Abstract
Background
Whereas insurance status has been previously associated with care patterns, little is currently known about the association between Medicaid insurance and the clinical characteristics, treatment, or outcomes of patients with atrial fibrillation (AF).
Methods and Results
We used data from adults with AF enrolled in the Outcomes Registry for Better Informed Treatment of AF (ORBIT‐AF), a national outpatient registry conducted at 176 community, multispecialty sites. The primary outcome of interest was the proportion of patients prescribed any oral anticoagulation (OAC; warfarin or novel oral anticoagulants [NOAC]). Secondary outcomes of interest included the proportion of patients prescribed NOACs (dabigatran or rivaroxaban); time in therapeutic range (TTR) for warfarin users, all‐cause mortality, stroke/systemic embolism, and major bleed. Of 10 133 patients, N=470 (4.6%) had Medicaid insurance. Medicaid patients were similarly likely to receive OAC at baseline (72.8% vs 76.3%; unadjusted P=0.079), but less likely to receive NOAC at baseline or follow‐up (12.1% vs 16.3%; unadjusted P=0.019). After risk adjustment, Medicaid status was associated with lower use of OAC at baseline among patients with high stroke risk (odds ratio [OR]=0.68; 95% CI=0.49, 0.94), but was not associated with OAC use overall (OR=0.82; 95% CI=0.61, 1.09). Among warfarin users, median TTR was lower among Medicaid patients (60% vs 68%; P<0.0001; adjusted TTR difference, −2.9; 95% CI=−5.7, −0.2; P=0.04). Use of an NOAC over 2 years of follow‐up was not statistically different by insurance. Compared with non‐Medicaid patients, Medicaid patients had higher unadjusted rates of mortality, stroke/systemic embolism, and major bleeding; however, these differences were attenuated following adjustment for clinical characteristics.
Conclusions
In a contemporary AF cohort, use of OAC overall and use of NOACs were not significantly lower among Medicaid patients relative to others. However, among warfarin users, Medicaid patients spent less time in therapeutic range compared with those with other forms of insurance.
Keywords: anticoagulation, atrial fibrillation, Medicaid, quality of care, stroke prevention
Subject Categories: Atrial Fibrillation, Health Services, Quality and Outcomes, Primary Prevention
Introduction
Atrial fibrillation (AF) is a complex disease that requires appropriate oral anticoagulant therapy (OAC) to mitigate risks for stroke. Low socioeconomic status (SES) patients are at particular increased risk for stroke and may have a higher comorbidity burden, less access to care, and lower rates of optimal medication adherence relative to their higher SES counterparts.1, 2 Past studies have reported that patients insured by Medicaid, a social health care program for low‐income individuals in the United States, are less likely to receive evidence‐based cardiovascular therapies than those with private insurance.3, 4 With the marked expansion of individuals receiving coverage by Medicaid, it is increasingly important to understand how type of insurance may influence care and outcomes of AF patients.5 Recent analyses of administrative claims data have reported treatment disparities in stroke prophylaxis by insurance status, with Medicaid patients being significantly less likely to be taking OACs relative to those with private insurance.6, 7 However, because administrative claims data are limited by lack of detailed information on potentially important comorbidities, estimates of appropriate treatment may be imprecise. Additionally, beyond warfarin, novel OACs (NOACs) have recently become available and possess several advantages relative to warfarin, but are more expensive and therefore may be less accessible to lower‐income patients.7 Certain NOACs are also taken twice per day, which may increase the likelihood of noncompliance, yet no study to date has assessed use of NOACs by insurance status. Using the nation's largest clinical data registry of AF, our goal was to better characterize the clinical characteristics, anticoagulation patterns, and longitudinal outcomes among Medicaid patients with AF relative to those with other forms of insurance.
Methods
Study Population
We used data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). The ORBIT‐AF study design has been published.8 Briefly, ORBIT‐AF is a prospective, US‐based outpatient registry of AF management. Patients ages 18 years or older provided informed consent and were enrolled at 176 clinical sites. ORBIT‐AF sites represent a geographically diverse set of providers from multiple specialties, including cardiology, electrophysiology, and primary care. Information on demographics, medical comorbidities, AF history, procedures, medications, and provider characteristics are abstracted and entered into a web‐based data collection form. Prospective data on hospitalizations, disease progression, medications, procedures, and vital status is collected at ≈6 month intervals for 2 years after initial enrollment. The Duke Clinical Research Institute coordinates and manages the study.
The ORBIT‐AF Registry enrolled 10 135 AF patients between June 2010 and August 2011. After excluding patients who were missing information on insurance or OAC status (n=2), we were left with a final analytic population of N=10 133 patients who completed the baseline study visit.
Study Endpoints
The primary outcome of interest for this study was the proportion of patients who were using OAC (warfarin, dabigatran, or rivaroxaban) at baseline or during follow‐up. Current medications at each visit were abstracted from medication lists in the medical record and entered into online data collection forms. Because ORBIT‐AF enrolled some patients before the approval of rivaroxaban and Apixaban was not approved until after the enrollment period (2010–2011), OAC at baseline was defined as warfarin or dabigatran use. As a secondary endpoint, we also examined the proportion of patients who were using novel anticoagulants (dabigatran or rivaroxaban) at baseline or at any visit during the 2‐year follow‐up period. Secondary outcomes of interest included time in therapeutic range (TTR) for warfarin users, all‐cause mortality, first stroke/systemic embolism, and first major bleed. TTR was calculated using the Rosendaal method. Daily INR values were imputed between the first and last INR measured during follow‐up. For patients with 60 days or more between any 2 INR measurements, this time span was excluded from the TTR calculation. Using the Rosendaal method of linear interpolation between each pair of measured INR values, we calculated TTR as the percent of days with INR between 2 and 3 during this period. Stroke/systemic embolism was defined as the composite of all stroke (both ischemic and hemorrhagic) and systemic (non–central nervous system) embolism at any point during follow‐up. Major bleeding was defined as any bleeding event meeting International Society of Thrombosis and Hemostatis major bleeding criteria at any point during follow‐up.
Medicaid Status
Patients were classified as Medicaid or non‐Medicaid based on insurance status as recorded at the baseline visit. Because Medicaid eligibility is based on income and is therefore a proxy for lower SES,9 we classified Medicaid patients as any patient with Medicaid insurance at baseline, regardless of additional forms of insurance. Of 470 patients insured by Medicaid, N=47 (10%) also had private insurance, n=267 (56.8%) also had Medicare insurance, and n=5 (1.1%) also had military health care.
Statistical Analysis
We compared baseline characteristics by Medicaid insurance status at the baseline visit (yes or no) among those N=10 133 patients who completed the baseline visit. Continuous variables are presented as medians (interquartile range [IQR], 25th–75th percentiles) and categorical variables are presented as counts (proportions). Differences between Medicaid and non‐Medicaid patients were compared using Wilcoxon rank‐sum tests for continuous variables and chi‐square test for categorical variables. We used similar methods to compare unadjusted antithrombotic treatment rates at baseline and over 2 years of follow‐up among Medicaid and non‐Medicaid patients. To characterize OAC use over follow‐up, we used data from all available visits until the patient was censored because of death or loss‐to‐follow‐up. Of N=10 133 patients, N=6935 (68.4%) had 24 full months of follow‐up. As a sensitivity analysis, we examined unadjusted OAC and NOAC use among those patients with a full 24 months of follow‐up and found that patterns of OAC and NOAC treatment were similar. Therefore, we present estimates of OAC use over follow‐up using all available visit data over the 2‐year study period. We repeated these analyses stratifying by stroke risk as estimated by CHADS2 (<2, ≥2) and CHA2DS2‐VASc (<2, ≥2). The CHADS2 score is calculated by assigning 2 points for a history of past stroke or transient ischemic attack (TIA) and 1 point each for congestive heart failure (CHF)/left ventricular (LV) dysfunction, hypertension, older age (≥75 years), and diabetes (range, 0–6).10 The CHA2DS2‐VASc score assigns 2 points each for age ≥75 years and history of stroke or TIA, and 1 point each for CHF/LV dysfunction, hypertension, diabetes, female sex, 65≤age≤74, and vascular disease (range, 0–9).11 We also examined patterns of reported contraindications among those patients definitively indicated for OAC (CHADS2 ≥2) because of the possibility that reduced access to care and fewer resources may be associated with less‐consistent monitoring and/or medication nonadherence. We limited this analysis to those patients who were OAC indicated because of stroke risk because we were most interested in reasons for nontreatment among the subgroup of patients with the greatest potential stroke reduction benefit with OAC treatment. Among patients who were not treated with OAC at baseline, we compared frequencies of contraindications to OAC by Medicaid status overall and stratified by CHADS2 score using chi‐squared tests.
We assessed the association between receiving baseline OAC and Medicaid status using logistic regression with generalized estimating equations (GEEs) to account for the correlation of patients within sites. All multivariable models were adjusted for the following variables as documented at the baseline visit: demographics (age, race [African American/Hispanic/white/other], and sex); medical history (smoking, cancer, hypertension, osteoporosis/hip fracture, diabetes, hypothyroidism, gastrointestinal bleed, obstructive sleep apnea, insufficient kidney function [dialysis or estimated glomerular filtration rate <60 mL/min per 1.73 m2], hyperlipidemia, anemia, cognitive impairment/dementia, frailty, coronary artery disease, chronic obstructive pulmonary disease [COPD], alcohol or drug abuse, peripheral vascular disease, sinus node dysfunction, stroke/TIA, CHF, and valvular disease [all yes/no]), vital signs (heart rate [beats/min], blood pressure [mm Hg], and body mass index [BMI; kg/m2]), left atrial diameter, AF history (type of AF [new onset/paroxysmal AF/persistent AF/permanent AF], past cardioversions, past antiarrhythmic drugs, past AF interventional therapy), and functional status (living independently/living with assistance/residence in assisted living/residence in skilled nursing home/bedbound). All continuous variables were tested for linearity. Nonlinear relationships were found for age, systolic blood pressure, and BMI, which were accounted for using linear splines and truncation. Missing data (<0.2% except left atrial diameter enlargement type, which has 14% missing) on the covariates used in the modeling were handled using multiple imputation. Among patients who were taking OAC, we compared receipt of novel oral anticoagulants (dabigatran or rivaroxaban) versus warfarin by Medicaid status during 2 years of follow‐up using the same modeling approach. The association between TTR and Medicaid status was assessed using multivariable‐adjusted GEE linear regression modeling. Finally, we compared clinical outcomes by Medicaid status using Cox proportional hazards regression with a robust sandwich covariance estimate.
All P values presented are 2‐sided, and we considered P<0.05 to be statistically significant for all analyses. Statistical analysis was performed using SAS software (version 9.3; SAS Institute Inc., Cary, NC). All ORBIT‐AF study participants gave written informed consent before enrollment. The Duke Institutional Review Board (IRB) approved the ORBIT‐AF Registry, and all participating sites obtained approval from local IRBs before entering patient data.
Results
Baseline Characteristics
Among 10 133 patients enrolled in ORBIT‐AF, n=470 (4.6%) had Medicaid insurance at baseline; N=95 patients (0.98%) were uninsured. Distribution of baseline characteristics of the study population by Medicaid status is shown in Table 1. Compared with patients who did not have Medicaid insurance, Medicaid patients were, on average, younger, more likely to be female, less likely to be white, and more likely to have less than a high school education. Burden of comorbidities was greater in the Medicaid population, with higher rates of CHF, past stroke/ TIA, COPD, smoking, diabetes, and hypertension than among non‐Medicaid patients. High stroke risk as estimated by CHADS2 ≥2 was more common among Medicaid patients (78.7%) than non‐Medicaid patients (71.5%; P=0.0004).
Table 1.
Baseline Characteristics of the ORBIT‐AF Population by Medicaid Enrollment
| Variable | Non‐Medicaid (n=9663; 95.4%) | Medicaid (n=470; 4.6%) | P Valuea |
|---|---|---|---|
| Age, y, median (IQR) | 75.0 (67.0–82.0) | 70.0 (61.0–79.0) | <0.0001 |
| Female sex | 41.8 | 53.0 | <0.0001 |
| Race | <0.0001 | ||
| White | 90.7 | 58.7 | |
| Black or African American | 4.4 | 18.1 | |
| Hispanic | 3.5 | 19.4 | |
| Other | 1.3 | 3.4 | |
| Insurance provider | |||
| Other | 2.7 | — | <0.0001 |
| Medicare | 68.1 | — | |
| Private | 26.8 | — | |
| Military health care | 1.1 | — | |
| State‐specific plan (non‐Medicaid) | 0.3 | — | |
| None | 1.0 | — | |
| Education | |||
| Some school | 12.9 | 37.7 | <0.0001 |
| High school graduate | 51.6 | 41.9 | |
| College graduate | 22.9 | 15.5 | |
| Postgraduate | 8.5 | 2.8 | |
| Medical history | |||
| CHF | 31.8 | 47.5 | <0.0001 |
| Past stroke/TIA | 14.8 | 22.3 | <0.0001 |
| COPD | 15.9 | 25.7 | <0.0001 |
| Current smoker | 5.5 | 12.3 | <0.0001 |
| Diabetes | 29.0 | 38.5 | <0.0001 |
| Hypertension | 82.8 | 88.9 | 0.0005 |
| Obstructive sleep apnea | 18.1 | 20.0 | 0.29 |
| CHADS2, median | 2.0 | 2.0 | <0.0001 |
| IQR | 1.0, 3.0 | 2.0, 3.0 | |
| CHA2DS2‐VASc, median | 4.0 | 4.0 | <0.0001 |
| IQR | 3.0, 5.0 | 3.0, 6.0 | |
| CHADS2 ≥2 | 71.5 | 78.7 | <0.0001 |
| ATRIA, median | 3.0 | 3.0 | 0.20 |
| IQR | 1.0, 4.0 | 1.0, 4.0 | |
ATRIA indicates Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study cohort; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HS, high school; IQR, interquartile range; ORBIT‐AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; TIA, transient ischemic attack.
P values from Pearson chi‐square tests for categorical variables and Wilcoxon rank‐sum tests for continuous variables.
Stroke Prevention Therapy
The unadjusted rates of antithrombotic treatment at baseline and during follow‐up are shown in Table 2. The overall rate of OAC use was 76.1% at baseline and 82.0% over follow‐up. The overall rate of NOAC use at baseline or follow‐up was 16.1%. Medicaid patients were slightly less likely to receive OAC at baseline (72.8% vs 76.3%; P=0.08), but this difference was not statistically significant. Among patients with high stroke risk (CHADS2 ≥2), Medicaid status was associated with significantly lower rates of OAC receipt (73.2% vs 80.5%; P<0.001). This difference persisted when defining high stroke risk according to CHA2DS2‐VASc >2, with lower rates of OAC among Medicaid patients versus non‐Medicaid patients (73.7% vs 78.3%; P=0.02). Results from multivariable regression models of the association between Medicaid status and OAC receipt are shown in Table 2. Among patients with high stroke risk (CHADS2 ≥2), Medicaid patients were 32% less likely to receive OAC at baseline than non‐Medicaid patients after adjustment. Whereas Medicaid patients overall were less likely to receive OAC at baseline or during follow‐up, these differences were not statistically significant after adjustment for clinical covariates. Among warfarin users, TTR was higher for Non‐Medicaid patients than for Medicaid patients (median=68 vs 60; P<0.0001). This difference persisted after multivariable adjustment (TTR difference of −2.93; 95% CI, −5.67, −0.19).
Table 2.
Anticoagulation by Medicaid Status in the ORBIT‐AF Population*
| Overall (N=10 133) | Non‐Medicaid (N=9663) | Medicaid (N=470) | P Valuea | Unadjusted ORb (95% CI) | Adjusted ORb (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|
| OAC use | |||||||
| OAC at baseline | 76.1 | 76.3 | 72.8 | 0.08 | 0.80 (0.60, 1.07) | 0.82 (0.61, 1.09) | 0.17 |
| OAC (any follow‐up visit) | 82.0 | 82.2 | 79.6 | 0.16 | 0.93 (0.77, 1.13) | 0.99 (0.80, 1.23) | 0.92 |
| OAC at baseline among CHADS2 ≥2 | 80.1 | 80.5 | 73.2 | <0.001 | 0.65 (0.47, 0.89) | 0.68 (0.49, 0.94) | 0.02 |
| OAC at baseline among CHADS2‐VASc ≥2 | 78.1 | 78.3 | 73.7 | 0.02 | 0.74 (0.54, 1.01) | 0.77 (0.57, 1.04) | 0.09 |
| NOAC use | |||||||
| NOAC at baseline or any follow‐up visit | 16.1 | 16.3 | 12.1 | 0.02 | 0.87 (0.65, 1.17) | 0.96 (0.69, 1.32) | 0.80 |
| NOAC among CHADS2 ≥2 | 13.9 | 14.1 | 10.8 | 0.07 | 0.91 (0.64, 1.30) | 0.92 (0.63, 1.33) | 0.65 |
| NOAC among CHADS2‐VASc ≥2 | 15.5 | 15.6 | 11.9 | 0.03 | 0.90 (0.66, 1.23) | 0.97 (0.70, 1.36) | 0.87 |
| TTRc | 68 (54–79) | 60 (45–73.5) | −6.58 (−9.61, −3.54) | −2.93 (−5.67, −0.19) | 0.04 | ||
IQR indicates interquartile range; NOAC, novel oral anticoagulant; OAC, oral anticoagulation; ORBIT‐AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; TIA, transient ischemic attack; TTR, time in therapeutic range.
Estimates are percentages unless otherwise indicated.
Chi‐squared tests.
Generalized estimating equations logistic regression models. Adjusted models included age, race, sex, smoking, cancer, hypertension, osteoporosis/hip fracture, diabetes, hypothyroidism, gastrointestinal bleed, obstructive sleep apnea, insufficient kidney function, hyperlipidemia, anemia, cognitive impairment/dementia, frailty, coronary artery disease, chronic obstructive pulmonary disease, alcohol or drug abuse, peripheral vascular disease, sinus node dysfunction, stroke/transient ischemic attack, congestive heart failure, valvular disease, heart rate, blood pressure, body mass index, left atrial diameter, type of atrial fibrillation (AF), past cardioversions, past antiarrhythmic drugs, past AF interventional therapy, and functional status.
Calculated from the Rosendaal method and based on patients who were taking warfarin at baseline and had at least 5 international normalized ratio measurements during follow‐up (n=5322). Estimates shown are from linear regression.
Among patients who were taking OAC, Medicaid patients were less likely to receive NOACs than non‐Medicaid patients at baseline or during follow‐up (12.1% vs 16.3%; P=0.016). Medicaid patients were less likely to receive NOACs in unadjusted models, but this association was attenuated after adjustment. Similar results were found when stratifying patients by stroke risk according to CHA2DS2‐VASc, with lower rates of NOAC receipt in Medicaid patients (15.9% vs 17.6%; P=0.46) and attenuation of results after multivariable modeling adjustment.
Contraindications to Therapy
Figure displays the reported OAC contraindications by Medicaid status, among patients with high stroke risk (CHADS2 ≥2; n=1449). Medicaid patients were more commonly described as unable to adhere/monitor warfarin as a reported contraindication to OAC, whereas non‐Medicaid patients were more likely to have past bleed, patient refusal, and frequent falls/frailty listed as a contraindication to OAC.
Figure 1.
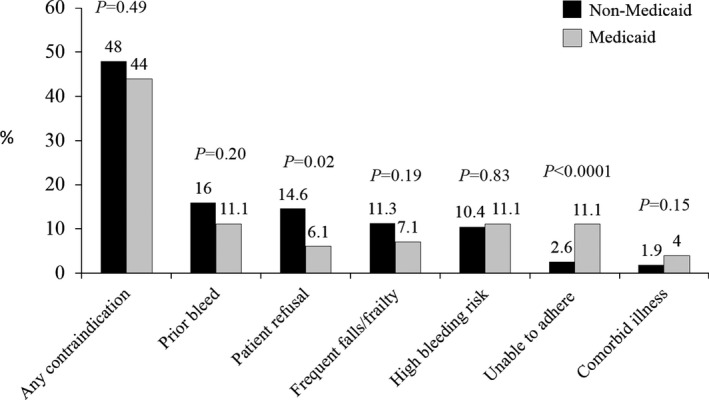
Documented contraindications to OAC by Medicaid status among untreated patients with CHADS 2 ≥2. This figure displays reported rates of contraindications to oral anticoagulant therapy among untreated patients with high estimated stroke risk by Medicaid insurance status.
Outcomes
Unadjusted and adjusted rates of adverse clinical outcomes by Medicaid status are shown in Table 3. Medicaid patients had higher unadjusted rates of adverse events compared with non‐Medicaid patients, including mortality (6.9% vs 5.6%), stroke/systemic embolism (1.5% vs 1.0%), and major bleeding (4.3% vs 3.8%). However, these differences were attenuated after adjustment for clinical characteristics.
Table 3.
Adverse Clinical Outcomesa by Medicaid Status in ORBIT‐AF
| Outcome | No. of Events: Non‐Medicaid (p‐years) | No. of Events Non‐Medicaid (p‐years) | Unadjusted Event Rate per 100 Patient Years | Unadjusted HR (95% CI) | Medicaid vs Non‐Medicaid | Adjusted P Value | ||
|---|---|---|---|---|---|---|---|---|
| Non‐Medicaid | Medicaid | Unadjusted P Value | Adjusted HRb (95% CI) | |||||
| All‐cause mortality | 1146 (20 585) | 61 (890) | 5.6 | 6.9 | 1.25 (0.98, 1.60) | 0.07 | 0.97 (0.75, 1.25) | 0.80 |
| Stroke/systemic embolism | 201 (20 439) | 13 (883) | 1.0 | 1.5 | 1.51 (0.90, 2.54) | 0.12 | 1.05 (0.63, 1.76) | 0.85 |
| Major bleeding | 755 (19 791) | 36 (846) | 3.8 | 4.3 | 1.11 (0.75, 1.65) | 0.60 | 0.97 (0.67, 1.38) | 0.85 |
ORBIT‐AF indicates Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
Over 2 years of follow‐up.
Adjusted models included age, race, sex, smoking, cancer, hypertension, osteoporosis/hip fracture, diabetes, hypothyroidism, gastrointestinal bleed, obstructive sleep apnea, insufficient kidney function, hyperlipidemia, anemia, cognitive impairment/dementia, frailty, coronary artery disease, chronic obstructive pulmonary disease, alcohol or drug abuse, peripheral vascular disease, sinus node dysfunction, stroke/transient ischemic attack, congestive heart failure, valvular disease, heart rate, blood pressure, body mass index, left atrial diameter, type of atrial fibrillation (AF), past cardioversions, past antiarrhythmic drugs, past AF interventional therapy, and functional status.
Discussion
We evaluated the association between Medicaid status and receipt of anticoagulation in a large, contemporary population of patients with AF across the United States. Our major findings are as follows: (1) Medicaid patients are younger, yet have higher comorbidity burden and stroke risk than non‐Medicaid patients; (2) despite having higher stroke risk and fewer absolute OAC contraindications, Medicaid patients were slightly, but not statistically significantly, less likely to receive OAC at baseline and follow‐up; (3) Medicaid patients treated with warfarin spent less time in therapeutic range than non‐Medicaid warfarin users; (4) Medicaid patients were less likely to receive NOAC than non‐Medicaid patients, but these differences did not persist after adjustment for clinical characteristics; and (5) Medicaid patients had higher rates of mortality, stroke/systemic embolism, and major bleeding, but rates were statistically similar to those of non‐Medicaid patients after adjustment.
Although paradoxical relationships for stroke prevention have been described, to our knowledge, ours is the first study to examine the association between insurance status, OAC receipt, and clinical outcomes using a large, national US cohort of AF. Although differences in OAC receipt by Medicaid status were attenuated after adjustment in our study, disparities in unadjusted treatment rates were consistent with those observed in past work. In a population of 12 000 patients in the Swedish Stroke Register, patients with higher income levels and university‐level education were ≈20% more likely to receive OAC compared to patients with lower income or educational attainment.12 Similar patterns have been observed in US‐based populations. In a recent analysis of claims data from multiple payer databases, Medicaid patients were significantly more likely to have elevated risk of stroke, but were less likely to be receiving OAC compared with patients with other types of insurance.7 In a state‐level analysis of Medicaid patients with AF in Ohio, fewer than 10% of Medicaid patients were receiving warfarin.13
There are several possibilities for the observed differences in adjusted rates of OAC use among Medicaid and non‐Medicaid patients in our study compared with past work. First, our analysis was based on data from a clinical outpatient registry that were limited to patients who consented to longitudinal follow‐up. Therefore, it is possible that patients who consent to participate in ORBIT‐AF have greater access to health care resources for anticoagulation management, regardless of health insurance status, which may reduce observed disparities by type of insurance. Second, past work demonstrating differences in OAC use by Medicaid status were conducted using administrative data sets. In our analysis, the observed disparities in OAC treatment were attenuated after adjustment for a set of detailed clinical covariates, some of which are unavailable in administrative claims (ie, vital signs and functional status). Therefore, we were able to adjust for potential confounders not available in claims‐only data sets.
In contrast to these past studies, we found that after adjusting for clinical factors, Medicaid patients were similarly likely to receive OAC and NOAC. However, Medicaid patients spent less time in therapeutic range compared to non‐Medicaid patients. This finding is consistent with past work suggesting that lower income is an independent predictor of poorer anticoagulant control.14 One case‐control analysis of AF patients in Australia reported that patients in the lowest income bracket were 2.7 times more likely to have an INR value of 6.0 or greater compared with those in the middle‐income bracket.15 The increased time spent out of therapeutic range may contribute to the higher unadjusted rates of stroke and major bleeding among Medicaid patients, a finding that has been reported in past work.16 Given that physicians in our study were more likely to list “inability to adhere/monitor” as an OAC contraindication among Medicaid patients, it is possible that concerns about increased propensity for suboptimal anticoagulation and corresponding event risk contribute to lower treatment rates.
Robust evidence for the mechanisms of the association between SES and suboptimal anticoagulation control is limited. Patient use of anticoagulation management resources is likely a function of access to care, which may be reduced in lower‐income populations. One study of telephone requests to family and general practice physicians in Ontario, Canada, reported that callers presenting themselves as high‐SES patients were 78% more likely to be offered an appointment than the same callers when presenting as low‐SES patients.17 Given that access to anticoagulation management systems of care is associated with increased propensity for anticoagulant receipt,18 better OAC management support may represent an opportunity to reduce disparities in OAC receipt across socioeconomic strata. Additional work is needed to identify the most appropriate strategies for increasing access to care and improving anticoagulation management in underserved populations.
Limitations
There are several limitations to our study. First, the number of Medicaid patients was somewhat limited, and thus we had limited power to detect small differences in clinical outcomes or treatment receipt by insurance groups. Second, the ORBIT‐AF registry is voluntary, and Medicaid patients in our study may not be representative of all Medicaid patients with AF. Consistent with past work,19, 20 we classified all patients with Medicaid insurance as “Medicaid,” regardless of additional forms of insurance. Because we were unable to further stratify the Medicaid population by other forms of insurance attributed to small numbers of patients in each strata, it is possible that treatment patterns differ within the Medicaid population by those who had additional insurance versus those who did not. Third, we did not have information on other socioeconomic variables that may influence treatment decisions, such as median household income. Residual measured and unmeasured confounding variables may have influenced these findings. Fourth, this study was conducted during the earlier period of NOAC approval (2009–2010). Because uptake of NOAC is a dynamic phenomenon, recent treatment patterns may differ. Additionally, because the ORBIT‐AF follow‐up period was limited to 2 years, it is possible that different results would have been observed with longer follow‐up. Finally, we did not have data on actual prescription drug and/or INR monitoring costs, which may differ by geographical region and could influence choice of OAC therapy.
Conclusions
Medicaid patients represented 5% of the population and had a greater comorbidity burden and higher stroke risk than patients with other forms of insurance in a contemporary, community‐based AF cohort. Whereas Medicaid patients were similarly likely to receive OAC compared with patients with other forms of insurance, their TTR was significantly lower. Use of NOAC was not different by insurance status. More research is needed to identify strategies to reduce socioeconomic barriers to optimal anticoagulation in AF populations.
Author Contributions
Dr O'Brien had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The ORBIT‐AF registry is sponsored by Janssen Scientific Affairs, LLC (Raritan, NJ). This project was supported (in part) by funding from the Agency of Healthcare Research and Quality through cooperative agreement number 1U19 HS021092.
Disclosures
O'Brien reports research support from Janssen Scientific, Bristol‐Myers Squibb, Novartis, Pfizer, and GlaxoSmithKline and consultant/advisory board fees from Portola. Fonarow reports consulting/advisory board; Modest; Ortho McNeil. Dr Kowey reports that he has served as a consultant/advisory board for Boehringer Ingelheim, Bristol‐Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo (all modest). Mahaffey reports research support from AstraZeneca, Amgen, Bayer, Boehringer Ingleheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Portola, POZEN Pharmaceutical, Schering‐Plough, and The Medicines Company and consulting agreements with Amgen, AstraZeneca, GlaxoSmithKline, Johnson & Johnson, and Merck. Piccini reports research support from Boston Scientific Corporation and Janssen and consultancies to Forest Laboratories, Janssen, and Medtronic. Peterson reports research support from Eli Lilly & Company and Janssen. The remaining authors have no disclosures.
Acknowledgments
The authors thank the ORBIT‐AF Registry staff and participants for their important contributions to this work.
(J Am Heart Assoc. 2016;5:e002721 doi: 10.1161/JAHA.115.002721)
References
- 1. Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, Wolfe CD, McKevitt C. Socioeconomic status and stroke: an updated review. Stroke. 2012;43:1186–1191. [DOI] [PubMed] [Google Scholar]
- 2. Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann Intern Med. 1998;129:412–416. [DOI] [PubMed] [Google Scholar]
- 3. Pamboukian SV, Funkhouser E, Child IG, Allison JJ, Weissman NW, Kiefe CI. Disparities by insurance status in quality of care for elderly patients with unstable angina. Ethn Dis. 2006;16:799–807. [PubMed] [Google Scholar]
- 4. Carlisle DM, Leake BD, Shapiro MF. Racial and ethnic disparities in the use of cardiovascular procedures: associations with type of health insurance. Am J Public Health. 1997;87:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carman KG, Eibner C, Paddock SM. Trends in health insurance enrollment, 2013–15. Health Aff (Millwood). 2015;34:1044–1048. [DOI] [PubMed] [Google Scholar]
- 6. Boulanger L, Hauch O, Friedman M, Foster T, Dixon D, Wygant G, Menzin J. Warfarin exposure and the risk of thromboembolic and major bleeding events among Medicaid patients with atrial fibrillation. Ann Pharmacother. 2006;40:1024–1029. [DOI] [PubMed] [Google Scholar]
- 7. Lang K, Bozkaya D, Patel AA, Macomson B, Nelson W, Owens G, Mody S, Schein J, Menzin J. Anticoagulant use for the prevention of stroke in patients with atrial fibrillation: findings from a multi‐payer analysis. BMC Health Serv Res. 2014;14:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011;162:606–612.e601. [DOI] [PubMed] [Google Scholar]
- 9. Foraker RE, Rose KM, Whitsel EA, Suchindran CM, Wood JL, Rosamond WD. Neighborhood socioeconomic status, Medicaid coverage and medical management of myocardial infarction: atherosclerosis risk in communities (ARIC) community surveillance. BMC Public Health. 2010;10:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 11. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 12. Sjolander M, Eriksson M, Asplund K, Norrving B, Glader EL. Socioeconomic inequalities in the prescription of oral anticoagulants in stroke patients with atrial fibrillation. Stroke. 2015;46:2220–2225. [DOI] [PubMed] [Google Scholar]
- 13. Wess ML, Schauer DP, Johnston JA, Moomaw CJ, Brewer DE, Cook EF, Eckman MH. Application of a decision support tool for anticoagulation in patients with non‐valvular atrial fibrillation. J Gen Intern Med. 2008;23:411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–1414. [DOI] [PubMed] [Google Scholar]
- 15. Diug B, Evans S, Lowthian J, Maxwell E, Dooley M, Street A, Wolfe R, Cameron P, McNeil J. The unrecognized psychosocial factors contributing to bleeding risk in warfarin therapy. Stroke. 2011;42:2866–2871. [DOI] [PubMed] [Google Scholar]
- 16. Cressman AM, Macdonald EM, Yao Z, Austin PC, Gomes T, Paterson JM, Kapral MK, Mamdani MM, Juurlink DN. Socioeconomic status and risk of hemorrhage during warfarin therapy for atrial fibrillation: a population‐based study. Am Heart J. 2015;170:133–140.e133. [DOI] [PubMed] [Google Scholar]
- 17. Olah ME, Gaisano G, Hwang SW. The effect of socioeconomic status on access to primary care: an audit study. CMAJ. 2013;185:E263–E269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burkiewicz JS. Effect of access to anticoagulation management services on warfarin use in patients with atrial fibrillation. Pharmacotherapy. 2005;25:1062–1067. [DOI] [PubMed] [Google Scholar]
- 19. Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163:1331–1337. [DOI] [PubMed] [Google Scholar]
- 20. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. [DOI] [PubMed] [Google Scholar]


