanisms can contribute to acquired resistance to a single epigenetic therapy; illustrating the need to study resistance mechanisms in multiple cancer models. Resistance to EZH2 inhibitors has also recently been described in EZH2 gain-of-function lymphoma cells (Gibaja et al., 2015). This resistance was shown to be mediated by the acquisition of distinct mutations in the mutant (Y661D) and wildtype (Y111L) EZH2 alleles, respectively. These mutations render the PRC2 complex insensitive to treatment with EZH2 inhibitors, potentially by reducing the ability of these compounds to bind EZH2 (Gibaja et al., 2015). Studying the underlying mechanisms of acquired resistance to epigenetic therapies is of the utmost importance as it will be essential for our ability circumvent these clinical barriers. Moreover, understanding these mechanisms may also provide a means of predicting therapeutic responses based on the identification of biomarkers of resistance/sensitivity.
Identifying additional therapeutic opportunities within the cancer epigenome
The majority of somatic mutations affecting chromatin regulatory proteins in cancer are loss-of-function mutations leading to a reduction, or complete loss of chromatin modifying activities (Garraway and Lander, 2013). However, our ability to intervene therapeutically in this context is currently limited since it is often unclear what mechanisms are driving oncogenesis. The demonstration of “epigenetic antagonism” between the SWI/SNF and PRC2 complexes in cancer cells provides an interesting concept to consider in this context (Knutson et al., 2013; Wilson et al., 2010). The SWI/SNF complex is an ATP-dependent chromatin-remodeling complex that primarily functions in transcriptional activation. SWI/SNF activity plays important tumor suppressive roles in vivo and many SWI/SNF components are inactivated in cancer (Kadoch and Crabtree, 2015; Kadoch et al., 2013). In Drosophila melanogaster SWI/SNF genes were discovered as antagonists of PRC2 mediated gene repression (Kennison and Tamkun, 1988; Tamkun et al., 1992). This antagonism is conserved in mammals where SWI/SNF components directly oppose PRC2 mediated gene repression (Kia et al., 2008). In cancer, loss of SNF5 (also known as SMARCB1 or BAF47), which encodes a SWI/SNF component, leads to elevated levels of H3K27me3 genome-wide, suggesting that SNF5-deficient cancers may rely on EZH2-mediated H3K27me3 to drive tumorigenesis. Roberts and colleagues demonstrated that this is indeed the case as concurrent loss of Ezh2 and Snf5 is not compatible with cancer development in mice (Wilson et al., 2010). This finding has been exploited therapeutically using EZH2 SET domain inhibitors to treat SNF5-deficient rhabdoid tumors, which are markedly more sensitive to this treatment than SNF5 wild type cancers (Knutson et al., 2013) (Figure 2). Moreover, EZH2 inhibitor treatment of non-small cell lung cancer cells with mutations in another SWI/SNF component gene BRG1 (also known as SMARCA4) sensitizes these cells to chemotherapy, an effect that was not apparent in BRG1 wild type cells (Fillmore et al., 2015). These are significant observations given the fact that approximately 20% of all cancers contain inactivating mutations in SWI/SNF component genes (Kadoch et al., 2013); and suggest that EZH2 inhibitors may have broad clinical utility outside of merely an EZH2 gain-of-function context. Following these observations, EZH2 inhibitors are now entering clinical trials for the treatment of SNF5-deficient tumors - NCT02601937 and NCT02601937 https://clinicaltrials.gov.
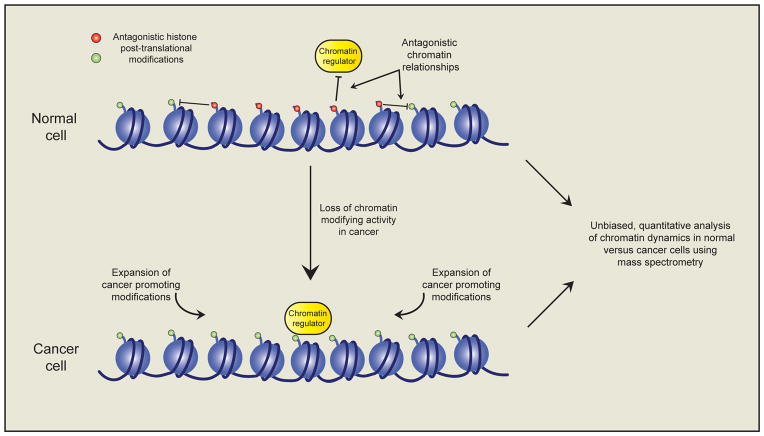
The central tenet that has emerged here is how loss of a single chromatin modifying activity may facilitate an increase in another, or perhaps multiple, functionally antagonistic activities which themselves promote oncogenesis (Figure 4). This highlights the need to expand our understanding of the inter-connectedness of the epigenome, and how loss (and gain) of a single activity in cancer cells impacts the epigenome at large. This knowledge will shed light on mechanisms of disease pathogenesis and allow us to identify additional therapeutic opportunities. Recent advancements in sensitivity and accuracy of mass spectrometry and the introduction of quantitative labeling techniques such as SILAC and iTRAQ is now allowing for the molecular characterization of global epigenetic signatures (Britton et al., 2011). The use of these quantitative techniques has already identified antagonistic relationships involving cancer relevant histone methylation at H3K27 and H3K36 (Jaffe et al., 2013; Pasini et al., 2010; Yuan et al., 2011). For example, loss of PRC2 mediated H3K27 methylation that occurs due to mutation or deletion of PRC2 components in certain hematopoietic malignancies, malignant peripheral nerve sheath tumors (MPNSTs), melanomas and glioblastomas; leads to global increases in H3K27 acetylation (H3K27ac) and H3K36me1/2/3 (De Raedt et al., 2014; Ernst et al., 2010; Jaffe et al., 2013; Lee et al., 2014; Nikoloski et al., 2010; Ntziachristos et al., 2012; Zhang et al., 2012, 2014). The functional role of increased H3K36 methylation in a PRC2-deficient context has yet to be addressed; however, ChIP-seq studies have demonstrated that the increases in H3K27ac typically occur at previously silenced gene enhancer elements, potentially leading to inappropriate enhancer activity and transcriptional activation of associated genes (Ferrari et al., 2014). Consistent with this, transcriptional profiling of PRC2-deficient MPNST and T-ALL cells demonstrates that these cancers have characteristic expression signatures involving aberrant activation of PRC2 target genes with associated increases in H3K27ac and H3K9ac (Lee et al., 2014; Ntziachristos et al., 2012). Re-introduction of PRC2 activity into PRC2-deficient MPNST cells leads to silencing of these aberrantly active genes, which is associated with loss of H3K27ac and cell growth arrest. Taken together, these findings suggest that increases in H3K27ac in response to PRC2-loss may be instrumental in driving oncogenic gene expression programs. This implicates H3K27ac as a potential therapeutic target for the treatment of PRC2-deficient cancers. The use of quantitative mass spectrometry to define global epigenomic changes in cancer cells will yield significant mechanistic insights into the role of altered epigenomic states in cancer development (Creech et al., 2014; Stunnenberg and Vermeulen, 2011) (Figure 4). Moreover, this will provide a rationale for therapeutically targeting such modifications.
Figure 4. Defining the epigenomic changes in cancer.
The alteration of a single chromatin modifying activity in cancer cells is known to have profound affects on the landscape of additional related chromatin modifications and other chromatin regulators. These alterations are likely instrumental in disease pathogenesis; however until recently our ability to systematically annotate such changes has been limited. The use of unbiased quantitative mass spectrometry techniques holds great promise for the annotation of chromatin dynamics in response to cancer promoting alterations in epigenetic pathways. This may facilitate the identification of additional therapeutic targets within the cancer epigenome.
Perspectives
The manipulation of cancer-promoting epigenetic states holds significant potential for the development of new cancer therapies. However, our understanding of the epigenomic alterations that promote oncogenesis and their influences on chromatin-based mechanisms are still limited. Additional effort is needed to deliver on this therapeutic promise. Unbiased analyses of the epigenomic changes in cancer cells will provide significant insights into the chromatin alterations that are instrumental in disease pathogenesis. Additionally, the use of chromatin-focused genomic screening techniques, particularly those utilizing the CRISPR/Cas9-mediated genome editing technology, are already facilitating the identification of epigenetic dependencies in cancer cells (Shi et al., 2015; Zuber et al., 2011). Application of these approaches will be important for the identification of additional therapeutic targets within the cancer epigenome. Moreover, their use in the context of drug resistance will facilitate the annotation of mechanisms related to drug sensitivity (Chen et al., 2015) and thereby aid the rational design of treatment regimens to circumvent the emergence of drug resistant cells. Structure based drug design towards the development of small-molecule therapeutics is often hampered by difficulties producing high-resolution structures for target molecules using traditional methods such as X-ray crystallography. However, recent advancements in cryogenic-electron microscopy (cryo-EM) techniques are revolutionizing the structural biology field and may well soon help alleviate this bottleneck (Nogales, 2016). Moreover, novel approaches to drug design such as the use of phthalimide conjugates to mediate proteosomal degradation of target proteins may overcome several limitations of conventional small-molecules (Winter et al., 2015). The mechanistic dissection of altered epigenomic states in cancer cells will undoubtedly accelerate the development of clinical strategies to manipulate these phenomena. In the next 10 years we will surely see the identification of new context-specific drug targets; complimented by the development of novel small-molecule therapeutics. As the field matures, this interface of basic and clinical research will hopefully allow us to thoroughly exploit the therapeutic potential of the cancer epigenome and provide curative options for cancer patients.
Acknowledgments
We thank members of the Armstrong laboratory for helpful discussions during the preparation of the manuscript. Work in the Armstrong lab is supported by grants from the National Institutes of Health (CA176745, CA140575 and CA66996) and the Leukemia and Lymphoma Society. Support is also provided from the National Institutes of Health grant P30CA008748 to Memorial Sloan Kettering Cancer Center. GLB is supported by an EMBO Long-Term Fellowship (ALTF 1235-2015) with additional support from Marie Curie Actions. DGV is supported by a CURE Childhood Cancer Research Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alekseyenko Aa, Walsh EM, Wang X, Grayson AR, Hsi PT, Kharchenko PV, Kuroda MI, French Ca. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. 2015:1507–1523. doi: 10.1101/gad.267583.115.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson AK, Ma J, Wang J, Chen X, Gedman AL, Dang J, Nakitandwe J, Holmfeldt L, Parker M, Easton J, Huether R, Kriwacki R, Rusch M, Wu G, Li Y, Mulder H, Raimondi S, Pounds S, Kang G, Shi L, Becksfort J, Gupta P, Payne-Turner D, Vadodaria B, Boggs K, Yergeau D, Manne J, Song G, Edmonson M, Nagahawatte P, Wei L, Cheng C, Pei D, Sutton R, Venn NC, Chetcuti A, Rush A, Catchpoole D, Heldrup J, Fioretos T, Lu C, Ding L, Pui CH, Shurtleff S, Mullighan CG, Mardis ER, Wilson RK, Gruber Ta, Zhang J, Downing JR. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet. 2015;47:1–147. doi: 10.1038/ng.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy S, Condon B, Druzina Z, Bonanno JB, Gheyi T, Zhang F, MacEwan I, Zhang A, Ashok S, Rodgers L, Russell M, Luz JG. Structural context of disease-associated mutations and putative mechanism of autoinhibition revealed by X-Ray crystallographic analysis of the EZH2-SET domain. PLoS One. 2013;8:1–15. doi: 10.1371/journal.pone.0084147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani Ia, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke J, Wilder-Romans K, Dhanireddy S, Engelke C, Iyer MK, Jing X, Wu YM, Cao X, Qin ZS, Wang S, Feng FY, Chinnaiyan AM. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K, Tsurutani J, Sakai K, Kaneda H, Fujisaka Y, Takeda M, Watatani M, Arao T, Satoh T, Okamoto I, Kurata T, Nishio K, Nakagawa K. Switching addictions between HER2 and FGFR2 in HER2-positive breast tumor cells: FGFR2 as a potential target for salvage after lapatinib failure. Biochem Biophys Res Commun. 2011;407:219–24. doi: 10.1016/j.bbrc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. A decade of exploring the cancer epigenome — biological and translational implications. Nucleus. 2011 doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguelin W, Popovic R, Teater M, Jiang Y, Bunting KL, Rosen M, Shen H, Yang SN, Wang L, Ezponda T, Martinez-Garcia E, Zhang H, Zheng Y, Verma SK, McCabe MT, Ott HM, Van Aller GS, Kruger RG, Liu Y, McHugh CF, Scott DW, Chung YR, Kelleher N, Shaknovich R, Creasy CL, Gascoyne RD, Wong KK, Cerchietti L, Levine RL, Abdel-Wahab O, Licht JD, Elemento O, Melnick AM. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–92. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, Kellis M, Marra MA, Beaudet AL, Ecker JR, Farnham PJ, Hirst M, Lander ES, Mikkelsen TS, Thomson JA. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol. 2010;28:1045–1048. doi: 10.1038/nbt1010-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, Pollock RM, Richon VM, Kung AL, Armstrong SA. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien GL, Bracken AP. Transcriptomics: unravelling the biology of transcription factors and chromatin remodelers during development and differentiation. Semin Cell Dev Biol. 2009;20:835–841. doi: 10.1016/j.semcdb.2009.07.010. S1084-9521(09)00162-1 [pii] [DOI] [PubMed] [Google Scholar]
- Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Müller U, Murakami M, Radimerski T, Bentires-Alj M. JAK2/STAT5 Inhibition Circumvents Resistance to PI3K/mTOR Blockade: A Rationale for Cotargeting These Pathways in Metastatic Breast Cancer. Cancer Cell. 2012;22:796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Britton LMP, Gonzales-Cope M, Zee BM, Garcia BA. Breaking the histone code with quantitative mass spectrometry. Expert Rev Proteomics. 2011;8:631–43. doi: 10.1586/epr.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Curry E, Magnani L, Wilhelm-Benartzi CS, Borley J. Poised epigenetic states and acquired drug resistance in cancer. Nat Rev Cancer. 2014;14:747–53. doi: 10.1038/nrc3819. [DOI] [PubMed] [Google Scholar]
- Cai SF, Chen CW, Armstrong SA. Drugging Chromatin in Cancer: Recent Advances and Novel Approaches. Mol Cell. 2015;60:561–570. doi: 10.1016/j.molcel.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. NSD2 momentum. Sci Exch. 2013;6 doi: 10.1038/scibx.2013.1083. doi: 10.1038/scibx.2013.1083. [DOI] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-W, Koche RP, Sinha AU, Deshpande AJ, Zhu N, Eng R, Doench JG, Xu H, Chu SH, Qi J, Wang X, Delaney C, Bernt KM, Root DE, Hahn WC, Bradner JE, Armstrong Sa. DOT1L inhibits SIRT1-mediated epigenetic silencing to maintain leukemic gene expression in MLL-rearranged leukemia. Nat Med. 2015:1–10. doi: 10.1038/nm.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesi M, Nardini E, Lim RS, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025–3034. [PubMed] [Google Scholar]
- Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A, Perez-Atayde A, Wong KK, Yuan GC, Gray NS, Young RA, George RE. CDK7 Inhibition Suppresses Super-Enhancer-Linked Oncogenic Transcription in MYCN-Driven Cancer. Cell. 2014;159:1126–1139. doi: 10.1016/j.cell.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T, Chipumuro E, Herter-Sprie GS, Akbay EA, Altabef A, Zhang J, Shimamura T, Capelletti M, Reibel JB, Cavanaugh JD, Gao P, Liu Y, Michaelsen SR, Poulsen HS, Aref AR, Barbie DA, Bradner JE, George RE, Gray NS, Young RA, Wong KK. Targeting Transcriptional Addictions in Small Cell Lung Cancer with a Covalent CDK7 Inhibitor. Cancer Cell. 2014;26:909–922. doi: 10.1016/j.ccell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E, Healy E, Bracken AP. PRC2 mediated H3K27 methylations in cellular identity and cancer. Curr Opin Cell Biol. 2015;37:42–48. doi: 10.1016/j.ceb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Coolen MW, Stirzaker C, Song JZ, Statham AL, Kassir Z, Moreno CS, Young AN, Varma V, Speed TP, Cowley M, Lacaze P, Kaplan W, Robinson MD, Clark SJ. Consolidation of the cancer genome into domains of repressive chromatin by long-range epigenetic silencing (LRES) reduces transcriptional plasticity. Nat Cell Biol. 2010;12:235–246. doi: 10.1038/ncb2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech AL, Taylor JE, Maier VK, Wu X, Feeney CM, Udeshi ND, Peach SE, Boehm JS, Lee JT, Carr Sa, Jaffe JD. Building the Connectivity Map of epigenetics: Chromatin profiling by quantitative targeted mass spectrometry. Methods. 2014;72:57–64. doi: 10.1016/j.ymeth.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle SR, Olhava EJ, Therkelsen Ca, Basavapathruni A, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, Waters NJ, Chesworth R, Moyer MP, Copeland Ra, Richon VM, Pollock RM. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–1025. doi: 10.1182/blood-2013-04-497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle SR, Olhava EJ, Therkelsen Ca, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, Jin L, Kuntz KW, Chesworth R, Moyer MP, Bernt KM, Tseng JC, Kung AL, Armstrong Sa, Copeland Ra, Richon VM, Pollock RM. Selective Killing of Mixed Lineage Leukemia Cells by a Potent Small-Molecule DOT1L Inhibitor. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung C, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, Beinke S, Chapman TD, Roberts EJ, Soden PE, Auger KR, Mirguet O, Doehner K, Delwel R, Burnett AK, Jeffrey P, Drewes G, Lee K, Huntly BJP, Kouzarides T. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–33. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt T, Beert E, Pasmant E, Luscan A, Brems H, Ortonne N, Helin K, Hornick JL, Mautner V, Kehrer-Sawatzki H, Clapp W, Bradner J, Vidaud M, Upadhyaya M, Legius E, Cichowski K. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature. 2014;514:247–251. doi: 10.1038/nature13561. [DOI] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AJ, Bradner J, Armstrong Sa. Chromatin modifications as therapeutic targets in MLL-rearranged leukemia. Trends Immunol. 2012;33:563–570. doi: 10.1016/j.it.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabali M, Selleri L, Parry P, Bower M, Young BD, Evans Ga. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992;2:113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, Hochhaus A, Drexler HG, Duncombe A, Cervantes F, Oscier D, Boultwood J, Grand FH, Cross NCP. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–91. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983;111:47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Ferrari KJ, Scelfo A, Jammula S, Cuomo A, Barozzi I, Stützer A, Fischle W, Bonaldi T, Pasini D. Polycomb-Dependent H3K27me1 and H3K27me2 Regulate Active Transcription and Enhancer Fidelity. Mol Cell. 2014;53:49–62. doi: 10.1016/j.molcel.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore CM, Xu C, Desai PT, Berry JM, Rowbotham SP, Lin YJ, Zhang H, Marquez VE, Hammerman PS, Wong KK, Kim CF. EZH2 inhibition sensitizes BRG1 and EGFR mutant lung tumours to TopoII inhibitors. Nature. 2015;520:239–42. doi: 10.1038/nature14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SGT, Liu K, Iyer SP, Bearss D, Bhalla KN. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014;28:2155–64. doi: 10.1038/leu.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CY, Gilan O, Lam EYN, Rubin AF, Ftouni S, Tyler D, Stanley K, Sinha D, Yeh P, Morison J, Giotopoulos G, Lugo D, Jeffrey P, Lee SCW, Carpenter C, Gregory R, Ramsay RG, Lane SW, Abdel-Wahab O, Kouzarides T, Johnstone RW, Dawson SJ, Huntly BJP, Prinjha RK, Papenfuss AT, Dawson MA. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538. doi: 10.1038/nature14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Pérez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MÁ, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- French Ca, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, Kutok JL, Toretsky Ja, Tadavarthy aK, Kees UR, Fletcher Ja, Aster JC. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- French Ca, Rahman S, Walsh EM, Kühnle S, Grayson AR, Lemieux ME, Grunfeld N, Rubin BP, Antonescu CR, Zhang S, Venkatramani R, Dal Cin P, Howley PM. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: Implications for a novel oncogenic mechanism. Cancer Discov. 2014;4:929–941. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CA. NUT midline carcinoma. Nat Rev Cancer. 2014;14:149–50. doi: 10.1016/j.cancergencyto.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Frigola J, Song J, Stirzaker C, Hinshelwood RA, Peinado MA, Clark SJ. Epigenetic remodeling in colorectal cancer results in coordinate gene suppression across an entire chromosome band. Nat Genet. 2006;38:540–549. doi: 10.1038/ng1781. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Lander ES. Lessons from the Cancer Genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Gibaja V, Shen F, Harari J, Korn J, Ruddy D, Saenz-Vash V, Zhai H, Rejtar T, Paris CG, Yu Z, Lira M, King D, Qi W, Keen N, Hassan aQ, Chan HM. Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene. 2015:1–9. doi: 10.1038/onc.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, Aserlind AB, Wang H, Evan GI, Kluk MJ, Bradner JE, Aster JC, French CA. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33:1736–1742. doi: 10.1038/onc.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. Infant leukaemia biology, aetiology and treatment. Leukemia. 1996;10:372–377. [PubMed] [Google Scholar]
- Greger V, Passarge E, Höpping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83:155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-A. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Lawton LN, Rozovskaia T, Frampton GM, Levine SS, Volkert TL, Croce CM, Nakamura T, Canaani E, Young Ra. Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev. 2008;22:3403–3408. doi: 10.1101/gad.1741408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y, Ciceri F, Blaser JG, Greystoke BF, Jordan AM, Miller CJ, Ogilvie DJ, Somervaille TCP. The Histone Demethylase KDM1A Sustains the Oncogenic Potential of MLL-AF9 Leukemia Stem Cells. Cancer Cell. 2012;21:473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Hayami S, Kelly JD, Cho HS, Yoshimatsu M, Unoki M, Tsunoda T, Field HI, Neal DE, Yamaue H, Ponder BAJ, Nakamura Y, Hamamoto R. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer. 2011;128:574–586. doi: 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Huang Z, Wu H, Chuai S, Xu F, Yan F, Englund N, Wang Z, Zhang H, Fang M, Wang Y, Gu J, Zhang M, Yang T, Zhao K, Yu Y, Dai J, Yi W, Zhou S, Li Q, Wu J, Liu J, Wu X, Chan H, Lu C, Atadja P, Li E, Wang Y, Hu M. NSD2 Is Recruited through Its PHD Domain to Oncogenic Gene Loci to Drive Multiple Myeloma. Cancer Res. 2013;73:6277–6288. doi: 10.1158/0008-5472.CAN-13-1000. [DOI] [PubMed] [Google Scholar]
- Issa JPJ, Kantarjian HM. Targeting DNA Methylation. Clin Cancer Res. 2009;15:3938–3946. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JD, Wang Y, Chan HM, Zhang J, Huether R, Kryukov GV, Bhang HC, Taylor JE, Hu M, Englund NP, Yan F, Wang Z, Robert McDonald E, Wei L, Ma J, Easton J, Yu Z, deBeaumount R, Gibaja V, Venkatesan K, Schlegel R, Sellers WR, Keen N, Liu J, Caponigro G, Barretina J, Cooke VG, Mullighan C, Carr SA, Downing JR, Garraway LA, Stegmeier F. Global chromatin profiling reveals NSD2 mutations in pediatric acute lymphoblastic leukemia. Nat Genet. 2013;45:1386–91. doi: 10.1038/ng.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19:523–34. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, Kornberg RD. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci U S A. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SY, Granowicz EM, Maillard I, Thomas D, Hess JL. Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood. 2011;117:4759–4768. doi: 10.1182/blood-2010-12-327668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci Adv. 2015;1:e1500447–e1500447. doi: 10.1126/sciadv.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, Crabtree GR. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45:592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schüle R, Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66:11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- Kauffman EC, Robinson BD, Downes MJ, Powell LG, Lee MM, Scherr DS, Gudas LJ, Mongan NP. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog. 2011;50:931–944. doi: 10.1002/mc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Reiman T, Maxwell Ca, Taylor BJ, Larratt LM, Mant MJ, Belch AR, Pilarski LM. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101:1520–1529. doi: 10.1182/blood-2002-06-1675. [DOI] [PubMed] [Google Scholar]
- Kennison Ja, Tamkun JW. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci U S A. 1988;85:8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–3464. doi: 10.1128/MCB.02019-07. MCB.02019-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoechel B, Roderick JE, Williamson KE, Zhu J, Lohr JG, Cotton MJ, Gillespie SM, Fernandez D, Ku M, Wang H, Piccioni F, Silver SJ, Jain M, Pearson D, Kluk MJ, Ott CJ, Shultz LD, Brehm MA, Greiner DL, Gutierrez A, Stegmaier K, Kung AL, Root DE, Bradner JE, Aster JC, Kelliher MA, Bernstein BE. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat Genet. 2014;46:364–370. doi: 10.1038/ng.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson SK, Warholic NM, Wigle TJ, Klaus CR, Allain CJ, Raimondi A, Porter Scott M, Chesworth R, Moyer MP, Copeland RA, Richon VM, Pollock RM, Kuntz KW, Keilhack H. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci. 2013;110:7922–7927. doi: 10.1073/pnas.1303800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, Sacks JD, Raimondi A, Majer CR, Song J, Scott MP, Jin L, Smith JJ, Olhava EJ, Chesworth R, Moyer MP, Richon VM, Copeland Ra, Keilhack H, Pollock RM, Kuntz KW. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007 doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. nrc2253 [pii] [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, Xia X, Jesneck J, Bracken AP, Silverman LB, Kutok JL, Kung AL, Armstrong SA. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008;14:355–68. doi: 10.1016/j.ccr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AJ, Cheung P, Chen K, Zee BM, Kioi M, Lauring J, Xi Y, Park BH, Shi X, Garcia BA, Li W, Gozani O. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol Cell. 2011;44:609–20. doi: 10.1016/j.molcel.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B, Jenkins CE, Hannett NM, McMillin D, Sanda T, Sim T, Kim ND, Look T, Mitsiades CS, Weng AP, Brown JR, Benes CH, Marto JA, Young RA, Gray NS. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511:616–620. doi: 10.1038/nature13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle S, Amat R, Glover-Cutter K, Sansó M, Zhang C, Allen JJ, Shokat KM, Bentley DL, Fisher RP. Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nat Struct Mol Biol. 2012;19:1108–1115. doi: 10.1038/nsmb.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach JP, Moreau P, San-Miguel JF, Richardson PG. Panobinostat for the Treatment of Multiple Myeloma. Clin Cancer Res. 2015:4767–4774. doi: 10.1158/1078-0432.CCR-15-0530. [DOI] [PubMed] [Google Scholar]
- Lee H, Kahn TG, Simcox A, Schwartz YB, Pirrotta V. Genome-wide activities of Polycomb complexes control pervasive transcription. Genome Res. 2015;25:1170–1181. doi: 10.1101/gr.188920.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, Tap WD, Fletcher Ja, Huberman KH, Qin LX, Viale A, Singer S, Zheng D, Berger MF, Chen Y, Antonescu CR, Chi P. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1227–32. doi: 10.1038/ng.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén J, Hoke Ha, Lin CY, Lau A, Orlando Da, Vakoc CR, Bradner JE, Lee TI, Young Ra. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–52. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- Mansour MR, Abraham BJ, Anders L, Gutierrez A, Durbin AD, Lawton L, Sallan SE, Silverman LB, Loh ML, Hunger SP, Sanda T, Richard A, Berezovskaya A, Gutierrez A, Durbin AD, Etchin J, Lawton L, Sallan SE, Silverman LB, Loh ML, Hunger SP, Sanda T, Young RA, Look AT. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science (80-) 2014;346:1373–1377. doi: 10.1126/science.1259037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marango J, Shimoyama M, Nishio H, Meyer JA, Min DJ, Sirulnik A, Martinez-Martinez Y, Chesi M, Bergsagel PL, Zhou MM, Waxman S, Leibovitch BA, Walsh MJ, Licht JD. The MMSET protein is a histone methyltransferase with characteristics of a transcriptional corepressor. Blood. 2008;111:3145–54. doi: 10.1182/blood-2007-06-092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. nature09784 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur PK, Herner A, Mello SS, Wirth M, Hausmann S, Sánchez-Rivera FJ, Lofgren SM, Kuschma T, Hahn SA, Vangala D, Trajkovic-Arsic M, Gupta A, Heid I, Noël PB, Braren R, Erkan M, Kleeff J, Sipos B, Sayles LC, Heikenwalder M, Heßmann E, Ellenrieder V, Esposito I, Jacks T, Bradner JE, Khatri P, Sweet-Cordero EA, Attardi LD, Schmid RM, Schneider G, Sage J, Siveke JT. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015;21:1163–71. doi: 10.1038/nm.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MT, Graves aP, Ganji G, Diaz E, Halsey WS, Jiang Y, Smitheman KN, Ott HM, Pappalardi MB, Allen KE, Chen SB, Della Pietra a, Dul E, Hughes aM, Gilbert Sa, Thrall SH, Tummino PJ, Kruger RG, Brandt M, Schwartz B, Creasy CL. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27) Proc Natl Acad Sci. 2012;109:2989–2994. doi: 10.1073/pnas.1116418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian X, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, Creasy CL. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AHFM, Günther T, Buettner R, Schüle R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HKa, Cheng C, Djabali M, Ernst P. The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis. Cell Rep. 2014;7:1239–1247. doi: 10.1016/j.celrep.2014.04.015. with supp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad H, Smitheman K, Kamat C, Soong D, Federowicz K, VanAller G, Schneck J, Carson J, Liu Y, Butticello M, Bonnette W, Gorman S, Degenhardt Y, Bai Y, McCabe M, Pappalardi M, Kasparec J, Tian X, McNulty K, Rouse M, McDevitt P, Ho T, Crouthamel M, Hart T, Concha N, McHugh C, Miller W, Dhanak D, Tummino P, Carpenter C, Johnson N, Hann C, Kruger R. A DNA Hypomethylation Signature Predicts Antitumor Activity of LSD1 Inhibitors in SCLC. Cancer Cell. 2015;28:57–69. doi: 10.1016/j.ccell.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. ng.518 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson Na, Severson TM, Chiu R, Field M, Jackman S, Krzywinski M, Scott DW, Trinh DL, Tamura-Wells J, Li S, Firme MR, Rogic S, Griffith M, Chan S, Yakovenko O, Meyer IM, Zhao EY, Smailus D, Moksa M, Chittaranjan S, Rimsza L, Brooks-Wilson A, Spinelli JJ, Ben-Neriah S, Meissner B, Woolcock B, Boyle M, McDonald H, Tam A, Zhao Y, Delaney A, Zeng T, Tse K, Butterfield Y, Birol I, Holt R, Schein J, Horsman DE, Moore R, Jones SJM, Connors JM, Hirst M, Gascoyne RD, Marra Ma. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntean AG, Hess JL. The Pathogenesis of Mixed-Lineage Leukemia. Annu Rev Pathol Mech Dis. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Taranova O, He J, Zhang Y. DOT1L, the H3K79 methyltransferase, is required for MLL-AF9 - Mediated leukemogenesis. Blood. 2011;117:6912–6922. doi: 10.1182/blood-2011-02-334359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25:1345–58. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoloski G, Langemeijer SMC, Kuiper RP, Knops R, Massop M, Tönnissen ERLTM, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–667. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- Nimura K, Ura K, Shiratori H, Ikawa M, Okabe M, Schwartz RJ, Kaneda Y. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf-Hirschhorn syndrome. Nature. 2009;460:287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- Nogales E. The development of cryo-EM into a mainstream structural biology technique. 2016;13:24–27. doi: 10.1038/nmeth.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos P, Tsirigos A, Van Vlierberghe P, Nedjic J, Trimarchi T, Flaherty MS, Ferres-Marco D, da Ros V, Tang Z, Siegle J, Asp P, Hadler M, Rigo I, De Keersmaecker K, Patel J, Huynh T, Utro F, Poglio S, Samon JB, Paietta E, Racevskis J, Rowe JM, Rabadan R, Levine RL, Brown S, Pflumio F, Dominguez M, Ferrando A, Aifantis I. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 2012;18:298–303. doi: 10.1038/nm.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldridge DA, Wood AC, Weichert-Leahey N, Crimmins I, Sussman R, Winter C, McDaniel LD, Diamond M, Hart LS, Zhu S, Durbin AD, Abraham BJ, Anders L, Tian L, Zhang S, Wei JS, Khan J, Bramlett K, Rahman N, Capasso M, Iolascon A, Gerhard DS, Guidry Auvil JM, Young RA, Hakonarson H, Diskin SJ, Thomas Look A, Maris JM. Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. Nature. 2015;528:418–421. doi: 10.1038/nature15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyer Ja, Huang X, Zheng Y, Shim J, Ezponda T, Carpenter Z, Allegretta M, Okot-Kotber CI, Patel JP, Melnick a, Levine RL, Ferrando a, Mackerell aD, Kelleher NL, Licht JD, Popovic R. Point mutation E1099K in MMSET/NSD2 enhances its methyltranferase activity and leads to altered global chromatin methylation in lymphoid malignancies. Leukemia. 2014;28:198–201. doi: 10.1038/leu.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T, Ferrando A. Therapeutic targeting of NOTCH1 signaling in T-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S205–S210. doi: 10.3816/CLM.2009.s.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Malatesta M, Jung HR, Walfridsson J, Willer A, Olsson L, Skotte J, Wutz A, Porse B, Jensen ON, Helin K. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010;38:4958–4969. doi: 10.1093/nar/gkq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic R, Martinez-Garcia E, Giannopoulou EG, Zhang Q, Zhang Q, Ezponda T, Shah MY, Zheng Y, Will CM, Small EC, Hua Y, Bulic M, Jiang Y, Carrara M, Calogero RA, Kath WL, Kelleher NL, Wang JP, Elemento O, Licht JD. Histone methyltransferase MMSET/NSD2 alters EZH2 binding and reprograms the myeloma epigenome through global and focal changes in H3K36 and H3K27 methylation. PLoS Genet. 2014;10:e1004566. doi: 10.1371/journal.pgen.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, Zeng J, Li M, Fan H. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci. 2012;109:21360–21365. doi: 10.1073/pnas.1210371110/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathert P, Roth M, Neumann T, Muerdter F, Roe JS, Muhar M, Deswal S, Cerny-Reiterer S, Peter B, Jude J, Hoffmann T, Boryń ŁM, Axelsson E, Schweifer N, Tontsch-Grunt U, Dow LE, Gianni D, Pearson M, Valent P, Stark A, Kraut N, Vakoc CR, Zuber J. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature. 2015;525:543–547. doi: 10.1038/nature14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexer BN, Ham a-JL, Rinehart C, Hill S, Granja-Ingram NDM, González-Angulo aM, Mills GB, Dave B, Chang JC, Liebler DC, Arteaga CL. Phosphoproteomic mass spectrometry profiling links Src family kinases to escape from HER2 tyrosine kinase inhibition. Oncogene. 2011;30:4163–74. doi: 10.1038/onc.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoird N, Schwartz BE, Delvecchio M, Sadoul K, Meyers D, Mukherjee C, Caron C, Kimura H, Rousseaux S, Cole PA, Panne D, French CA, Khochbin S. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–52. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Toguchida J, Ohtani N, Yandell DW, Rapaport JM, Dryja TP. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48:880–8. doi: 10.1007/BF02890404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Zhan F, Tian E, Barlogie B, JS Brief report A subset of multiple myeloma harboring the t (4 ; 14)(p16 ; q32) translocation lacks FGFR3 expression but maintains an IGH / MMSET fusion transcript. 2003;101:2374–2376. doi: 10.1182/blood-2002-09-2801.Supported. [DOI] [PubMed] [Google Scholar]
- Schenk T, Chen WC, Göllner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, Casero Ra, Marton L, Woster P, Minden MD, Dugas M, Wang JCY, Dick JE, Müller-Tidow C, Petrie K, Zelent A. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012;18:605–11. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitges FW, Prusty AB, Faty M, Stutzer A, Lingaraju GM, Aiwazian J, Sack R, Hess D, Li L, Zhou S, Bunker RD, Wirth U, Bouwmeester T, Bauer A, Ly-Hartig N, Zhao K, Chan H, Gu J, Gut H, Fischle W, Muller J, Thoma NH. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011;42:330–341. doi: 10.1016/j.molcel.2011.03.025. S1097-2765(11)00287-5 [pii] [DOI] [PubMed] [Google Scholar]
- Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L, Kuhfittig-Kulle S, Metzger E, Schüle R, Eggert A, Buettner R, Kirfel J. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: Implications for therapy. Cancer Res. 2009;69:2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Ipsaro JJ, Shi J, Milazzo JP, Wang E, Roe JS, Suzuki Y, Pappin DJ, Joshua-Tor L, Vakoc CR. NSD3-Short Is an Adaptor Protein that Couples BRD4 to the CHD8 Chromatin Remodeler. Mol Cell. 2015;60:847–859. doi: 10.1016/j.molcel.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Vakoc CR. The Mechanisms behind the Therapeutic Activity of BET Bromodomain Inhibition. Mol Cell. 2014;54:72–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol. 2015:1–10. doi: 10.1038/nbt.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole Pa, Casero Ra, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, Janiszewska M, Jin Huh S, Liang Y, Ryan J, Doherty E, Mohammed H, Guo H, Stover DG, Ekram MB, Peluffo G, Brown J, D’Santos C, Krop IE, Dillon D, McKeown M, Ott C, Qi J, Ni M, Rao PK, Duarte M, Wu S-Y, Chiang C-M, Anders L, Young RA, Winer EP, Letai A, Barry WT, Carroll JS, Long HW, Brown M, Shirley Liu X, Meyer CA, Bradner JE, Polyak K. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016:1–24. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneeringer CJ, Scott MP, Kuntz KW, Knutson SK, Pollock RM, Richon VM, Copeland RA. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci U S A. 2010;107:20980–5. doi: 10.1073/pnas.1012525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Stratikopoulos EE, Dendy M, Szabolcs M, Khaykin AJ, Lefebvre C, Zhou MM, Parsons R. Kinase and BET Inhibitors Together Clamp Inhibition of PI3K Signaling and Overcome Resistance to Therapy. Cancer Cell. 2015;27:837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Miller SM, Zawistowski JS, Nakamura K, Beltran AS, Duncan JS, Angus SP, Collins KAL, Granger DA, Reuther RA, Graves LM, Gomez SM, Kuan PF, Parker JS, Chen X, Sciaky N, Carey LA, Earp HS, Jin J, Johnson GL. Inhibition of Lapatinib-Induced Kinome Reprogramming in ERBB2-Positive Breast Cancer by Targeting BET Family Bromodomains. Cell Rep. 2015;11:390–404. doi: 10.1016/j.celrep.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunnenberg HG, Vermeulen M. Towards cracking the epigenetic code using a combination of high-throughput epigenomics and quantitative mass spectrometry-based proteomics. BioEssays. 2011;33:547–551. doi: 10.1002/bies.201100044. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci aM, Kaufman TC, Kennison Ja. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-E. [DOI] [PubMed] [Google Scholar]
- Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703–708. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer Genome Landscapes. Science (80-) 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Hackanson B, Lübbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, You J. Mechanistic analysis of the role of bromodomain-containing protein 4 (BRD4) in BRD4-NUT oncoprotein-induced transcriptional activation. J Biol Chem. 2015;290:2744–58. doi: 10.1074/jbc.M114.600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang T, Kwiatkowski N, Young RA, Gray NS, Zhao JJ, Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S. CDK7-Dependent Transcriptional Addiction in Triple-Negative Breast Cancer Article CDK7-Dependent Transcriptional Addiction in Triple-Negative Breast Cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle TJ, Knutson SK, Jin L, Kuntz KW, Pollock RM, Richon VM, Copeland RA, Scott MP. The Y641C mutation of EZH2 alters substrate specificity for histone H3 lysine 27 methylation states. FEBS Lett. 2011;585:3011–4. doi: 10.1016/j.febslet.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, Koellhoffer EC, Pomeroy SL, Orkin SH, Roberts CWM. Epigenetic Antagonism between Polycomb and SWI/SNF Complexes during Oncogenic Transformation. Cancer Cell. 2010;18:316–328. doi: 10.1016/j.ccr.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, Bradner JE. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science (80-) 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SHK, Goode DL, Iwasaki M, Wei MC, Kuo HP, Zhu L, Schneidawind D, Duque-Afonso J, Weng Z, Cleary ML. The H3K4-Methyl Epigenome Regulates Leukemia Stem Cell Oncogenic Potential. Cancer Cell. 2015;28:198–209. doi: 10.1016/j.ccell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yik JHN, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–45. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, Morin RD, Mungall AJ, Meissner B, Boyle M, Marquez VE, Marra MA, Gascoyne RD, Humphries RK, Arrowsmith CH, Morin GB, Aparicio SA. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–2459. doi: 10.1182/blood-2010-11-321208. blood-2010-11-321208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro-Ohtani Y, Wang H, Zang C, Arnett KL, Bailis W, Ho Y, Knoechel B, Lanauze C, Louis L, Forsyth KS, Chen S, Chung Y, Schug J, Blobel Ga, Liebhaber Sa, Bernstein BE, Blacklow SC, Liu XS, Aster JC, Pear WS. Long-range enhancer activity determines Myc sensitivity to Notch inhibitors in T cell leukemia. Proc Natl Acad Sci U S A. 2014;111:E4946–53. doi: 10.1073/pnas.1407079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem. 2011;286:7983–7989. doi: 10.1074/jbc.M110.194027. M110.194027 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang Y, Jones S, Sausen M, McMahon K, Sharma R, Wang Q, Belzberg AJ, Chaichana K, Gallia GL, Gokaslan ZL, Riggins GJ, Wolinksy JP, Wood LD, Montgomery EA, Hruban RH, Kinzler KW, Papadopoulos N, Vogelstein B, Bettegowda C. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1170–2. doi: 10.1038/ng.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li T, Price DH. RNA Polymerase II Elongation Control. Annu Rev Biochem. 2012;81:119–143. doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa R, Patel Y, Harden a, Rubinelli P, Smith SD, LeBeau MM, Rowley JD. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991;88:10735–10739. doi: 10.1073/pnas.89.9.4220f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]