Abstract
Background
Remnant lipoproteins (RLPs), the triglyceride‐enriched precursors to low‐density lipoprotein, are an emerging risk factor for coronary heart disease (CHD). We sought to determine the association of RLP cholesterol (RLP‐C) levels with incident CHD in 2 diverse, prospective, longitudinal observational US cohorts.
Methods and Results
We analyzed cholesterol levels from serum lipoprotein samples separated via density gradient ultracentrifugation in 4114 US black participants (mean age 53.8 years, 64% women) from the Jackson Heart Study and a random sample of 818 predominantly white participants (mean age 57.3 years, 52% women) from the Framingham Offspring Cohort Study. Multivariable‐adjusted hazard ratios (HRs) for RLP‐C (the sum of very low‐density lipoprotein3 cholesterol and intermediate‐density lipoprotein cholesterol) were derived to estimate associations with incident CHD events consisting of myocardial infarction, CHD death, and revascularizations for each cohort separately and as a combined population. There were 146 CHD events in the combined population. After adjustments for age, sex, body mass index, smoking, blood pressure, diabetes, and lipid‐lowering therapy for the combined population, RLP‐C (HR 1.23 per 1‐SD increase, 95% CI 1.06–1.42, P<0.01) and intermediate‐density lipoprotein cholesterol (HR 1.26 per 1‐SD increase, 95% CI 1.08–1.47, P<0.01) predicted CHD during an 8‐year follow‐up. Associations were attenuated by high‐density lipoprotein cholesterol and ultimately lost significance with inclusion of real low‐density lipoprotein cholesterol, which excludes Lp(a) and IDL cholesterol fractions. Similar associations were seen in multivariable analyses within each cohort.
Conclusion
RLP‐C levels are predictive of incident CHD in this diverse group of primary prevention subjects. Interventions aimed at reducing RLP‐C to prevent CHD warrant further intensive investigation.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT00415415.
Keywords: coronary heart disease, lipids, primary prevention, remnant lipoprotein cholesterol, triglycerides
Subject Categories: Risk Factors, Epidemiology, Cardiovascular Disease, Clinical Studies, Lipids and Cholesterol
Introduction
Despite remarkable capacity to lower low‐density lipoprotein cholesterol (LDL‐C) with the use of statins and other drugs, coronary heart disease (CHD) persists as a leading cause of morbidity and mortality in the United States.1, 2 Apolipoprotein B (apoB) and non–high‐density lipoprotein cholesterol (non–HDL‐C) levels are superior predictors of CHD risk compared with LDL‐C.3 The excess risk for CHD captured by apoB or non–HDL‐C suggests a need for more granularity among lipoproteins, such as triglyceride‐rich remnant lipoproteins (RLPs) and their associations with CHD risk.
In the fasting state, RLPs consist of very low density lipoproteins (VLDL) and intermediate‐density lipoproteins (IDL) and are estimated by triglyceride (TG) levels in the absence of advanced lipoprotein testing. Though not as well recognized as LDL particles for their role in atherogenesis, RLPs have several proatherogenic properties, including proinflammatory and prothrombotic effects; in addition, resident macrophages scavenge and retain RLPs in the subendothelial space.4, 5, 6, 7
Multiple prospective cohorts from around the world consistently suggest a link between RLPs, often reflected by serum TG measurements, and CHD.8, 9, 10, 11 Historically, some studies have suggested that this association is attenuated by the inclusion of other potentially causal factors such as HDL‐C, thus raising the question of whether RLPs may represent a surrogate for other causative factors in atherosclerotic disease.12, 13 Recent Mendelian randomization studies have served as the strongest evidence that RLPs are etiologic for atherosclerosis.6, 14, 15, 16 Further, TGs are predictive of residual risk in statin‐treated individuals.17 However, this extensive recent work relies on surrogate measures of RLP‐C and mainly stems from European populations and thus is not necessarily generalizable to the ethnically diverse US population.8, 14, 15, 16, 18
Therefore, we sought to evaluate the association of directly measured RLP‐C and its components, VLDL3‐C and IDL‐C, with risk for incident CHD in US populations of black men and women from the Jackson Heart Study (JHS) and in a subset of men and women from the predominantly white Framingham Offspring Cohort Study (FOCS).
Methods
The present study reports results of the RLP‐C analysis from the 2 primary prevention cohorts within the Lipoproteins Investigators Collaborative, which is described in Appendix S1.
Study Populations
The JHS methods have been described previously.19 The study included 5301 black adults from the Jackson, MS, region with the primary aim to investigate causes of cardiovascular disease in US blacks. Lipoprotein cholesterol subfractions were measured by using density gradient ultracentrifugation (Vertical Auto Profile [VAP], Atherotech) of fasting samples from the baseline visit available for 4722 participants from 2000 to 2004. After excluding 464 participants for missing covariate data and 144 participants with self‐reported prevalent cardiovascular disease, we included 4114 JHS participants with follow‐up from 2000 to 2008. Based on multiple imputations and assuming covariate data were missing at random, the results were robust to exclusions.
The FOCS began in 1971 and includes 5129 offspring and spouses of offspring from the Framingham Heart Study.20 We analyzed fasting serum by using density gradient ultracentrifugation from the cycle 6 examination of 902 randomly selected FOCS participants. After excluding 80 participants with prevalent cardiovascular disease and 4 others with missing covariate data, 818 participants were followed for up to 8 years for the analysis.
Each study was reviewed and approved by the corresponding institutional review boards, and informed consent was obtained from all participants.
CHD events were a combination of myocardial infarction (MI), CHD death, and revascularization including coronary artery bypass graft surgery or angioplasty. Events were actively and passively ascertained, and all events were adjudicated by independent reviewers.19, 21
Measurement of Lipoproteins
From both JHS and FOCS, frozen (−70°C) serum samples were sent on dry ice via overnight express to the testing laboratory (Atherotech in Birmingham, AL), where they were kept at −70°C until measurement. Cholesterol was measured from subfractions of the major lipoprotein classes, including LDL, IDL, VLDL, and HDL, after separation via single vertical spin density gradient ultracentrifugation (VAP; Appendix S1) as previously described.22 Although “direct” LDL‐C includes IDL‐C and lipoprotein (a) cholesterol [Lp(a)‐C], real LDL‐C excludes these 2 fractions and consists only of LDL‐C. Fasting RLP‐C was defined as the sum of the densest VLDL subfraction (VLDL3‐C) and IDL‐C.23, 24, 25
Statistical Analyses
Mean and SD values were determined for normally distributed continuous variables and compared by use of t tests. Medians with 25th and 75th percentile values were determined for non–normally distributed variables, and the groups were compared by the use of Wilcoxon rank sum testing. Proportions were determined for categorical variables and compared by using Fisher exact and χ2 testing. Restricted cubic spline curves adjusted for age, sex, body mass index, smoking, systolic and diastolic blood pressures, lipid‐altering medications, and diabetes were created to assess linearity assumptions of the relationship between lipids and CHD.
After verifying proportional hazards assumptions by using the Kolmogorov‐type supremum test (P>0.10), we examined associations with incident CHD over 8 years by using multivariable‐adjusted Cox proportional hazards models to estimate hazard ratios (HRs) per 1‐SD increase in RLP‐C and its components, VLDL3‐C and IDL‐C. In the JHS, follow‐up began at the baseline examination, while in the FOCS, follow‐up began at the cycle 6 examination; covariates were measured at the start of follow‐up. Each cohort was analyzed separately and then as a combined group for a patient‐level multivariable adjusted analysis. The primary model 1 included adjustments for age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures, lipid‐altering medications, and diabetes. Sequential models were created by using model 1 variables in combination with HDL‐C and real LDL‐C, which excludes IDL‐C and Lp(a)‐C and consists only of LDL‐C.
Forest plots were created to visualize HRs and 2‐sided 95% CIs for a 1‐SD increase in each of the RLP‐C variables for each study individually and for the combined cohort. In our prior analysis from these 2 cohorts, we found a significant association with HDL subclasses and incident CHD.26 The interactions between HDL‐C, HDL subclasses, and LDL‐C with RLP‐C and its components were assessed in multivariate‐adjusted Cox models and by using 3‐dimensional CHD risk plots.
Statistical analyses were coordinated across centers within the LIC study group by using SAS v9.3 (SAS Institute). All P‐values are considered significant at a 2‐sided α of .05.
Results
Baseline Characteristics
The baseline characteristics show the populations were predominantly female and middle aged on average, though the JHS study participants were slightly younger (Table 1). The JHS also had more than twice the baseline rate of diabetes compared with FOCS and a higher average body mass index of its participants. Smoking rates were similar in the 2 studies at <15%. Systolic blood pressure values were comparable, although diastolic blood pressure was significantly higher among the JHS participants. There was slightly greater use of lipid‐altering medications in the more contemporary JHS population.
Table 1.
Baseline Cardiometabolic Risk Factors of Jackson Heart Study and Framingham Offspring Cohort Study Participants
| Variable | Jackson Heart Study (n=4114) | Framingham Offspring Cohort (n=818) | P Value | Combined Populations (n=4932) |
|---|---|---|---|---|
| Males | 1463 (36%) | 395 (48%) | <0.0001 | 1858 (38%) |
| Age, y | 53.8 (12.8) | 57.3 (9.5) | <0.0001 | 54.4 (12.3) |
| Diabetes | 678 (16%) | 58 (7%) | <0.0001 | 736 (15%) |
| Current smokers | 512 (12%) | 118 (14%) | 0.12 | 630 (13%) |
| Body mass index, kg/m2 | 31.7 (7.3) | 27.7 (5.0) | <0.0001 | 31.1 (7.1) |
| Systolic blood pressure, mm Hg | 126.5 (18.0) | 126.2 (18.1) | 0.67 | 126.4 (18.0) |
| Diastolic blood pressure, mm Hg | 79.1 (10.4) | 74.8 (9.3) | <0.0001 | 78.4 (10.4) |
| Lipid‐altering medicationsa | 443 (11%) | 66 (8%) | 0.02 | 509 (10%) |
Values are n (%) or mean (SD) where appropriate.
Lipid‐altering medications include statins, bile sequestrants, niacin derivatives, and fibric acid derivatives.
Baseline lipids were significantly different between the 2 populations (Table 2). Notable differences included much lower TGs, slightly higher RLP‐C (due to higher IDL‐C), lower direct and real LDL‐C, higher Lp(a)‐C, higher HDL‐C, and lower non–HDL‐C among JHS participants. The correlations between VLDL3‐C and IDL‐C in the FOCS, JHS, and combined cohort were 0.75, 0.77, and 0.76, respectively. The correlations between VLDL3‐C and real LDL‐C in the FOCS, JHS, and combined cohort were 0.44, 0.18, and 0.21, respectively. Finally, the correlations between IDL‐C and real LDL‐C in the FOCS, JHS, and combined cohort were 0.67, 0.48, and 0.50, respectively.
Table 2.
Baseline Lipids of the Jackson Heart Study and Framingham Offspring Cohort Study Participants
| Variable | Jackson Heart Study (n=4114) | Framingham Offspring Cohort (n=818) | P Value | Combined Populations (n=4932) |
|---|---|---|---|---|
| Total cholesterol | 198.8 (40.5) | 201.5 (37.2) | 0.07 | 199.3 (40.0) |
| Triglycerides | 90 (67, 126) | 155 (126, 187) | 0.0001 | 100 (72, 143) |
| RLP‐C (VLDL3‐C+IDL‐C) | 29.7 (12.0) | 28.1 (11.6) | 0.0004 | 29.4 (12.0) |
| IDL‐C | 16.4 (8.0) | 15.1 (8.5) | <0.0001 | 16.2 (8.1) |
| VLDL‐C | 22.4 (9.7) | 23.2 (6.9) | 0.02 | 22.5 (9.3) |
| VLDL1+2‐C | 9.1 (5.5) | 10.3 (3.5) | <0.0001 | 9.3 (5.2) |
| VLDL3‐C | 13.3 (4.8) | 13.0 (3.8) | 0.08 | 13.2 (4.6) |
| Direct LDL‐C | 122.8 (36.2) | 128.0 (32.0) | <0.0001 | 123.7 (35.6) |
| LDL1‐C | 18.5 (8.8) | 21.2 (7.4) | <0.0001 | 18.9 (8.7) |
| LDL2‐C | 21.4 (14.6) | 27.4 (15.7) | <0.0001 | 22.4 (15.0) |
| LDL3‐C | 41.6 (17.1) | 45.4 (16.6) | <0.0001 | 42.2 (17.1) |
| LDL4‐C | 16.3 (11.4) | 12.1 (11.0) | <0.0001 | 15.6 (11.5) |
| Real LDL‐C a | 97.8 (32.9) | 106.1 (26.7) | <0.0001 | 99.1 (32.1) |
| Lp(a)‐C | 8.7 (5.2) | 6.8 (3.6) | <0.0001 | 8.4 (5.1) |
| Non–HDL‐C | 145.2 (38.6) | 151.2 (35.0) | <0.0001 | 146.2 (38.1) |
| HDL‐C | 53.6 (14.4) | 50.4 (13.2) | <0.0001 | 53.1 (14.3) |
| HDL2‐C | 13.6 (6.4) | 11.1 (5.0) | <0.0001 | 13.1 (6.3) |
| HDL3‐C | 40.1 (8.7) | 39.2 (8.7) | 0.01 | 39.9 (8.7) |
All values are in mg/dL. Values shown are means (SD) or median (25th, 75th percentile). Direct LDL‐C indicates low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; IDL‐C, intermediate‐density lipoprotein cholesterol; RLP‐C, remnant lipoprotein cholesterol; VLDL‐C, very low‐density lipoprotein cholesterol.
Real LDL‐C refers to cholesterol derived solely from LDL particles, excluding IDL and Lp(a).
CHD Events
There were 146 CHD events among the combined JHS and FOCS population. There were 63 MIs, 21 CHD deaths, and 28 revascularizations, for a total of 112 CHD events, among 4114 participants from the JHS during a mean of 5.6 years. There were 18 MIs, 1 CHD death, and 15 revascularizations, for a total of 34 CHD events, among 818 participants from the FOCS during a mean of 7.5 years. The association between RLP‐C and its components with CHD was linear in both studies (Figure S1).
Remnant Cholesterol and CHD in JHS
In unadjusted analyses, JHS participants experiencing incident CHD had a higher risk profile than did those without CHD during follow‐up (Table 3). Those experiencing CHD were older with a higher average systolic blood pressure, higher rates of diabetes, and a higher use of lipid‐lowering medications. The majority of the standard lipid profile measures of total cholesterol, HDL‐C, and non–HDL‐C, as well as directly measured LDL‐C, did not differ significantly with the exception of TGs, which were significantly higher in those experiencing incident CHD. Of note, while non–HDL‐C did not significantly differ, both IDL‐C and VLDL3‐C and their sum, RLP‐C, were significantly higher in the JHS participants experiencing incident CHD.
Table 3.
Unadjusted Baseline Cardiometabolic Risk Factors and Lipids Between Those With and Those Without Incident CHD in the Jackson Heart Study and the Framingham Offspring Cohort Study
| Variable | Jackson Heart Study | Framingham Offspring Cohort Study | ||||
|---|---|---|---|---|---|---|
| No CHD (n=4002) | CHD (n=112) | P Value | No CHD (n=784) | CHD (n=34) | P Value | |
| Males | 1417 (35%) | 46 (41%) | 0.22 | 371 (47%) | 24 (71%) | 0.008 |
| Age, y | 53.5 (12.7) | 64.8 (9.9) | <0.0001 | 57.2 (9.5) | 61.6 (8.3) | 0.007 |
| Diabetes | 629 (16%) | 49 (44%) | <0.0001 | 52 (7%) | 6 (18%) | 0.03 |
| Current smoking status | 498 (12%) | 14 (13%) | 0.99 | 110 (14%) | 8 (24%) | 0.13 |
| Body mass index, kg/m2 | 31.8 (7.3) | 29.8 (6.1) | 0.001 | 27.7 (5.0) | 29.2 (4.1) | 0.07 |
| Systolic blood pressure, mm Hg | 126.2 (17.9) | 135.1 (20.8) | <0.0001 | 125.8 (18.1) | 134.1 (16.8) | 0.009 |
| Diastolic blood pressure, mm Hg | 79.1 (10.4) | 76.6 (11.9) | 0.03 | 74.8 (9.3) | 76.4 (9.2) | 0.33 |
| Lipid‐altering medicationsa | 416 (10%) | 27 (24%) | <0.0001 | 59 (8%) | 7 (21%) | 0.02 |
| Total cholesterol, mg/dL | 198.7 (40.3) | 202.5 (47.5) | 0.32 | 201.0 (36.8) | 213.7 (44.8) | 0.05 |
| Triglycerides, mg/dL | 90 (67, 126) | 106 (81, 143) | 0.0002 | 154 (126, 185) | 175 (143, 207) | 0.003 |
| RLP‐C, mg/dL (VLDL3‐C+IDL‐C) | 29.6 (12.0) | 33.7 (13.0) | 0.0004 | 27.9 (11.5) | 31.5 (11.3) | 0.08 |
| IDL‐C, mg/dL | 16.3 (8.0) | 19.0 (8.5) | 0.0005 | 15.0 (8.5) | 17.3 (8.6) | 0.13 |
| VLDL‐C, mg/dL | 22.3 (9.6) | 24.7 (11.4) | 0.01 | 23.1 (6.9) | 25.4 (5.8) | 0.06 |
| VLDL1+2‐C, mg/dL | 9.1 (5.5) | 10.1 (6.6) | 0.06 | 10.2 (3.5) | 11.1 (3.1) | 0.14 |
| VLDL3‐C, mg/dL | 13.3 (4.7) | 14.7 (5.3) | 0.002 | 12.9 (3.8) | 14.2 (3.4) | 0.05 |
| Direct LDL‐C, mg/dL | 122.8 (36.1) | 125.5 (40.2) | 0.42 | 127.2 (31.5) | 145.3 (37.1) | 0.001 |
| LDL1‐C, mg/dL | 18.4 (8.8) | 20.6 (10.2) | 0.009 | 21.1 (7.3) | 23.1 (9.2) | 0.13 |
| LDL2‐C, mg/dL | 21.4 (14.6) | 20.8 (16.2) | 0.64 | 27.5 (15.6) | 25.9 (18.0) | 0.56 |
| LDL3‐C, mg/dL | 41.7 (17.1) | 38.8 (18.0) | 0.08 | 45.0 (16.5) | 56.3 (16.3) | <0.0001 |
| LDL4‐C, mg/dL | 16.3 (11.4) | 16.8 (11.5) | 0.65 | 11.9 (10.9) | 16.4 (12.8) | 0.02 |
| Real LDL‐C, mg/dL | 97.8 (32.8) | 97.0 (36.8) | 0.80 | 105.4 (26.4) | 121.7 (30.5) | 0.0005 |
| Lp(a)‐C, mg/dL | 8.7 (5.2) | 9.6 (5.8) | 0.06 | 6.8 (3.6) | 6.3 (4.3) | 0.45 |
| Non–HDL‐C, mg/dL | 145.0 (38.5) | 150.2 (42.2) | 0.16 | 150.3 (34.6) | 170.8 (39.8) | 0.0008 |
| HDL‐C, mg/dL | 53.7 (14.4) | 52.3 (15.3) | 0.33 | 50.7 (13.2) | 42.9 (10.3) | 0.0007 |
| HDL2‐C, mg/dL | 13.5 (6.4) | 13.9 (6.6) | 0.58 | 11.2 (5.1) | 8.8 (3.0) | 0.005 |
| HDL3‐C, mg/dL | 40.1 (8.7) | 38.5 (9.4) | 0.05 | 39.4 (8.7) | 34.1 (7.5) | 0.0005 |
Values are n (%), mean (SD), or median (25th, 75th percentile) where appropriate. CHD indicates coronary heart disease; direct LDL‐C indicates low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; IDL‐C, intermediate‐density lipoprotein cholesterol; RLP‐C, remnant lipoprotein cholesterol; VLDL‐C, very low‐density lipoprotein cholesterol.
Lipid‐altering medications include statins, bile sequestrants, niacin derivatives, and fibric acid derivatives.
In unadjusted models (Figure S2), increasing RLP‐C was associated with a 34% increased risk of CHD (HR 1.34 per 1‐SD increase, 95% CI 1.15–1.56, P<0.001). After adjustment for traditional cardiovascular risk factors in model 1, the HR remained significant (HR 1.18, 95% CI 1.00–1.39, P=0.049). When adjustment was made for model 1 variables and HDL‐C, the relationship was attenuated slightly and lost significance (HR 1.15, 95% CI 0.97–1.37, P=0.11). When further adjustment was made for real LDL‐C in the model, the relationship was similar (HR 1.13; 0.95–1.35; P=0.17).
The association of IDL‐C with CHD was borderline significant in the JHS model adjusted for risk factors and HDL‐C levels (HR 1.19, 95% CI 1.00–1.42, P=0.052). While VLDL3‐C was significantly associated with CHD in unadjusted models (HR 1.27, 95% CI 1.10–1.47, P<0.001), it was not independent of cardiovascular risk factors (HR 1.11, 95% CI 0.95–1.30, P=0.20).
Remnant Cholesterol and CHD in FOCS
In unadjusted analyses, FOCS participants experiencing incident CHD had a higher risk profile than those without CHD during follow‐up (Table 3). The group of participants experiencing CHD had a higher proportion of men, were older, and had higher rates of diabetes, a higher body mass index, a higher average systolic blood pressure, and a higher use of lipid‐lowering medications. In contrast to the JHS, multiple standard lipid parameters were less favorable among those experiencing CHD. The total cholesterol, TGs, LDL‐C, and non–HDL‐C values were significantly higher, while the HDL‐C was significantly lower, among those experiencing events. Notably, there was a trend for higher RLP‐C in those with incident CHD (P=0.08).
In unadjusted models (Figure S3), increasing RLP‐C was associated with a trend toward a 32% increased risk of CHD (HR 1.32, 95% CI 0.98–1.79, P=0.07). The association was strengthened after adjustment for traditional cardiovascular risk factors, particularly sex, as discussed later, in model 1 (HR 1.46, 95% CI 1.05–2.04, P=0.026). When adjustment was made for HDL‐C, the association of RLP‐C with CHD remained robust (HR 1.43, 95% CI 1.02–2.01, P=0.037). When adding real LDL‐C to the model, the association is lost (HR 1.08, 95% CI 0.71–1.64, P=0.717).
There was an association of IDL‐C with CHD in models adjusted for risk factors and HDL‐C levels (HR 1.48, 95% CI 1.05–2.08, P=0.03). However, the association of VLDL3‐C with CHD was attenuated in model 1 and when further adjusted for HDL‐C (HR 1.27, 95% CI 0.89–1.81, P=0.18).
Sex Differences in RLP‐C and CHD
In unadjusted models in FOCS, RLP‐C trended toward higher risk for CHD for men and women combined but did not meet statistical significance (HR 1.32, P=0.07). After further inspection, once sex is adjusted for, the association between RLP‐C and CHD events becomes significant (model 1: HR 1.46, P=0.026). The reason is that RLP‐C is higher for women than for men (mean of 29.2 versus 26.9, P=0.004), while the proportion experiencing CHD is significantly lower for women than for men (2% versus 6%, P=0.008). This inverse relationship is causing RLP‐C to become more significant when adjusted for sex in FOCS with or without model 1 covariates. Among JHS participants, sex differences did not exist (RLP‐C 30.5 mg/dL versus 29.2 mg/dL, CHD 2.5% versus 3.1% for women versus men).
In FOCS, there is significant interaction (P<0.05) across all models by sex such that the relationship between RLP‐C with CHD is significant in men but not in women (Table S1). In model 1, the RLP‐C hazard is significant in men (HR 1.78, P=0.001) but not in women (HR 0.71, P=0.35). In the fully adjusted model inclusive of HDL‐C and real LDL‐C, there is an inverse association in FOCS women suggesting that RLP‐C (driven by IDL‐C) is protective for CHD (HR 0.37, P=0.040). However, several factors suggest this is s statistical artifact. First, there were a limited number of events among FOCS women (10 events in 423 women). Second, RLP‐C is similarly distributed in FOCS women with and without events (29.2±12.5 versus 29.0±7.0, respectively). Finally, there was no significant interaction across sex in the well‐powered JHS for the association between RLP‐C and its components with CHD in any model (P>0.3, Table S2).
Combined Cohorts
Given similar findings in JHS and FOCS for RLP‐C, VLDL3‐C, and IDL‐C, we combined the 2 populations for a patient‐level, multivariable‐adjusted analysis. After adjustment for cardiovascular risk factors (Figure 1), there was a 23% increase in CHD risk per 1‐SD increase in RLP‐C (HR 1.23, 95% CI 1.06–1.42, P<0.01) and IDL‐C (HR 1.26, 95% CI 1.08–1.47, P<0.01). With further adjustment for HDL‐C, there was a slight attenuation of CHD risk with RLP‐C (HR 1.20, 95% CI 1.03–1.40, P=0.02). The association of RLP‐C with CHD after adjustment for risk factors and HDL‐C was driven by IDL‐C contributing a 25% increase in CHD risk (HR 1.25, 95% CI 1.07–1.46, P<0.001) rather than VLDL3‐C (HR 1.11, 95% CI 0.95–1.29, P=0.20). All associations were attenuated when including real LDL‐C in the model.
Figure 1.
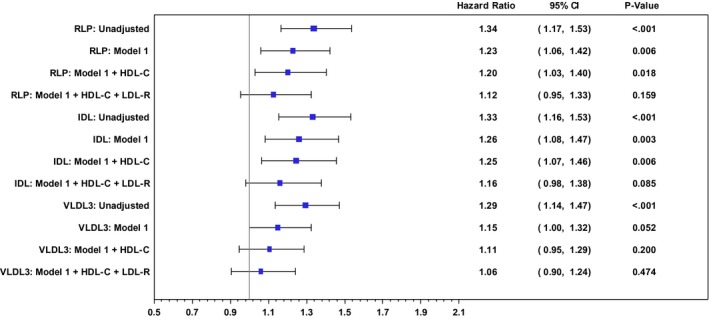
Forest plot of hazard ratios for CHD events in a combined population from the Jackson Heart and Framingham Offspring Cohort Studies. Hazard ratios are for a 1‐SD increase in remnant lipoprotein cholesterol (RLP‐C) and its components, IDL‐C and VLDL 3‐C, in unadjusted models, risk factor–adjusted models (model 1), and models inclusive of HDL‐C and real LDL‐C [LDL‐R; excludes IDL and Lp(a)]. Model 1 variables were age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures (SBP and DBP, respectively), lipid‐altering medications, and diabetes. HDL‐C indicates high‐density lipoprotein cholesterol; IDL‐C, intermediate‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Lp(a)‐C, lipoprotein(a) cholesterol; real LDL‐C, XXX; VLDL‐C, very low density lipoprotein cholesterol.
Interactions Among HDL‐C, LDL‐C, and RLP‐C
In the combined group, there is a trend toward an interaction between HDL‐C and RLP‐C levels such that the highest risk was found in those with the lowest HDL‐C and highest RLP‐C levels (P=0.07, Figure 2). This trend was largely driven by a significant interaction among JHS participants between the HDL2‐C subclass and RLP‐C (P=0.041, Figure S4). There were no significant interactions between real LDL‐C and RLP‐C (P=0.593, Figure S5).
Figure 2.
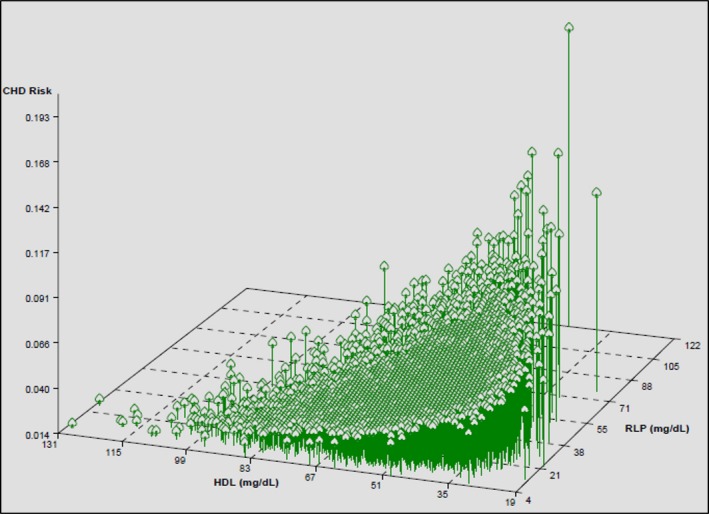
Three‐dimensional plot of modeled risk for coronary heart disease (CHD) by high‐density lipoprotein cholesterol (HDL‐C) and remnant lipoprotein cholesterol (RLP‐C) levels showing a trend toward the highest risk in those with the lowest HDL‐C and highest RLP‐C levels (P for interaction 0.066).
Discussion
In a diverse population of US adults free of prior CHD, our main finding was the association of RLP‐C with incident CHD during an 8‐year follow‐up. This risk was primarily attributable to IDL‐C levels. This is the largest prospective study of the association of remnants and incident CHD in US blacks, in whom standard lipid parameters including LDL‐C and HDL‐C were not predictive of CHD. This finding in US blacks is strikingly different from that in the predominantly white FOCS participants. In FOCS, traditional lipids, including LDL‐C, HDL‐C, and non–HDL‐C, were predictive of events, with a trend for RLP‐C to predict events.
Remnants and Events
Our primary findings of an association of RLP‐C levels with cardiovascular events are expected based on the results of prior cross‐sectional and prospective studies. In a prospective study of 135 patients with coronary artery disease who presented to a hospital in Japan and were followed for up to 3 years, higher RLP‐C levels were an independent predictor of recurrent coronary events.27 A prospective analysis of >1100 older Japanese American men from the Honolulu Heart Study found that RLP levels were independently predictive of incident CHD during 17 years.11 In a case‐control study of Korean patients presenting with stroke, RLP‐C levels were also associated with large artery atherosclerotic stroke.28 These initial studies suggested a link between RLPs and cardiovascular disease.
Building on this link, compelling evidence suggesting a causative role for RLPs stems from several elegant Mendelian randomization studies. In a cohort of >50 000 Danish participants, Jorgensen et al identified genetic variants that contributed to extreme elevations in TG levels.29 The investigators showed that these genetic elevations in calculated RLP‐C based on nonfasting TG levels were associated with an 87% increased odds of MI. In a slightly expanded study population inclusive of the same Danish cohort, Varbo et al analyzed genetic variants affecting single lipoprotein classes including nonfasting remnants, HDL‐C, and LDL‐C.16 The investigators found a causal odds ratio for ischemic heart disease of 2.8 for each 39‐mg/dL increase in nonfasting remnant cholesterol levels (again, as calculated from TG measurements). They went on to show that this effect may be mediated through inflammation.6 Importantly, in contrast to our analysis, these studies used surrogate measures of RLP‐C (TGs) rather than direct measures and recognized the limited ability to distinguish the causality of RLP‐C from TGs.24, 30, 31
In this context, our results demonstrating the association of directly measured RLP‐C levels with incident CHD validate the findings of these prior studies though with a more direct measure of RLPs than TGs. Further, these results extend prior findings concentrated in European or Asian populations to a diverse primary prevention population of middle‐aged men and women from the United States. In contrast to prior work, this is the first demonstration of this association in a large, prospective study of US blacks.
While there was a suggestion of no association of RLP‐C with CHD among FOCS women in our study, we were underpowered to make any conclusions, as there were only 10 CHD events in this group. In a cross‐sectional analysis from >1500 women in the Framingham Heart Study, RLP‐C levels were independently associated with prevalent cardiovascular disease.10 Careful attention should be paid to sex‐based differences in future analyses of remnants‐related risk.
Remnants and Atherosclerosis
The growing body of observational evidence suggesting a causative role for remnants in atherosclerotic disease compels consideration of biological plausibility. It is well established that LDL particles are able to traverse the arterial endothelium where they are retained and can initiate and propagate atherosclerosis.32 Due to their larger size, there has been skepticism over the capability of remnants and their precursors (chylomicrons, large VLDL) to contribute directly to atherosclerosis. However, type III hyperlipidemia and its characteristic excess of remnants with normal LDL particle levels are linked to premature atherosclerotic disease.33 Our study found that the RLP association was primarily driven by IDL. Prior studies have linked IDL to angiographic coronary atherosclerosis and carotid intima‐media thickness.34, 35, 36
Further, animal and human studies have demonstrated the retention of remnant particles in arterial walls.37, 38, 39 The atherogenic effects of RLPs on the endothelium include upregulation of proinflammatory cytokines, monocyte recruitment and activation, and increased production of prothrombotic factors.4 Changes in RLPs in the postprandial state may lead to endothelial dysfunction as measured by flow‐mediated dilation.40 In an immunohistochemical analysis of carotid plaque after endarterectomy, RLPs were significantly associated with carotid plaque macrophage content, a surrogate for plaque instability.41 In a small study correlating RLP‐C levels with subclinical atherosclerosis, RLP‐C was significantly associated with increased carotid intima‐media thickness in asymptomatic middle‐aged men.42
These studies suggest that RLPs are capable of orchestrating a variety of proatherogenic effects, including inflammation, thrombus formation, and endothelial dysfunction. Our findings add to the considerable body of evidence suggesting a causative role for RLPs in atherosclerosis.
Future Directions
Notably, there are strikingly different RLP‐C levels across studies.10, 27, 28, 43 These differences strongly suggest that the methods used (ie, immunoseparation, ultracentrifugation) have significantly different profiles or sensitivities for separating and measuring remnant lipoproteins. In the present study, the measured parameter reflects cholesterol from the dense VLDL3 subclass and IDL. In light of compelling emerging evidence suggesting the causal role of RLPs in atherosclerosis, a standardized definition of which lipoproteins constitute RLPs is urgently needed.
An important and necessary next step is to develop and test strategies targeted against RLPs to reduce cardiovascular disease. Recent Mendelian randomization studies suggested a prominent role of apoCIII activity in atherosclerotic disease by evaluating the effects of lifelong exposure to genetically reduced apoCIII function and reduced TG levels.14, 15 apoCIII is an integral component of RLPs and inhibits the function of lipoprotein lipase and hepatic lipase, while potentially promoting VLDL assembly.44, 45 Development of an antisense oligonucleotide inhibitor of apoCIII is under way, and phase 3 studies are anticipated.46 Our findings suggest targeting participants with elevated RLP‐C in such studies, as RLP‐C levels are likely a more specific measure of RLP burden compared with less specific surrogates such as TGs, which were not predictive in this population.
Limitations
There are limitations to our analysis. External generalizability to blacks throughout the United States and world is limited as the JHS only includes those from 1 geographic region. However, the limitation to the Jackson, MS, region has allowed for a rigorously conducted cohort study with strengthened internal validity for the JHS. Further, this allowed for the largest study of RLP‐C and incident CHD in US blacks. Similar results were seen in male participants of the predominantly white population of the FOCS supporting the broad applicability of the RLP‐C association with CHD; however, we were underpowered to appropriately assess this association in white women from the FOCS.
Another limitation is the use of fasting samples. In the fasting state and in type III hyperlipidemia, dense VLDL (ie, VLDL3) and IDL are the dominant constituents of circulating RLPs and so we focused on these classes in our fasting serum study.47 However, it remains plausible that chylomicron remnants are partially responsible for RLP‐associated risk and the method used in this study does not distinguish cholesterol from chylomicron remnants. Regardless of the measurement technique used, the association of RLP‐C with CHD has been remarkably consistent.
Finally, in contrast to the elegant Mendelian randomization studies that represent lifetime exposure to risk factors, the present study of population‐based cohorts uses one‐time measurements of potentially volatile risk factors that fluctuate over time. Despite this potential volatility, these one‐time measurements still captured a CHD risk association and represent a more clinically relevant approach.
Conclusion
In US population‐based studies of a diverse group of primary prevention subjects including black men and women, we found a consistent association of RLP‐C (mostly in the form of IDL‐C) with the risk for incident CHD. Importantly, our findings add to the considerable body of evidence stemming from European and Asian populations linking RLPs with CHD. The consistent observational evidence supports ongoing development of therapies to intervene regarding RLPs to reduce cardiovascular events.
Sources of Funding
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities, with additional support from the National Institute on Biomedical Imaging and Bioengineering. The Framingham Heart Study (FHS) is supported by the National Institutes of Health/NHLBI (Contract N01‐HC‐25195‐06). The FHS is conducted and supported by the NHLBI in collaboration with Boston University.
Disclosures
Drs Joshi and Martin have received support from the Pollin Cardiovascular Prevention Fellowship and NIH Training Grants T32HL007227 and T32HL07024, respectively. Dr Martin has also received support from the Marie‐Josée and Henry R. Kravis fellowship. Drs Martin and Jones are coinventors on a pending patent filed by Johns Hopkins University for a method of LDL‐C estimation. Drs Massaro and D'Agostino were funded by Atherotech Research for this work. Dr Blaha served on the advisory board for Pfizer. Dr Kulkarni is Atherotech research director and receives royalty from the University of Alabama at Birmingham. Dr Toth is consultant for Amgen, AstraZeneca, Kowa, Merck, and Novartis and on the speaker's bureau for Amarin, AstraZeneca, Genzyme GlaxoSmithKline, Kowa, and Merck. There are no disclosures for Mr Khokhar, Mr Lirette, Dr Griswold, and Dr Correa.
Supporting information
Appendix S1. Lipoprotein Investigators Collaborative (LIC) Study Group
Table S1. Framingham Offspring Cohort Study
Table S2. Jackson Heart Study
Figure S1. Splines demonstrating approximately linear association between remnant lipoprotein cholesterol (RLP‐C) and coronary heart disease (CHD) risk in the Jackson Heart and Framingham Offspring Cohort Studies. There were no significant inflection points in the correlations. Similar correlations were seen with RLP components (IDL‐C and VLDL3‐C).
Figure S2. Forest plot of hazard ratios for coronary heart disease (CHD) events in the Jackson Heart Study. Hazard ratios are associated with 1‐SD increase in remnant lipoprotein cholesterol (RLP‐C) and its components, IDL‐C and VLDL3‐C, in unadjusted models, risk factor–adjusted models (model 1), and models inclusive of HDL‐C and real LDL‐C [LDL‐R; excludes IDL and Lp(a)]. Model 1 variables were age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures (SBP and DBP), lipid‐altering medications, and diabetes.
Figure S3. Forest plot of hazard ratios for coronary heart disease (CHD) events in the Framingham Offspring Cohort Study. Hazard ratios are associated with 1‐SD increase in remnant lipoprotein cholesterol (RLP‐C) and its components, IDL‐C and VLDL3‐C, in unadjusted models, risk factor–adjusted models (model 1), and models inclusive of HDL‐C and real LDL‐C [LDL‐R; excludes IDL and Lp(a)]. Model 1 variables were age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures (SBP and DBP), lipid‐altering medications, and diabetes.
Figure S4. Three‐dimensional plot of modeled risk for coronary heart disease (CHD) by HDL subclasses and remnant lipoprotein cholesterol (RLP‐C) levels in combined populations from the Jackson Heart and sample of Framingham Offspring Cohort Studies. There is significant interaction between HDL2‐C and RLP‐C levels to predict CHD (P for interaction 0.023). The interaction between HDL3‐C and RLP‐C does not meet significance (P for interaction 0.169).
Figure S5. Three‐dimensional plot of modeled risk for coronary heart disease (CHD) by real LDL‐C [excludes IDL‐C and Lp(a)‐C] and remnant lipoprotein cholesterol (RLP‐C) levels in combined populations from the Jackson Heart and sample of Framingham Offspring Cohort Studies (P for interaction 0.593).
(J Am Heart Assoc. 2016;5:e002765 doi: 10.1161/JAHA.115.002765)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, De Ferranti S, Despres J, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, Mcguire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics‐2015 update: a report from the American Heart Association. Circulation. 2015;131:E29–E322. [DOI] [PubMed] [Google Scholar]
- 2. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris‐Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; American Heart Association Clinical Lipidology T, Prevention Committee Of The Council On Nutrition Pa, Metabolism, Council On Arteriosclerosis T, Vascular B, Council On Cardiovascular N And Council On The Kidney In Cardiovascular D . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. [DOI] [PubMed] [Google Scholar]
- 3. Sniderman AD, Williams K, Contois JH, Monroe HM, Mcqueen MJ, De Graaf J, Furberg CD. A meta‐analysis of low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. [DOI] [PubMed] [Google Scholar]
- 4. Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16:145–154. [DOI] [PubMed] [Google Scholar]
- 5. Moyer MP, Tracy RP, Tracy PB, Van't Veer C, Sparks CE, Mann KG. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arterioscler Thromb Vasc Biol. 1998;18:458–465. [DOI] [PubMed] [Google Scholar]
- 6. Varbo A, Benn M, Tybjaerg‐Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. [DOI] [PubMed] [Google Scholar]
- 7. Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. [DOI] [PubMed] [Google Scholar]
- 8. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 9. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 10. McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant‐like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–236. [DOI] [PubMed] [Google Scholar]
- 11. Imke C, Rodriguez BL, Grove JS, McNamara JR, Waslien C, Katz AR, Willcox B, Yano K, Curb JD. Are remnant‐like particles independent predictors of coronary heart disease incidence? The Honolulu Heart Study Arterioscler Thromb Vasc Biol. 2005;25:1718–1722. [DOI] [PubMed] [Google Scholar]
- 12. Emerging Risk Factors C , Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. [DOI] [PubMed] [Google Scholar]
- 14. Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG, Tybjaerg‐Hansen A. Loss‐of‐function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 15. TG, HDL Working Group Of The Exome Sequencing Project NHL, Blood I , Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, Depristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho‐Melander M, Ardissino D, Loos RJ, Mcpherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss‐of‐function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varbo A, Benn M, Tybjaerg‐Hansen A, Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz GG, Abt M, Bao W, Demicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–2275. [DOI] [PubMed] [Google Scholar]
- 18. Joshi PH, Martin SS, Blumenthal RS. The remnants of residual risk. J Am Coll Cardiol. 2015;65:2276–2278. [DOI] [PubMed] [Google Scholar]
- 19. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6‐4‐17. [PubMed] [Google Scholar]
- 20. Feinleib M, Kannel WB, Garrison RJ, Mcnamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 21. D'agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 22. Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26:787–802. [DOI] [PubMed] [Google Scholar]
- 23. Hegele RA. Monogenic dyslipidemias: window on determinants of plasma lipoprotein metabolism. Am J Hum Genet. 2001;69:1161–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones SR, Martin SS, Brinton EA. Letter by Jones et al. regarding article, “Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation”. Circulation. 2014;129:E655. [DOI] [PubMed] [Google Scholar]
- 25. Martin SS, Faridi KF, Joshi PH, Blaha MJ, Kulkarni KR, Khokhar AA, Maddox TM, Havranek EP, Toth PP, Tang F, Spertus JA, Jones SR. Remnant lipoprotein cholesterol and mortality after acute myocardial infarction: further evidence for a hypercholesterolemia paradox from the TRIUMPH Registry. Clin Cardiol. 2015;38:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, Blaha MJ, Kulkarni KR, Khokhar AA, Correa A, D'agustino RB Sr, Jones SR; On Behalf Of The Lipoprotein Investigators Collaborative Study G . Association of high‐density lipoprotein subclasses and incident coronary heart disease: the Jackson Heart and Framingham Offspring Cohort Studies. Eur J Prev Cardiol. 2016;23:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99:2858–2860. [DOI] [PubMed] [Google Scholar]
- 28. Kim JY, Park JH, Jeong SW, Schellingerhout D, Park JE, Lee DK, Choi WJ, Chae SL, Kim DE. High levels of remnant lipoprotein cholesterol is a risk factor for large artery atherosclerotic stroke. J Clin Neurol. 2011;7:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jorgensen AB, Frikke‐Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg‐Hansen A. Genetically elevated non‐fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–1833. [DOI] [PubMed] [Google Scholar]
- 30. Wurtz P, Kangas AJ, Soininen P, Lehtimaki T, Kahonen M, Viikari JS, Raitakari OT, Jarvelin MR, Davey Smith G, Ala‐Korpela M. Lipoprotein subclass profiling reveals pleiotropy in the genetic variants of lipid risk factors for coronary heart disease: a note on Mendelian randomization studies. J Am Coll Cardiol. 2013;62:1906–1908. [DOI] [PubMed] [Google Scholar]
- 31. Varbo A, Benn M, Tybjaerg‐Hansen A, Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Response: lipoprotein subclass profiling reveals pleiotropy in the genetic variants of lipid risk factors for coronary heart disease: a note on Mendelian randomization studies. J Am Coll Cardiol. 2013;62:1908–1909. [DOI] [PubMed] [Google Scholar]
- 32. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 33. Fung M, Hill J, Cook D, Frohlich J. Case series of type III hyperlipoproteinemia in children. BMJ Case Rep. 2011; doi: 10.1136/bcr.02.2011.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krauss RM, Lindgren FT, Williams PT, Kelsey SF, Brensike J, Vranizan K, Detre KM, Levy RI. Intermediate‐density lipoproteins and progression of coronary artery disease in hypercholesterolaemic men. Lancet. 1987;2:62–66. [DOI] [PubMed] [Google Scholar]
- 35. Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS). Treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol. 1996;16:697–704. [DOI] [PubMed] [Google Scholar]
- 36. Hodis HN, Mack WJ, Dunn M, Liu C, Liu C, Selzer RH, Krauss RM. Intermediate‐density lipoproteins and progression of carotid arterial wall intima‐media thickness. Circulation. 1997;95:2022–2026. [DOI] [PubMed] [Google Scholar]
- 37. Daugherty A, Lange LG, Sobel BE, Schonfeld G. Aortic accumulation and plasma clearance of beta‐VLDL and HDL: effects of diet‐induced hypercholesterolemia in rabbits. J Lipid Res. 1985;26:955–963. [PubMed] [Google Scholar]
- 38. Proctor SD, Mamo JC. Intimal retention of cholesterol derived from apolipoprotein B100‐ and apolipoprotein B48‐containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2003;23:1595–1600. [DOI] [PubMed] [Google Scholar]
- 39. Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie‐Hardman J, Kotite L, Kunitake ST, Havel RJ, Kane JP. Triglyceride‐rich lipoproteins isolated by selected‐affinity anti‐apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14:1767–1774. [DOI] [PubMed] [Google Scholar]
- 40. Maggi FM, Raselli S, Grigore L, Redaelli L, Fantappie S, Catapano AL. Lipoprotein remnants and endothelial dysfunction in the postprandial phase. J Clin Endocrinol Metab. 2004;89:2946–2950. [DOI] [PubMed] [Google Scholar]
- 41. Zambon A, Puato M, Faggin E, Grego F, Rattazzi M, Pauletto P. Lipoprotein remnants and dense LDL are associated with features of unstable carotid plaque: a flag for non‐HDL‐C. Atherosclerosis. 2013;230:106–109. [DOI] [PubMed] [Google Scholar]
- 42. Karpe F, Boquist S, Tang R, Bond GM, De Faire U, Hamsten A. Remnant lipoproteins are related to intima‐media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res. 2001;42:17–21. [PubMed] [Google Scholar]
- 43. Mcnamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant lipoprotein cholesterol and triglyceride reference ranges from the Framingham Heart Study. Clin Chem. 1998;44:1224–1232. [PubMed] [Google Scholar]
- 44. Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very‐low‐density lipoprotein and low‐density lipoprotein containing apolipoprotein C‐III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, Hussain MM, Parks RJ, Wang Y, Yao Z. Expression of apolipoprotein C‐III in McA‐RH7777 cells enhances VLDL assembly and secretion under lipid‐rich conditions. J Lipid Res. 2010;51:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Graham MJ, Lee RG, Bell TA III, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, Baker BF, Burkey J, Crooke ST, Crooke RM. Antisense oligonucleotide inhibition of apolipoprotein C‐III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–1490. [DOI] [PubMed] [Google Scholar]
- 47. Wang T, Nakajima K, Leary ET, Warnick GR, Cohn JS, Hopkins PN, Wu LL, Cilla DD, Zhong J, Havel RJ. Ratio of remnant‐like particle‐cholesterol to serum total triglycerides is an effective alternative to ultracentrifugal and electrophoretic methods in the diagnosis of familial type III hyperlipoproteinemia. Clin Chem. 1999;45:1981–1987. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Lipoprotein Investigators Collaborative (LIC) Study Group
Table S1. Framingham Offspring Cohort Study
Table S2. Jackson Heart Study
Figure S1. Splines demonstrating approximately linear association between remnant lipoprotein cholesterol (RLP‐C) and coronary heart disease (CHD) risk in the Jackson Heart and Framingham Offspring Cohort Studies. There were no significant inflection points in the correlations. Similar correlations were seen with RLP components (IDL‐C and VLDL3‐C).
Figure S2. Forest plot of hazard ratios for coronary heart disease (CHD) events in the Jackson Heart Study. Hazard ratios are associated with 1‐SD increase in remnant lipoprotein cholesterol (RLP‐C) and its components, IDL‐C and VLDL3‐C, in unadjusted models, risk factor–adjusted models (model 1), and models inclusive of HDL‐C and real LDL‐C [LDL‐R; excludes IDL and Lp(a)]. Model 1 variables were age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures (SBP and DBP), lipid‐altering medications, and diabetes.
Figure S3. Forest plot of hazard ratios for coronary heart disease (CHD) events in the Framingham Offspring Cohort Study. Hazard ratios are associated with 1‐SD increase in remnant lipoprotein cholesterol (RLP‐C) and its components, IDL‐C and VLDL3‐C, in unadjusted models, risk factor–adjusted models (model 1), and models inclusive of HDL‐C and real LDL‐C [LDL‐R; excludes IDL and Lp(a)]. Model 1 variables were age, sex, body mass index (BMI), current smoking, systolic and diastolic blood pressures (SBP and DBP), lipid‐altering medications, and diabetes.
Figure S4. Three‐dimensional plot of modeled risk for coronary heart disease (CHD) by HDL subclasses and remnant lipoprotein cholesterol (RLP‐C) levels in combined populations from the Jackson Heart and sample of Framingham Offspring Cohort Studies. There is significant interaction between HDL2‐C and RLP‐C levels to predict CHD (P for interaction 0.023). The interaction between HDL3‐C and RLP‐C does not meet significance (P for interaction 0.169).
Figure S5. Three‐dimensional plot of modeled risk for coronary heart disease (CHD) by real LDL‐C [excludes IDL‐C and Lp(a)‐C] and remnant lipoprotein cholesterol (RLP‐C) levels in combined populations from the Jackson Heart and sample of Framingham Offspring Cohort Studies (P for interaction 0.593).


