Abstract
Background
Short‐term exposure to elevated air pollution has been associated with higher risk of acute cardiovascular diseases, with systemic oxidative stress induced by air pollution hypothesized as an important underlying mechanism. However, few community‐based studies have assessed this association.
Methods and Results
Two thousand thirty‐five Framingham Offspring Cohort participants living within 50 km of the Harvard Boston Supersite who were not current smokers were included. We assessed circulating biomarkers of oxidative stress including blood myeloperoxidase at the seventh examination (1998–2001) and urinary creatinine‐indexed 8‐epi‐prostaglandin F2α (8‐epi‐PGF 2α) at the seventh and eighth (2005–2008) examinations. We measured fine particulate matter (PM 2.5), black carbon, sulfate, nitrogen oxides, and ozone at the Supersite and calculated 1‐, 2‐, 3‐, 5‐, and 7‐day moving averages of each pollutant. Measured myeloperoxidase and 8‐epi‐PGF 2α were loge transformed. We used linear regression models and linear mixed‐effects models with random intercepts for myeloperoxidase and indexed 8‐epi‐PGF 2α, respectively. Models were adjusted for demographic variables, individual‐ and area‐level measures of socioeconomic position, clinical and lifestyle factors, weather, and temporal trend. We found positive associations of PM 2.5 and black carbon with myeloperoxidase across multiple moving averages. Additionally, 2‐ to 7‐day moving averages of PM 2.5 and sulfate were consistently positively associated with 8‐epi‐PGF 2α. Stronger positive associations of black carbon and sulfate with myeloperoxidase were observed among participants with diabetes than in those without.
Conclusions
Our community‐based investigation supports an association of select markers of ambient air pollution with circulating biomarkers of oxidative stress.
Keywords: air pollution, isoprostanes, myeloperoxidase
Subject Categories: Epidemiology, Risk Factors
Introduction
Increasing evidence indicates that short‐term exposure to elevated air pollution is associated with higher risk of incident ischemic stroke, myocardial infarction, and other acute cardiovascular events.1, 2, 3 Oxidative stress, an imbalance between the production of the reactive oxygen species and the human body's antioxidant defense mechanism,4 has been proposed as an important underlying biological mechanism mediating this association.3, 5, 6, 7 Increased oxidative stress may induce endothelial dysfunction, which is characterized by increased endothelial permeability, altered vascular tone, platelet adhesion and aggregation, and enhanced thrombogenicity.8, 9
Myeloperoxidase is an enzyme that is abundantly stored in inflammatory cells such as neutrophils, macrophages, and monocytes and is involved in a wide range of activities that generate reactive oxygen and nitrogen species.10, 11, 12, 13 Prior studies have yielded mixed results.14, 15, 16, 17, 18 In a recent study, positive associations of short‐term exposure to fine particulate matter (diameter ≤2.5 μm [PM2.5]), black carbon (BC), and nitrogen oxides (NOx) with myeloperoxidase were found in a group of potentially genetically susceptible participants.14
8‐Epi‐prostaglandin F2α (8‐epi‐PGF2α) is formed from peroxidation of arachidonic acid19 and is detectable in human plasma and urine. The quantification of 8‐epi‐PGF2α has been widely used as a noninvasive method to assess lipid peroxidation.20, 21 Higher short‐term air pollution has been associated with higher 8‐epi‐PGF2α sampled from exhaled breath condensate in children, adolescents, and healthy young adults22, 23, 24, 25; however, few studies have assessed the relationship between exposure to ambient air pollution and urinary 8‐epi‐PGF2α 26, 27 or in older populations at increased risk of cardiovascular events.
Epidemiologic studies conducted in the Boston area have reported positive associations of short‐term exposure to air pollution with acute stroke onset,28 atrial fibrillation,29 and myocardial infarction onset.30 In the present study, we evaluated whether short‐term (1–7 days) ambient air pollution exposure is associated with systemic levels of oxidative stress, measured by plasma myeloperoxidase and urinary creatinine‐indexed 8‐epi‐PGF2α, in the community‐based Framingham Heart Study. Our study catchment region and study period largely overlap with the above‐mentioned studies, and a closer look at the relationship may enable us to elucidate underlying biologic pathways that could in part explain previous findings.
Methods
Study Sample
The study participants were from the Framingham Heart Study Offspring cohort living within 50 km of the Harvard Supersite air pollution monitor in Boston, Massachusetts.31 The study design and selection criteria of the Framingham Offspring cohort has been described elsewhere.32 We included 2035 participants from the Offspring cohort seventh examination (1998–2001) and/or eighth examination (2005–2008) who were not current smokers and had at least one valid measurement of plasma myeloperoxidase or urinary creatinine‐indexed 8‐epi‐PGF2α (3386 observations in total). At each examination, physical examinations were performed according to standardized protocols, and data on demographics, medication history, smoking history, and alcohol intake were collected via questionnaires. All participants provided written informed consent for the Framingham Heart Study examinations, and institutional review boards at Beth Israel Deaconess Medical Center and Boston University Medical Center approved the study.
Biomarkers of Oxidative Stress
Fasting morning plasma samples and urine samples were collected at the examination visits. Plasma myeloperoxidase (ng/mL) was measured in duplicate in examination 7 by using the commercially available Enzyme Immunoassay Kit (OXIS Health Products), and 8‐epi‐PGF2α (pg/mL) was measured in duplicate with the Enzyme Immunoassay Kit (Cayman Chemical) in examinations 7 and 8. Measured 8‐epi‐PGF2α was adjusted for urinary creatinine and was expressed in nanograms per millimole of creatinine. The levels of myeloperoxidase and indexed 8‐epi‐PGF2α were loge transformed.
Air Pollution and Meteorological Variables
Air pollution levels were measured at the Harvard Supersite, located on the rooftop of the Francis A. Countway Library of Medicine (5 stories above ground level) and 50 m from the nearest street. Measurement methods have been described previously.31 PM2.5 (μg/m3) was measured by using a tapered element oscillating microbalance (Model 1400A; Rupprecht & Patashnick Co Inc), and BC (μg/m3) was measured by using an aethalometer (Model AE‐16; Magee Scientific Corp). Ozone (O3, ppm) and NOx were estimated by averaging available data from local state monitors within the greater Boston area. Daily sulfate (SO4 2−, μg/m3) was calculated from elemental sulfur measured with x‐ray fluorescence analysis of the PM2.5 filter samples. On days when SO4 2− x‐ray fluorescence measurements were not available, an SO4 2− analyzer (Model 5020; Thermo Electron Corp) was used. Temperature and relative humidity were monitored at the Boston Logan International Airport Weather Station, located 12 km from the Supersite.
Statistical Methods
We calculated 1‐, 2‐, 3‐, 5‐, and 7‐day moving averages for measured pollutants based on the daily means. For each moving average of a pollutant, we fit multivariable linear regression models (for plasma myeloperoxidase) and multivariable linear mixed‐effects models with subject‐specific random intercepts (for indexed urinary 8‐epi‐PGF2α). We adjusted for individual‐ and area‐level covariates in the models, including centered age, and (centered age)2; sex; body mass index; smoking status (former or never smoker); pack years; alcohol intake; educational level; and the quartile of median household income in the participant's census tract from the 2000 US Census. An examination identifier (examination 7 or 8) was added to the linear mixed models. We additionally adjusted for season, linear time trend, temperature, and relative humidity.
In secondary analyses, we explored the associations within current US Environmental Protection Agency (EPA) National Ambient Air Quality Standards by excluding observations with any of the 7 days before the examination date that had a 24‐hour PM2.5 >35 μg/m3. We also explored whether associations differed when we included current smokers. Additionally, we repeated our analyses after restricting the study population to participants who lived within 40 km of the Harvard Supersite air pollution monitor. Further, we examined whether associations varied by age (>65/≤65 years), sex, obesity (31.8%), diabetes (16.8%), cardiovascular disease (15.0%), antihypertensive medication use (46.8%), statin use (31.5%), and season (warm [April to September] versus cold [October to March]) by adding an interaction term to these models.
Analyses were scaled to 5 μg/m3 for PM2.5, 0.4 μg/m3 for BC, 2 μg/m3 for SO4 2−, and 0.01 ppm for NOx and O3, which approximated the IQR.
Estimated percent changes were reported with 95% CIs. For primary analyses, we focused on describing the association patterns between pollutants and the biomarkers. For sensitivity analyses in which effect modification was explored, the 2‐tailed P‐value from the Wald test of the interaction term was used to decide whether the observed association differed between subgroups; however, only consistent association patterns were considered important and highlighted. A 2‐tailed P<0.05 value was considered statistically significant in these analyses. Primary analyses were performed using PROC GLM and PROC Mixed in SAS 9.4 (SAS Institute, Inc). Figures were plotted using Stata 13 (StataCorp LP).
Results
Table 1 shows the population characteristics. PM2.5 was strongly correlated with BC and SO4 2−. NOx was moderately correlated with BC and negatively correlated with O3 (Table 2). The correlation structure was similar for longer‐term moving averages. Figure 1 shows the distributions of myeloperoxidase and indexed urinary 8‐epi‐PGF2α, and Figure 2 shows the distribution of the daily concentrations of each air pollutant.
Table 1.
Characteristics of the 3386 Observations From the Framingham Offspring Cohort Examination 7 (1998–2001) and/or 8 (2005–2008) Participants
| No. (%) or Mean [SD] | |
|---|---|
| Examination cycle 7 | 1878 (55.5%) |
| Age, y | 64.1 [9.7] |
| Women | 1789 (52.8%) |
| BMI, kg/m2 | 28.5 [5.4] |
| Alcohol, drinks/wk | 4.2 [6.9] |
| Diabetes | 569 (16.8%) |
| Former smoker | 2018 (59.6%) |
| Education | |
| <High school | 161 (4.8%) |
| High School | 1051 (31.0%) |
| Some college | 1050 (31.0%) |
| College graduate | 1094 (32.3%) |
| Antihypertensive medication use | 1583 (46.8%) |
| Statins | 1066 (31.5%) |
| Plasma myeloperoxidasea, ng/mL | 40.6 [22.5] |
| Urinary 8‐epi‐PGF2α a, pg/mL | 897.9 [842.3] |
| Urine creatinine, mg/100 mL | 115.2 [69.1] |
| Indexed urinary 8‐epi‐PGF2α a, ng/mmol creatinine | 108.7 [69.6] |
BMI indicates body mass index; 8‐epi‐PGF2α, 8‐epi‐prostaglandin F2α.
Geometric mean [SD of the geometric mean].
Table 2.
Characteristics of the 1‐Day Moving Averages of Air Pollutants Previous to the Examination Date in the Study Population (1998–2001, 2005–2008)
| Pollutant | No. of Observations | Mean (SD) | IQR | BC | SO4 2− | NOx | O3 |
|---|---|---|---|---|---|---|---|
| PM2.5, μg/m3 | 3380 | 9.86 (5.34) | 6.28 | 0.76 | 0.79 | 0.47 | −0.05 |
| BC, μg/m3 | 3376 | 0.84 (0.46) | 0.57 | — | 0.53 | 0.61 | −0.25 |
| SO4 2−, μg/m3 | 2758 | 2.98 (2.25) | 2.22 | — | — | 0.33 | 0.05 |
| NOx, ppm | 3081 | 0.04 (0.02) | 0.02 | — | — | — | −0.52 |
| O3, ppm | 3377 | 0.02 (0.01) | 0.01 | — | — | — | — |
BC indicates black carbon; NOx, nitrogen oxides; O3, ozone; PM2.5, fine particulate matter; SO4 2−, sulfate.
Figure 1.
Histograms of (A) myeloperoxidase, (B) loge transformed myeloperoxidase, (C) indexed 8‐epi‐prostaglandin F2α (8‐epi‐PGF 2α), and (D) loge transformed indexed 8‐epi‐PGF 2α among the Framingham Offspring cohort examination 7 (1998–2001) and/or 8 (2005–2008) participants. Solid line indicates the normal‐density plot; dashed line indicates the kernel‐density plot.
Figure 2.
Histograms of the 1‐day moving average concentrations of air pollutants previous to the examination date in the study population (1998–2001, 2005–2008): (A) fine particulate matter (PM 2.5), (B) black carbon (BC), (C) sulfate (SO 4 2−), (D) nitrogen oxides (NO x), and (E) ozone (O3). Solid line indicates the normal‐density plot; dashed line indicates the kernel‐density plot.
We found positive associations of PM2.5 and BC with plasma myeloperoxidase across multiple moving averages (Figure 3A). Additionally, 3‐ to 7‐day moving averages of SO4 2− were weakly associated with plasma myeloperoxidase; however, 95% CIs were rather wide.
Figure 3.
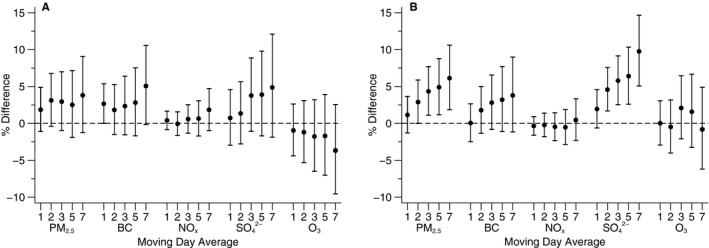
Associations of moving averages of air pollutants with (A) myeloperoxidase and (B) indexed 8‐epi‐prostaglandin F2α (8‐epi‐PGF 2α). Scaled to 5 μg/m3 for fine particulate matter (PM 2.5), 0.4 μg/m3 for black carbon (BC), 2 μg/m3 for sulfate (SO 4 2−), and 0.01 ppm for nitrogen oxides (NO x) and ozone (O3). Models are adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, education level, quartile of median household income in the participants’ census tracts from the 2000 US Census, sine and cosine of the day of year, examination date, day of the week, temperature, and relative humidity, and an examination identifier is added to models with indexed 8‐epi‐PGF 2α as the dependent variable. Error bars indicate the 95% CIs.
We also observed consistent positive associations for PM2.5 and SO4 2− with indexed urinary 8‐epi‐PGF2α, with stronger associations appearing in 3‐ to 7‐day moving averages of PM2.5 and 2‐ to 7‐day moving averages of SO4 2− (Figure 3B). Similar but weaker positive associations were observed for 2‐ to 7‐day moving averages of BC.
Excluding observations with any 24‐hour average PM2.5 above the EPA National Ambient Air Quality Standards (19 observations for plasma myeloperoxidase and 38 observations for urinary 8‐epi‐PGF2α) did not change our findings substantially. As before, 3‐ to 7‐day moving averages of PM2.5 and 2‐ to 7‐day moving averages of SO4 2− were positively associated with indexed urinary 8‐epi‐PGF2α with 95% CIs that did not overlap the null. Results were not materially altered after we included current smokers and adjusted for smoking status and pack years in the primary analyses or after we restricted study participants to those who lived within 40 km of the Harvard Supersite air pollution monitor. We tested the robustness of our results by including BC and SO4 2− simultaneously; the associations were slightly attenuated but without any substantial change.
There was no consistent evidence of differing associations between pollutants and either biomarker by age, sex, obesity, cardiovascular disease, antihypertensive medication use, statin use, or season. However, stronger associations of BC and SO4 2− with plasma myeloperoxidase were observed among participants with diabetes than those without (Figure 4A).
Figure 4.
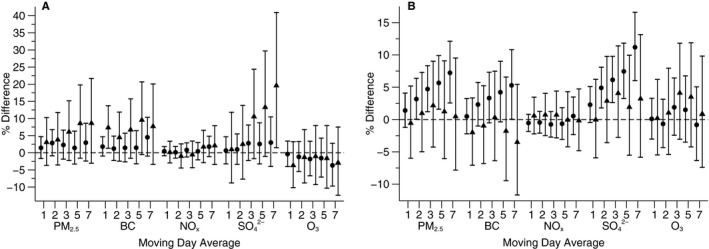
Associations of moving averages of air pollutants with (A) myeloperoxidase and (B) indexed 8‐epi‐prostaglandin F2α (8‐epi‐PGF 2α) among participants with diabetes and those without (triangle, participants with diabetes; circle, participants without diabetes). Scaled to 5 μg/m3 for fine particulate matter (PM 2.5), 0.4 μg/m3 for black carbon (BC), 2 μg/m3 for sulfate (SO 4 2−), and 0.01 ppm for nitrogen oxides (NO x) and ozone (O3). Models are adjusted for centered age, (centered age)2, sex, bpdy mass index, smoking status, pack years, alcohol intake, education level, quartile of median household income in the participants’ census tracts from the 2000 US Census, sine and cosine of the day of year, examination date, day of the week, temperature, and relative humidity, and an examination identifier is added to models with indexed 8‐epi‐PGF 2α as the dependent variable. Error bars indicate the 95% CIs.
Discussion
In our community‐based study, we found positive associations of PM2.5 and BC with plasma myeloperoxidase and of PM2.5 and SO4 2− with urinary 8‐epi‐PGF2α across multiple moving averages. The association of BC and SO4 2− with plasma myeloperoxidase appeared to be stronger among participants with diabetes. To our knowledge, we report the largest community‐based study to date on the association of short‐term ambient air pollution with oxidative stress biomarkers.
Myeloperoxidase can be involved in diverse oxidation reactions, including lipid peroxidation by acting as an enzyme in generating multiple reactive oxygen and nitrogen species, and may promote endothelial dysfunction.10 Accumulation of lipid peroxidation products in vascular walls promotes disruption of vulnerable plaques,33, 34 which likely contributes to the risk of acute cardiovascular events. Some,14, 15, 16 but not all,17, 18 prior studies have found an association between short‐term air pollution and plasma myeloperoxidase. Ruckerl et al found higher myeloperoxidase levels were associated with the BC, NO, NO2, and PM2.5 within 5 days in a group of potentially genetically susceptible participants who were free of type 2 diabetes or impaired glucose tolerance.14 However, Delfino et al reported no association between measured air pollutants and myeloperoxidase among 29 nonsmoking elderly participants with a history of coronary artery disease.17
Urinary 8‐epi‐PGF2α is a reliable and stable biomarker of lipid peroxidation that may promote vasoconstriction and platelet activation.20 Prior studies have found increased 8‐epi‐PGF2α in exhaled breath condensate after exposure to air pollutants.22, 24, 25 However, systemic oxidative stress may be better reflected by 8‐epi‐PGF2α measured in plasma or urine. Mixed results have been seen between air pollution and 8‐epi‐PGF2α 26, 35, 36 or other oxidative stress markers.37
Prior studies of short‐term ambient air pollution exposure with acute cardiovascular outcomes38, 39, 40, 41 and markers of vascular reactivity42 and inflammation43 suggest that individuals with diabetes are more sensitive to air pollution, as a result of baseline chronic inflammation and endothelial dysfunction.44 We observed tendencies for participants with diabetes to have higher levels of myeloperoxidase in relation to BC and SO4 2−. There was no evidence suggesting differing associations between pollutants and 8‐epi‐PGF2α.
In this study region, local traffic sources and regional pollution both contribute to PM2.5 mass concentrations.45 Locally emitted or transported BC is a product of incomplete combustion and is associated with different sources such as traffic, residential heating and cooking, and biomass burning. SO4 2− is primarily from regional sulfur‐related pollution sources such as coal‐fired power plants, and some is generated from local diesel exhaust.46 When we included both BC and SO4 2− in the models, we observed potential positive association between BC and myeloperoxidase but not SO4 2−, suggesting that local sources may play an important role, whereas for 8‐epi‐PGF2α, the stronger association with SO4 2− suggests that the transported pollutants may play a stronger role, consistent with the finding of Ren et al.47
There are several limitations that should be noted. We assigned the ambient air pollution level measured by a central monitoring site to all participants, which may decrease precision of our estimates and induce exposure measurement error. Prior studies in our region have demonstrated moderate correlation between PM2.5 measured at the Supersite and personal exposure level.48 In daily time series, most of the variability in exposure within the study region is related to temporal, rather than spatial, variability,45 which supports assigning regional average concentrations to study participants. In the present investigation, the distribution of exposure of the participants was primarily related to the date that participants came for their examination appointment. Thus, we expect the exposure measurement error caused by assignment to be nondifferential, leading to attenuated point estimates and wider CIs. The participants of the Framingham Offspring Study were predominantly white individuals of European ancestry and middle‐aged to older adults, which limits the generalizability of our findings to other ethnicities and to age groups not studied. We acknowledge that we cannot exclude the possibility of residual confounding and that we cannot prove causal relations.
There are also several strengths. First, our study sample was from a large community‐based cohort with standardized protocols for physical examinations and biomarker assessments. Second, we adjusted for demographic characteristics, lifestyle, individual‐ and area‐level of socioeconomic position, weather, and temporal trend. Third, assessments of air pollutants and biomarkers were performed separately. Fourth, we conducted the study in a region that has pollution levels in compliance with current air quality standards, and our findings still suggested adverse associations. Future studies in regions with higher levels of ambient air pollution are needed to determine if these associations are stronger in such regions. Additionally, participants of the Framingham Heart Study scheduled the date of their examination visit months in advance, and this was not likely related to the air pollution level on the days leading up to that prescheduled appointment.
Conclusions
Our findings suggest positive associations of short‐term exposure to PM2.5 and BC with plasma myeloperoxidase and of short‐term exposure to PM2.5 and SO4 2− with urinary 8‐epi‐PGF2α. The associations of BC and SO4 2− with plasma myeloperoxidase appear stronger among participants with diabetes. Our findings provide evidence suggesting potential intermediate biological mechanisms that may in part explain the observed associations between transiently higher air pollution levels and the increase of acute cardiovascular events.
Sources of Funding
This publication was made possible by USEPA grant RD‐83479801. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. This work was further supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health contracts and grants HHSN268201500001I, N01‐HC 25195, 1RO1HL64753, R01HL076784, 1R01AG028321, and T32HL007575 and National Institutes of Environmental Health Sciences grants 1F32ES023352‐01 and P30ES000002.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002742 doi: 10.1161/JAHA.115.002742)
Results were presented at the 48th Annual Meeting of the Society for Epidemiologic Research, June 16–19, 2015 in Denver, CO.
References
- 1. Yang WS, Wang X, Deng Q, Fan WY, Wang WY. An evidence‐based appraisal of global association between air pollution and risk of stroke. Int J Cardiol. 2014;175:307–313. [DOI] [PubMed] [Google Scholar]
- 2. Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta‐analysis. JAMA. 2012;307:713–721. [DOI] [PubMed] [Google Scholar]
- 3. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition PA, Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 4. Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. [DOI] [PubMed] [Google Scholar]
- 5. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ljungman PL, Mittleman MA. Ambient air pollution and stroke. Stroke. 2014;45:3734–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Bornstadt D, Kunz A, Endres M. Impact of particulate matter exposition on the risk of ischemic stroke: epidemiologic evidence and putative mechanisms. J Cereb Blood Flow Metab. 2014;34:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. 2011;13:200–207. [DOI] [PubMed] [Google Scholar]
- 9. Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. [DOI] [PubMed] [Google Scholar]
- 10. Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. [DOI] [PubMed] [Google Scholar]
- 12. Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. [DOI] [PubMed] [Google Scholar]
- 13. Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–359. [DOI] [PubMed] [Google Scholar]
- 14. Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, Dailey L, Devlin RB, Diaz‐Sanchez D, Koenig W, Phipps R, Silbajoris R, Soentgen J, Soukup J, Peters A, Schneider A. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. [DOI] [PubMed] [Google Scholar]
- 15. Huttunen K, Siponen T, Salonen I, Yli‐Tuomi T, Aurela M, Dufva H, Hillamo R, Linkola E, Pekkanen J, Pennanen A, Peters A, Salonen RO, Schneider A, Tiittanen P, Hirvonen MR, Lanki T. Low‐level exposure to ambient particulate matter is associated with systemic inflammation in ischemic heart disease patients. Environ Res. 2012;116:44–51. [DOI] [PubMed] [Google Scholar]
- 16. Scannell C, Chen L, Aris RM, Tager I, Christian D, Ferrando R, Welch B, Kelly T, Balmes JR. Greater ozone‐induced inflammatory responses in subjects with asthma. Am J Respir Crit Care Med. 1996;154:24–29. [DOI] [PubMed] [Google Scholar]
- 17. Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, Farin F, Peretz A, Kaufman JD. A randomized cross‐over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol. 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ II. A series of prostaglandin F2‐like compounds are produced in vivo in humans by a non‐cyclooxygenase, free radical‐catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts LJ, Morrow JD. Measurement of F(2)‐isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. [DOI] [PubMed] [Google Scholar]
- 21. Tacconelli S, Capone ML, Patrignani P. Measurement of 8‐iso‐prostaglandin F2alpha in biological fluids as a measure of lipid peroxidation. Methods Mol Biol. 2010;644:165–178. [DOI] [PubMed] [Google Scholar]
- 22. De Prins S, Dons E, Van Poppel M, Int Panis L, Van de Mieroop E, Nelen V, Cox B, Nawrot TS, Teughels C, Schoeters G, Koppen G. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ Int. 2014;73:440–446. [DOI] [PubMed] [Google Scholar]
- 23. Rosa MJ, Yan B, Chillrud SN, Acosta LM, Divjan A, Jacobson JS, Miller RL, Goldstein IF, Perzanowski MS. Domestic airborne black carbon levels and 8‐isoprostane in exhaled breath condensate among children in New York City. Environ Res. 2014;135:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. Traffic‐related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013;121:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman‐Strickland P, Diehl SR, Zhu P, Tong J, Gong J, Zhu T, Zhang J. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allen J, Trenga CA, Peretz A, Sullivan JH, Carlsten CC, Kaufman JD. Effect of diesel exhaust inhalation on antioxidant and oxidative stress responses in adults with metabolic syndrome. Inhalation Toxicol. 2009;21:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nuernberg AM, Boyce PD, Cavallari JM, Fang SC, Eisen EA, Christiani DC. Urinary 8‐isoprostane and 8‐OHdG concentrations in boilermakers with welding exposure. J Occup Environ Med. 2008;50:182–189. [DOI] [PubMed] [Google Scholar]
- 28. Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Link MS, Luttmann‐Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol. 2013;62:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. [DOI] [PubMed] [Google Scholar]
- 31. Kang CM, Koutrakis P, Suh HH. Hourly measurements of fine particulate sulfate and carbon aerosols at the Harvard‐U.S. Environmental Protection Agency Supersite in Boston. J Air Waste Manag Assoc. 2010;60:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 33. Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8:577–602. [DOI] [PubMed] [Google Scholar]
- 34. Miller MR. The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochemical Society transactions. 2014;42:1006–1011. [DOI] [PubMed] [Google Scholar]
- 35. Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, Stigendal L. Experimental exposure to wood‐smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhalation Toxicol. 2006;18:845–853. [DOI] [PubMed] [Google Scholar]
- 37. Liu L, Urch B, Poon R, Szyszkowicz M, Speck M, Gold DR, Wheeler AJ, Scott JA, Brook JR, Thorne PS, Silverman FS. Effects of ambient coarse, fine, and ultrafine particles and their biological constituents on systemic biomarkers: a controlled human exposure study. Environ Health Perspect. 2015;123:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles? Am J Respir Crit Care Med. 2001;164:831–833. [DOI] [PubMed] [Google Scholar]
- 39. Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–592. [DOI] [PubMed] [Google Scholar]
- 40. Villeneuve PJ, Johnson JY, Pasichnyk D, Lowes J, Kirkland S, Rowe BH. Short‐term effects of ambient air pollution on stroke: who is most vulnerable? Sci Total Environ. 2012;430:193–201. [DOI] [PubMed] [Google Scholar]
- 41. O'Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution‐associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. [DOI] [PubMed] [Google Scholar]
- 43. Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gold DR. Vulnerability to cardiovascular effects of air pollution in people with diabetes. Curr Diab Rep. 2008;8:333–335. [DOI] [PubMed] [Google Scholar]
- 45. Lee HJ, Gent JF, Leaderer BP, Koutrakis P. Spatial and temporal variability of fine particle composition and source types in five cities of Connecticut and Massachusetts. Sci Total Environ. 2011;409:2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee HJ, Kang CM, Coull BA, Bell ML, Koutrakis P. Assessment of primary and secondary ambient particle trends using satellite aerosol optical depth and ground speciation data in the New England region, United States. Environ Res. 2014;133:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren C, Fang S, Wright RO, Suh H, Schwartz J. Urinary 8‐hydroxy‐2’‐deoxyguanosine as a biomarker of oxidative DNA damage induced by ambient pollution in the Normative Aging Study. Occup Environ Med. 2011;68:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407:3754–3765. [DOI] [PubMed] [Google Scholar]


