Abstract
Background
A higher milk consumption may be associated with a lower stroke risk. We conducted a comprehensive systematic review and dose–response meta‐analysis of milk and other dairy products in relation to stroke risk.
Methods and Results
Through a systematic literature search, prospective cohort studies of dairy foods and incident stroke in stroke‐free adults were identified. Random‐effects meta‐analyses with summarized dose–response data were performed, taking into account sources of heterogeneity, and spline models were used to systematically investigate nonlinearity of the associations. We included 18 studies with 8 to 26 years of follow‐up that included 762 414 individuals and 29 943 stroke events. An increment of 200 g of daily milk intake was associated with a 7% lower risk of stroke (relative risk 0.93; 95% CI 0.88–0.98; P=0.004; I2=86%). Relative risks were 0.82 (95% CI 0.75–0.90) in East Asian and 0.98 (95% CI 0.95–1.01) in Western countries (median intakes 38 and 266 g/day, respectively) with less but still considerable heterogeneity within the continents. Cheese intake was marginally inversely associated with stroke risk (relative risk 0.97; 95% CI 0.94–1.01 per 40 g/day). Risk reductions were maximal around 125 g/day for milk and from 25 g/day onwards for cheese. Based on a limited number of studies, high‐fat milk was directly associated with stroke risk. No associations were found for yogurt, butter, or total dairy.
Conclusions
Milk and cheese consumption were inversely associated with stroke risk. Results should be placed in the context of the observed heterogeneity. Future epidemiological studies should provide more details about dairy types, including fat content. In addition, the role of dairy in Asian populations deserves further attention.
Keywords: dairy products, meta‐analysis, prospective cohort study, stroke, systematic review
Subject Categories: Cardiovascular Disease
Introduction
Stroke is the second‐leading global cause of death, accounting for 11% of total deaths worldwide,1 and a major cause of long‐term disability.2 East Asian countries such as Japan and China have greater mortality and morbidity from stroke than from coronary heart disease, whereas it is the opposite in Western countries.3 A healthy diet is important for the primary prevention of stroke.4, 5 In Western as well as Asian countries, dairy consumption is recommended as part of a healthy diet.6, 7, 8, 9 For example, in the United States, 3 daily servings of dairy, mainly low‐fat or fat‐free, is recommended.7 The Chinese and Japanese recommendations are 300 mL of daily dairy8 and 2 daily servings of milk and dairy products, respectively.9
In 2011, we observed a nonsignificant inverse association of milk with stroke risk with a relative risk (RR) of 0.87 (95% CI: 0.72–1.07) per 200 mL of daily intake in a meta‐analysis.10 This meta‐analysis was, however, based on only 6 cohort studies and showed large heterogeneity, partly due to a strong inverse association in a Japanese cohort.11 In a more recent meta‐analysis of dairy consumption and stroke risk, the pooled RR was 0.91 (95% CI 0.82–1.01) for high versus low milk intake with large heterogeneity, based on 9 studies,12 including 1 study in children.13 Based on 6 studies, the association was nonlinear.12
Several new prospective cohort studies14, 15, 16, 17 have become available on the association between dairy consumption and stroke risk, amounting to a total of 18 studies. Most evidence of dairy in relation to stroke risk relates to milk consumption. However, a considerable amount of heterogeneity was present in 2 previous meta‐analyses in relation to the results of milk.10, 12 Previous investigations on heterogeneity were limited based on the number of available studies10 or were limited to the analysis of total dairy only.12 We conducted a comprehensive dose–response meta‐analysis, taking into account potential nonlinear associations and sources of heterogeneity (such as continent, type of stroke, and fat content), for which additional or unpublished data were obtained from investigators.
Methods
Literature Search and Selection
This review was conducted in accordance with the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.18 Published articles, without language restrictions, up to October 2015 were retrieved from PubMed, EMBASE, and SCOPUS (search strategy and MOOSE checklist are shown in Data S1), complemented by hand searches of reference lists and correspondence with researchers in the field. Based on titles and abstract, we excluded studies on animals, children aged <18 years, and patient populations. Eligible studies were selected using predefined criteria (ie, prospective design and reported data on dairy consumption in relation to incident fatal or total stroke). For 21 eligible articles, the full text was retrieved. Several authors provided additional information upon request.14, 15, 19, 20, 21, 22, 23, 24, 25 One of the authors (A.P.) additionally provided unpublished data from the Singapore Chinese Health Study, a population‐based cohort of 63 257 Chinese adults (A.P., unpublished results, 2015).
Two articles were excluded because the available data did not allow dose–response calculations.15, 18 In case of duplicate results,26, 27 we included the most updated23 or comprehensive27 results. For the Nurses’ Health Study we used 2 articles: 1 on low‐fat and high‐fat dairy23 and 1 on other types of dairy.28 One article provided results for 2 studies (Nurses’ Health Study and the Health Professionals Follow up Study)23 and 2 presented results for men and women separately,19, 29 resulting in 18 studies (see flow chart in Figure 1).11, 14, 15, 16, 17, 19, 20, 21, 22, 23, 24, 25, 28, 29, 30, 31, 32, 33
Figure 1.
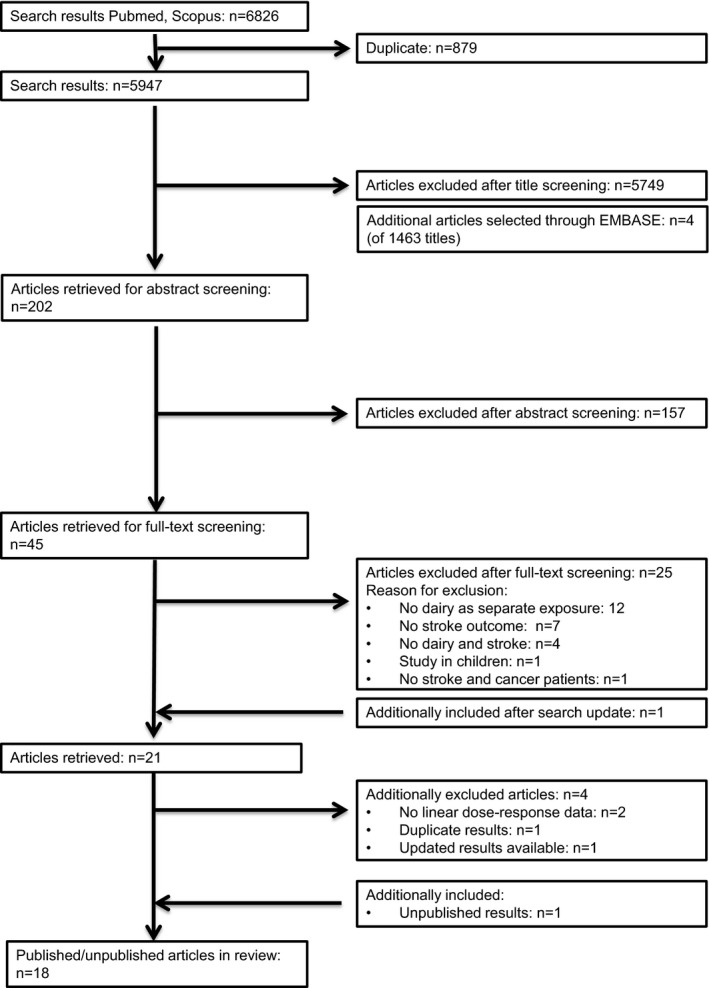
Flow chart of literature search for meta‐analysis on dairy intake and incident stroke.
Data Extraction
The selection and data extraction process was conducted by the first author (J.G.) and checked by a coauthor (S.S.S.‐M.) using a structured extraction form. We extracted descriptive study data as well as ranges of intake, medians or midpoints, numbers of subjects and stroke events, person‐years at risk, and RRs with the corresponding 95% CIs for each category of dairy intake (ie, total dairy, low‐fat dairy, high‐fat dairy, fermented dairy, milk, low‐fat milk, high‐fat milk, cheese, yogurt, and butter).
If dairy intake was only reported in portions,11, 23, 28, 31, 33 we used portion sizes of 177 g for total, low‐fat, and high‐fat dairy; 244 g for total, low‐fat, and high‐fat milk; 244 g for yogurt; and 43 g for cheese to estimate grams per day34, 35 or we used previously reported serving sizes of the same cohort.23, 28 For open‐ended upper limits of intake, we applied the same width as the adjacent category, whereas for open‐ended lowest categories a zero was assigned.
Categorization of dairy types was primarily based on the categorization in the original studies (see Data S1). For the meta‐analysis on fermented dairy we included studies that combined at least 2 of the products cheese, yogurt, and sour milk. If results on total stroke (primary outcome) were not available, we used ischemic stroke.17, 28 Two reviewers (J.G. and S.S.S.‐M.) independently evaluated the quality of the studies by using the Newcastle‐Ottawa quality assessment scale (Data S1).36 The rating system scores studies from 0 (highest degree of bias) to 9 (lowest degree of bias).
Statistical Analysis
We performed meta‐analyses for dairy types reported upon by 3 or more studies. If studies presented several statistical models, we included the model that included most confounders. Median intakes for each dairy type were estimated as the average of medians, midpoints, or means per study, weighted by study size. For 4 or more studies, linearity of associations was investigated using spline analysis and dose–response meta‐regression (Generalized Least‐Square Trend). Three studies could not be used for nonlinear analyses, because they only provided linear results.16, 17, 29
Splined variables were created using MKSPLINE in STATA in order to select the most appropriate knot points of nonlinear associations based on goodness‐of‐fit tests and χ2 statistics. Linear and nonlinear associations were further analyzed using dose–response (Generalized Least‐Square Trend) meta‐regression analysis. Random‐effects meta‐regression trend estimation of summarized dose–response data37 was used to derive the incremental dose–response RRs.
Forest plots were created to visualize linear dose–response slopes and corresponding 95% CIs across studies with increments of 200 g/day for total, low‐fat, and high‐fat dairy, fermented dairy, total milk, low‐fat and high‐fat milk, per 100 g/day for yogurt, 40 g/day for cheese, and 10 g/day for butter. The shape of the associations within individual studies was visualized by means of Ding's spaghetti plots, as described previously.38
To explore between‐study heterogeneity, the Cochrane Q test was conducted and the I2 statistic was calculated.39 Meta‐regression and subgroup analyses to explore sources of heterogeneity based on the linear analyses were performed for percentage of men, the Newcastle‐Ottawa Quality score (<7 versus ≥7), continent (East Asian versus Western including Australia), outcome (fatal versus total incident stroke), stroke subtype (ischemic and hemorrhagic) versus total stroke, follow‐up time (≥15 years versus <15 years), and whether analyses were adjusted for age, sex, body mass index, smoking, total energy intake, physical activity, and dietary factors. Potential publication bias was assessed by the Eggers test and symmetry of the funnel plot40 if at least 10 studies were available. Statistical analyses were performed using STATA version 11.0 (StataCorp, College Station, TX).
Results
Table 1 shows the characteristics of the 18 prospective cohort studies11, 15, 16, 17, 19, 20, 21, 22, 23, 24, 25, 28, 29, 30, 31, 32, 33 including 1 case‐cohort study,29 which comprised 762 414 participants and 29 943 stroke events. Two studies presented sex‐specific results,19, 29 2 comprised only women,17, 28 and 3 only men.20, 30, 32 Eight studies were from Europe,15, 16, 20, 21, 22, 29, 30, 32 5 were from east Asia (China and Japan),11, 19, 24, 31 3 were from the United States, 17, 23, 28 and 1 was from Australia.33 The Asian populations mainly consumed milk. The sample size of the studies ranged from 2061 to 223 170 and the duration of follow‐up from 8 to 26 years. Six studies reported fatal stroke,11, 19, 29, 30, 33 10 total stroke,16, 17, 20, 22, 23, 24, 25, 28, 32 and 1 both outcomes.15 All studies adjusted for age, sex, and smoking. Eight studies additionally adjusted for body mass index, physical activity, total energy intake, and other dietary factors.15, 20, 22, 23, 25, 29 Five studies scored <7 points for study quality.11, 16, 24, 30, 31
Table 1.
Characteristics of 18 Prospective Cohort Studies on Dairy Consumption and Stroke Risk
| Study, Country | Men (%); Mean Age (Range) | Follow‐Up Time (Years) (Range) | No. Events (Cohort Size) | Dairy Types | Dietary Method, Years | Outcome; Source | Adjustments |
|---|---|---|---|---|---|---|---|
|
Bernstein 2012,23
Health Professionals Follow‐up Study |
100; (30–55) | 19.3 | 1397 (43 150) |
Low‐fat dairy High‐fat dairy |
1986, 131 item FFQ with updated information in 1990, 1994, 1998, 2002, 2006. | Total stroke; Registries and medical records | Stratified on age, time period, adjusted for BMI, cigarette smoking, PA, parental history of early MI, multivitamin use, vitamin E supplement use, aspirin use, intake of total energy, cereal fiber, alcohol, trans fat, fruits and vegetables, other protein sources |
|
Bernstein 2012,23
Nurses’ Health Study |
0; (30–55) | 24.3 | 2633 (84 010) |
Low‐fat dairy High‐fat dairy |
1980, 61 item FFQ with updated and extended information in 1986, 1990, 1994, 1998, 2002. | Total stroke; Registries and medical records | Stratified on age, time period, adjusted for BMI, cigarette smoking, PA, parental history of early MI, menopausal state, multivitamin use, vitamin E supplement use, aspirin use, intake of total energy, cereal fiber, alcohol, trans fat, fruits and vegetables, other protein sources |
|
Dalmeijer 2013,22
EPIC‐NL, The Netherlands |
26; 49 | 13.1 | 531 (33 625) |
Total dairy Low‐fat dairy High‐fat dairy milk products Fermented dairy Cheese |
1993–1997, 178‐item FFQ | Total stroke; Registries | Sex, age, total energy, PA, smoking, education, BMI, intake of ethanol, coffee, fruit, vegetables, fish, meat, bread |
| Elwood 2004,32 Caerphilly, UK | 100; 52 (45–59) | 22a (20–24) | 185 (2403) | Milk | 1979–1983, FFQ | Total stroke; GP and hospital records | Age, total energy, smoking, social class, BMI, SBP, consumption of alcohol, fat, prior vascular disease |
|
Goldbohm 2011,29 Netherlands Cohort Study, The Netherlands Men Women |
100; 0; (55–69) | 10a |
657 (12 912) 397 (7870) |
Low‐fat dairy milk products Low‐fat milk (nonfermented) High‐fat milk (nonfermented) fermented dairyb Cheese Butter |
1986, 150‐item FFQ | Fatal stroke; National registries | Age, education, smoking (cigarette, cigar, pipe), nonoccupational PA, occupational PA, BMI, multivitamin use, alcohol, total energy, MUFA, PUFA, vegetables, fruits |
|
Iso 1999,28
Nurses’ Health Study, USA |
0; 46 | 13.6 |
347 (85 764) |
Milk Low‐fat milk High‐fat milk Yogurt Cheese |
1980, 61‐item FFQ | Ischemic stroke; Medical records, national registries, relatives, death certificates | Age, smoking, time period, BMI, alcohol, menopausal status (including hormone replacement therapy), PA, multivitamin use, vitamin E supplementation, history of hypertension, DM, and hypercholesterolemia |
| Kinjo 1999,11 Six prefectures, Japan | 46c; (40–69) | 15a |
Total stroke: 11 030; Ischemic stroke: 4084 (223 170) |
Milk | 1965, 1‐page questionnaire | Fatal total, ischemic, and hemorrhagic stroke; Death registry (ICD‐7) | Sex, age, follow‐up interval, prefecture, alcohol drinking, smoking, occupation |
|
Kondo, 2013,19
NIPPON, Japan Men Women |
100 0 50.3 (>30) |
24a |
217 (4045) 200 (5198) |
Milk and dairy productsd | 1980, weighed diet records during 3 consecutive weekdays | Fatal stroke; death registry (ICD‐9; 430–438) | Age, BMI, smoking, alcohol drinking habit, history of DM, use of antihypertensives, work category, total energy intake |
|
Larsson 200920
ATBC, Finland |
100; 57.6 (50–69) | 13.6 | 3365 (26 556) |
Total dairy Milk Low‐fat milk High‐fat milk Yogurt Cheese Butter |
1985–1988, 276‐item FFQ | Total, ischemic, and hemorrhagic stroke; national registries (ICD8: 430–434, 436 or ICD9 or ICD10) | Age, supplementation group, education, cigarettes, BMI, serum total cholesterol, serum HDL‐cholesterol, history of DM and heart disease, leisure‐time PA, total energy, alcohol, caffeine, sugar, red meat, poultry, fish, fruit, fruit juices, vegetables, potatoes, whole grains, refined grains |
|
Larsson 2012,21
Swedish Mammography Cohort, Cohort of Swedish Men, Sweden |
54; 60.3 (45–83) | 10.2 | 4089 (74 961) |
Total dairy Low‐fat dairy High‐fat dairy Milk Fermented dairyb Cheese |
1997, 96‐item FFQ | Total, ischemic, or hemorrhagic stroke; Swedish national hospital discharge registry (ICD10: I60, I61, I63, I64) | Age, sex, smoking status, smoking pack years, education, BMI, PA, aspirin use, history of hypertension, DM, family history of MI, total energy, alcohol, coffee, fresh red meat, processed meat, fish, fruits, vegetables, mutually adjusted for other dairy types |
|
Lin 2013,24
CVDFACTS, China |
23; 45.8 | 10.5a (9–12) | 123 (2061) | Total dairyd | 1990–1993, 49‐item FFQ | Total stroke; self‐report confirmed by medical records, death certificate (ICD9: 430–438) | Sex, age, urinary sodium/creatinine, smoking status, drinking status, PA, BMI, SBP change, DBP change, hypertension medication |
| Louie 2013,33 BMES, Australia | 44; 65.4 | 15a | 158 (2662) |
Total dairy Low‐fat dairy High‐fat dairy |
1992–1994, 145‐item FFQ | Fatal stroke; National death registry (ICD9 and ICD10; I60–I69) | Age, sex, total energy, BMI, weight change, PA, previous MI, previous stroke, smoking, stage II hypertension, type 2 DM, use of antihypertensive medication, use of statins, change in dairy intake |
| Misirli 2012,16 EPIC Greece, Greece | 41; NR | 10.6 | 395 (23 601) | Total dairy | 1994–1999, 150‐item FFQ, validated, but no information on validation provided | Total stroke, fatal stroke; I60 to I69, G45, G46; Self‐report verified by pathology reports, medical records, discharge diagnosis, or death certificates. | Sex, age, education, smoking, BMI, PA, hypertension, DM, total energy intake |
|
Ness 200130
Collaborative Study, Scotland |
100; 48.3 (35–64) | 25a | 196 (5765) | Milk | 1970–1973, FFQ validated, 1 question on usual milk intake | Fatal stroke; Death registry | Age, smoking, DBP, cholesterol, BMI, adjusted forced expiratory volume, social class, father's social class, education, deprivation category, siblings, car user, angina, electrocardiogram ischemia, bronchitis, and alcohol consumption |
| Pan, Singapore Chinese Cohort Study, Singapore (unpublished data) | 45; 60a (45–74) | 14.7 |
1098 (total stroke) 579 (ischemic stroke) (57 078) |
Total dairy Milk |
1993–1998, 165‐item FFQ | Fatal total, ischemic, and hemorrhagic stroke; Registry linkage | Age, sex, dialect, year of interview, educational level, BMI, PA, smoking status, alcohol use, baseline history of self‐reported DM, hypertension, and total energy intake, dietary intakes of red meat, poultry, fish, vegetables, fruit, all grains, tea and coffee |
|
Praagman 201515
Rotterdam Study, The Netherlands |
38; 66.9 (>55) | 17.3 | 182 (4235) |
Total dairy Low‐fat dairy High‐fat dairy Milk Fermented dairy Cheese Yogurt |
1990–1993, validated 170‐item FFQ | Total stroke, fatal stroke; Medical records, validated by a specialist and (incident stroke) by linkage with GP and medical specialists (ICD10: I60–I69) | Sex, age, total energy intake, BMI, smoking, education, alcohol, vegetables, fruit, meat, bread, fish, coffee, tea |
|
Sauvaget 200331
Life‐span study, Japan |
38; 56 | 15.5a (15–16) | 1094 (31 831)e | Milk | 1979–1981, 22‐item FFQ, validated for animal products | Fatal stroke; National registration (ICD9) and death certificates | Stratified on sex, birth cohort, adjusted for city, radiation dose, self‐reported BMI, smoking status, alcohol habits, education level, history of DM or hypertension |
|
Sonestedt 201125
Malmö, diet and cancer cohort, Sweden |
38; 57.3 (44–74) | 12 | 1176 (26 445) |
Total dairy Milk Fermented dairyb Low‐fat milk High‐fat milk Cheese Butter |
1991–1996, modified diet history method (7 days) and 168‐item questionnaire | Total stroke; Hospital discharge register and cause‐of‐death register, local stroke register of Malmö (ICD9; 430, 431, 434, 436) | Age, sex, season, method, energy intake, BMI, smoking, alcohol consumption, leisure‐time PA, education |
|
Yaemsiri 201217
WHI‐OS, USA |
0; 63.5 |
7.6 (7–11) |
1049 (87 025) | Total dairy | 1994–1998, 120‐item FFQ at baseline and after 3 years | Incident ischemic stroke | Age, race, education, family income, smoking years, hormone replacement therapy, metabolic equivalent task hours per week, alcohol intake, history of coronary heart disease, atrial fibrillation, DM, use of aspirin, antihypertensive medication, cholesterol‐lowering medication, BMI, SBP, total energy intake |
BMI indicates body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FFQ, food frequency questionnaire; GP, general practitioner; ICD, International Classification of Diseases; MI, myocardial infarction; MUFA, monounsaturated fatty acids; NR, not reported; PA, physical activity; PUFA, polyunsaturated fatty acids; SBP, systolic blood pressure; WHI‐OS, Women's Health Initiative Observational Study.
Midpoint of range.
Fermented dairy comprised yogurt and sour milk,25 fermented sour cream and yogurt,21 or fermented low‐fat milk,29 which were the largest contributors to total fermented dairy in these studies.
Retrieved from other publications of this cohort.
According to the authors, total dairy is mainly milk: 93% of total dairy19 or almost all dairy (personal communication Dr Lin) comprised milk and is therefore included in milk analysis.
Based on milk as exposure.
Milk
An increment of 200 g of daily milk intake was associated with a 7% lower risk of stroke (RR 0.93; 95% CI 0.88–0.98; n=14)1 including 603 920 participants and 25 269 stroke events (Table 2 and Figure 2). The median milk intake was 147 g/day (range 0–1051 g/day). Considerable heterogeneity was present (I2=86%, P<0.001), which could be partly attributed to continent. The RR per 200 g/day milk was 0.82 (95% CI 0.75–0.90, n=5) in Asian populations (median intake 38 g/day) and 0.98 (95% CI 0.95–1.01; n=9) in Western populations (median intake 266 g/day). Based on multivariable meta‐regression, degree of adjustment, study quality, and outcome event also explained some heterogeneity, but not significantly in addition to the continent variable. The association of milk with total stroke was nonlinear, with the strongest inverse association around 125 g/day (RR 0.86; 95% CI 0.82–0.89; n=13; Figure 3). For milk intake in the range of 125 to 750 g/day the inverse association remained significant, but was attenuated.
Table 2.
Results of Linear and Nonlinear Dose–Response Meta‐Analyses
| Dairy Type (Increment g/day) | Studies | No Studies (Results) | RR (95% CI), P Value | Heterogeneity I2 (%), P Value | No Events; Total n | Median Intake (g/day),a Range | Knot, P Nonlinearity RR (95% CI) at Knot |
|---|---|---|---|---|---|---|---|
| Milk (200) | 11, 15, 19, 20, 21, 22, 24, 25, 28, 29, 30, 31, 32 | 14 (16) | 0.93 (0.88–0.98), 0.004 | 86.0; <0.001 | 25 269; 603 920 | 147, 0 to 1051 |
125 g/day, <0.001 0.86 (0.82–0.89) |
| Western countries15, 20, 21, 22, 25, 28, 29, 30, 32 | 9 (10) | 0.98 (0.95–1.01), 0.18 | 47.2; 0.048 | 11 507; 280 536 | 266, 0 to 1051 | ||
| East Asian countries11, 19, 24, 31 | 5 (6) | 0.82 (0.75–0.90), <0.0001 | 46.4; 0.10 | 13 762; 323 384 | 38, 0 to 232 |
165 g/day, <0.0001 0.79 (0.75–0.84) |
|
| Ischemic stroke11, 20, 21, 28 | 5 | 0.95 (0.89–1.01), 0.09 | 83.6; <0.001 | 10 871; 467 529 | 115, 0 to 1051 |
115 g/day, 0.03 0.90 (0.85–0.96) |
|
| Hemorrhagic stroke11, 20, 21 | 4 | 0.90 (0.74–1.09), 0.29 | 94.4; <0.001 | 1237; 158 595 | 197, 0 to 1051 |
125 g/day, <0.0001 0.76 (0.70–0.82) |
|
| Fatal stroke11, 15, 19, 29, 30, 31 | 7 (9) | 0.88 (0.81–0.96), 0.002 | 65.1; 0.003 | 15 071; 352 105 | 66, 0 to 757 |
150 g/day, <0.0001 0.81 (0.77–0.85) |
|
| Low‐fat milk (200) | 20, 25, 28, 29 | 4 (5) | 0.96 (0.90–1.03), 0.26 | 68.2; 0.01 | 5942; 159 547 | 150, 0 to 783 | b |
| High‐fat milk (200) | 20, 25, 28, 29 | 4 (5) | 1.04 (1.02–1.06), 0.001 | 0.0; 0.65 | 5942; 159 547 | 102, 0 to 850 | b |
| Cheese (40) | 15, 20, 21, 22, 25, 28, 29 | 7 (8) | 0.97 (0.94–1.01), 0.12 | 31.2; 0.18 | 11 126; 272 368 | 26, 0 to 112 |
25 g/day, 0.002 0.91 (0.86–0.96) |
| Yogurt (100) | 15, 20, 28 | 3 | 1.02 (0.90–1.17), 0.73 | 47.8; 0.15 | 4276; 116 555 | 22, 0 to 214 | b |
| Fermented dairy (200) | 15, 21, 22, 25, 29 | 5 (6) | 0.91 (0.82–1.01), 0.08 | 64.5; 0.02 | 7414; 160 048 | 110, 0 to 436 | |
| Total dairy (200) | 15, 16, 17, 20, 21, 22, 25, 33 | 9 | 0.99 (0.96–1.02); 0.42 | 65.6; 0.003 | 12 425; 336 118 | 305, 0 to 2078 | |
| Ischemic stroke20, 21 | 3 | 1.00 (0.96–1.04); 0.95 | 67.6; 0.046 | 6440; 158 595 | 347, 2 to 1296 | ||
| Hemorrhagic stroke20, 21 | 3 | 1.02 (0.98–1.06); 0.41 | 0; 0.37 | 1237; 158 595 | 347, 2 to 1296 | ||
| Fatal stroke15, 16, 33 | 4 | 0.97 (0.85–1.11); 0.65 | 65.3; 0.04 | 1652; 87 576 | 99, 2 to 571 | ||
| Low‐fat dairy (200) | 15, 21, 22, 23, 29, 33 | 7 (8) | 0.97 (0.95–0.99); 0.005 | 0.0; 0.65 | 9372; 242 643 | 179, 0 to 632 |
75 g/day, 0.01 0.94 (0.89–1.004) |
| High‐fat dairy (200) | 15, 21, 22, 23, 33 | 6 | 0.96 (0.93–0.99); 0.02 | 0.0; 0.84 | 9372; 262 643 | 163, 18 to 497 |
55 g/day, 0.01 0.94 (0.89–1.004) |
| Butter (10) | 20, 25, 29 | 3 (4) | 1.00 (0.99–1.01), 0.66 | 0.0; 0.70 | 2230; 47 227 | 11, 0 to 79 | b |
RR indicates relative risk.
Weighted according to study size.
Not investigated because the number of available studies was ≤3.
Figure 2.
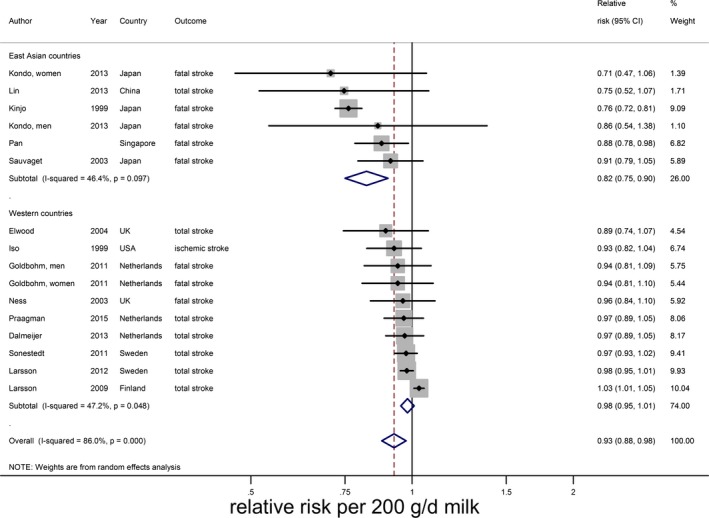
Relative risks of total stroke for an increment of 200 g of daily milk intake, by continent. Squares represent relative risks and square sizes study‐specific statistical weight; horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 3.
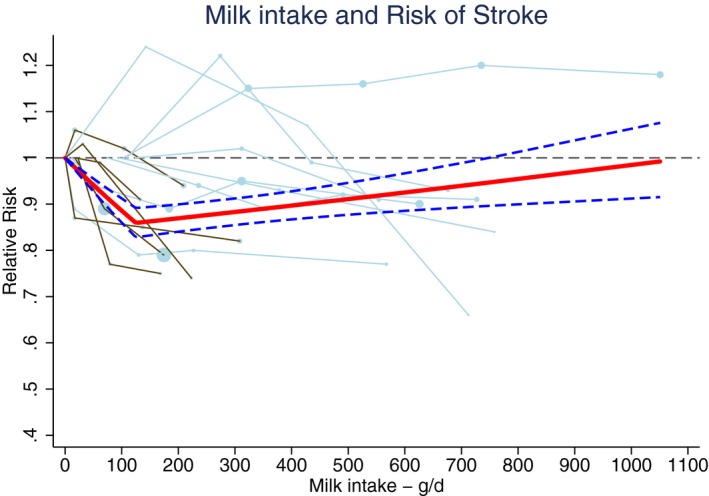
Ding's spaghetti plot for the nonlinear association between milk intake and total stroke (n=13). Light blue lines represent Western and brown lines East Asian studies. Circles are placed at study‐specific relative risks related to the corresponding quantity of intake. Circle areas are proportional to the study‐specific statistical weight. The solid red line represents the pooled RR at each quantity of intake and the 2 dashed dark blue lines the corresponding 95% CI.
There were indications of publication bias (small study effect) based on asymmetry of the funnel plot, especially in Western countries (Eggers test, P=0.06 for all studies and 0.02 for Western studies; Figure 4). However, removal of 2 small studies19, 24 did not change the results. For ischemic stroke, hemorrhagic stroke, and fatal stroke RRs were 0.95 (95% CI 0.89–1.01; n=5), 0.90 (0.74–1.09; n=4), and 0.88 (95% CI 0.81–0.96; n=7), respectively, with considerable heterogeneity for each end point (Figure 5). Nonlinear associations were observed for total stroke in Asian countries, ischemic stroke, hemorrhagic stroke, and fatal stroke, with the strongest inverse associations until 165, 115, 125, and 155 g/day, respectively (Figure 6). Within Western countries, the percentage of men predicted the effect size of milk in relation to stroke risk (P=0.02). However, the results were dominated by 1 large Finnish cohort20 (Figure 7).
Figure 4.
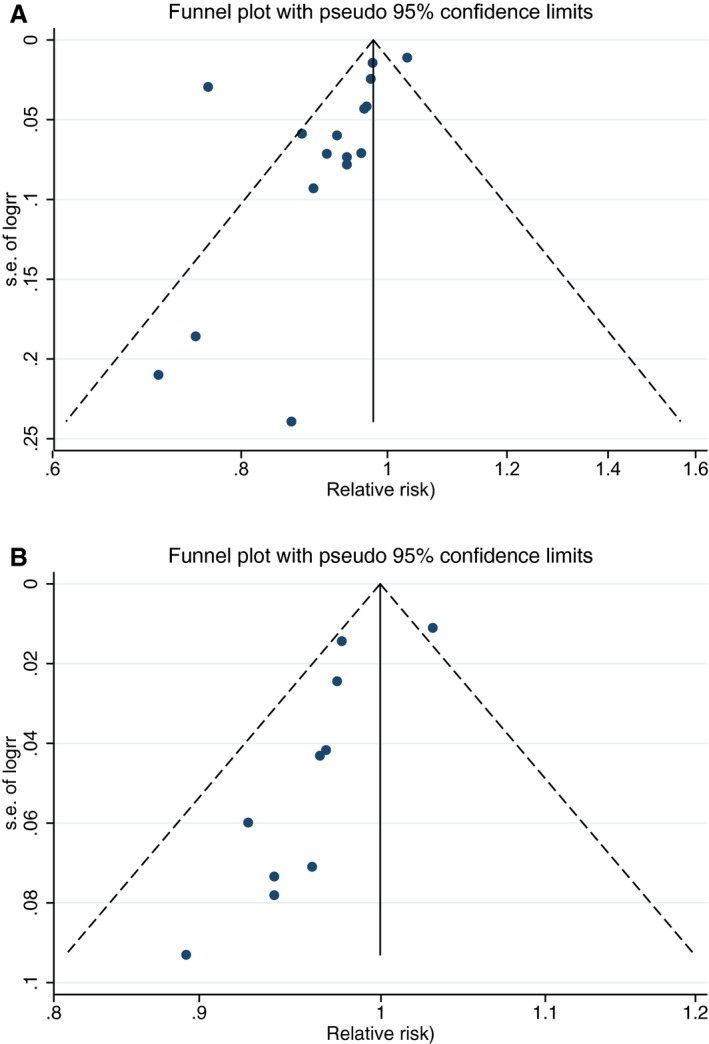
Funnel plots for studies of the association between milk intake and stroke risk based on dose–response slopes; Egger's test, P=0.06 (A) and funnel plot for studies in Western countries of the association between milk intake and stroke risk (B) based on dose–response slopes; Egger's test, P=0.02.
Figure 5.
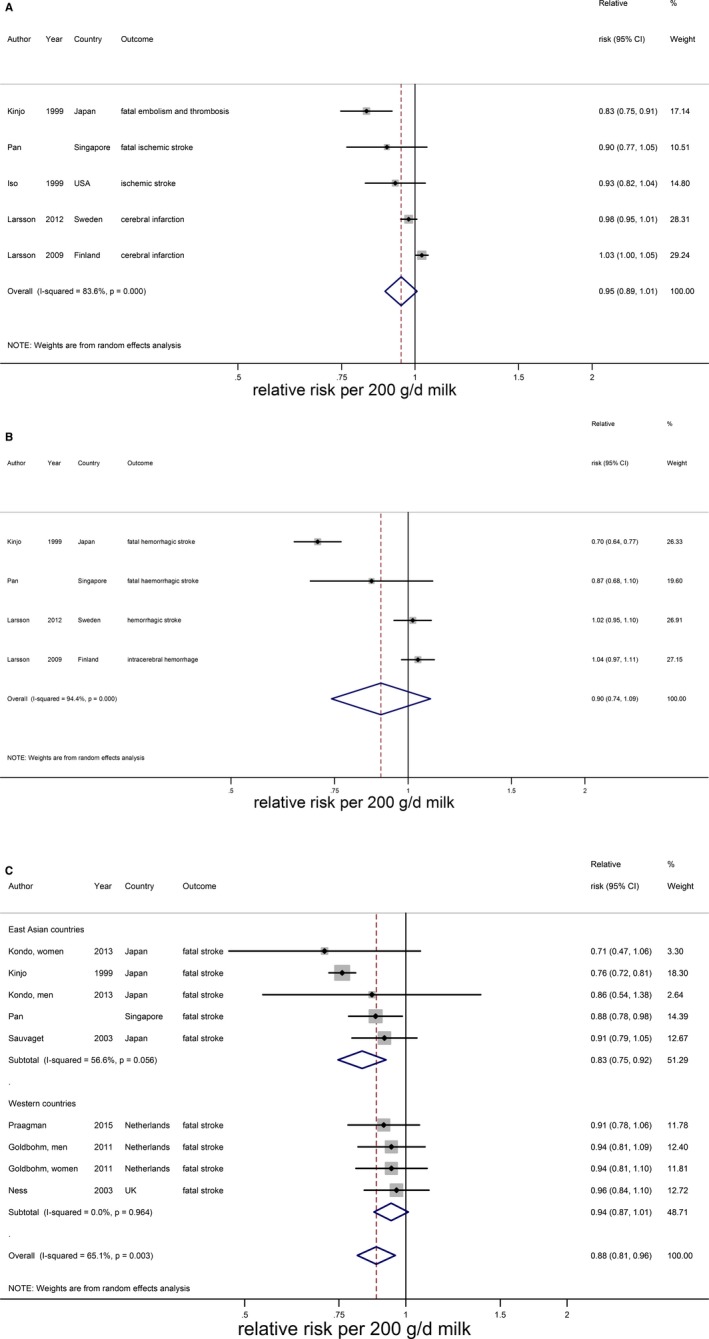
Relative risks of ischemic stroke (A), hemorrhagic stroke (B), and fatal stroke stratified by continent (C) for an increment of 200 g/day in milk intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 6.
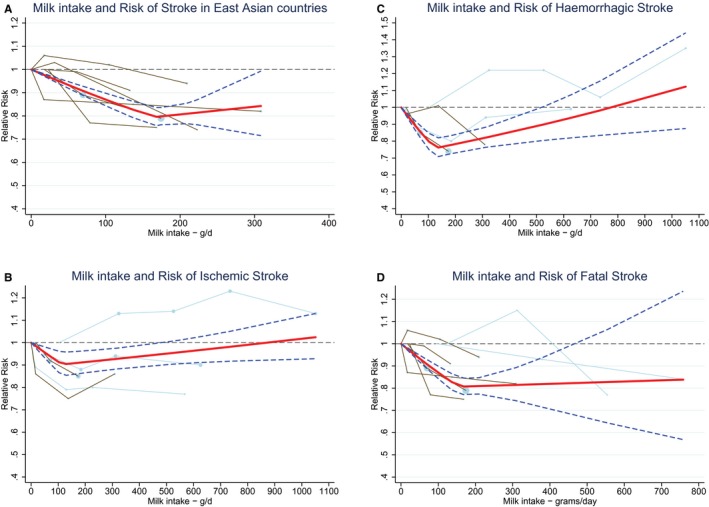
Ding's spaghetti plots for (A) the nonlinear association (P for nonlinearity=0.01, knot at 165 g/day) between milk intake and total stroke in East Asian countries (n=5). B, For the nonlinear association (P for nonlinearity=0.03; knot at 115 g/day) between milk intake and ischemic stroke (n=5). C, For the nonlinear association (P for nonlinearity <0.0001; knot at 125 g/day) between milk intake and hemorrhagic stroke (n=4). D, For the nonlinear association (P for nonlinearity <0.0001; knot at 150 g/day) between milk intake and fatal stroke (n=6). Light blue lines represent Western studies and brown lines represent East Asian studies. The circles are placed at the study‐specific relative risks that are related to the corresponding quantity of intake. The area of the circles is proportional to the study‐specific statistical weight. The solid red line represents the pooled relative risks at each quantity of intake and the 2 dashed dark blue lines the corresponding 95% CI.
Figure 7.
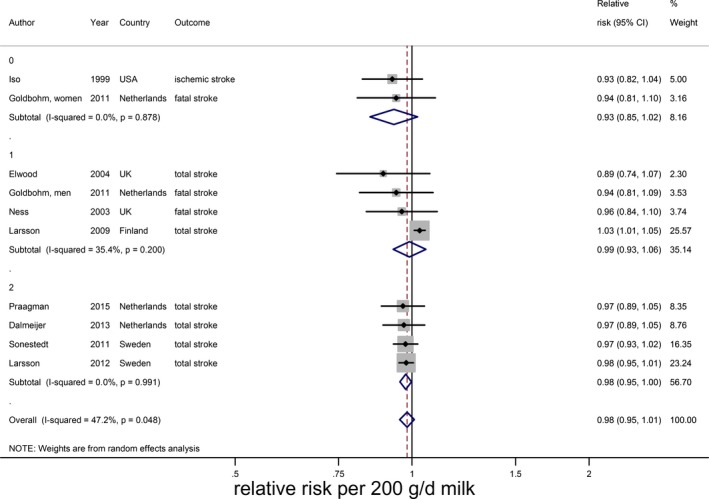
Relative risks of total stroke for an increment of 200 g/day in milk intake in Western countries, stratified for sex. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs (0=women, 1=men, 2=mixed).
The RR of low‐fat milk consumption (4 Western studies, median intake: 150 g/day)20, 25, 28, 29 was 0.96 (95% CI 0.90–1.03) per 200 g/day (Figure 8A) with considerable heterogeneity (I2=68%, P=0.01), which was explained by sex (meta‐regression: P=0.04). Based on the same studies, high‐fat milk (median intake 102 g/day) was significantly associated with a higher risk of stroke (RR 1.04; 95% CI 1.02–1.06) per 200 g/day with no heterogeneity (Figure 8B).
Figure 8.
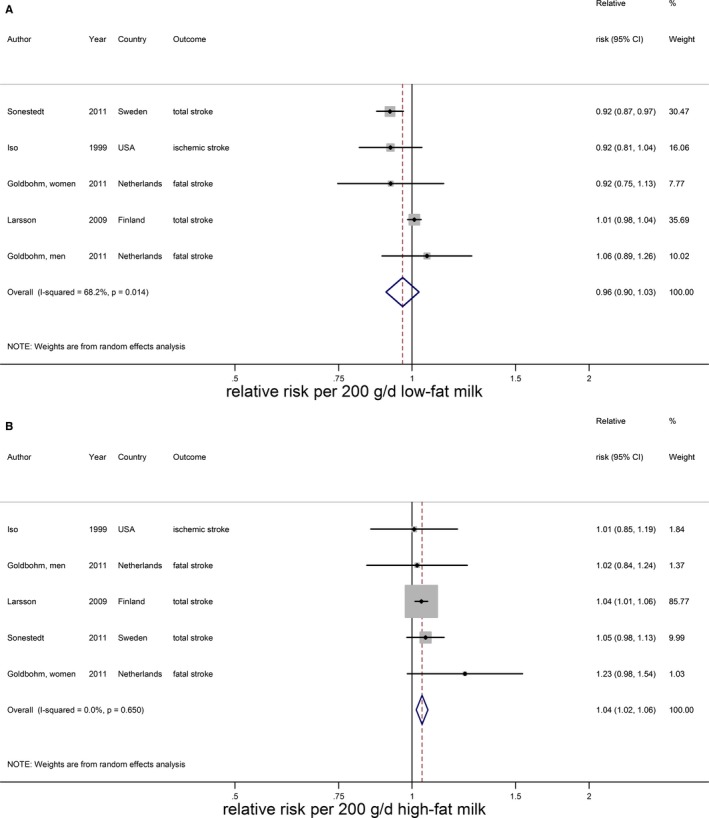
Relative risks of total stroke for an increment of 200 g/day in low‐fat milk intake (A) and high‐fat milk intake (B). Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Cheese, Yogurt, and Total Fermented Dairy
Cheese intake (weighted median intake 26 g/day) was marginally associated with a lower stroke risk, with a RR of 0.97 (95% CI 0.94–1.01) per 40 g/day with low heterogeneity (I2=31%, P=0.18; n=7; Figure 9).15, 20, 21, 22, 25, 28, 29 The inverse association was nonlinear and most pronounced above 25 g/day (RR 0.91; 95% CI 0.86–0.96; P for nonlinearity<0.002; Figure 10). Yogurt intake (n=3) was not associated with stroke risk (RR: 1.02; 95% CI 0.90–1.17 per 100 g/day; Figure 11).15, 20, 28 Total fermented dairy intake was borderline significantly associated with a 9% (RR of 0.91; 95% CI 0.82–1.01, n=5) lower risk of stroke per 200 g/day, with no indications of a nonlinear association (Figure 12A).15, 20, 22, 25, 29 The considerable heterogeneity (I2=65%, P=0.02) was absent for the subset of 3 studies with results on fatal stroke (RR: 0.80; 95% CI: 0.67–0.95; Figure 12B).
Figure 9.
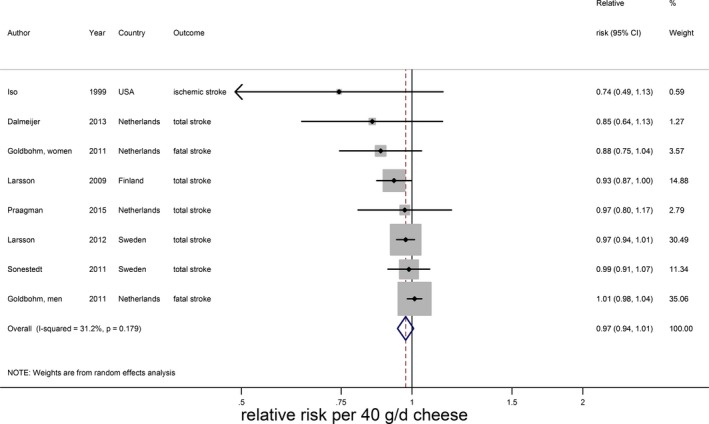
Relative risks of total stroke for an increment of 40 g/day in cheese intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 10.
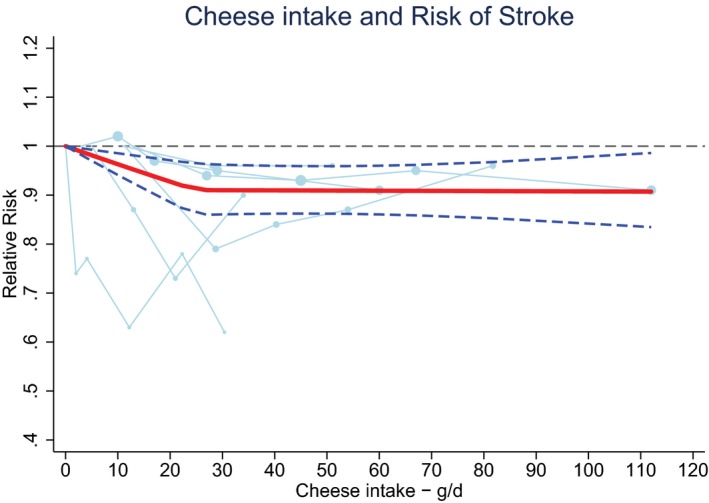
Ding's spaghetti plot for the nonlinear association between cheese intake and total stroke (n=6). Circles are placed at the study‐specific relative risks related to the corresponding quantity of intake. The area of the circles is proportional to the study‐specific statistical weight. The solid red line represents the pooled RR at each quantity of intake and the 2 dashed dark blue lines the corresponding 95% CI.
Figure 11.
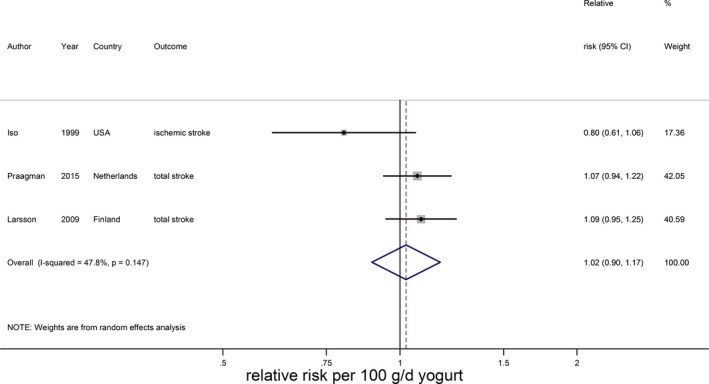
Relative risks of total stroke for an increment of 100 g/day in yogurt intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 12.
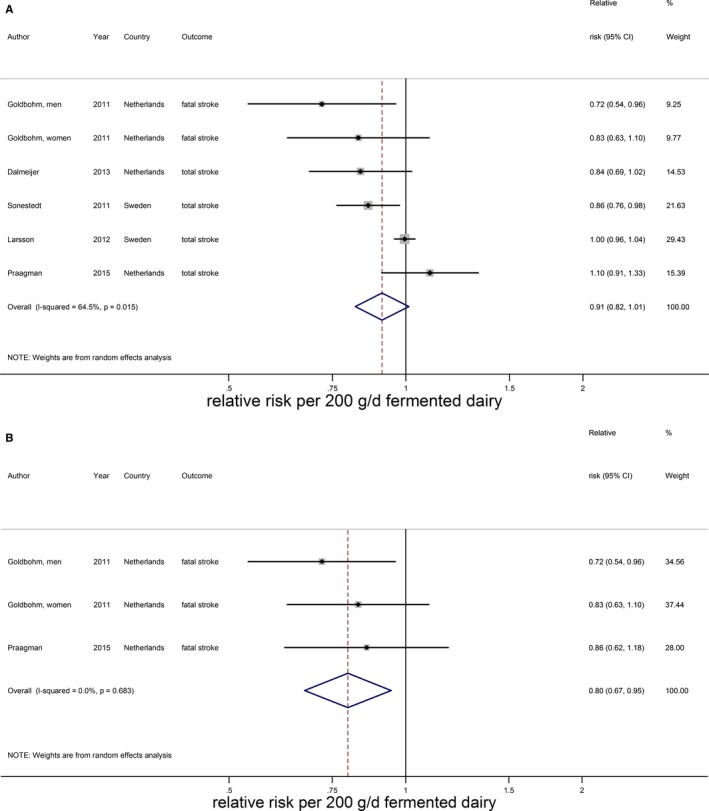
Relative risks of total stroke for an increment of 200 g/day in fermented dairy intake (A) and fatal stroke for an increment of 200 g/day in fermented dairy intake (B). Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Total Dairy, Low‐Fat Dairy, High‐Fat Dairy, and Butter
Total dairy was not associated with stroke risk (RR 0.99; 95% CI 0.96–1.02 per 200 g/day; n=9) with no evidence for a nonlinear association (Figure 13A).15, 16, 17, 20, 21, 22, 25, 33 The considerable heterogeneity (I2=66%, P<0.005) could not be explained by end point, study quality, degree of adjustment, and duration of follow‐up. The percentage of males influenced the RR (P=0.049), but this was driven by 1 large Finnish cohort study with only men.20 Total dairy was also not associated with ischemic stroke (n=3), hemorrhagic stroke (n=3), and fatal stroke (n=4), with considerable heterogeneity for ischemic and fatal stroke (Figure 13B through 13D). Nonlinear associations could not be investigated. Both low‐fat15, 21, 22, 23, 29, 33 and high‐fat dairy15, 21, 22, 23, 33 were significantly associated with a lower stroke risk per 200 g/day. RRs (95% CI) were 0.97 (0.95–0.99) for low‐fat dairy and 0.96 (0.93–0.99) for high‐fat dairy with no heterogeneity (Figure 14). Both associations were nonlinear with stronger inverse associations above 75 g/day for low‐fat dairy and 55 g/day for high‐fat dairy (Figure 15). Butter intake20, 25, 29 was not associated with stroke risk (RR: 1.00; 95% CI 0.99–1.01 per 10 g/day; Figure 16).
Figure 13.
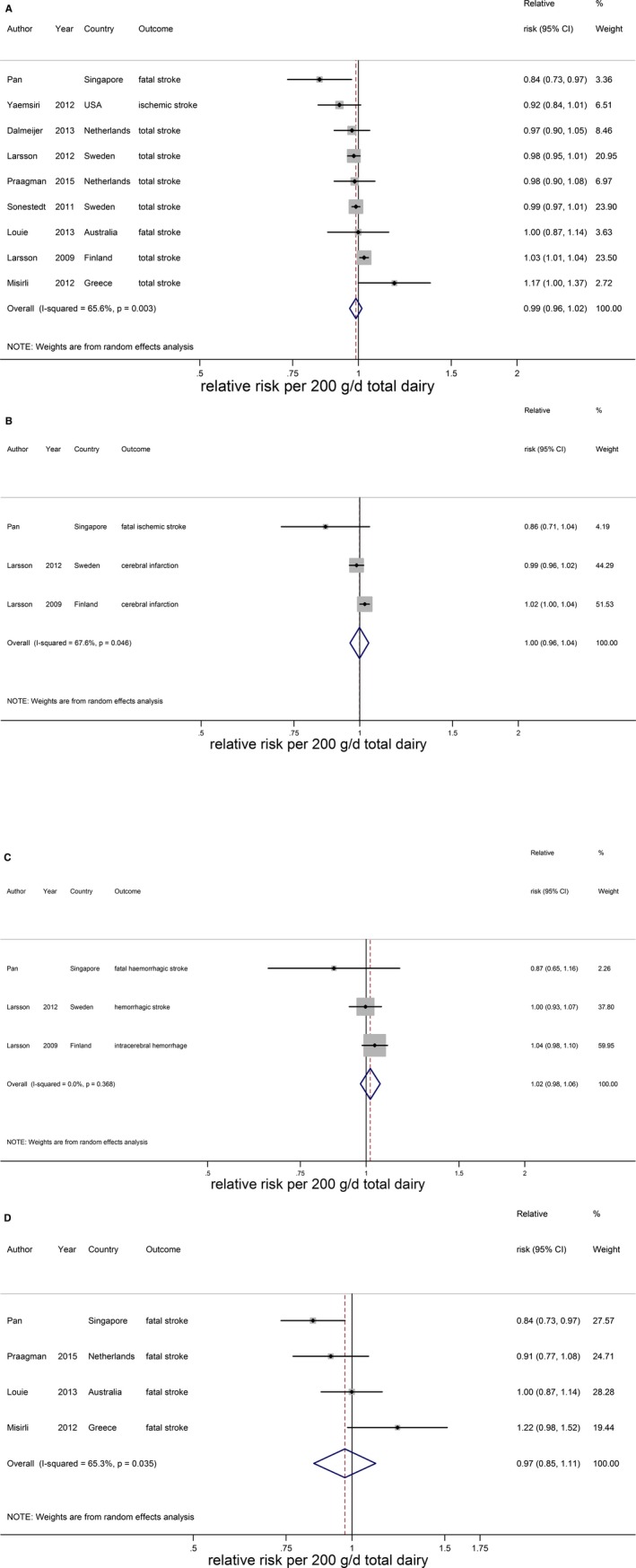
Relative risks of total stroke (A), ischemic stroke (B), hemorrhagic stroke (C), and fatal stroke (D) for an increment of 200 g/day in total dairy intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 14.
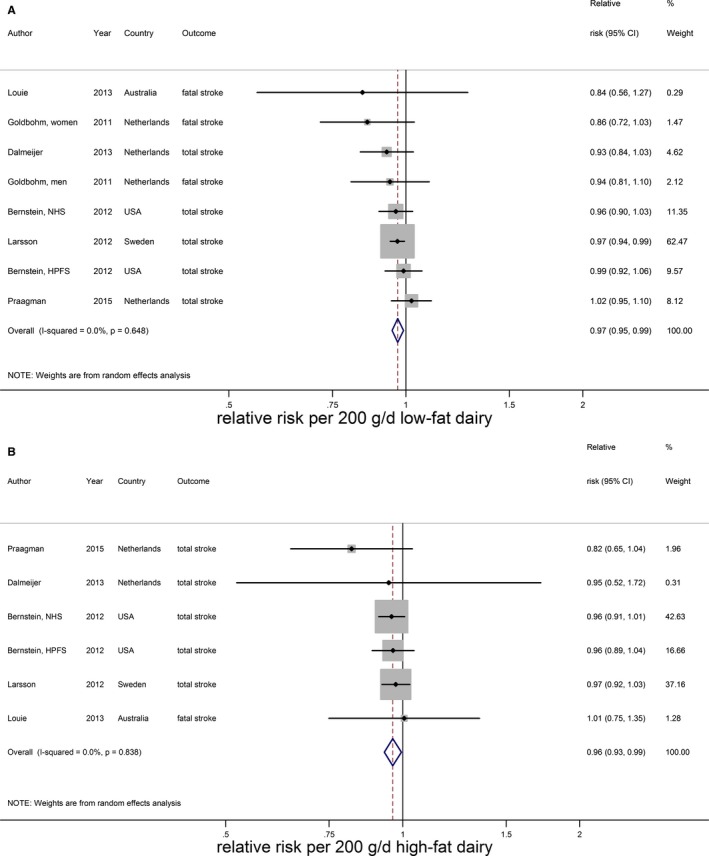
Relative risks of total stroke for an increment of 200 g/day in low‐fat dairy (A) and high‐fat dairy (B) intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with 95% CIs.
Figure 15.
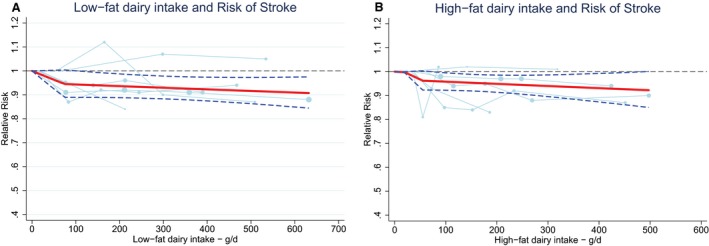
Ding's spaghetti plots for (A) the nonlinear association (P for nonlinearity=0.01; knot at 75 g/day) between low‐fat dairy intake and total stroke (n=6) and (B) the nonlinear association (P for nonlinearity=0.01; knot: 55 g/day) between high‐fat dairy intake and total stroke (n=6). Light blue lines represent Western studies. The circles are placed at the study‐specific relative risks that are related to the corresponding quantity of intake. The area of the circles is proportional to the study‐specific statistical weight. The solid red line represents the pooled relative risk at each quantity of intake and the 2 dashed dark blue lines the corresponding 95% CI.
Figure 16.
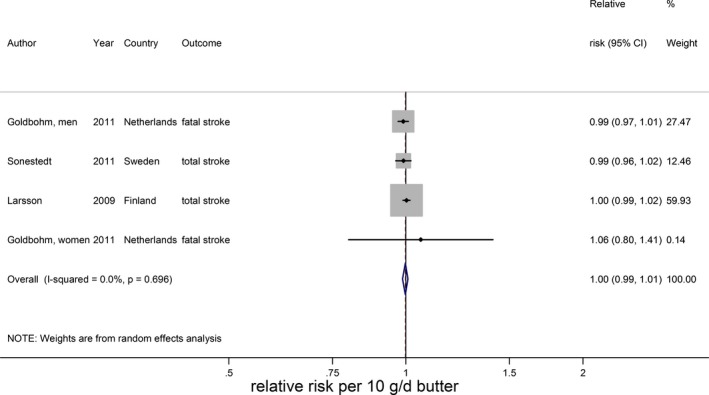
Relative risks of total stroke for an increment of 10 g/day in butter intake. Squares represent study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight, ie, the inverse of the variance); horizontal lines represent 95% CIs; diamond represents summary relative risk estimates with 95% CIs.
Discussion
This meta‐analysis showed an inverse association of milk consumption with risk of stroke, with a maximal reduction around 125 g/day. The high amount of heterogeneity was partly explained, but not completely absent, after stratifying for continent. Based on a limited number of studies, high‐fat milk was directly associated with stroke risk. Cheese intake was marginally inversely associated with stroke risk, with a maximal risk reduction from 25 g/day onwards for cheese. For yogurt, butter, and total dairy no associations were observed. The role of fat content of dairy products is not yet clear.
Dairy is a heterogeneous food group of (semi) solid and liquid fermented or nonfermented foods, differing in nutrients such as fat and sodium. We therefore separately analyzed specific dairy types. Based on 14 studies, we found a significant 7% lower risk of stroke per 200 g/day of milk, which was smaller than the 13% (based on 6 studies) that we showed in our previous meta‐analysis.10 Our findings are in line with the meta‐analysis by Hu et al,12 who reported a 9% lower risk for a high versus a low milk intake. The association in our study was nonlinear, with the lowest risk observed at 125 g/day. Heterogeneity was partly attributable to continent, with milk being inversely associated with stroke in East Asia, but not in Europe. Hu et al12 studied heterogeneity for total dairy in relation to stroke risk, and also observed stronger associations in Asian studies.12 It should be noted that some Asian studies were based on multivariable models that contained only a few covariates.11, 24, 31 However, the results provided by A.P., which were adjusted for many potential confounders, supported the lower risk of stroke with increasing milk intake making residual confounding unlikely as an explanation for the difference between continents. The difference in results between continents may reflect differences in types of stroke as in Asia hemorrhagic stroke is more common than in Western countries.41 Our results on hemorrhagic stroke, however, did not provide an explanation for the observed heterogeneity.
Cheese consumption was inversely associated with stroke risk, with a 3% lower risk of stroke per 40 g/day of cheese and a maximum reduction above 25 g/day. Our results regarding cheese were in agreement with Hu et al12 and Qin et al,42 who also reported inverse associations for a high versus a low cheese consumption. They did not investigate whether the association was nonlinear. Yogurt was not associated with stroke risk, based on 3 studies, whereas total fermented dairy showed a borderline inverse association with stroke risk. In general, results for fermented dairy products were only based on Western populations.
Limited information was available regarding the role of dairy fat in relation to stroke risk. For high‐fat milk a direct association was observed, whereas for low‐fat milk the association was (nonsignificantly) inverse. However, the number of studies that reported on types of milk was limited and only comprised Western populations. In addition, results for low‐fat total dairy did not differ from those of high‐fat total dairy.
In a previous meta‐analysis of 9 prospective cohort studies, we reported that milk and low‐fat dairy were inversely associated with hypertension, a major risk factor for stroke.43 Apart from butter, dairy is an important source of calcium, which was inversely associated with stroke risk in a meta‐analysis of 11 prospective cohort studies including populations with low‐to‐moderate calcium intakes and Asian populations.44 That meta‐analysis also suggested protective effects of dairy calcium rather than nondairy calcium against stroke.44 A recent update of these meta‐analyses including updated results from the Nurses’ Health Study I and II, however, showed no association between dietary (dairy and other sources) calcium and stroke risk with a RR (95% CI) of 0.98 (0.94–1.02) for a 300 mg/day increase of calcium intake.45 Calcium may play a role in the inverse association of milk and cheese with stroke risk. However, we only found inverse associations for milk and cheese, and not for the other calcium‐containing sources of dairy. Milk and dairy also contain other minerals, such as potassium and magnesium, which were also found to be inversely associated with stroke risk.45, 46, 47 On the other hand, cheese contains a lot of sodium, which is directly associated with hypertension. A meta‐analysis of prospective cohort studies, however, did not show a relation of cheese consumption to hypertension.43 The mechanism of how dairy would protect against stroke is therefore not yet clear.
Strengths of our meta‐analysis are our comprehensive dose–response analyses and the careful evaluation of nonlinearity of the associations based on a large number of studies from Asian and Western countries by contacting authors for additional information and by contacting researchers in the field for inclusion of additional cohorts. A limitation is that it is difficult to disentangle the effects of the dosages of milk intake from Western compared to those of East Asian countries, because consumption levels in East Asia were considerably lower than those in Western countries. In addition, reported dairy types differed between studies, which complicates direct comparison of results between dairy types. Another limitation was that the number of studies, except for milk intake, was rather low (n≤8). Therefore, options for meta‐regression or subgroup analyses were limited for several exposure categories. The quality of a meta‐analysis is dependent on the quality of the included studies. The degree of adjustment for confounders varied widely between studies. Regarding the results on milk, 3 of 6 Asian studies had a low study quality score11, 24, 31 as opposed to 1 of 10 Western studies.30 Therefore, influences of study quality and continent cannot easily be separated. However, the (unpublished) results of Pan et al, which were fully adjusted for potential confounders, supported the inverse association that we observed for other East Asian countries. We therefore believe that our results on milk consumption are not solely due to confounding. Residual confounding will, as in any cohort study, always be of concern.
In conclusion, this meta‐analysis of 18 prospective observational studies indicates a possible role for milk and cheese consumption in stroke prevention. Results should be placed in the context of the observed heterogeneity. Future epidemiological studies should provide more details about types of dairy, including fat content. In addition, the role of milk and dairy in Asian populations deserves further attention.
Sources of Funding
This meta‐analysis was partly funded by an unrestricted grant from the Dutch Dairy Association.
Disclosures
Soedamah‐Muthu received funding from the Global Dairy Platform, Dairy Research Institute, and Dairy Australia for a meta‐analysis on cheese and blood lipids (2012) and a meta‐analysis of dairy and mortality (2015). Gijsbers, Geleijnse, Pan, and de Goede reported no conflicts of interest. The sponsors had no role in design and conduct of the study, data collection and analysis, interpretation of the data, decision to publish, or preparation of the manuscript.
Supporting information
Data S1. Supplemental methods.
(J Am Heart Assoc. 2016;5:e002787 doi: 10.1161/JAHA.115.002787)
Note
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- 3. Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77:1923–1932. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris‐Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie‐Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 6. Dietary Guidelines for Americans, 2010. Washington, DC: US Department of Agriculture and US Department of Health and Human Services; 2010. [Google Scholar]
- 7. U.S. Department of Health and Human Services and U.S. Department of Agriculture 2015 – 2020 Dietary Guidelines for Americans. 8th ed Available at: http://health.gov/dietaryguidelines/2015/guidelines/..
- 8. Ge K. The transition of Chinese dietary guidelines and food guide pagoda. Asia Pac J Clin Nutr. 2011;20:439–446. [PubMed] [Google Scholar]
- 9. Japanese food guide spinning top. Available at: http://www.mhlw.go.jp/bunya/kenkou/pdf/eiyou-syokuji4.pdf. Accessed July 1, 2015.
- 10. Soedamah‐Muthu SS, Ding EL, Al‐Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all‐cause mortality: dose‐response meta‐analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinjo Y, Beral V, Akiba S, Key T, Mizuno S, Appleby P, Yamaguchi N, Watanabe S, Doll R. Possible protective effect of milk, meat and fish for cerebrovascular disease mortality in Japan. J Epidemiol. 1999;9:268–274. [DOI] [PubMed] [Google Scholar]
- 12. Hu D, Huang J, Wang Y, Zhang D, Qu Y. Dairy foods and risk of stroke: a meta‐analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:460–469. [DOI] [PubMed] [Google Scholar]
- 13. van der Pols JC, Gunnell D, Williams GM, Holly JM, Bain C, Martin RM. Childhood dairy and calcium intake and cardiovascular mortality in adulthood: 65‐year follow‐up of the Boyd Orr cohort. Heart. 2009;95:1600–1606. [DOI] [PubMed] [Google Scholar]
- 14. Huang LY, Wahlqvist ML, Huang YC, Lee MS. Optimal dairy intake is predicated on total, cardiovascular, and stroke mortalities in a Taiwanese cohort. J Am Coll Nutr. 2014;33:426–436. [DOI] [PubMed] [Google Scholar]
- 15. Praagman J, Franco OH, Ikram MA, Soedamah‐Muthu SS, Engberink MF, van Rooij FJ, Hofman A, Geleijnse JM. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam Study. Eur J Nutr. 2015;54:981–990. [DOI] [PubMed] [Google Scholar]
- 16. Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012;176:1185–1192. [DOI] [PubMed] [Google Scholar]
- 17. Yaemsiri S, Sen S, Tinker L, Rosamond W, Wassertheil‐Smoller S, He K. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol. 2012;72:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 19. Kondo I, Ojima T, Nakamura M, Hayasaka S, Hozawa A, Saitoh S, Ohnishi H, Akasaka H, Hayakawa T, Murakami Y, Okuda N, Miura K, Okayama A, Ueshima H; Group NDR . Consumption of dairy products and death from cardiovascular disease in the Japanese general population: the NIPPON DATA80. J Epidemiol. 2013;23:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dairy foods and risk of stroke. Epidemiology. 2009;20:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson SC, Virtamo J, Wolk A. Dairy consumption and risk of stroke in Swedish women and men. Stroke. 2012;43:1775–1780. [DOI] [PubMed] [Google Scholar]
- 22. Dalmeijer GW, Struijk EA, van der Schouw YT, Soedamah‐Muthu SS, Verschuren WM, Boer JM, Geleijnse JM, Beulens JW. Dairy intake and coronary heart disease or stroke—a population‐based cohort study. Int J Cardiol. 2013;167:925–929. [DOI] [PubMed] [Google Scholar]
- 23. Bernstein AM, Pan A, Rexrode KM, Stampfer M, Hu FB, Mozaffarian D, Willett WC. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin PH, Yeh WT, Svetkey LP, Chuang SY, Chang YC, Wang C, Pan WH. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr. 2013;22:482–491. [PubMed] [Google Scholar]
- 25. Sonestedt E, Wirfalt E, Wallstrom P, Gullberg B, Orho‐Melander M, Hedblad B. Dairy products and its association with incidence of cardiovascular disease: the Malmo diet and cancer cohort. Eur J Epidemiol. 2011;26:609–618. [DOI] [PubMed] [Google Scholar]
- 26. He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elwood PC, Strain JJ, Robson PJ, Fehily AM, Hughes J, Pickering J, Ness A. Milk consumption, stroke, and heart attack risk: evidence from the Caerphilly cohort of older men. J Epidemiol Community Health. 2005;59:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30:1772–1779. [DOI] [PubMed] [Google Scholar]
- 29. Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10‐y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93:615–627. [DOI] [PubMed] [Google Scholar]
- 30. Ness AR, Smith GD, Hart C. Milk, coronary heart disease and mortality. J Epidemiol Community Health. 2001;55:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sauvaget C, Nagano J, Allen N, Grant EJ, Beral V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003;32:536–543. [DOI] [PubMed] [Google Scholar]
- 32. Elwood PC, Pickering JE, Fehily AM, Hughes J, Ness AR. Milk drinking, ischaemic heart disease and ischaemic stroke I. Evidence from the Caerphilly cohort. Eur J Clin Nutr. 2004;58:711–717. [DOI] [PubMed] [Google Scholar]
- 33. Louie JC, Flood VM, Burlutsky G, Rangan AM, Gill TP, Mitchell P. Dairy consumption and the risk of 15‐year cardiovascular disease mortality in a cohort of older Australians. Nutrients. 2013;5:441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bodner‐Montville J, Ahuja JKC, Ingwersen LA, Haggerty ES, Enns CW, Perloff BP. USDA Food and Nutrient Database for Dietary Studies: released on the web. J Food Compost Anal. 2006;19(suppl):S100–S107. [Google Scholar]
- 35. Food Portion Sizes. Norwich, London: TSO; 2005. [Google Scholar]
- 36. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 1, 2015.
- 37. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 38. Bauer SR, Hankinson SE, Bertone‐Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose‐response meta‐analysis of prospective studies. Medicine (Baltimore). 2013;92:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 40. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, Barker‐Collo S, Connor M, Roth GA, Sacco R, Ezzati M, Naghavi M, Murray CJ, Feigin VL; Global Burden of Diseases I, Risk Factors Study Stroke Expert G . The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart. 2014;9:101–106. [DOI] [PubMed] [Google Scholar]
- 42. Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta‐analysis of prospective cohort studies. Asia Pac J Clin Nutr. 2015;24:90–100. [DOI] [PubMed] [Google Scholar]
- 43. Soedamah‐Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose‐response meta‐analysis of prospective cohort studies. Hypertension. 2012;60:1131–1137. [DOI] [PubMed] [Google Scholar]
- 44. Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose‐response meta‐analysis. Am J Clin Nutr. 2013;97:951–957. [DOI] [PubMed] [Google Scholar]
- 45. Adebamowo SN, Spiegelman D, Willett WC, Rexrode KM. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta‐analyses. Am J Clin Nutr. 2015;101:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larsson SC, Orsini N, Wolk A. Dietary potassium intake and risk of stroke: a dose‐response meta‐analysis of prospective studies. Stroke. 2011;42:2746–2750. [DOI] [PubMed] [Google Scholar]
- 47. Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta‐analysis of prospective studies. Am J Clin Nutr. 2012;95:362–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.


