Abstract
Background
We evaluated the association of carotid intima‐media thickness (cIMT), carotid plaque, carotid distensibility coefficient (DC), and aortic pulse wave velocity (PWV) with incident atrial fibrillation (AF) and their role in improving AF risk prediction beyond the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)‐AF risk score.
Methods and Results
We analyzed data from 3 population‐based cohort studies: Atherosclerosis Risk in Communities (ARIC) Study (n=13 907); Multi‐Ethnic Study of Atherosclerosis (MESA; n=6640), and the Rotterdam Study (RS; n=5220). We evaluated the association of arterial indices with incident AF and computed the C‐statistic, category‐based net reclassification improvement (NRI), and relative integrated discrimination improvement (IDI) of incorporating arterial indices into the CHARGE‐AF risk score (age, race, height weight, systolic and diastolic blood pressure, antihypertensive medication use, smoking, diabetes, previous myocardial infarction, and previous heart failure). Higher cIMT (meta‐analyzed hazard ratio [95% CI] per 1‐SD increment, 1.12 [1.08–1.16]) and presence of carotid plaque (1.30 [1.19–1.42]) were associated with higher AF incidence after adjustment for CHARGE‐AF risk‐score variables. Lower DC and higher PWV were associated with higher AF incidence only after adjustment for the CHARGE‐AF risk‐score variables excepting height, weight, and systolic and diastolic blood pressure. Addition of cIMT or carotid plaque marginally improved CHARGE‐AF score prediction as assessed by the relative IDI (estimates, 0.025–0.051), but not when assessed with the C‐statistic and NRI.
Conclusions
Higher cIMT, presence of carotid plaque, and greater arterial stiffness are associated with higher AF incidence, indicating that atherosclerosis and arterial stiffness play a role in AF etiopathogenesis. However, arterial indices only modestly improve AF risk prediction.
Keywords: arterial stiffness, atherosclerosis, atrial fibrillation, carotid intima‐media thickness
Subject Categories: Atrial Fibrillation, Epidemiology
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, causing considerable morbidity and mortality. Previous studies suggest that structural and functional changes in arteries may play an important role in the pathogenesis of AF.1, 2, 3, 4, 5, 6, 7 However, some knowledge gaps remain. First, although higher pulse pressure (a surrogate measure for increased proximal aortic stiffness) had been reported to be independently associated with greater risk of AF,1 it is unclear whether aortic pulse wave velocity (PWV)—the gold standard measure of aortic stiffness8—and carotid distensibility are associated with AF. Second, although higher carotid intima‐media thickness (cIMT) is associated with higher risk of AF,2 whether consideration of cIMT or indices of arterial stiffness would improve risk prediction of AF is unknown. Thus far, 3 risk scores that predict occurrence of AF in community‐dwelling individuals have been published.9, 10, 11 The ARIC‐AF risk score11 and Framingham‐AF risk score9 were both derived from single‐cohort studies. By contrast, the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) AF risk score is a simple clinical risk score that was derived from 3 large cohorts in the United States and validated in 2 European cohorts.11
We hypothesized that (1) higher cIMT, presence of carotid plaque, higher PWV, and lower carotid distensibility are associated with higher incidence of AF and (2) these factors will improve risk prediction of AF, over and above the CHARGE‐AF risk score. We tested our hypotheses in 3 large community‐based cohort studies in the United States and Europe: the Atherosclerosis Risk in Communities (ARIC) Study; the Multi‐Ethnic Study of Atherosclerosis (MESA); and the Rotterdam Study (RS).
Methods
Study Populations
Atherosclerosis Risk in Communities
The ARIC cohort is a biracial study population, consisting of 15 792 men and women, 45 to 64 years of age at baseline (1987–1989), from 4 communities in North Carolina, Mississippi, Minnesota, and Maryland.12 After the baseline examination, participants had 4 additional exams, the last in 2011–2013. The present study is based on data obtained from visit 2 (1990–1992) through December 31, 2009. We analyzed arterial indices data from visit 2 because it was at this visit that cIMT, carotid plaque, and carotid distensibility data were available in most participants. There were 14 348 participants who attended the visit 2 examination. We excluded participants with prevalent AF (n=77), race other than white or black because of small sample size (n=91), missing covariates (n=56), and without arterial measurements (n=217). The analysis cohort of participants with at least one arterial measurement comprised 13 907 individuals.
Multi‐Ethnic Study of Atherosclerosis
The MESA study population has been previously described in detail.13 Briefly, between July 2000 and August 2002, 6814 men and women (2622 whites, 1893 African Americans, 1496 Hispanics, and 803 Chinese Americans), ages 45 to 84, without clinical cardiovascular disease, were recruited from 6 communities in Maryland, Illinois, North Carolina, California, New York, and Minnesota. After the baseline examination, participants had 4 additional exams, the last in 2010–2012. The present study is based on data obtained from the baseline examination (2000–2002) through February 22, 2012. We excluded participants with prevalent AF (n=58), no follow‐up information (n=25), missing covariates (n=37), and without arterial measurements (n=54). The analysis cohort of participants with at least 1 arterial measurement comprised 6640 individuals.
Rotterdam Study
The RS is a prospective cohort study of individuals ≥55 years of age living in the Ommoord district in Rotterdam, The Netherlands. Details regarding the rationale and design of the RS have been previously described.14 The baseline examination for the first RS cohort (RS‐I) in 1990–1993 recruited 7983 individuals. In 2000–2001, 3011 participants who had become 55 years of age or moved into the study district since the start of the study were added to the cohort (RS‐II). Since the baseline examinations, follow‐up visits were conducted every 3 to 4 years. The present analysis is based on data obtained from the third visit for RS‐I (RS‐I‐3, 1997–1999) and the baseline examination for RS‐II (RS‐II‐1, 2000–2001) through 2008. We analyzed arterial indices data from RS‐I‐3 and RS‐II‐1 because it was at these visits that cIMT, carotid plaque, carotid distensibility data, and PWV were measured in participants. There were 6938 participants at RS‐I‐3 and RS‐II‐1 examinations. We excluded participants with no informed consent for follow‐up data collection from medical records (n=38), incomplete data on AF prevalence or incidence (n=654), prevalent AF (n=359), missing covariates (n=434), race other than white because of small sample size (n=116), age >94 years (n=9), creatinine ≥2 mg/dL (n=9), and without arterial measurements (n=99). The analysis cohort consisted of 5220 participants.
The study complies with the Declaration of Helsinki. The ARIC and MESA study protocols were approved by the institutional review board of each participating center, and informed consent was obtained from each study participant. The RS has been approved by the medical ethics committee according to the Wet Bevolkingsonderzoek: ERGO (Population Screening Act: Rotterdam Study), executed by the Ministry of Health, Welfare, and Sports of The Netherlands. All participants provided written informed consent to participate in the study and obtain information from their treating physicians.
Ascertainment of AF
Atherosclerosis Risk in Communities
AF diagnoses were obtained from electrocardiograms (ECGs) at study visits, hospital discharge diagnoses codes, and death certificates through December 31, 2009.15 All study ECGs automatically coded as AF were visually rechecked by a cardiologist to confirm the diagnosis.16 For all hospital discharges, a trained abstractor obtained and recorded all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), discharge diagnoses. AF was defined as the presence of ICD‐9‐CM code 427.31 (AF) or 427.32 (atrial flutter) in the discharge codes.
Multi‐Ethnic Study of Atherosclerosis
Hospitalizations are reported by MESA participants every 9 to 12 months during phone and clinic follow‐up contacts. Incident AF was identified from hospital discharge diagnoses codes for AF or atrial flutter (classified as ICD‐9‐CM diagnosis codes 427.31 and 427.32), ascertained by the MESA events detection protocol, or from Medicare inpatient claims data.
Rotterdam Study
AF or atrial flutter at baseline and during follow‐up was ascertained through direct linkage of the study base with the medical files of participating general practitioners, which included the results of their own diagnoses and those of physicians practicing in hospitals and outpatient clinics. Additional cases were obtained from the national registration of all hospital discharge diagnoses and study ECGs. All potential AF cases were adjudicated by 2 independent study physicians and subsequently confirmed by a medical specialist as described in more detail previously.17
Measurement of Arterial Indices
cIMT and carotid plaque
In ARIC, cIMT was assessed in 3 segments: the distal common carotid artery (CCA; 1 cm proximal to dilation of the carotid bulb), the carotid artery bifurcation (1 cm proximal to the flow divider), and the proximal internal carotid arteries (1‐cm section of the internal carotid arteries immediately distal to the flow divider). At each of these segments, 11 measurements of the far wall (in 1‐mm increments) were attempted. The mean of the mean measurements across these segments of both right and the left sides were estimated. Trained readers adjudicated carotid plaque presence or absence if 2 of the following 3 criteria were met: abnormal wall thickness (defined as cIMT >1.5 mm), abnormal shape (protrusion into the lumen, loss of alignment with adjacent arterial wall boundary), and abnormal wall texture (brighter echoes than adjacent boundaries).
The ultrasound procedure to measure cIMT in the ARIC study has been previously described.18, 19, 20 A Biosound 2000IISA system was used and images recorded on a VHS tape. cIMT was measured centrally by trained readers at the ARIC Ultrasound Reading Center. The site‐specific reliability coefficients was estimated as 0.77, 0.73, and 0.70 for the mean carotid far wall IMT at the carotid bifurcation, internal carotid arteries, and CCAs, respectively.20, 21 For the presence or absence of plaque, the intrareader agreement was associated with a κ statistic of 0.76, whereas the inter‐reader agreement was 0.56, which suggests good agreement beyond chance.20, 21
In MESA, cIMT measurements were made on near and far walls of the CCA (1 projection), and the internal carotid artery IMT measurements were centered on the bulb (3 projections) using hand‐drawn continuous tracings of the intima‐lumen and media‐adventitia interfaces.22 These tracings were then used to calculate mean of the maximum cIMT. The presence of carotid plaque was graded with a semiquantitative scale of 0% and 1% to 24% (plaque absent) and ≥25% (plaque present). cIMT measurement was performed using a matrix array probe (M12L; General Electric, Fairfield, CT) with the frequency set at 13 MHz and at 32 frames per second. A super‐VHS videotape recording was then made for 20 seconds. Images were digitized at 30 frames per second, and automated diameter measurements were made from this video segment using customized software. End‐diastolic images (smallest diameter of the artery) were captured. Reproducibility was assessed by blinded replicate readings of cIMT performed by 2 readers. One reader reread 66 studies, for a between‐reader correlation coefficient of 0.84 (n=66), and the other reread 48 studies, for a correlation coefficient of 0.86.23
In RS, CCA IMT was measured for a 1‐cm length that was proximal to the bulb. The maximal CCA IMT, summarized as the mean of the maximal measurements from the near and far walls on both the left and right sides, was used for analysis. The left and right CCAs, bifurcations, and internal carotid arteries were evaluated for the presence of atherosclerotic plaques. A plaque was defined as a focal broadening of the intima‐media relative to the adjacent segments, with protrusion into the lumen. The CCA, the bifurcation, and the internal carotid artery on both sides were visualized with an Ultramark IV device (Advanced Technology Laboratories, Bethel, WA) using a 7.5‐MHz linear array transducer. A carotid plaque was defined as a subjective 1.5‐fold focal broadening of the intima‐media relative to the adjacent segments, with protrusion into the lumen. The left and right CCAs, left and right carotid bifurcation, and left and right internal carotid arteries were examined for the presence of plaques. A weighted plaque score was obtained by counting the sites where a plaque was visible and dividing this number by the total number of sites for which images were available. The result was multiplied by 6, the maximum number of sites. A score of 0, 1, 2, and ≥3 were considered to have no, mild, moderate, and severe carotid atherosclerosis, respectively. For the current analysis, a score of ≥2 was classified as plaque present. Reproducibility of cIMT and carotid plaque measurements have been described in detail previously.24, 25
Carotid distensibility
In all 3 cohorts, the cross‐sectional arterial wall distensibility coefficient (DC) was calculated according to the following equation: DC=2ΔD/(D×pulse pressure) (10−3/kPa), where ΔD denotes the absolute change in diameter during systole and D denotes the end‐diastolic diameter.
In ARIC, B‐mode ultrasound scans of the left CCA with ECG gating and echo tracking of the arterial diameter were used to assess carotid distensibility. Methods for the acquisition of B‐mode ultrasound scans that were ECG gated and for the echo tracking of the arterial diameter in the ARIC study have been described.12, 26, 27 Participants were asked to refrain from smoking, vigorous exercise, and caffeine‐containing beverages beginning the night before ultrasound imaging. There was an average of 5.6 cardiac cycles of adequate quality for readers to measure arterial diameter whose changes through the cardiac cycle were used in the determination of the arterial wall characteristics. Assessment of arterial diameter variation was conducted using a standardized protocol by readers at the ARIC Ultrasound Reading Center using computer software developed by the Reading Center.28 The continuous variation of the interadventitial arterial diameter throughout the cardiac cycle was measured and recorded from the left CCA using an electronic tracking device designed for ARIC (Autrec 4881‐AWT; Autrec, Winston‐Salem, NC).29 The interadventitial arterial diameter was defined as the distance between the near wall and far wall of the longitudinally imaged distal left CCA. The diameter was automatically calculated, digitized, and displayed on a strip chart for immediate review by the sonographer and stored electronically for offline reader analysis.
In MESA, the wall motion of the right CCA was imaged according to a common scanning protocol using high‐resolution B‐mode ultrasonography with a Logiq 700 machine (General Electric Medical Systems, Waukesha, WI). The right and left carotid arteries were imaged according to a common scanning protocol using high‐resolution B‐mode ultrasonography with a Logiq 700 machine (General Electric Medical Systems). Data necessary for calculating DC were obtained from a separate 20‐second‐long acquisition of longitudinal images of the right distal CCA. All of the images were interpreted at a central MESA ultrasound reading center (Tufts Medical Center, Boston, MA) by readers blinded to all of the clinical information. For each participant, an edge detector was used to process the images and extract carotid arterial diameter curves. Diastolic and systolic diameters were determined as the smallest and largest diameter values during a cardiac cycle. Blood pressure measurements were taken by upper arm sphygmomanometry (DINAMAP System; GE Medical Systems) at the time of the carotid artery ultrasound.
In RS, vessel wall motion of the right CCA was measured by means of an Ultramark IV duplex scanner (Advanced Technology Laboratories) with a 7.5‐MHz linear array transducer, connected to a vessel wall movement detector system. Common carotid distensibility was assessed with the participants in the supine position, with the head tilted slightly to the contralateral side for the measurement in the CCA. Vessel wall motion of the right common carotid artery was measured by means of a duplex of an Ultramark IV scanner (Advanced Technology Laboratories) with a 7.5‐MHz linear array transducer, connected to a vessel wall movement detector system. The details of this technique have been described elsewhere.30 After 5 minutes of rest, a region at 1.5 cm proximal to the origin of the bulb of the carotid artery was identified with the use of B‐mode ultrasound. Displacement of arterial walls was obtained by processing the radiofrequency signals originating from 2 selected sample volumes positioned over the anterior and posterior walls. End‐diastolic diameter (D), the absolute stroke change in diameter during systole (ΔD), and the relative stroke change in diameter (ΔD/D) were computed as the mean of 4 cardiac cycles of 3 successive recordings.
Pulse Wave Velocity
Aortic PWV data were only available in the RS. PWV was assessed with an automatic device (Complior Artech Medicla, Pantin, France).31
Covariates
All variables in the CHARGE‐AF risk score were considered: age (continuous); race (white vs nonwhite); height (continuous); weight (continuous); systolic blood pressure (continuous); diastolic blood pressure (continuous); use of antihypertensive medication; smoking status (current vs noncurrent); diabetes; history of heart failure; and history of myocardial infarction.11 Details on measurement methods are available in Data S1.
Statistical Analyses
We report means±SDs for continuous variables and counts with percentages for categorical variables. Person‐years at risk were calculated from the date of baseline until the date of incident AF, death, loss to follow‐up, or end of follow‐up, whichever occurred first.
To estimate the association of each arterial index with incident AF, we used Cox proportional hazards models to calculate hazard ratios and 95% CIs per 1‐SD and per‐quintile increment in arterial index (cIMT, DC, and PWV) and for presence of carotid plaque. Separate models were fit for each cohort and for each arterial index. We generated 2 models: In model 1, we adjusted for age and race. In model 2, we additionally adjusted for other baseline covariates in the CHARGE‐AF risk score. The CHARGE‐AF risk score comprises age (continuous), race (white vs nonwhite), height (continuous), weight (continuous), systolic blood pressure (continuous), diastolic blood pressure (continuous), use of antihypertensive medication, smoking status (current vs noncurrent), diabetes, history of heart failure, and history of myocardial infarction. An Excel spreadsheet (available as a supplemental file in reference 11) allows calculation of AF risk using this predictive model. Given that adjusting for height, weight, systolic blood pressure, and diastolic blood pressure may result in overadjustment for indices of arterial stiffness, we constructed a model 3 for DC and PWV, which adjusted for all covariates in the CHARGE‐AF risk score except for the 4 aforementioned variables.
ARIC, MESA, and RS results were meta‐analyzed using an inverse variance weighting method.32 Because incident AF cases in ARIC and MESA were mostly ascertained from hospitalizations, health care utilization may be a potential confounder. To address this issue, we performed a sensitivity analysis by additionally adjusting for time‐dependent incident hospitalization.
The refitted CHARGE‐AF risk score was used as the benchmark (base model) to assess the role of each arterial index in enhancing risk prediction of AF. We added cIMT, DC, and PWV to the model as continuous predictors and carotid plaque as a dichotomous predictor. To determine improvement in model discrimination with addition of each arterial index, we calculated the Harrell's C‐statistic for AF risk (10‐year risk in ARIC, 9.25‐year risk in MESA, and 10‐year risk in RS) using methods that accounted for censoring33 for the base model and the base model plus arterial index (expanded model). Because the 3 cohort studies had different follow‐up times, we computed slightly different year‐risk for the 3 studies. We present optimism‐corrected C‐statistics and 95% CIs obtained by bootstrapping for internal validation.34
Using Cox proportional hazards, the AF risk (for 10 years in ARIC, 9.25 years in MESA, and 10 years in RS) was calculated, and individuals were classified into <5%, 5% to 10%, and >10% risk categories. To evaluate reclassification, we calculated category‐based net reclassification improvement (NRI), taking into account censored observations.35 In addition, we estimated relative integrated discrimination improvement (IDI), which is the ratio of absolute difference in discrimination slopes of the 2 models over the discrimination slope of the model without the arterial index.36 To test model calibration, we compared the “goodness of fit” of the observed and expected number of events within estimated risk decile groups using the Grønnesby‐Borgan statistic.37 Finally, for indices of arterial stiffness, we also benchmarked against an alternative base model, which was a score comprising the CHARGE‐AF variables except for height, weight, systolic blood pressure, and diastolic blood pressure.
Sex‐ and race‐stratified analyses were conducted in ARIC because it is the largest cohort that is also biracial. In addition, we evaluated the ability of arterial indices in improving prediction of AF in these subgroups: participants without heart failure or history of myocardial infarction, and participants with body mass index <30 and ≥30 kg/m2. The proportional hazard assumption was verified using Schoenfeld's residuals. Statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC). All P values reported were 2‐sided.
Results
Study Participants
The cohort at risk for AF consisted of 6193 men and 7714 women in ARIC, 3136 men and 3504 women in MESA, and 2264 men and 2956 women in RS. There were 23.8% and 39.4% nonwhite participants in ARIC and MESA, respectively. Mean age of participants was highest in RS (69.0±7.8 years), followed by MESA (62.0±10.2 years) and ARIC (57.0±5.7 years). Prevalence of carotid plaque was highest in RS (52.6%) and lowest in MESA (12.8%; Table 1).
Table 1.
Baseline Characteristics of Study Participants According to Incident Atrial Fibrillation: ARIC, MESA, and RS
| ARIC | MESA | RS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=13 907) | No AF (n=12 276) | AF (n=1631) | All (n=6640) | No AF (n=6330) | AF (n=310) | All (n=5220) | No AF (n=4921) | AF (n=299) | |
| Age, y (SD) | 57.0 (5.7) | 56.7 (5.7) | 59.8 (5.4) | 62.0 (10.2) | 61.6 (10.1) | 70.4 (8.1) | 69.0 (7.8) | 68.8 (7.8) | 73.2 (7.3) |
| Women | 7714 (55.5) | 6907 (56.8) | 744 (45.6) | 3504 (52.8) | 3385 (53.5) | 119 (38.4) | 2956 (56.6) | 2803 (57.0) | 153 (51.2) |
| Nonwhite race | 3311 (23.8) | 3037 (24.7) | 274 (16.8) | 2614 (39.4) | 2526 (39.9) | 88 (28.4) | NA | NA | NA |
| Height, cm (SD) | 168.5 (9.3) | 168.2 (9.3) | 170.3 (9.5) | 166.4 (10.0) | 166.3 (10.0) | 168.6 (10.4) | 167.3 (9.3) | 167.3 (9.2) | 168.2 (10.3) |
| Weight, kg (SD) | 79.3 (16.7) | 78.6 (16.4) | 84.6 (17.7) | 78.6 (17.3) | 78.5 (17.3) | 81.7 (17.5) | 75.7 (13.0) | 75.5 (12.9) | 78.0 (13.3) |
| Current smoker | 3113 (22.4) | 2702 (22.0) | 411 (25.2) | 861 (13.0) | 825 (13.0) | 36 (11.6) | 1025 (19.6) | 979 (19.9) | 46 (15.4) |
| Diabetes | 2049 (14.7) | 1692 (13.8) | 357 (21.9) | 836 (12.6) | 790 (12.5) | 46 (14.8) | 664 (12.7) | 616 (12.5) | 48 (16.1) |
| HTN medication use | 4528 (32.6) | 3720 (30.3) | 808 (49.5) | 2449 (36.9) | 2273 (35.9) | 176 (56.8) | 1760 (33.7) | 1606 (32.6) | 154 (51.5) |
| Systolic BP, mm Hg (SD) | 121.4 (18.7) | 120.7 (18.4) | 126.8 (20.2) | 126.5 (21.5) | 126.0 (21.3) | 135.6 (22.0) | 143.4 (21.1) | 143.1 (20.9) | 149.5 (24.2) |
| Diastolic BP, mm Hg (SD) | 72.0 (10.2) | 72.0 (10.1) | 72.2 (10.9) | 71.9 (10.3) | 71.9 (10.3) | 72.0 (10.3) | 76.9 (11.1) | 76.9 (11.0) | 76.6 (11.7) |
| History of MI | 140 (1.0) | 113 (0.9) | 27 (1.7) | NA | NA | NA | 319 (6.1) | 289 (5.9) | 30 (10.0) |
| History of heart failure | 69 (0.5) | 52 (0.4) | 17 (1.0) | NA | NA | NA | 125 (2.4) | 112 (2.3) | 13 (4.3) |
| Carotid plaquea | 4748 (34.4) | 4001 (32.9) | 747 (46.2) | 846 (12.8) | 760 (12.1) | 86 (27.9) | 2595 (52.6) | 2413 (51.9) | 182 (64.3) |
| cIMT, mm (SD)b | 0.75 (0.20) | 0.74 (0.19) | 0.82 (0.24) | 0.87 (0.19) | 0.86 (0.19) | 0.99 (0.24) | 1.04 (0.19) | 1.04 (0.19) | 1.11 (0.19) |
| DC, 10−3/kPa (SD)c | 16.8 (6.8) | 17.0 (6.9) | 15.1 (6.1) | 18.8 (8.1) | 19.0 (8.2) | 15.4 (7.0) | 11.5 (4.7) | 11.6 (4.7) | 10.2 (4.1) |
| PWV, m/s (SD)d | NA | NA | NA | NA | NA | NA | 13.1 (3.1) | 13.1 (3.1) | 14.4 (3.7) |
Data are presented as No. (%) of participants unless otherwise stated. There were 13 907, 6640, and 5220 participants who had data on at least one arterial measurement in ARIC, MESA, and RS, respectively. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; BP, blood pressure; cIMT, carotid intima‐media thickness; DC, distensibility coefficient; HTN, hypertension; MESA, Multi‐Ethnic Study of Atherosclerosis; MI, myocardial infarction; NA, not applicable; PWV, pulse wave velocity; RS, Rotterdam Study.
No. of participants in ARIC, 13 796; MESA, 6595; RS, 4929.
No. of participants in ARIC, 13 595; MESA, 6605; RS, 4839.
No. of participants in ARIC, 10 300; MESA, 6417; RS, 4015.
No. of participants in RS, 4639.
During follow‐up in ARIC (median, 17.8 years), 1631 AF events occurred, of which 63 (3.9%) were atrial flutter events. In MESA, there were 310 AF events (atrial flutter, 21 [6.8%]) after median follow‐up of 8.5 years. During follow‐up in RS (median, 7.5 years), there were 299 AF events.
Association of Arterial Indices With Risk of AF
In all 3 cohorts, higher cIMT was associated with higher incidence of AF. Hazard ratios per 1‐SD increment in cIMT for AF were 1.12, 1.16, and 1.09 in ARIC, MESA, and RS, respectively, after full adjustment for variables in the CHARGE‐AF risk score (Table 2). Compared with participants in the lowest quintile of cIMT, participants in the highest quintile had a risk of AF that was 1.3‐, 1.9‐, and 1.6‐fold higher in ARIC, MESA, and RS, respectively, after full adjustment for variables in the CHARGE‐AF risk score (Table 2). In the fully adjusted model, the meta‐analyzed hazard ratio (95% CI) for AF per 1‐SD increment in cIMT was 1.12 (1.08–1.16) and 1.37 (1.17–1.61) comparing participants in the highest quintile with the lowest quintile. The presence of carotid plaque was associated with a 1.3‐, 1.4‐, and 1.3‐fold increased risk of AF, in ARIC, MESA, and RS, respectively, after full adjustment (Figure). The corresponding meta‐analyzed hazard ratio (95% CI) of carotid plaque for incident AF was 1.30 (1.19–1.42; Figure). To account for potential confounding effect of hospitalization, we additionally adjusted for time‐dependent hospitalization. The association of higher CIMT with higher incidence of AF remained essentially the same (Table S1).
Table 2.
Hazard Ratios (95% CIs) of Carotid Intima‐Media Thickness, Distensibility Coefficent, and Pulse Wave Velocity for Atrial Fibrillation: ARIC, MESA, and RS
| ARIC | ||||||||
|---|---|---|---|---|---|---|---|---|
| cIMT Quintiles (mm) (n=13 595) | P fora Trend | cIMT Continuous, per 1 SD (0.20 mm) | P Value | |||||
| <0.60 | 0.60 to <0.67 | 0.67 to <0.75 | 0.75 to <0.86 | ≥0.86 | ||||
| AF cases, n | 197 | 234 | 284 | 366 | 499 | |||
| Person‐years | 45 101 | 44 809 | 44 173 | 43 260 | 38 135 | |||
| AF incidenceb | 4.4 (3.8–5.0) | 5.2 (4.6–5.9) | 6.4 (5.7–7.2) | 8.5 (7.6–9.4) | 13.1 (12.0–14.3) | |||
| Model 1 | 1 (ref.) | 1.06 (0.88–1.29 | 1.21 (1.00–1.45) | 1.50 (1.26–1.79) | 2.07 (1.74–2.45) | <0.001 | 1.26 (1.21–1.30) | <0.001 |
| Model 2 | 1 (ref.) | 0.93 (0.77–1.13) | 0.93 (0.78–1.12) | 1.06 (0.88–1.26) | 1.29 (1.08–1.54) | <0.001 | 1.12 (1.08–1.17) | <0.001 |
| DC Quintiles (10−3/kPa) (n=10 300) | P for Trend | DC Continuous, per 1 SD (6.8×10−3/kPa) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <11.0 | 11.0 to <14.0 | 14.0 to <17.4 | 17.4 to <21.5 | ≥21.5 | ||||
| AF cases, n | 275 | 238 | 231 | 176 | 137 | |||
| Person‐years | 29 308 | 31 723 | 34 827 | 33 866 | 35 929 | |||
| AF incidenceb | 9.4 (8.3–10.5) | 7.5 (6.6–8.5) | 6.6 (5.8–7.5) | 5.2 (4.5–6.0) | 3.8 (3.2–4.5) | |||
| Model 1 | 1 (ref.) | 0.90 (0.76–1.07) | 0.86 (0.72–1.03) | 0.75 (0.61–0.91) | 0.63 (0.51–0.78) | <0.001 | 0.84 (0.78–0.91) | <0.001 |
| Model 2 | 1 (ref.) | 1.02 (0.85–1.22) | 1.04 (0.86–1.25) | 0.97 (0.79–1.29) | 0.89 (0.70–1.13) | 0.38 | 0.95 (0.88–1.03) | 0.25 |
| Model 3 | 1 (ref.) | 1.00 (0.84–1.19) | 0.98 (0.82–1.18) | 0.89 (0.72–1.08) | 0.74 (0.59–0.93) | 0.01 | 0.90 (0.83–0.97) | 0.004 |
| MESA | ||||||||
|---|---|---|---|---|---|---|---|---|
| cIMT Quintiles (mm) (n=6605) | P for Trend | cIMT Continuous, per 1 SD (0.19 mm) | P Value | |||||
| <0.71 | 0.71 to <0.80 | 0.80 to <0.89 | 0.89 to <1.01 | ≥1.01 | ||||
| AF cases (n) | 18 | 40 | 43 | 88 | 121 | |||
| Person‐years | 10 613 | 10 410 | 10 538 | 10 300 | 9803 | |||
| AF incidenceb | 1.7 (1.1–2.7) | 3.8 (2.8–5.2) | 4.1 (3.0–5.5) | 8.5 (6.9–10.5) | 12.3 (10.3–14.8) | |||
| Model 1 | 1 (ref.) | 1.67 (0.95–2.92) | 1.37 (0.78–2.40) | 2.34 (1.39–3.96) | 2.66 (1.57–4.49) | <0.001 | 1.25 (1.15–1.37) | <0.001 |
| Model 2 | 1 (ref.) | 1.61 (0.92–2.82) | 1.21 (0.69–2.11) | 1.90 (1.13–3.21) | 1.94 (1.15–3.28) | 0.006 | 1.16 (1.06–1.27) | 0.002 |
| DC Quintiles (10−3/kPa) (n=6417) | P For Trend | DC continuous, per 1 SD (10.9×10−3/kPa) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <12.4 | 12.4 to <15.9 | 15.9 to <19.4 | 19.4 to <24.2 | ≥24.2 | ||||
| AF cases (n) | 105 | 85 | 52 | 33 | 29 | |||
| Person‐years | 9544 | 10 042 | 10 147 | 10 278 | 10 207 | |||
| AF incidenceb | 11.0 (9.1–13.3) | 8.5 (6.8–10.5) | 5.1 (3.9–6.7) | 3.2 (2.3–4.5) | 2.84 (2.0–4.1) | |||
| Model 1 | 1 (ref.) | 0.97 (0.72–1.29) | 0.73 (0.52–1.02) | 0.58 (0.39–0.87) | 0.78 (0.49–1.22) | 0.01 | 0.81 (0.69–0.96) | 0.02 |
| Model 2 | 1 (ref.) | 1.03 (0.76–1.38) | 0.79 (0.56–1.12) | 0.65 (0.43–0.99) | 0.91 (0.57–1.45) | 0.10 | 0.86 (0.73–1.02) | 0.09 |
| Model 3 | 1 (ref.) | 1.01 (0.75–1.34) | 0.75 (0.54–1.06) | 0.62 (0.41–0.04) | 0.81 (0.51–1.27) | 0.03 | 0.83 (0.70–0.98) | 0.03 |
| RS | ||||||||
|---|---|---|---|---|---|---|---|---|
| cIMT Quintiles (mm) (n=4839) | P for Trend | cIMT Continuous, per 1 SD (0.19 mm) | P Value | |||||
| <0.88 | 0.88 to <0.97 | 0.97 to <1.07 | 1.07 to <1.18 | ≥1.18 | ||||
| AF cases (n) | 24 | 48 | 49 | 69 | 90 | |||
| Person‐years | 7198 | 7323 | 7291 | 7116 | 6748 | |||
| AF incidenceb | 3.3 (2.1–5.0) | 6.5 (4.8–8.7) | 6.7 (5.0–8.9) | 9.7 (7.5–12.3) | 13.3 (10.7–16.4) | |||
| Model 1 | 1 (ref.) | 1.56 (0.95–2.56) | 1.46 (0.89–2.40) | 1.94 (1.20–3.13) | 2.36 (1.47–3.81) | <0.001 | 1.21 (1.09–1.35) | <0.001 |
| Model 2 | 1 (ref.) | 1.37 (0.83–2.24) | 1.18 (0.71–1.94) | 1.47 (0.90–2.40) | 1.61 (0.99–2.64) | 0.05 | 1.09 (0.97–1.23) | 0.16 |
| DC Quintiles (10−3/kPa) (n=4015) | P for Trend | DC Continuous, per 1 SD (4.7×10−3/kPa) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <7.4 | 7.4 to <9.7 | 9.7 to <12.1 | 12.1 to <15.2 | ≥15.2 | ||||
| AF cases (n) | 66 | 58 | 49 | 38 | 26 | |||
| Person‐years | 5738 | 6071 | 6086 | 6022 | 6002 | |||
| AF incidenceb | 11.5 (8.9–14.6) | 9.6 (7.3–12.4) | 8.1 (6.0–10.6) | 6.3 (4.5–8.7) | 4.3 (2.8–6.3) | |||
| Model 1 | 1 (ref.) | 1.10 (0.77–1.58) | 1.05 (0.71–1.55) | 0.96 (0.62–1.48) | 0.76 (0.46–1.25) | 0.31 | 0.94 (0.80–1.10) | 0.46 |
| Model 2 | 1 (ref.) | 1.17 (0.81–1.69) | 1.17 (0.79–1.75) | 1.09 (0.70–1.71) | 0.88 (0.52–1.50) | 0.77 | 1.00 (0.85–1.19) | 0.97 |
| Model 3 | 1 (ref.) | 1.14 (0.80–1.65) | 1.13 (0.76–1.67) | 1.03 (0.67–1.60) | 0.84 (0.51–1.39) | 0.58 | 0.98 (0.83–1.15) | 0.78 |
| PWV Quintiles (m/s) (n=4639) | P for Trend | PWV Continuous, per 1 SD (3.1 m/s) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <10.6 | 10.6 to <11.9 | 11.9 to <13.4 | 13.4 to <15.4 | ≥15.4 | ||||
| AF cases (n) | 30 | 34 | 47 | 72 | 84 | |||
| Person‐years | 7040 | 6993 | 7061 | 6728 | 6446 | |||
| AF incidenceb | 4.3 (2.9–6.1) | 4.9 (3.4–6.8) | 6.7 (4.9–8.9) | 10.7 (8.4–13.5) | 13.0 (10.4–16.1) | |||
| Model 1 | 1 (ref.) | 0.97 (0.59–1.60) | 1.18 (0.74–1.88) | 1.64 (1.05–2.55) | 1.63 (1.04–2.57) | 0.003 | 1.18 (1.06–1.32) | 0.004 |
| Model 2 | 1 (ref.) | 0.79 (0.48–1.30) | 0.93 (0.58–1.49) | 1.18 (0.74–1.86) | 1.03 (0.63–1.67) | 0.33 | 1.05 (0.93–1.19) | 0.46 |
| Model 3 | 1 (ref.) | 0.92 (0.56–1.50) | 1.12 (0.70–1.78) | 1.54 (0.99–2.41) | 1.47 (0.93–2.32) | 0.010 | 1.15 (1.03–1.29) | 0.016 |
Model 1: Cox proportional hazard model adjusted for age and race (model 1 in RS was adjusted for age only). Model 2: model 1+adjusted for height, weight, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. Model 3: model 1+use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. Incidence rates (95% CI) of AF were 7.3 (7.0–7.7), 6.0 (5.4–6.7), and 7.8 (7.0–8.8) per 1000 person‐years in ARIC, MESA, and RS, respectively. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; cIMT, carotid intima‐media thickness; DC, distensibility coefficient; MESA, Multi‐Ethnic Study of Atherosclerosis; PWV, pulse wave velocity; RS, Rotterdam Study.
P for trend was obtained from a linear term in quintile number.
Crude incidence rate per 1000 person‐years.
Figure 1.
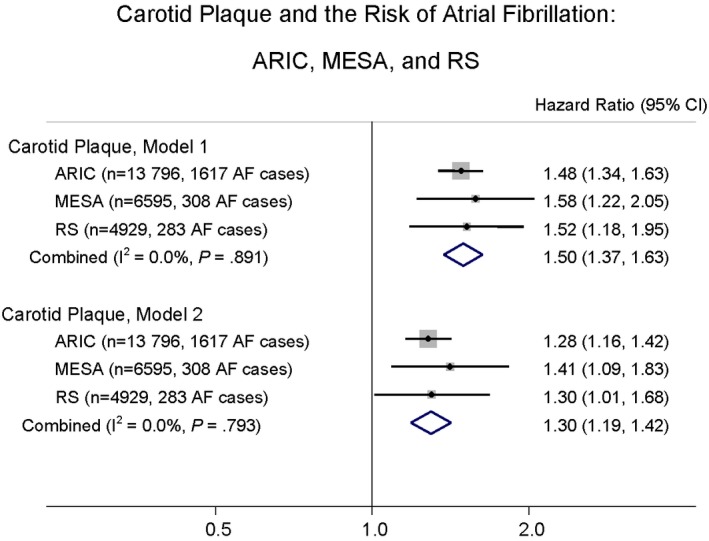
Carotid plaque and the risk of atrial fibrillation: ARIC, MESA, and RS. Model 1: Cox proportional hazard model adjusted for age and race. Model 2: model 1+adjusted for height, weight, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; MESA, Multi‐Ethnic Study of Atherosclerosis; RS, Rotterdam Study.
Although in model 1 higher DC was associated with a lower incidence of AF in ARIC and MESA, after full adjustment in model 2, DC was no longer associated with AF (Table 2). Similarly, in model 1, higher PWV was associated with higher incidence of AF in RS, but no longer after full adjustment in model 2 (Table 2). However, in model 3 (CHARGE‐AF risk score variables excepting height, weight, systolic blood pressure, and diastolic blood pressure), higher DC was associated with a lower incidence of AF in ARIC and MESA. Similarly, in model 3, higher PWV was associated with higher incidence of AF in RS (Table 2).
We conducted sex‐ and race‐stratified analyses in ARIC. We did not find evidence of a difference in the association between higher cIMT and incident AF by sex (P for sex interaction=0.51) or by race (P for race interaction=0.61; Table 3). Similarly, no evidence of difference in the association of carotid plaque with incident AF by sex or race was observed (P for sex interaction=0.90; P for race interaction=0.13; Table 4).
Table 3.
Sex‐ and Race‐Stratified Hazard Ratios of Carotid Intima‐Media Thickness and Distensibility Coefficient for Atrial Fibrillation in the ARIC Studya
| Sex‐Stratified | ||||||||
|---|---|---|---|---|---|---|---|---|
| cIMT Quintiles (mm) | P for Trend | Continuous, per 1 SD (0.20 mm) | P Value | |||||
| <0.60 | 0.60 to <0.67 | 0.67 to <0.75 | 0.75 to <0.86 | ≥0.86 | ||||
| Women | ||||||||
| Model 1 | 1 (ref.) | 0.97 (0.76–1.25) | 1.18 (0.92–1.51) | 1.49 (1.17–1.90) | 1.96 (1.54–2.50) | <0.001 | 1.29 (1.21–1.37) | <0.001 |
| Model 2 | 1 (ref.) | 0.88 (0.69–1.13) | 0.99 (0.77–1.26) | 1.14 (0.89–1.45) | 1.30 (1.01–1.66) | 0.005 | 1.13 (1.06–1.21) | 0.0003 |
| Men | ||||||||
| Model 1 | 1 (ref.) | 1.07 (0.80–1.45) | 1.02 (0.77–1.36) | 1.21 (0.92–1.59) | 1.65 (1.27–2.15) | <0.001 | 1.19 (1.13–1.25) | <0.001 |
| Model 2 | 1 (ref.) | 0.98 (0.73–1.32) | 0.86 (0.65–1.15) | 0.98 (0.74–1.28) | 1.25 (0.96–1.63) | 0.007 | 1.12 (1.06–1.18) | <0.001 |
| DC Quintiles (10−3/kPa) | P for Trend | Continuous, per 1 SD (6.8×10−3/kPa) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <11.0 | 11.0 to <14.0 | 14.0 to <17.4 | 17.4 to <21.5 | ≥21.5 | ||||
| Women | ||||||||
| Model 1 | 1 (ref.) | 0.92 (0.72–1.20) | 0.82 (0.63–1.06) | 0.65 (0.49–0.87) | 0.56 (0.41–0.77) | <0.001 | 0.79 (0.71–0.88) | <0.001 |
| Model 2 | 1 (ref.) | 1.06 (0.82–1.37) | 0.99 (0.76–1.31) | 0.90 (0.66–1.22) | 0.79 (0.56–1.13) | 0.16 | 0.90 (0.80–1.02) | 0.09 |
| Model 3 | 1 (ref.) | 1.06 (0.82–1.37) | 0.94 (0.72–1.23) | 0.83 (0.61–1.11) | 0.68 (0.49–0.95) | 0.01 | 0.86 (0.77–0.96) | 0.005 |
| Men | ||||||||
| Model 1 | 1 (ref.) | 0.85 (0.67–1.09) | 0.88 (0.69–1.12) | 0.82 (0.63–1.07) | 0.71 (0.53–0.95) | 0.03 | 0.90 (0.82–0.99) | 0.05 |
| Model 2 | 1 (ref.) | 0.97 (0.76–1.24) | 1.05 (0.82–1.36) | 1.02 (0.77–1.36) | 0.97 (0.70–1.34) | 0.95 | 1.00 (0.90–1.12) | 0.98 |
| Model 3 | 1 (ref.) | 0.92 (0.71–1.17) | 0.97 (0.76–1.24) | 0.91 (0.70–1.20) | 0.82 (0.61–1.11) | 0.28 | 0.95 (0.85–1.05) | 0.28 |
| Race‐Stratified | ||||||||
|---|---|---|---|---|---|---|---|---|
| cIMT Quintiles (mm) | P for Trend | Continuous, per 1 SD (0.20 mm) | P Value | |||||
| <0.60 | 0.60 to <0.67 | 0.67 to <0.75 | 0.75 to <0.86 | ≥0.86 | ||||
| White | ||||||||
| Model 1 | 1 (ref.) | 1.12 (0.91–1.38) | 1.23 (1.01–1.50) | 1.54 (1.27–1.86) | 2.16 (1.79–2.60) | <0.001 | 1.22 (1.17–1.28) | <0.001 |
| Model 2 | 1 (ref.) | 0.97 (0.79–1.19) | 0.93 (0.76–1.14) | 1.05 (0.87–1.28) | 1.32 (1.09–1.60) | <0.001 | 1.12 (1.07–1.17) | <0.001 |
| Black | ||||||||
| Model 1 | 1 (ref.) | 0.76 (0.45–1.27) | 1.03 (0.64–1.64) | 1.27 (0.81–2.00) | 1.57 (1.00–2.47) | 0.003 | 1.22 (1.10–1.34) | <0.001 |
| Model 2 | 1 (ref.) | 0.74 (0.44–1.24) | 0.90 (0.56–1.43) | 1.02 (0.64–1.61) | 1.13 (0.72–1.79) | 0.15 | 1.15 (1.04–1.28) | 0.01 |
| DC Quintiles (10−3/kPa) | P for Trend | Continuous, per 1 SD (6.8×10−3/kPa) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <11.0 | 11.0 to <14.0 | 14.0 to <17.4 | 17.4 to <21.5 | ≥21.5 | ||||
| White | ||||||||
| Model 1 | 1 (ref.) | 0.96 (0.79–1.17) | 0.92 (0.75–1.12) | 0.83 (0.67–1.02) | 0.67 (0.53–0.85) | <0.001 | 0.87 (0.80–0.94) | 0.0004 |
| Model 2 | 1 (ref.) | 1.09 (0.89–1.33) | 1.09 (0.89–1.35) | 1.05 (0.84–1.32) | 0.94 (0.72–1.22) | 0.72 | 0.97 (0.89–1.06) | 0.51 |
| Model 3 | 1 (ref.) | 1.07 (0.88–1.32) | 1.05 (0.85–1.29) | 0.97 (0.78–1.21) | 0.80 (0.62–1.02) | 0.08 | 0.92 (0.85–0.99) | 0.04 |
| Black | ||||||||
| Model 1 | 1 (ref.) | 0.72 (0.50–1.05) | 0.68 (0.45–1.01) | 0.44 (0.26–0.75) | 0.49 (0.28–0.87) | <0.001 | 0.70 (0.57–0.85) | 0.0004 |
| Model 2 | 1 (ref.) | 0.80 (0.55–1.18) | 0.83 (0.54–1.26) | 0.61 (0.35–1.07) | 0.72 (0.39–1.33) | 0.12 | 0.84 (0.68–1.05) | 0.13 |
| Model 3 | 1 (ref.) | 0.76 (0.52–1.13) | 0.75 (0.49–1.13) | 0.53 (0.31–0.92) | 0.60 (0.33–1.08) | 0.02 | 0.77 (0.63–0.95) | 0.01 |
Model 1: Cox proportional hazard model adjusted for age and race. Model 2: model 1+adjusted for height, weight, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. Model 3: model 1+use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. P for sex interaction (cIMT, model 2)=0.51, P for race interaction (cIMT, model 2)=0.61. P for sex interaction (DC, model 2)=0.65, P for race interaction (DC, model 2)=0.30. ARIC indicates Atherosclerosis Risk in Communities; cIMT, carotid intima‐media thickness; DC, distensibility coefficient.
Analyses for cIMT and DC were based on 13 595 and 10 300 participants, respectively.
Table 4.
Sex‐ and Race‐Stratified Hazard Ratios of Carotid Plaque for Atrial Fibrillation in the ARIC Studya
| Sex‐Stratified | Women | Men | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | 1.47 (1.26–1.70) | <0.001 | 1.36 (1.19–1.56) | <0.001 |
| Model 2 | 1.28 (1.10–1.50) | 0.002 | 1.28 (1.12–1.47) | <0.001 |
| Race‐Stratified | White | Black | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | 1.52 (1.37–1.70) | <0.001 | 1.24 (0.96–1.60) | 0.09 |
| Model 2 | 1.33 (1.19–1.48) | <0.001 | 1.06 (0.81–1.38) | 0.68 |
Model 1: Cox proportional hazard model adjusted for age and race. Model 2: model 1+adjusted for height, weight, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, current smoking, diabetes, history of heart failure, and history of myocardial infarction. P for sex interaction (model 2)=0.90, P for race interaction (model 2)=0.13. ARIC indicates Atherosclerosis Risk in Communities; HR, hazard ratio.
Analysis was based on 13 796 participants.
Model Discrimination, Calibration, and Reclassification With Addition of Arterial Indices to the CHARGE‐AF Risk Score
Model discrimination of the refitted CHARGE‐AF risk score (base model) in the 3 cohorts was good (C‐statistic ranging from 0.708 to 0.788; Table 5). The addition of cIMT to the base model increased the C‐statistic (95% CI) marginally from 0.769 (0.751–0.786) to 0.773 (0.756–0.790) in ARIC, 0.786 (0.764–0.809) to 0.791 (0.769–0.813) in MESA, and 0.709 (0.680–0.739) to 0.711 (0.682–0.740) in RS. Similarly, in all 3 cohorts, addition of carotid plaque to the base model or addition of carotid plaque and cIMT to the base model did not meaningfully improve discrimination. However, in ARIC, cIMT, carotid plaque, and cIMT plus carotid plaque led to small increments in predictive ability as measured with the relative IDI (0.051 [95% CI, 0.016–0.095], 0.025 [95% CI, 0.004–0.044], and 0.058 [95% CI, 0.022–0.097], respectively). Improvements in prediction as measured with relative IDI were also observed in MESA for carotid plaque and cIMT plus carotid plaque (0.034 [0.002–0.064] and 0.058 [0.012–0.113], respectively). In ARIC, compared with the base model (χ2 statistic, 15.8), model calibration also improved with addition of cIMT (χ2 statistic, 13.7) or carotid plaque (χ2 statistic, 11.6). The category‐based NRI for cIMT, carotid plaque, or cIMT plus carotid plaque, however, was of negligible magnitude in all 3 cohorts.
Table 5.
C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model: ARIC, MESA, and RS
| C‐Statistic (95% CI) | Calibration χ2 P Value | Category‐Based NRI (95% CI) | Relative IDI (95% CI) | |
|---|---|---|---|---|
| ARIC | ||||
| BM 1 | 0.769 (0.751, 0.786) | 0.07 | NA | NA |
| BM 1+cIMT | 0.773 (0.756, 0.790) | 0.15 | 0.013 (−0.009, 0.034) | 0.051 (0.016, 0.095) |
| BM 2 | 0.769 (0.752, 0.786) | 0.07 | NA | NA |
| BM 2+plaque | 0.771 (0.754, 0.788) | 0.24 | 0.001 (−0.021, 0.022) | 0.025 (0.004, 0.044) |
| BM 3 | 0.769 (0.751, 0.786) | 0.07 | NA | NA |
| BM 3+plaque+cIMT | 0.773 (0.756, 0.790) | 0.11 | 0.008 (−0.016, 0.033) | 0.058 (0.022, 0.097) |
| BM 4 | 0.761 (0.740, 0.782) | 0.10 | NA | NA |
| BM 4+DC | 0.761 (0.740, 0.783) | 0.07 | −0.012 (−0.026, 0.001) | 0.0001 (−0.007, 0.006) |
| BM 4a | 0.720 (0.695, 0.745) | 0.40 | NA | NA |
| BM 4a+DC | 0.723 (0.698, 0.747) | 0.09 | 0.001 (−0.157, 0.018) | 0.017 (0.004, 0.031) |
| MESA | ||||
| BM 1 | 0.786 (0.764, 0.809) | 0.65 | NA | NA |
| BM 1+cIMT | 0.791 (0.769, 0.813) | 0.59 | −0.011 (−0.076, 0.057) | 0.038 (−0.004, 0.089) |
| BM 2 | 0.787 (0.765, 0.809) | 0.22 | NA | NA |
| BM 2+plaque | 0.791 (0.769, 0.813) | 0.36 | 0.001 (−0.053, 0.046) | 0.034 (0.002, 0.064) |
| BM 3 | 0.788 (0.765, 0.810) | 0.58 | NA | NA |
| BM 3+plaque+cIMT | 0.794 (0.772, 0.816) | 0.59 | −0.012 (−0.058, 0.032) | 0.058 (0.012, 0.113) |
| BM 4 | 0.788 (0.766, 0.810) | 0.54 | NA | NA |
| BM 4+DC | 0.789 (0.767, 0.811) | 0.64 | −0.014 (−0.060, 0.018) | 0.006 (−0.007, 0.019) |
| BM 4a | 0.772 (0.749, 0.795) | 0.29 | NA | NA |
| BM 4a+DC | 0.773 (0.750, 0.796) | 0.32 | 0.0004 (−0.026, 0.028) | 0.012 (−0.003, 0.027) |
| RS | ||||
| BM 1 | 0.709 (0.680, 0.739) | 0.60 | NA | NA |
| BM 1+cIMT | 0.711 (0.682, 0.740) | 0.47 | 0.015 (−0.001, 0.037) | 0.009 (−0.012, 0.032) |
| BM 2 | 0.711 (0.681, 0.740) | 0.78 | NA | NA |
| BM 2+plaque | 0.712 (0.682, 0.741) | 0.68 | 0.026 (−0.008, 0.064) | 0.010 (−0.016, 0.034) |
| BM 3 | 0.708 (0.679, 0.738) | 0.48 | NA | NA |
| BM 3+plaque+cIMT | 0.711 (0.681, 0.740) | 0.46 | 0.033 (−0.003, 0.071) | 0.017 (−0.014, 0.046) |
| BM 4 | 0.711 (0.679, 0.742) | 0.52 | NA | NA |
| BM 4+DC | 0.711 (0.679, 0.742) | 0.41 | −0.003 (−0.011, 0.002) | −0.000 (−0.002, 0.002) |
| BM 4a | 0.678 (0.644, 0.711) | 0.002 | NA | NA |
| BM 4a+DC | 0.678 (0.644, 0.712) | 0.003 | 0.007 (−0.002, 0.022) | 0.000 (−0.002, 0.002) |
| BM 5 | 0.720 (0.690, 0.749) | 0.63 | NA | NA |
| BM 5+PWV | 0.720 (0.690, 0.749) | 0.63 | −0.001 (−0.014, 0.014) | 0.005 (−0.006, 0.017) |
| BM 5a | 0.692 (0.660, 0.724) | 0.0005 | NA | NA |
| BM 5a+PWV | 0.697 (0.666, 0.728) | 0.018 | 0.014 (−0.012, 0.040) | 0.047 (0.009, 0.083) |
BM 1, base model for participants with complete cIMT data; BM 2, base model for participants with complete carotid plaque data; BM 3, base model for participants with complete cIMT and carotid plaque data; BM 4, base model for participants with complete DC data; BM 4a, base model (excluding height, weight, systolic blood pressure, and diastolic blood pressure) for participants with complete DC data; BM 5, base model for participants with complete PWV data; BM 5a, base model (excluding height, weight, systolic blood pressure, and diastolic blood pressure) for participants with complete PWV data. The base model is the refitted CHARGE‐AF model in the 3 different cohorts. NRI categories are <5%, 5% to 10%, and >10% risk of AF in 10, 9.25, and 10 years in ARIC, MESA, and RS, respectively. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; cIMT, carotid intima‐media thickness; DC, distensibility coefficient; IDI, integrated discrimination improvement; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not applicable; NRI, net reclassification improvement; PWV, pulse wave velocity; RS, Rotterdam Study.
Addition of DC to the base model did not meaningfully alter model discrimination or improve model calibration in all 3 cohorts. Similarly, reclassification measured with the NRI and relative IDI was negligible for DC in all 3 cohorts. However, addition of DC to the alternative base model (Table 5, model 4a) resulted in a modest improvement in prediction as measured with relative IDI in ARIC, and not MESA and RS. PWV was only evaluated in RS and did not meaningfully improve any performance measure compared with the base model. However, compared with the alternative base model (Table 5, model 5a), addition of PWV resulted in a modest improvement of prediction as measured with relative IDI.
In sex‐ and race‐stratified analyses in ARIC, addition of cIMT, carotid plaque, cIMT plus carotid plaque, or DC resulted in relative IDIs in the same direction and of similar magnitude to the overall analysis (Table 6). Among participants without heart failure or history of myocardial infarction, addition of cIMT, carotid plaque, cIMT plus carotid plaque, or DC yielded results that were similar to the entire sample in all 3 cohorts (Table S2). In RS, addition of PWV to the base model did not improve prediction of AF (Table S2).
Table 6.
Sex‐ and Race‐Stratified C‐Statistic, Category‐Based NRI, and Relative IDI for Atrial Fibrillation When Carotid Intima‐Media Thickness and Carotid Plaque Are Added to the Refitted CHARGE‐AF Model in the ARIC Studya
| Model | C‐Statistic (95% CI) | Calibration χ2 P Value | NRI (95% CI) | Relative IDI (95% CI) |
|---|---|---|---|---|
| Women | ||||
| BM 1 | 0.787 (0.761, 0.813) | 0.73 | NA | NA |
| BM 1+cIMT | 0.791 (0.764, 0.817) | 0.57 | 0.010 (−0.023, 0.046) | 0.068 (−0.013, 0.164) |
| BM 2 | 0.788 (0.762, 0.814) | 0.72 | NA | NA |
| BM 2+plaque | 0.790 (0.764, 0.816) | 0.92 | 0.008 (−0.026, 0.040) | 0.031 (0.001, 0.060) |
| BM 3 | 0.787 (0.761, 0.813) | 0.73 | NA | NA |
| BM 3+plaque+cIMT | 0.791 (0.765, 0.817) | 0.86 | 0.026 (−0.013, 0.062) | 0.078 (0.006, 0.162) |
| BM 4 | 0.777 (0.743, 0.812) | 0.39 | NA | NA |
| BM 4+DC | 0.778 (0.744, 0.812) | 0.38 | −0.022 (−0.047, −0.002) | 0.002 (−0.012, 0.018) |
| BM 4a | 0.735 (0.695, 0.776) | 0.02 | NA | NA |
| BM 4a+DC | 0.741 (0.701, 0.780) | 0.66 | −0.007 (−0.027, 0.012) | 0.045 (0.011, 0.082) |
| Men | ||||
| BM 1 | 0.730 (0.706, 0.755) | 0.73 | NA | NA |
| BM 1+cIMT | 0.734 (0.710, 0.758) | 0.67 | 0.014 (−0.015, 0.043) | 0.049 (0.004, 0.092) |
| BM 2 | 0.730 (0.706, 0.754) | 0.48 | NA | NA |
| BM 2+plaque | 0.732 (0.707, 0.756) | 0.72 | 0.017 (−0.008, 0.042) | 0.027 (0.004, 0.051) |
| BM 3 | 0.730 (0.706, 0.755) | 0.73 | NA | NA |
| BM 3+plaque+cIMT | 0.734 (0.710, 0.758) | 0.25 | 0.013 (−0.014, 0.040) | 0.281 (0.228, 0.337) |
| BM 4 | 0.720 (0.689, 0.751) | 0.74 | NA | NA |
| BM 4+DC | 0.720 (0.690, 0.751) | 0.77 | 0.003 (−0.003, 0.012) | −0.001 (−0.002, 0.002) |
| BM 4a | 0.703 (0.671, 0.736) | 0.92 | NA | NA |
| BM 4a+DC | 0.705 (0.673, 0.737) | 0.36 | −0.003 (−0.027, 0.022) | 0.005 (−0.007, 0.017) |
| White | ||||
| BM 1 | 0.761 (0.742, 0.780) | 0.03 | NA | NA |
| BM 1+cIMT | 0.764 (0.745, 0.782) | 0.27 | 0.007 (−0.018, 0.031) | 0.046 (0.005, 0.090) |
| BM 2 | 0.761 (0.742, 0.779) | 0.03 | NA | NA |
| BM 2+plaque | 0.762 (0.743, 0.781) | 0.08 | 0.014 (−0.016, 0.039) | 0.025 (0.004, 0.048) |
| BM 3 | 0.761 (0.742, 0.780) | 0.03 | NA | NA |
| BM+plaque+cIMT | 0.764 (0.745, 0.783) | 0.10 | 0.018 (−0.007, 0.044) | 0.054 (0.013, 0.094) |
| BM 4 | 0.753 (0.729, 0.776) | 0.41 | NA | NA |
| BM 4+DC | 0.753 (0.729, 0.776) | 0.31 | −0.005 (−0.012, −0.001) | −0.001 (−0.003, 0.001) |
| BM 4a | 0.707 (0.678, 0.735) | 0.02 | NA | NA |
| BM 4a+DC | 0.708 (0.680, 0.736) | 0.06 | −0.026 (−0.046, −0.006) | 0.010 (−0.001, 0.022) |
| Black | ||||
| BM 1 | 0.782 (0.740, 0.825) | 0.62 | NA | NA |
| BM 1+cIMT | 0.788 (0.747, 0.830) | 0.79 | 0.022 (−0.037, 0.083) | 0.068 (−0.036, 0.205) |
| BM 2 | 0.780 (0.738, 0.822) | 0.90 | NA | NA |
| BM 2+plaque | 0.785 (0.743, 0.826) | 0.73 | −0.035 (−0.084, 0.011) | 0.029 (−0.014, 0.077) |
| BM 3 | 0.782 (0.740, 0.825) | 0.62 | NA | NA |
| BM 3+plaque+cIMT | 0.790 (0.749, 0.831) | 0.65 | 0.017 (−0.045, 0.083) | 0.074 (−0.026, 0.201) |
| BM 4 | 0.786 (0.733, 0.839) | 0.73 | NA | NA |
| BM 4+DC | 0.790 (0.738, 0.842) | 0.31 | 0.026 (−0.009, 0.079) | 0.026 (−0.028, 0.079) |
| BM 4a | 0.758 (0.703, 0.813) | 0.65 | NA | NA |
| BM 4a+DC | 0.771 (0.717, 0.824) | 0.95 | −0.022 (−0.063, 0.001) | 0.083 (−0.035, 0.183) |
BM 1, base model for participants with complete cIMT data; BM 2, base model for participants with complete carotid plaque data; BM 3, base model for participants with complete cIMT and carotid plaque data; BM 4, base model for participants with complete DC data; BM 4a, base model (excluding height, weight, systolic blood pressure, and diastolic blood pressure) for participants with complete DC data. ARIC indicates Atherosclerosis Risk in Communities; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; cIMT, carotid intima‐media thickness.
Analyses for cIMT, and plaque+cIMT were based on 13 595 participants, and analyses for plaque only were based on 13 796 participants. The base model is the refitted CHARGE‐AF model in the ARIC study.
Among participants with body mass index <30 kg/m2, addition of cIMT plus plaque yielded a modest improvement in prediction as measured with relative IDI in ARIC and MESA. Reclassification measured with NRI and relative IDI with respect to the base model was negligible for DC in all 3 cohorts and PWV in RS (Table S3). Among participants with body mass index ≥30 kg/m2, addition of cIMT and cIMT plus plaque yielded a modest improvement in prediction as measured with relative IDI in ARIC and RS. Reclassification measured with NRI and relative IDI with respect to the base model was negligible for DC all 3 cohorts and PWV in RS (Table S4).
Discussion
In this investigation comprising 3 large, population‐based cohort studies, we evaluated the association of arterial structural and functional alterations with incident AF and their potential roles in improving risk prediction of AF. We found that higher cIMT and presence of carotid plaque were associated with higher incidence of AF. Furthermore, we observed that addition of markers of atherosclerosis (cIMT, carotid plaque, or cIMT plus carotid plaque) to the CHARGE‐AF risk score marginally improved risk prediction of AF. In contrast, indices of arterial stiffness (DC and PWV) were not associated with AF and did not improve its risk prediction compared with the CHARGE‐AF risk score. However, when we excluded height, weight, systolic blood pressure, and diastolic blood pressure from the multivariable model, we observed that higher DC was associated with a lower incidence of AF and higher PWV was associated with higher incidence of AF. Furthermore, our findings were consistent in various subgroups: women and men; blacks and whites; participants without heart failure or history of myocardial infarction; and those with body mass index <30 and ≥30 kg/m2. Collectively, our findings underscore the role of atherosclerosis and arterial stiffness in the etiopathogenesis of AF, but do not support the use of carotid ultrasound or measurement of arterial stiffness in refining prediction of AF.
Association and Predictive Value of Arterial Indices for AF
Our study is consistent with previous studies that had reported that higher cIMT2, 3, 6, 7 and severity of carotid plaques2 were associated with higher risk of AF. By contrast, carotid distensibility was not associated with AF in all 3 cohorts after adjustment for variables in the CHARGE‐AF risk score. Of note, measures of arterial stiffness (DC and PWV) already incorporate information that is provided by height, weight, systolic blood pressure, and diastolic blood pressure; therefore, adjustment for these variables may lead to overadjustment. Indeed, when we excluded these variables from the multivariable model, we found that higher DC was associated with a lower incidence of AF in ARIC and MESA, but not RS. Additionally, higher PWV was associated with higher incidence of AF in RS. Our findings therefore are consistent with a previous Framingham Heart Study report that indicated that higher pulse pressure—a surrogate measure for increased proximal aortic stiffness38—independently predicts incident AF.1 Of note, this Framingham Heart Study analysis adjusted for mean arterial pressure, and not systolic and diastolic pressure; even after adjusting for mean arterial pressure, higher pulse pressure was associated with higher risk of AF.1 However, pulse pressure is, after all, an indirect surrogate measure for increased proximal aortic stiffness. To the best of our knowledge, our study is the first to evaluate PWV—the gold‐standard measure of aortic stiffness8—in relation to incident AF in a population‐based setting, and we found an independent association in RS.
A previous study had evaluated whether coronary artery calcium improves prediction of AF.39 To the best of our knowledge, our study is the first to evaluate the role of arterial stiffness indices in improving prediction of AF, benchmarked against the CHARGE‐AF risk score. Our findings do not support the role of measuring cIMT, carotid plaque, DC, or PWV to enhance prediction of AF: Absolute improvements in risk prediction in our study are unlikely to be of major clinical importance (maximum relative IDI, 0.058; 95% CI, 0.022–0.097; category‐based NRI all close to 0) and are similar in magnitude to the relative IDI of coronary artery calcium as benchmarked against the CHARGE‐AF risk score (0.077; 95% CI, 0.040–0.110).39 Enhancing prediction of AF is an important public health imperative,40 and our findings point to investigation of other risk factors, and not markers of atherosclerosis.
Mechanisms Underlying Association of Arterial Indices With AF
Given that cIMT and carotid plaque are markers of systemic atherosclerosis, the association of these indices with AF may be explained by the underlying role of atherosclerosis in the etiopathogenesis of AF. Indeed, myocardial infarction is a strong risk factor for AF.41
Myocardial damage, often in association with heart failure, contributes to onset of AF in patients with a history of myocardial infarction. Alternatively, subclinical atherosclerosis may cause gradual ischemic damage to myocardial tissue, resulting in premature apoptosis of myocytes, fibrosis of atrial wall, formation of areas with reduced or blocked conduction, and subsequent facilitation of reentrant atrial arrhythmias.42 The role of atherosclerosis in the development of AF is supported by results of studies in which atrial tissues were histologically investigated.43, 44 In addition, atherosclerosis and its risk factors are associated with structural and electrical remodeling of the atria that forms the substrate leading to AF development and progression.45, 46, 47 Increased arterial stiffness, on the other hand, has been proposed to increase AF risk by causing left ventricular hypertrophy,48 impaired ventricular relaxation,49, 50 and left atrial enlargement.51
Clinical and Public Health Implications
Although our findings do not support the use of carotid ultrasound to improve prediction of AF, they underscore the role of atherosclerosis and arterial stiffness in the etiopathogenesis of AF. Current guidelines on the treatment of AF emphasize a 3‐pronged strategy (anticoagulation to prevent stroke, rhythm control, and rate control) and make no recommendation regarding the importance of optimizing the control of atherosclerosis risk factors to prevent AF.52, 53 Our findings support a comprehensive management strategy that additionally includes optimizing the control of atherosclerosis risk factors (eg, weight reduction and intensive systolic blood pressure control) to prevent AF. This comprehensive strategy is particularly timely and important given recent evidence from clinical trials that supports the central role of atherosclerosis risk factors in the etiology and burden of AF: A randomized controlled trial by Sanders et al. showed that, as compared with patients randomized to general lifestyle advice, those randomized to weight management with intensive risk factor management experienced significant reduction in AF symptom burden and severity.54 In a follow‐up trial, compared with patients who did not undergo atherosclerosis risk factor management, those who opted for risk factor management had greater reduction in AF frequency, duration, and symptom severity after AF ablation.55 Furthermore, recent data from randomized, controlled trials have shown that compared with standard blood pressure control (systolic blood pressure target of 140 mm Hg), intensive blood pressure control (systolic blood pressure target <12056 or 130 mm Hg57) is associated with lower AF incidence.
Strengths and Limitations
The principal strength of this study is the reproducibility of findings across independent population‐based cohorts. Other strengths include the consistency of findings in various subgroups, long follow‐up, inclusion of nonwhite participants, extensive standardized measurement of covariates and arterial indices, and the large number of AF cases. However, several limitations should be noted. First, incident AF was identified mostly from hospitalization discharges in ARIC and MESA, and we could not include asymptomatic AF or AF managed exclusively in an outpatient setting. However, we have previously shown that the validity of AF ascertainment using hospitalizations in ARIC is acceptable,15 and that incidence rates of AF in ARIC and MESA are consistent with other population‐based studies. In addition, RS has direct digital linkage to general practitioners' files with a flagging system to notify new diagnoses of AF. All files were checked by hand every 3 to 4 years by research assistants to make sure that AF diagnoses were not missed. Furthermore, given that health care utilization may be a potential confounder, we adjusted our analyses for time‐dependent incident hospitalizations, which did not change our observations. Second, exclusion of participants with missing data on arterial indices could have introduced selection biases into our analyses. However, missing information was primarily attributed to logistic reasons, which was likely to be random and thus would not have significantly biased our results. Third, for computation of category‐based NRI, we used predefined risk categories that may not correspond to clinically meaningful risk cutoffs. Finally, we could not eliminate residual confounding by other measurable and nonmeasurable risk factors.
Conclusions
We have shown, in 3 population‐based cohort studies, that higher cIMT, presence of carotid plaque, and greater arterial stiffness are associated with higher risk of AF. However, the addition of these arterial indices only minimally improves the prediction of AF, over and above the CHARGE‐AF risk score. Our findings add to the growing body of evidence that atherosclerosis and arterial stiffness play an important role in development of AF, underscoring the potential role of treating atherosclerosis risk factors to prevent AF.
Sources of Funding
This work was supported by a research grant from the National Institute on Aging (R21AG042660‐01A1) awarded to Dr Chen. In addition, Dr Chen receives support from the American Heart Association (10SDG3420031) and the ARIC and MESA contracts. Dr Alonso received support from the National Heart, Lung, and Blood Institute (RC1 HL099452) and the American Heart Association (16EIA26410001). Dr Heckbert receives support from the National Heart, Lung, and Blood Institute (R01 HL102214). Drs Leening and Stricker are supported by the Netherlands Organization for Health Research and Development (ZonMw HTA 80‐82500‐98‐10208); Dr Witteman is supported by the Netherlands Organization for Scientific Research (NWO Vici 918‐76‐619). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The Multi‐Ethnic Study of Atherosclerosis is supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐RR‐024156 and UL1‐RR‐025005 from the National Center for Research Resources (NCRR). The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO); the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
Disclosures
None.
Supporting information
Data S1. Methods.
Table S1. Hazard Ratios (95% CIs) of Carotid Intima‐Media Thickness for Atrial Fibrillation Adjusted for Hospitalization: ARIC and MESA
Table S2. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants Without Heart Failure or Myocardial Infarction: ARIC, MESA, and RS
Table S3. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants With BMI <30 kg/m2: ARIC, MESA, and RS
Table S4. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants With BMI ≥30 kg/m2: ARIC, MESA, and RS
Acknowledgments
The authors thank the staff and participants of the ARIC study, MESA, and RS for their important contributions.
(J Am Heart Assoc. 2016;5:e002907 doi: 10.1161/JAHA.115.002907)
References
- 1. Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new‐onset atrial fibrillation. JAMA. 2007;297:709–715. [DOI] [PubMed] [Google Scholar]
- 2. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Rooij FJ, Lip GY, Witteman JC. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2007;167:382–387. [DOI] [PubMed] [Google Scholar]
- 3. Chen LY, Foo DC, Wong RC, Seow SC, Gong LL, Benditt DG, Ling LH. Increased carotid intima‐media thickness and arterial stiffness are associated with lone atrial fibrillation. Int J Cardiol. 2013;168:3132–3134. [DOI] [PubMed] [Google Scholar]
- 4. Larstorp ACK, Ariansen I, Gjesdal K, Olsen MH, Ibsen H, Devereux RB, Okin PM, Dahlof B, Kjeldsen SE, Wachtell K. Association of pulse pressure with new‐onset atrial fibrillation in patients with hypertension and left ventricular hypertrophy the losartan intervention for endpoint (LIFE) reduction in hypertension study. Hypertension. 2012;60:347–353. [DOI] [PubMed] [Google Scholar]
- 5. Valbusa F, Bonapace S, Bertolini L, Zenari L, Arcaro G, Targher G. Increased pulse pressure independently predicts incident atrial fibrillation in patients with type 2 diabetes. Diabetes Care. 2012;35:2337–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Proietti M, Calvieri C, Malatino L, Signorelli S, Corazza GR, Perticone F, Vestri AR, Loffredo L, Davi G, Violi F, Basili S, Fibrillation AA. Relationship between carotid intima‐media thickness and non valvular atrial fibrillation type. Atherosclerosis. 2015;238:350–355. [DOI] [PubMed] [Google Scholar]
- 7. Adamsson Eryd S, Ostling G, Rosvall M, Persson M, Smith JG, Melander O, Hedblad B, Engstrom G. Carotid intima‐media thickness is associated with incidence of hospitalized atrial fibrillation. Atherosclerosis. 2014;233:673–678. [DOI] [PubMed] [Google Scholar]
- 8. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; Non‐invasive EN . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 9. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102 doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 14. Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW. The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol. 2013;28:889–926. [DOI] [PubMed] [Google Scholar]
- 15. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in Whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leening MJ, Kavousi M, Heeringa J, van Rooij FJ, Verkroost‐van Heemst J, Deckers JW, Mattace‐Raso FU, Ziere G, Hofman A, Stricker BH, Witteman JC. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. High‐resolution B‐mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC). The ARIC Study Group. J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 19. Li R, Cai J, Tegeler C, Sorlie P, Metcalf PA, Heiss G. Reproducibility of extracranial carotid atherosclerotic lesions assessed by B‐mode ultrasound: the Atherosclerosis Risk in Communities Study. Ultrasound Med Biol. 1996;22:791–799. [DOI] [PubMed] [Google Scholar]
- 20. Li R, Duncan BB, Metcalf PA, Crouse JR III, Sharrett AR, Tyroler HA, Barnes R, Heiss G. B‐mode‐detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. [DOI] [PubMed] [Google Scholar]
- 21. Chambless LE, Zhong MM, Arnett D, Folsom AR, Riley WA, Heiss G. Variability in B‐mode ultrasound measurements in the Atherosclerosis Risk in Communities (ARIC) Study. Ultrasound Med Biol. 1996;22:545–554. [DOI] [PubMed] [Google Scholar]
- 22. Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge‐detected and manual‐traced common carotid intima‐media thickness measurements with Framingham risk factors the Multi‐Ethnic Study of Atherosclerosis. Stroke. 2011;42:1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima‐media thickness progression as a predictor of stroke in Multi‐Ethnic Study of Atherosclerosis. Stroke. 2011;42:3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bots ML, Mulder PG, Hofman A, van Es GA, Grobbee DE. Reproducibility of carotid vessel wall thickness measurements. The Rotterdam Study. J Clin Epidemiol. 1994;47:921–930. [DOI] [PubMed] [Google Scholar]
- 25. Bots ML, Hofman A, deJong PTVM, Grobbee DE. Common carotid intima media thickness as an indicator of atherosclerosis at other sites of the carotid artery—the Rotterdam Study. Ann Epidemiol. 1996;6:147–153. [DOI] [PubMed] [Google Scholar]
- 26. Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal‐medial thickness: the ARIC Study. Ultrasound Med Biol. 1997;23:157–164. [DOI] [PubMed] [Google Scholar]
- 27. Arnett DK, Boland LL, Evans GW, Riley W, Barnes R, Tyroler HA, Heiss G. Hypertension and arterial stiffness: the Atherosclerosis Risk in Communities Study. ARIC Investigators. Am J Hypertens. 2000;13:317–323. [DOI] [PubMed] [Google Scholar]
- 28. Arnett DK, Chambless LE, Evans GW, Riley W. Variability in ultrasonic measurements of arterial stiffness in the Atherosclerosis Risk in Communities Study. Ultrasound Med Biol. 1999;25:175–180. [DOI] [PubMed] [Google Scholar]
- 29. Riley WA, Barnes ME, Evans GW, Smith SO, Heiss G. Measurement of arterial distensibility in the Atherosclerosis Risk in Communities (ARIC) cohort In: Borgatti F, ed. Follow‐Up and Prevention of Atherosclerotic Plaque. Torino, Italy: Centro Scientifico Editore; 1992:45–54. [Google Scholar]
- 30. Hoeks APG, Brands PJ, Smeets FAM, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. [DOI] [PubMed] [Google Scholar]
- 31. Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse‐wave velocity‐measurement—validation and clinical‐application studies. Hypertension. 1995;26:485–490. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Smith G, Altman D. Systematic Reviews in Health Care: Meta‐Analysis in Context. London: BMJ Publishing Group; 2001. [Google Scholar]
- 33. Chambless LE, Diao G. Estimation of time‐dependent area under the roc curve for long‐term risk prediction. Stat Med. 2006;25:3474–3486. [DOI] [PubMed] [Google Scholar]
- 34. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 35. Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pencina MJ, D'Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. May S, Hosmer DW. A simplified method of calculating an overall goodness‐of‐fit test for the cox proportional hazards model. Lifetime Data Anal. 1998;4:109–120. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle‐aged and older subjects with uncomplicated systolic hypertension—the role of proximal aortic diameter and the aortic pressure‐flow relationship. Circulation. 2003;108:1592–1598. [DOI] [PubMed] [Google Scholar]
- 39. O'Neal WT, Efird JT, Dawood FZ, Yeboah J, Alonso A, Heckbert SR, Soliman EZ. Coronary artery calcium and risk of atrial fibrillation (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;114:1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd‐Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TSM, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 42. Anderson KR, Sutton MGS, Lie JT. Histo‐pathological types of cardiac fibrosis in myocardial‐disease. J Pathol. 1979;128:79–85. [DOI] [PubMed] [Google Scholar]
- 43. Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Falk RH. Etiology and complications of atrial fibrillation: insights from pathology studies. Am J Cardiol. 1998;82:10n–16n. [DOI] [PubMed] [Google Scholar]
- 45. Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Brooks AG, Worthington M, Rajendram A, Kelly DR, Zhang YA, Kuklik P, Nelson AJ, Wong CX, Worthley SG, Rao M, Faull RJ, Edwards J, Saint DA, Sanders P. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010;7:1282–1290. [DOI] [PubMed] [Google Scholar]
- 46. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 47. Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, Antic R, Kalman JM, Sanders P. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. [DOI] [PubMed] [Google Scholar]
- 48. Gardin JM, Arnold A, Gottdiener JS, Wong ND, Fried LP, Klopfenstein HS, Oleary DH, Tracy R, Kronmal R. Left ventricular mass in the elderly—the Cardiovascular Health Study. Hypertension. 1997;29:1095–1103. [DOI] [PubMed] [Google Scholar]
- 49. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 50. Leite‐Moreira AF, Correia‐Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res. 1999;43:344–353. [DOI] [PubMed] [Google Scholar]
- 51. Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood‐pressure on left atrial size—the Framingham Heart Study. Hypertension. 1995;25:1155–1160. [DOI] [PubMed] [Google Scholar]
- 52. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines ESCCfP . 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 53. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 54. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 55. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 56. Chen LY, Bigger JT, Hickey KT, Chen H, Lopez‐Jimenez C, Banerji MA, Evans G, Fleg JL, Papademetriou V, Thomas A, Woo V, Seaquist ER, Soliman EZ. Effect of intensive blood pressure lowering on incident atrial fibrillation and P‐wave indices in the ACCORD Blood Pressure Trial. Am J Hypertens. 2015; doi: 10.1093/ajh/hpv172. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Okin PM, Hille DA, Larstorp ACK, Wachtell K, Kjeldsen SE, Dahlof B, Devereux RB. Effect of lower on‐treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension. 2015;66:368–373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Methods.
Table S1. Hazard Ratios (95% CIs) of Carotid Intima‐Media Thickness for Atrial Fibrillation Adjusted for Hospitalization: ARIC and MESA
Table S2. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants Without Heart Failure or Myocardial Infarction: ARIC, MESA, and RS
Table S3. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants With BMI <30 kg/m2: ARIC, MESA, and RS
Table S4. C‐Statistic, Categorical‐Based NRI, and Relative IDI for Atrial Fibrillation When Each Arterial Index Is Added to the Refitted CHARGE‐AF Model in Participants With BMI ≥30 kg/m2: ARIC, MESA, and RS


