Abstract
Background
Inflammation of the aortic wall is recognised as a key pathogenesis of abdominal aortic aneurysm (AAA). This study was undertaken to determine whether inflammatory cytokines could be used as biomarkers for the presence of AAA.
Methods and Results
Tissue profiles of 27 inflammatory cytokine were examined in AAA (n=14) and nonaneurysmal (n=14) aortic tissues. Three cytokines, regulated upon activation normally T‐cell expressed and secreted (RANTES), eotaxin, and macrophage inflammatory protein 1 beta (MIP‐1b), had increased expression in AAA, particularly within the adventitial layer of the aortic wall. Basic fibroblast growth factor (bFGF) had reduced expression in all layers of the AAA wall. Examination of the circulating plasma profiles of AAA (n=442) and AAA‐free controls (n=970) suggested a (risk factor adjusted) AAA‐association with eotaxin, RANTES, and high sensitivity C‐reactive protein (hsCRP). A plasma inflammatory cytokine score, calculated using these three markers, suggested a strong risk association with AAA (odds ratio, 4.8; 95% CI, 3.5–6.7; P<0.0001), independent of age, sex, history of ischemic heart disease, and smoking.
Conclusions
Contrary to reports suggesting a distinct T helper 2–associated inflammatory profile in AAA, this current study suggests a more‐generalized pattern of inflammation, albeit with some potentially distinct features, including elevated plasma eotaxin and decreased plasma RANTES. In combination with hsCRP, these markers may have potential utility as AAA biomarkers.
Keywords: abdominal aortic aneurysm, aorta, C‐reactive protein, eotaxin, inflammation, plasma, RANTES
Subject Categories: Aneurysm, Inflammation, Biomarkers
Introduction
Abdominal aortic aneurysm (AAA [MIM 100070]) is a common condition, particularly in men over the age of 65 years, with a reported prevalence of between 2% and 8%.1 The demographic risk factor profile for AAA is well described and includes smoking, family history of AAA, older age, male sex, and concurrent atherosclerotic diseases, particularly coronary artery disease (CAD).2, 3, 4 Though historically considered to be a consequence of atherosclerosis, AAA is now considered to be a distinct clinical entity, albeit one that shares many risk factors with occlusive atherosclerotic vascular disease.2, 5
Periaortic inflammation is a key distinguishing feature of AAA,6, 7 and several studies have suggested an association between circulating inflammatory cytokines and AAA.8, 9, 10, 11 Nevertheless, to date only a relatively small number of circulating biomarkers have convincingly been implicated in AAA and none have been translated into AAA‐specific clinical practice.
This study aimed to determine infrarenal abdominal aortic tissue inflammatory cytokine profiles in both aneurysm patients and nonaneurysm controls. The resulting AAA tissue profile was then compared with the plasma profile from a large series of well‐characterized AAA and ultrasound confirmed nonaneurysmal study participants. As such, this study represents the largest number of samples and simultaneously analyzed inflammatory cytokines published to date.
Materials and Methods
Tissue and Plasma Samples
Infrarenal aortic tissue was obtained from 14 AAA patients undergoing elective open repair at the Vascular Surgery Unit, Dunedin Hospital (Dunedin, New Zealand). These were compared with nonaneurysmal aortic tissues, obtained less than 24 hours postmortem, from 14 age‐ and sex‐matched cadaveric controls (Table 1).
Table 1.
Aortic Tissue Population Demographics
| Controls, n=14 | AAA, n=14 | P Value | |
|---|---|---|---|
| Age, y | 68.0±11.4 | 70.9±6.6 | 0.3581 |
| Male sex, n (%) | 10 (71.4) | 10 (71.4) | 1.0 |
| Max infrarenal diameter, mm | — | 54.3 (47.5–56.6) | — |
AAA indicates abdominal aortic aneurysm; Max, maximum.
Plasma samples from 442 AAA patients and 970 AAA‐free controls (Table 2) were recruited from the Otago region of New Zealand. AAA patients were either under active aneurysm surveillance or recruited before elective aneurysm surgical repair. All controls were recruited from the same community as part of a local AAA ultrasound screening program (inclusion criteria age greater than 60 years of age and both males and females). Those screened individuals with an infrarenal aorta greater than 25 mm in diameter were excluded from this control group.
Table 2.
Blood Plasma Population Demographics
| Controls, n=970 | AAA, n=442 | P Value | |
|---|---|---|---|
| Age, y | 68.5±7.6 | 75.0±7.9 | <0.0001 |
| Male sex (%) | 741 (76.4) | 334 (75.6) | 0.76 |
| Max infrarenal diameter, mm | 19.7 (18.0–21.2) | 52.0 (42.9–60.8) | <0.0001 |
| Male |
20.2 (18.6–21.7) n=741 |
53.0 (44.1–63.2) n=334 |
|
| Female |
17.9 (16.7–19.1) n=229 |
46.7 (39.9–56.0) n=108 |
|
| Smoking pack‐years | 1.4 (0–17.0) | 22.5 (7.5–42.7) | <0.0001 |
| Hypertension (%) | 31.6 | 59.3 | <0.0001 |
| Diabetes (%) | 6.8 | 11.4 | 0.004 |
| Ischemic heart disease (%) | 21.4 | 36.3 | <0.0001 |
| Peripheral artery disease (%) | 1.6 | 16.4 | <0.0001 |
AAA indicates abdominal aortic aneurysm; Max, maximum.
Written informed consent was obtained from all participants or relevant next of kin (for material collected postmortem), and the study was undertaken with the approval of the regional ethics committee.
Tissue and Plasma Preparation
Anterior infrarenal aortic wall biopsies were collected as soon as possible from the operating theatre or postmortem room and immediately transferred into RNA‐later solution. Immediately after collection, each (thrombus‐free) specimen was divided into 2 equivalent pieces. The first half was divided into small (≈50 mg) full‐wall thickness pieces. The second half was carefully microdissected into discrete (≈50 mg) adventitial and intima+medial layers specimens for separate processing. Tissue samples were immersed in RNA‐later solution (Life Technologies, Carlsbad, CA) for 24 hours at 4°C and then stored at −80°C until processed.
Separate total wall, adventitia, and intima+medial specimens from each individual were thawed and washed in buffer before being homogenized (PT2100; Polytron; Kinetica AG, Lausanne, Switzerland) in ice‐cold cell lysis buffer (10 μL/mg of tissue; Bio‐Rad, Hercules, CA). The resulting homogenate was centrifuged at 2000 g for 20 minutes at 4°C. Lysate aliquots were stored at −80°C until assayed.
Case and control EDTA plasma samples were collected, centrifuged, separated into 500‐μL aliquots, and stored at −80°C within 30 minutes. The average length of storage time before being assayed was 5.6 years, and despite a lack of significant differences between cases and controls (P=0.87), storage times were nevertheless included in the regression analysis to ensure that this was not a confounding factor.
Cytokine Assay
Tissues and plasma inflammatory cytokines levels were determined using the Bio‐Plex Pro human cytokine 27‐Plex Panel (Bio‐Rad), according to the manufacturer's instructions. Data were analyzed using Bio‐Plex Manager software (version 4.1; Bio‐Rad).
High sensitivity C‐reactive protein (hsCRP; Tina‐quant high sensitivity assay; Roche Diagnostics, Indianapolis, IN) and nonfasting triglycerides and high‐density lipoprotein (HDL; enzymatic‐colorimetric method; Roche) levels were also measured for each plasma sample.
Tissue mRNA Expression
Quantitative polymerase chain reaction (qPCR) probes were used to determine relative expression of eotaxin 1 (chemokine [C‐C motif] ligand [CCL]11), eotaxin 2 (CCL24), eotaxin 3 (CCL26), regulated upon activation normally T‐cell expressed and secreted (RANTES; CCL5), and macrophage inflammatory protein 1 beta (MIP‐1b; CCL4) referenced to GAPDH (Table 3) in 6 AAA and 11 control aortic biopsies. Total RNA was extracted using Norgen Total RNA kits (Norgen Biotek Corporation, Thorold, Ontario, Canada), according to the manufacturers’ instructions. RNA quantity and purity were assessed using a NanoDrop spectrophotometer (Implen GmbH, München, Germany). Relative quantification of target gene probe intensities was calculated using the ΔCt (threshold cycle) method (GAPDH as the housekeeper gene).
Table 3.
Taqman Probes Used for qPCR
| Genes | Type | Sequences/Taqman Probe ID | Control Mean Ct | Case Mean Ct |
|---|---|---|---|---|
| Eotaxin 1 (CCL11) | SYBR Green |
Forward: 5′ AAAAGGTCTCCGCAGCACTTCTGT 3′ Reverse: 5′ AAAAGACAGAAGCTGGCCCAGCGA 4′ |
29.7 (±2.1) | 30.2 (±1.2) |
| Eotaxin 2 (CCL24) | TaqMan | Hs00171082_m1 | Undetectable >35 | 33.0 (±1.0) |
| Eotaxin 3 (CCL26) | TaqMan | Hs01099415_m1 | Undetectable >35 | 33.6 (±1.4) |
| MIP‐1b (CCL4) | TaqMan | Hs99999148_m1 | 34.9 (±0.3) | 30.0 (±1.5) |
| MIP‐1b (CCL4) alternate probe | SYBR Green |
Forward: 5′ ACCAATACCATGAAGCTCTGCGTG 3′ Reverse: 5′ TGCTTCTTTTGGTTTGGAATTTGG 3′ |
33.7 (±1.2) | 30.8 (±1.6) |
| RANTES (CCL5) | TaqMan | Hs00982282_m1 | 32.1 (±1.2) | 27.5 (±1.6) |
| GAPDH | TaqMan | Hs02758991_g1 | 23.2 (±0.6) | 24.5 (±1.0) |
Taqman probes were used to confirm localized tissue gene expression of the proteins identified in the inflammatory cytokine bioplex experiments. Ct values are means±1 SD. CCL indicates chemokine (C‐C motif) ligand; Ct, threshold cycle; MIP‐1b, macrophage inflammatory protein 1 beta; qPCR, quantitative polymerase chain reaction; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Statistical Analysis
StatView software (version 5.01; SAS Institute Inc., Cary, NC) was used to perform statistical analysis. The distribution of continuous variables (kurtosis and skewness) was assessed and analyzed with either the Mann–Whitney U test or ANOVA with Fisher's protected least significant difference test. Multiple (step‐wise) logistic regression was used to evaluate the interactions between cytokine biomarkers and confounding demographic variables. Network relationships between variables were examined using variable principal component analysis (Omics Explorer 3.1; Qlucore, Lund, Sweden) with log‐transformed data and linking each marker with its 2 nearest neighbors in the network (Euclidean distance threshold, 65%). Spearman's rank correlations were used to assess plasma biomarker and aneurysm size correlations.
Receiver operating characteristic (ROC) curves were constructed to determine the optimal binary cut‐off value of each differentially expressed cytokine. This value was calculated using the maximum of the Youden index J=max [SEi+SPi−1], where SEi and SPi are the sensitivity and specificity over all possible threshold values.
Results were expressed as medians and interquartile ranges or mean±SD for normally distributed variables. P value significance thresholds were conservatively adjusted for multiple testing (Bonferroni correction) to determine statistically significant differentially expressed cytokines in AAA patients.
Results
Aortic Tissue Inflammatory Cytokine Profiles
Tissue inflammatory cytokine profiles were assessed in 14 AAA and 14 control total wall biopsies (Table 4). In the tissue analysis, 90.1% of IL‐2 values were below the assay detectable range and therefore this cytokine was excluded from the tissue biomarker analysis. In all 8 cytokines (interleukin [IL]‐1b, IL‐10, IL‐12p70, basic fibroblast factor [bFGF], vascular endothelial growth factor [VEGF], MIP=1a/CCL3, MIP‐1b, and RANTES) appear to have suggestive (P<0.05) differential case‐control expression in total wall biopsies; however, only bFGF (decreased in AAA compared to controls) and RANTES (increased in AAA) fell below the multiple testing threshold (Table 4). In 12 AAA and 12 control samples, matching isolated intima+media and adventitial specimens were available. When isolated intima+media layers (Table 5) were compared, a similar pattern was observed to that of total wall specimens, but with IL‐6 also showing a suggestive association (increased in AAA). While generally matching both total wall and intima+media specimens, adventitial tissue appeared to show the greatest AAA versus control cytokine differences (Table 6). Four markers reached multiple testing significance between adventitial AAA and control specimens. Three cytokines (eotaxin, MIP‐1b, and RANTES) were increased, whereas bFGF was decreased, in AAA adventitia.
Table 4.
Total Aortic Wall Tissue Protein Biomarkers
| Control Total Wall, n=14 | AAA Total Wall, n=14 | P Value | |
|---|---|---|---|
| IL‐1b | 401 (234–655) | 857 (683–1153) | 0.0112 |
| IL1‐Ra | 7836 (5769–10 701) | 9737 (6538–12 435) | 0.5816 |
| IL‐4 | 311 (206–489) | 395 (252–478) | 0.9034 |
| IL‐5 | 1755 (878–2517) | 2342 (1917–3168) | 0.1325 |
| IL‐6 | 3182 (2349–7885) | 9479 (5825–16 019) | 0.0803 |
| IL‐7 | 1218 (899–1642) | 1819 (1270–2553) | 0.1573 |
| IL‐8/CXCL8 | 3883 (1963–6904) | 6821 (3934–13 158) | 0.1415 |
| IL‐9 | 3208 (2336–3328) | 2863 (2304–3483) | 0.9566 |
| IL‐10 | 2328 (1730–2615) | 1467 (1279–1744) | 0.0108 |
| IL‐12p70 | 5692 (3565–6891) | 3213 (2589–4427) | 0.0088 |
| IL‐13 | 792 (677–1298) | 798 (656–1513) | 0.9566 |
| IL‐15b | 949 (0–1376) | 487 (0–1346) | 0.3581 |
| IL17A | 2716 (2025–4803) | 3601 (2127–4484) | 0.8843 |
| bFGF | 39 910 (34 115–41 726) | 25 941 (20 487–31 659) | 0.0006a |
| VEGF | 7335 (5126–9281) | 4347 (3308–5161) | 0.0101 |
| Eotaxin | 2414 (2110–3200) | 2694 (2289–4416) | 0.1901 |
| G‐CSF | 2809 (493–6405) | 3786 (3076–4303) | 0.4347 |
| GM‐CSF | 3514 (2618–4184) | 4264 (3801–5095) | 0.0896 |
| IFN‐γ | 3530 (1689–5483) | 4222 (2516–5452) | 0.6459 |
| IP‐10/CXCL10 | 29 938 (21 888–38 431) | 23 939 (19 724–29 939) | 0.1585 |
| MCP‐1/CCL2 | 7104 (3605–8082) | 9768 (6284–10 914) | 0.1455 |
| MIP‐1a/CCL3 | 113 (56–197) | 226 (188–358) | 0.0076 |
| MIP‐1b/CCL4 | 2423 (1871–3483) | 4989 (3846–6260) | 0.0101 |
| PDGFβ | 5806 (5172–10 346) | 8360 (7056–9446) | 0.5496 |
| RANTES/CCL5 | 13 765 (11 557–15 785) | 21 280 (18 859–24 370) | 0.0010a |
| TNF‐αb | 1896 (1505–3161) | 1593 (0–3411) | 0.4146 |
Aortic wall tissue biomarkers were assessed in total wall biopsies, as well as microdissected (Table 5) intima and media and (Table 6) adventitia. AAA indicates abdominal aortic aneurysm; bFGF, basic fibroblast growth factor; CCL, chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte macrophage colony‐stimulating factor; IFN‐γ, interferon‐gamma; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MCP‐1, monocyte chemoattractant protein 1; MIP, macrophage inflammatory protein; PDGFβ, platelet‐derived growth factor beta; RANTES, regulated on activation, normal T‐cell expressed and secreted; TNF‐α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
In the total aortic wall samples, only bFGF and RANTES were significantly different after correction for multiple testing.
Some samples recorded IL‐15 values below the detectable range of this assay and were assigned a rank order of 0. All inflammatory cytokines are measured in pg/100 mg tissue.
Table 5.
Intima and Media Aortic Wall Tissue Protein
| Control Intima+Media, n=12 | AAA Intima+Media, n=12 | P Value | |
|---|---|---|---|
| IL‐1b | 526 (234–914) | 1685 (1092–1892) | 0.0026 |
| IL1‐Ra | 7848 (4819–12 157) | 11 508 (10 568–27 464) | 0.0940 |
| IL‐4 | 364 (326–543) | 414 (316–535) | 0.8525 |
| IL‐5 | 2080 (1537–3098) | 2610 (2020–3272) | 0.1891 |
| IL‐6 | 4149 (2064–6744) | 11 022 (8610–19 028) | 0.0092 |
| IL‐7 | 1939 (1319–2273) | 1616 (1236–2233) | 0.7440 |
| IL‐8/CXCL8 | 6382 (1572–8276) | 15 220 (12 050–17 346) | 0.0696 |
| IL‐9 | 3116 (2678–4191) | 2957 (2561–3728) | 0.6924 |
| IL‐10 | 2459 (1968–2682) | 2087 (1660–3010) | 0.7583 |
| IL‐12p70 | 5873 (4513–6944) | 3500 (2728–5681) | 0.0671 |
| IL‐13 | 1040 (940–1540) | 1378 (980–1748) | 0.2184 |
| IL‐15b | 1225 (0–1599) | 702 (0–1159) | 0.4386 |
| IL‐17A | 3331 (2744–5148) | 3689 (3166–4367) | 0.9738 |
| bFGF | 39 529 (34 285–43 898) | 20 667 (18 759–26 183) | 0.0012a |
| VEGF | 8068 (6942–9679) | 5860 (4437–8056) | 0.0646 |
| Eotaxin | 2893 (2297–3945) | 3192 (3096–3975) | 0.3258 |
| G‐CSF | 2401 (493–8789) | 3361 (2962–4816) | 0.4118 |
| GM‐CSF | 3858 (3367–4678) | 3985 (3431–4305) | 0.8603 |
| IFN‐γ | 2951 (2264–5453) | 4344 (2536–4868) | 0.9476 |
| IP‐10/CXCL10 | 27 815 (22 667–50 709) | 33 094 (26 638–55 247) | 0.2623 |
| MCP‐1/CCL2 | 7749 (3236–10 146) | 11 087 (8768–11 837) | 0.0966 |
| MIP‐1a/CCL3 | 184 (138–493) | 559 (370–972) | 0.0284 |
| MIP‐1b/CCL4 | 2649 (1484–4500) | 7426 (6819–8199) | 0.0019 |
| PDGFβ | 7775 (5199–10 871) | 8414 (7558–12 910) | 0.3248 |
| RANTES/CCL5 | 12 592 (10 194–14 215) | 25 331 (21 570–29 229) | 4.9×10−5 a |
| TNF‐αb | 2076 (0–2410) | 2635 (1396–2969) | 0.3643 |
Microdissected intima and media aortic wall tissue biomarkers. AAA indicates abdominal aortic aneurysm; bFGF, basic fibroblast growth factor; CCL, chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte macrophage colony‐stimulating factor; IFN‐γ, interferon‐gamma; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MCP‐1, monocyte chemoattractant protein 1; MIP, macrophage inflammatory protein; PDGFβ, platelet‐derived growth factor beta; RANTES, regulated on activation, normal T‐cell expressed and secreted; TNF‐α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
As observed in the total wall samples, only bFGF and RANTES were significantly different after correction for multiple testing.
Some samples recorded IL‐15 values below the detectable range of this assay and were assigned a rank order of 0. All inflammatory cytokines are measured in pg/100 mg tissue.
Table 6.
Adventitia Aortic Wall Tissue Protein Biomarkers
| Control Adventitia, n=12 | AAA Adventitia, n=12 | P Value | |
|---|---|---|---|
| IL‐1b | 356 (196–492) | 939 (634–1283) | 0.0084 |
| IL1‐Ra | 7643 (3841–10 426) | 9441 (5647–10 677) | 0.3559 |
| IL‐4 | 283 (106–443) | 290 (122–446) | 0.8432 |
| IL‐5 | 2119 (1186–2690) | 3153 (2040–3450) | 0.0848 |
| IL‐6 | 3465 (1635–5930) | 9878 (5662–15 003) | 0.0290 |
| IL‐7 | 1599 (685–2128) | 1930 (1464–2179) | 0.3088 |
| IL‐8/CXCL8 | 2213 (1731–3794) | 5104 (2175–6823) | 0.1879 |
| IL‐9 | 3208 (2313–3707) | 3017 (2469–3626) | 0.9439 |
| IL‐10 | 1307 (1073–1556) | 1479 (1266–1997) | 0.1213 |
| IL‐12p70 | 1847 (180–2670) | 2534 (2080–3460) | 0.0671 |
| IL‐13 | 1082 (864–1550) | 1121 (970–1595) | 0.5383 |
| IL‐15b | 470 (0–916) | 533 (0–983) | 0.8951 |
| IL‐17A | 3334 (1970–4056) | 2879 (1668–3528) | 0.4237 |
| bFGF | 43 819 (39 739–48 884) | 32 588 (26 826–35 877) | 0.0014a |
| VEGF | 4082 (2898–5001) | 4493 (3010–4953) | 0.7728 |
| Eotaxin | 1680 (1182–1869) | 3074 (2777–4402) | 0.0004a |
| G‐CSF | 493 (480–3362) | 3903 (2406–4547) | 0.0346 |
| GM‐CSF | 3962 (3445–4358) | 4066 (3823–4337) | 0.7416 |
| IFN‐γ | 1443 (1017–3482) | 3222 (1935–4537) | 0.1131 |
| IP‐10/CXCL10 | 14 629 (13 125–16 341) | 16 686 (13 244–29 077) | 0.2786 |
| MCP‐1/CCL2 | 4590 (2542–5626) | 9095 (6280–11 674) | 0.0115 |
| MIP‐1a/CCL3 | 65 (50–133) | 248 (158–356) | 0.0116 |
| MIP‐1b/CCL4 | 1815 (1511–1999) | 5076 (4000–6878) | 0.0014a |
| PDGFβ | 6514 (5429–7793) | 6297 (5552–7633) | 0.8951 |
| RANTES/CCL5 | 11 149 (10 100–14 543) | 22 021 (16 661–24 664) | 0.0003a |
| TNF‐αb | 1617 (0–2486) | 1821 (0–3169) | 0.9461 |
Microdissected adventitia aortic wall tissue biomarkers. The greatest case/control differences were observed in this layer of the wall, in which eotaxin, MIP‐1b, and RANTES were significantly elevated, and bFGF was significantly decreased, in AAA. AAA indicates abdominal aortic aneurysm; bFGF, basic fibroblast growth factor; CCL, chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte macrophage colony‐stimulating factor; IFN‐γ, interferon‐gamma; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MCP‐1, monocyte chemoattractant protein 1; MIP, macrophage inflammatory protein; PDGFβ, platelet‐derived growth factor beta; RANTES, regulated on activation, normal T‐cell expressed and secreted; TNF‐α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Markers below the multiple testing threshold (P<0.0019).
Some samples recorded IL‐15 values below the detectable range of this assay and were assigned a rank order of 0. All inflammatory cytokines are measured in pg/100 mg tissue.
Plasma Inflammatory Cytokine Profiles
In plasma samples, 15 of the 27 assayed cytokines were significantly (multiple testing threshold, P<0.0017) differentially expressed in AAA patients compared to AAA‐free controls (Table 7). In addition, significant differences were also observed in plasma hsCRP, HDL, and the atherogenic index in plasma (AIP; log triglycerides [Trig]/high‐density lipoprotein [HDL]). Plasma IL‐15 was below the assay detectable range in the majority of samples (1179 of 1412 [83.5%]), and, although an analysis indicated a potential difference (90th percentile values of 17.1 vs 0.7 pg/mL in cases and controls, respectively; P=5.3×10−7), this cytokine was nevertheless conservatively excluded from further analysis.
Table 7.
Plasma Protein Biomarkers
| AAA Free Controls, n=970 | AAA, n=442 | P Value | |
|---|---|---|---|
| hsCRP, mg/L | 1.6 (0.8–3.1) | 3.7 (1.9–8.7) | 2.1×10−43 a |
| HDL, mmol/L | 1.34±0.45 | 1.19±0.39 | 3.8×10−10 a |
| Triglycerides, mmol/L | 1.4 (1.0–2.0) | 1.5 (1.1–2.0) | 2.4×10−3 |
| AIP (log Trig/HDL) | 0.03 (−0.15 to 0.24) | 0.13 (−0.07 to 0.33) | 1.5×10−7 a |
| AIP >1 (% positive) | 51.9% | 67.1% | 1.3×10−6 a |
| IL‐1b, pg/mL | 13.7 (9.4–19.1) | 14.0 (9.8–19.8) | 0.362 |
| IL1‐Ra, pg/mL | 448.8 (299.8–791.5) | 426.3 (310.2–639.5) | 0.113 |
| IL‐2, pg/mL | 25.2 (6.1–50.6) | 36.9 (14.5–63.7) | 1.9×10−6 a |
| IL‐4, pg/mL | 14.3 (10.0–21.2) | 17.0 (12.5–24.4) | 7.9×10−8 a |
| IL‐5, pg/mL | 28. 4 (21.1–40.8) | 35.6 (24.9–51.1) | 2.9×10−11 a |
| IL‐6, pg/mL | 48.9 (34.4–69.8) | 53.7 (39.8–80.8) | 4.7×10−5 a |
| IL‐7, pg/mL | 43.1 (29.0–61.3) | 44.2 (31.8–64.1) | 0.131 |
| IL‐8/CXCL8, pg/mL | 135.2 (91.9–200.4) | 174.7 (125.8–253.4) | 4.1×10−17 a |
| IL‐9, pg/mL | 41.7 (25.7–66.6) | 44.8 (31.0–71.3) | 0.024 |
| IL‐10, pg/mL | 67.3 (42.4–105.4) | 80.4 (54.9–125.9) | 1.2×10−6 a |
| IL‐12p70, pg/mL | 108.7 (67.7–167.5) | 122.7 (72.8–195.2) | 5.8×10−4 a |
| IL‐13, pg/mL | 24.0 (17.9–33.1) | 26.5 (19.4–35.5) | 2.4×10−3 |
| IL‐17A, pg/mL | 311.4 (224.1–455.5) | 338.2 (221.2–496.3) | 0.088 |
| bFGF, pg/mL | 146.0 (98.8–208.8) | 154.0 (108.3–228.0) | 0.093 |
| VEGF, pg/mL | 36.5 (13.7–77.4) | 39.7 (14.7–78.6) | 0.505 |
| Eotaxin, pg/mL | 93.8 (58.6–137.7) | 140.1 (100.1–189.7) | 2.1×10−30 a, b |
| G‐CSF, pg/mL | 358.0 (263.0–464.3) | 349.2 (268.0–443.8) | 0.239 |
| GM‐CSF, pg/mL | 0.87 (0–23.2) | 2.9 (0–21.6) | 0.902 |
| IFN‐γ, pg/mL | 421.5 (299.6–598.5) | 417.2 (300.0–600.1) | 0.856 |
| IP‐10/CXCL10, pg/mL | 459.0 (354.4–589.3) | 577.7 (430.9–776.4) | 3.9×10−18 a |
| MCP‐1/CCL2, pg/mL | 85.5 (54.7–132.6) | 85.8 (61.7–124.5) | 0.566 |
| MIP‐1a/CCL3, pg/mL | 19.2 (14.1–26.7) | 24.1 (17.3–34.6) | 2.1×10−15 a |
| MIP‐1b/CCL4, pg/mL | 73.1 (57.4–91.6) | 79.7 (62.7–103.2) | 1.6×10−5 a, b |
| PDGFβ, pg/mL | 1377 (937–2007) | 1237 (760–1800) | 2.4×10−4 a |
| RANTES/CCL5, pg/mL | 17 750 (12 279–38 468) | 13 940 (10 799–19 020) | 9.5×10−17 a, b |
| TNF‐α, pg/mL | 288.4 (206.0–422.9) | 321.2 (229.9–458.2) | 2.8×10−4 a |
AAA indicates abdominal aortic aneurysm; AIP, atherogenic index of plasma; bFGF, basic fibroblast growth factor; CCL, chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte macrophage colony‐stimulating factor; HDL, high‐density lipoprotein; hsCRP, high sensitivity C‐reactive protein; IFN‐γ, interferon‐gamma; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MCP‐1, monocyte chemoattractant protein 1; MIP, macrophage inflammatory protein; PDGFβ, platelet‐derived growth factor beta; RANTES, regulated on activation, normal T‐cell expressed and secreted; TNF‐α, tumor necrosis factor alpha; Trig, troglycerides; VEGF, vascular endothelial growth factor.
Markers below the multiple testing threshold (P<0.0017).
Eotaxin, MIP‐1b, and RANTES were significantly different between cases and controls in both tissue and plasma samples.
Eotaxin, RANTES, and MIP‐1b levels were as significantly different between AAA cases and controls in both tissue and plasma samples, although RANTES showed an inverse direction of effect in the two sample types.
Several cytokines that showed suggestive (P<0.05, P>0.002) tissue differences (IL‐6, MIP‐1a, IL‐12p70, and IL‐10) were also significantly (P<0.0017) different in plasma (although IL‐12p70 and IL‐10 showed inverse directions of effect in tissue and plasma).
The plasma markers of strongest effect (P<5×10−6), including hsCRP and HDL, were taken forward for logistic regression analysis. The optimal binary cut‐off value for each marker was used to calculate odds ratios (ORs) for plasma marker associations with AAA (Table 8). All 13 markers had significant associations as unadjusted variables (Table 9); however, when adjusted for concurrent risk factors, the IL‐6, IL‐10, and MIP‐1b associations became nonsignificant. When the model also included those cytokines that were significantly differentially expressed in both tissue and plasma, only 5 markers (in order of significance; eotaxin, hsCRP, RANTES, HDL, and IL‐8/chemokine [C‐X‐C motif] ligand [CXCL]‐8) remained significantly associated with AAA.
Table 8.
Plasma Protein Biomarker ROC Curves
| Marker | AUC (95% CI) | Binary Cutoff |
|---|---|---|
| hsCRP | 0.73 (0.71–0.76) | 2.5 mg/L |
| HDL | 0.61 (0.57–0.64) | 1.25 mmol/L |
| IL‐2 | 0.58 (0.55–0.61) | 30 pg/mL |
| IL‐5 | 0.61 (0.58–0.64) | 32 pg/mL |
| IL‐4 | 0.59 (0.56–0.62) | 14.9 pg/mL |
| IL‐6 | 0.57 (0.54–0.60) | 50 pg/mL |
| IL‐8/CXCL8 | 0.64 (0.61–0.67) | 155 pg/mL |
| IL‐10 | 0.58 (0.55–0.61) | 75 pg/mL |
| Eotaxin | 0.69 (0.66–0.72) | 125 pg/mL |
| IP‐10/CXCL10 | 0.65 (0.62–0.68) | 525 pg/mL |
| MIP‐1a/CCL3 | 0.63 (0.60–0.66) | 22 pg/mL |
| MIP‐1b/CCL4 | 0.57 (0.54–0.60) | 75 pg/mL |
| RANTES/CCL5 | 0.64 (0.61–0.67) | 14 500 pg/mL |
| Smoking pack‐years | 0.72 (0.69–0.75) |
Area under the (non‐parametric) receiver operating characteristic (ROC) curves (AUC). AUC and the optimal binary cutoff for each significant (unadjusted) plasma biomarker along with smoking pack‐years for comparison. CCL indicates chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; HDL, high‐density lipoprotein; hsCRP, high sensitivity C‐reactive protein; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Table 9.
Plasma Protein Biomarker, Logistic Regression
| Biomarker/Binary Threshold | Odds Ratio (95% CI, P Value) | ||
|---|---|---|---|
| AAA | Adjusted AAA (Model 1) | Adjusted AAA (Model 2) | |
| hsCRP >2.5 mg/L | 4.0 (3.2–5.2), P<0.0001 | 2.2 (1.6–3.1), P<0.0001 | 2.1 (1.5–2.9), P<0.0001a |
| HDL >1.25 mmol/L | 0.62 (0.49–0.78), P<0.0001 | 0.64 (0.47–0.85), P=0.006b | 0.67 (0.47–0.95), P=0.026b |
| IL‐2 >30 pg/mL | 1.8 (1.4–2.2), P<0.0001 | 1.9 (1.4–2.6), P=0.0001 | 1.0 (0.68–1.6), P=0.89 |
| IL‐5 >32 pg/mL | 2.1 (1.6–2.6), P<0.0001 | 2.3 (1.7–3.2), P<0.0001 | 1.7 (1.1–2.6), P=0.0148 |
| IL‐4 >14.9 pg/mL | 1.7 (1.4–2.2), P<0.0001 | 1.7 (1.2–2.3), P<0.002 | 1.2 (0.81–1.8), P=0.35 |
| IL‐6 >50 pg/mL | 1.4 (1.1–1.8), P=0.002 | 1.3 (0.9–1.8), P=0.110 | 0.80 (0.53–1.2) P=0.28 |
| IL‐8/CXCL8 >155 pg/mL | 2.4 (1.9–3.0), P<0.0001 | 2.6 (1.8–3.5), P<0.0001 | 1.6 (1.1–2.5), P=0.0212 |
| IL‐10 >75 pg/mL | 1.5 (1.2–1.9), P<0.001 | 1.4 (1.0–1.9), P=0.049 | 0.84 (0.56–1.3), P=0.39 |
| Eotaxin >125 pg/mL | 3.1 (2.4–3.9), P<0.0001 | 3.4 (2.5–4.8), P<0.0001 | 3.3 (2.3–4.8), P<0.0001a |
| IP‐10/CXCL10 >525 pg/mL | 2.7 (2.1–3.4), P<0.0001 | 1.8 (1.3–2.5), P=0.0002 | 1.3 (0.89–1.8), P=0.19 |
| MIP‐1a/CCL3 >22 pg/mL | 2.2 (1.8–2.8), P<0.0001 | 2.2 (1.6–3.0), P<0.0001 | 1.3 (0.83–1.9), P=0.28 |
| MIP‐1b/CCL4 >75 pg/mL | 1.5 (1.2–1.9) P=0.0003 | 1.4 (1.0–1.9) P=0.055 | 1.2 (0.80–1.7), P=0.44 |
| RANTES/CCL5 >14 500 pg/mL | 0.55 (0.44–0.69), P<0.0001 | 0.48 (0.34–0.66), P<0.0001 | 0.43 (0.30–0.63), P<0.0001a |
| Ischemic heart disease | 2.1 (1.6–2.7), P<0.0001 | 2.2 (1.6–3.2), P<0.0001 | 3.0 (2.0–54.6), P<0.0001 |
| Smoking, per 10 pack‐years | 1.3 (1.2–1.4), P<0.0001 | 1.4 (1.3–1.5), P<0.0001 | 1.3 (1.2–1.4), P<0.0001 |
Adjusted model 1 included age, sex, history of ischemic heart disease (IHD), hypertension, diabetes, atherogenic index in plasma (AIP), smoking (pack‐years), and sample storage time. Adjusted model 2 included age, sex, history of IHD, smoking (pack‐years), and sample storage time plus the other significant (adjusted) markers (RANTES, eotaxin, MIP‐1b, HDL, and hsCRP). AAA indicates abdominal aortic aneurysm; CCL, chemokine (C‐C motif) ligand; CXCL, chemokine (C‐X‐C motif) ligand; HDL, high‐density lipoprotein; hsCRP, high sensitivity C‐reactive protein; IL, interleukin; IP‐10, interferon‐gamma‐induced protein 10; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Markers with statistical significance below the multiple testing threshold (P<0.0042).
AIP excluded from the HDL odds ratios adjusted models. When AIP was included, adjusted models for HDL >1.25 mmol/L became nonsignificant (model 2: 0.90 (0.58–1.4); P=0.657).
A combined AAA‐associated inflammation marker risk score was calculated by assigning 1 point for each of the following: RANTES <14 500 pg/mL, eotaxin >125 pg/mL, and hsCRP >2.5 mg/L, thereby scoring each individual between 0 and 3 (Table 10). The ROC curves for these three markers are shown in Figure 1. In all, 65.0% of AAA patients had a score greater than or equal to 2 compared to 25.9% of controls, with a resulting (adjusted) AAA OR of 4.8. Examination of the control cohort suggested that several conventional cardiovascular risk factors, including age, sex, smoking, hypertension, and past history of heart disease, were potential confounders of the plasma levels of the putative AAA‐associated cytokines (Table 11). Of note, the AAA‐associated inflammation marker risk score remained significant when these potentially confounding interactions were also included in the risk model.
Table 10.
Percentage Distribution and Logistic Regression for AAA‐Associated Inflammation Risks
| AAA‐Associated Inflammation Risk Score | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Control (n=970) | 25.5% | 46.6% | 21.5% | 4.4% |
| AAA (n=442) | 6.4% | 28.6% | 44.8% | 20.2% |
| AAA OR | 0.43 (0.27–0.68)‡ | 1 | 3.5 (2.7–4.7)† | 7.8 (5.1–12.1)† |
| Adjusted ORa | 0.36 (0.20–0.64)‡ | 1 | 3.5 (2.4–5.1)† | 5.3 (3.1–9.1)† |
| Adjusted ORa | 0.36 (0.20–0.64)‡ | 1 | 3.8 (2.7–5.4)† | |
| Adjusted ORa | 1 | 4.8 (3.5–6.7)† | ||
The combined AAA inflammation marker risk score assigned 1 point for each of the following: hsCRP >2.5 mg/L, RANTES <14 500 pg/mL, and eotaxin >125 pg/mL (scoring each individual between 0 and 3). AAA‐associated inflammation risk scores were significantly higher in the AAA group (χ2, P<0.0001). Using an AAA inflammation score of 1 (the most common score among controls) as the reference value, logistic regression suggested a protective association for those with a 0 score and a staged positive risk association with higher (2 or 3) scores. AAA indicates abdominal aortic aneurysm; hsCRP, high sensitivity C‐reactive protein; OR, odds ratio; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Adjusted for age, sex, history of ischemic heart disease, smoking (pack‐years), and sample storage time.
† P<0.0001; ‡ P<0.0005.
Figure 1.

Receiver operating characteristic (ROC) curves for hsCRP, eotaxin, and RANTES. ROC curves were constructed to determine the optimal binary cut‐off value of each differentially expressed cytokine. Cytokines were compared case/controls status (group) as the gold standard. CCL5 indicates chemokine (C‐C motif) ligand 5; hsCRP, high sensitivity C‐reactive protein; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Table 11.
Confounding Interactions With Conventional AAA Risk Factors in Nonaneurysmal Controls
| Sex | Female, n=229 | Male, n=741 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 19 516 (13 313–53 009) | 17 391 (11 968–34 932) | 0.013 |
| Eotaxin, pg/mL | 78 (48–136) | 98 (65–139) | 0.0014 |
| MIP‐1b, pg/mL | 76 (60–93) | 72 (57–92) | 0.17 |
| hsCRP, mg/L | 1.8 (0.8–3.3) | 1.5 (0.7–3.1) | 0.13 |
| HDL, mmol/L | 1.45 (1.20–1.71) | 1.23 (1.02–1.48) | <0.0001 |
| Age | <69 years, n=491 | >69 years, n=479 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 21 130 (12 075–50 154) | 15 933 (11 634–25 663) | <0.0001 |
| Eotaxin, pg/mL | 85 (49–126) | 104 (69–148) | <0.0001 |
| MIP‐1b, pg/mL | 72 (57–92) | 74 (59–92) | 0.81 |
| hsCRP, mg/L | 1.5 (0.7–3.1) | 1.7 (0.8–3.2) | 0.23 |
| HDL, mmol/L | 1.25 (1.03–1.54) | 1.29 (1.08–1.55) | 0.08 |
| Clinical History of IHD | Without IHD, n=718 | IHD, n=196 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 14 608 (11 060–20 333) | 60 755 (49 415–85 193) | <0.0001 |
| Eotaxin, pg/mL | 99 (63–144) | 76 (44–116) | <0.0001 |
| MIP‐1b, pg/mL | 68 (54–87) | 87 (72–114) | <0.0001 |
| hsCRP, mg/L | 1.5 (0.7–2.8) | 2.3 (1.2–4.0) | <0.0001 |
| HDL, mmol/L | 1.30 (1.08–1.59) | 1.15 (0.95–1.39) | <0.0001 |
| Clinical History of Hypertension | Without Hypertension, n=663 | Hypertension, n=306 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 16 956 (11 754–30 660) | 20 754 (13 160–52 990) | 0.0003 |
| Eotaxin, pg/mL | 92 (58–135) | 96 (60–144) | 0.34 |
| MIP‐1b, pg/mL | 70 (55–89) | 79 (62–101) | <0.0001 |
| hsCRP, mg/L | 1.5 (0.7–2.9) | 1.9 (1.0–3.4) | <0.0001 |
| Smoking History | Nonsmoker, n=457 | >10 Pack‐Years, n= 310 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 16 601 (11 739–32 181) | 20 750 (13 371–51 793) | 0.0004 |
| Eotaxin, pg/mL | 90 (57–138) | 102 (66–143) | 0.046 |
| MIP‐1b, pg/mL | 73 (57–93) | 75 (60–95) | 0.21 |
| hsCRP, mg/L | 1.3 (0.7–2.7) | 2.1 (1.0–4.0) | <0.0001 |
| HDL, mmol/L | 1.29 (1.07–1.54) | 1.23 (1.02–1.54) | 0.15 |
| AIP | Negative, n=392 | Positive, n=458 | P Value |
|---|---|---|---|
| RANTES, pg/mL | 16 497 (11 669–24 434) | 17 889 (12 345–44 462) | 0.003 |
| Eotaxin, pg/mL | 93 (59–144) | 92 (58–132) | 0.20 |
| MIP‐1b, pg/mL | 71 (56–87) | 74 (58–92) | 0.06 |
| hsCRP, mg/L | 1.3 (0.6–2.6) | 1.9 (0.9–3.7) | <0.0001 |
The potential confounding interactions between the lead circulating inflammatory cytokines (RANTES, eotaxin, MIP1b, hsCRP, and HDL) was investigated in the control cohort (to exclude case‐control interactions). AAA indicates abdominal aortic aneurysm; AIP, atherogenic index in plasma; HDL, high‐density lipoprotein; hsCRP, high sensitivity C‐reactive protein; IHD, ischemic heart disease; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T‐cell expressed and secreted.
In order to determine relatedness between markers, and how this might influence the adjusted logistic regression model, variable principal component analysis (vPCA) was performed. The vPCA indicated that eotaxin, RANTES, MIP‐1b, hsCRP, and HDL were more‐remote members of the correlation network (Figure 2A). In contrast, markers such as IL‐6, tumor necrosis factor alpha (TNF‐α), IL‐10, IL‐8, IL‐4, IL‐5, MIP‐1a, and IL‐1RA were central members of the network, with a higher degree of corelatedness (Figure 2B). Correlations between the three inflammatory cytokines differentially expressed in both tissue and plasma suggested a modest degree of correlation between MIP‐1b and both eotaxin and RANTES (ρ=0.35, P=6.3×10−27 and ρ=0.46, P=2.5×10−42, respectively). No significant correlation was observed between eotaxin and RANTES (ρ=−0.073). Although a significant correlation between hsCRP and RANTES has been previously described in 300 individuals,12 this was not observed in this current investigation (ρ=−0.02, P=0.56). Only modest correlations were observed between hsCRP and both eotaxin and MIP‐1b (ρ=0.12, P=1.6×10−6 and ρ=0.13, P=1.1×10−6, respectively). HDL was significantly inversely correlated with hsCRP (ρ=−0.20, P=2.2×10−13) and, more modestly, with MIP‐1b (ρ=−0.09, P<0.0017).
Figure 2.
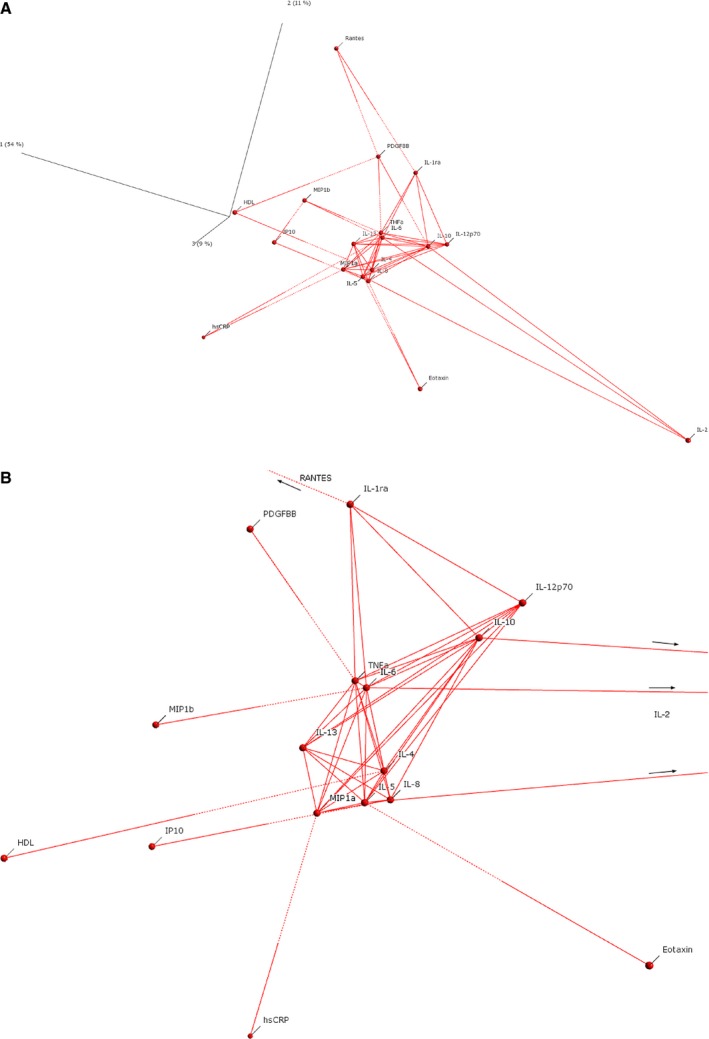
Variable principal component plots of plasma biomarkers. A, Log‐transformed AAA‐associated markers (P<0.05), with each linked to its nearest two neighbors. Solid lines (Euclidean distance threshold >65%) indicate those markers with a stronger degree of relatedness. Notice that the AAA‐associated markers, which showed independent association in multiple logistic regression (eotaxin, hsCRP, RANTES, and HDL), were more‐peripheral members of the network with a lesser degree of relatedness (dotted connection lines) to the central hub of the network. B, Central hub of the network shown in (A), with each linked to its nearest neighbor or displaying a stronger degree of relatedness (Euclidean distance threshold >65%). Notice that markers such as IL‐6, TNF‐α, IL‐10, IL‐8, IL‐4, Il‐5, IL‐12p70, MIP‐1a, and IL‐1RA were central members of the network. AAA indicates abdominal aortic aneurysm; HDL, high‐density lipoprotein; hsCRP, high sensitivity C‐reactive protein; IL, interleukin; MIP‐1a, macrophage inflammatory protein 1 alpha; RANTES, regulated on activation, normal T‐cell expressed and secreted; TNF‐α, tumor necrosis factor alpha.
Plasma marker correlations with aneurysm size were determined in the patient group. A relatively weak effect, but statistically significant, correlation was observed for hsCRP (ρ=0.278, P=1.2×10−8) and AAA size. Associations of weaker effect and (suggestive) significance were observed for IL‐8 (ρ=−0.15, P=0.0026) and eotaxin/CCL11 (ρ=−0.09 P=0.0574). RANTES was not associated with AAA size (ρ=0.02, P=0.7316).
mRNA Expression of AAA‐Associated Inflammatory Cytokines in Aortic Wall Tissue
To determine whether the differentially expressed inflammatory proteins detected in aortic tissue were produced with the aortic wall, mRNA was extracted from 6 AAA and 11 control aortic wall tissue homogenates.
Transcripts for eotaxin 1/CCL11, RANTES/CCL5, and MIP‐1b/CCL4 were detected in all AAA aortic wall tissue, confirming localized expression of these cytokines. Eotaxin 2/CCL24, eotaxin 3/CCL26, and both of the MIP‐1b/CCL4 probes had very low or no levels of expression in control tissue. In contrast, eotaxin 1/CCL11 and RANTES/CCL5 had detectable signal in all control samples (Table 3). GAPDH had slightly stronger expression in controls than cases (Ct values 23.2±0.6 and 24.5±1.1, respectively; P=0.02). Both RANTES/CCL5 and MIP‐1b/CCL4 had significantly greater mRNA transcript levels in AAA compared with control aortic wall tissue (Figure 3).
Figure 3.
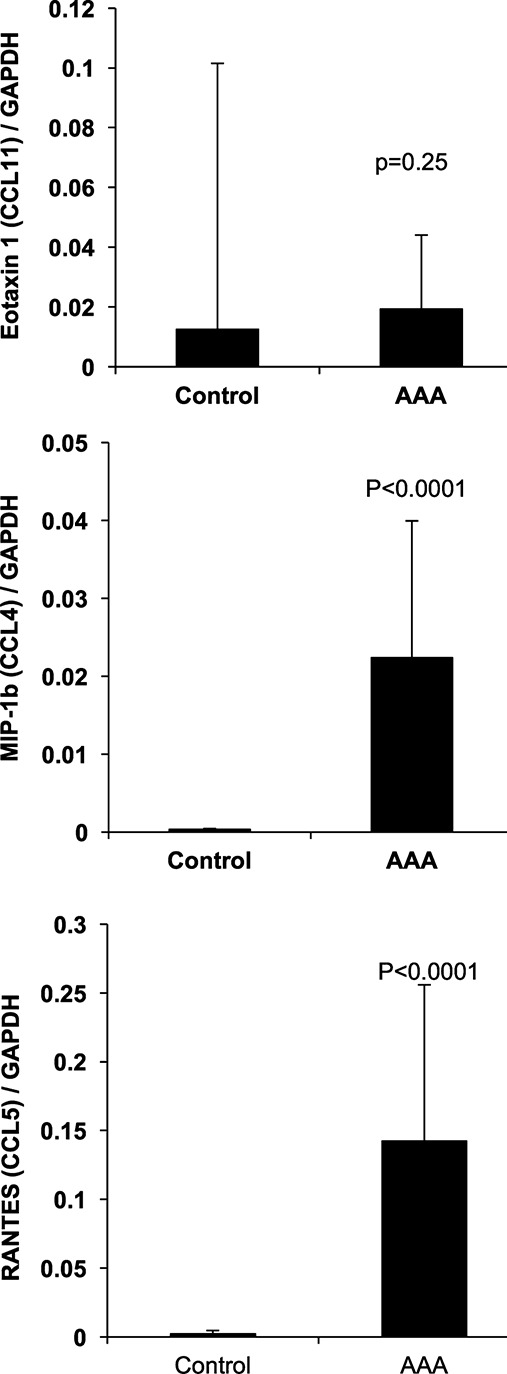
Quantitative PCR results comparing AAA total wall with nonaneurysmal control total wall biopsies for eotaxin 1/CCL11, MIP‐1b/CCL4, and RANTES/CCL5. Transcripts for eotaxin 1/CCL11, RANTES/CCL5, and MIP‐1b/CCL4 were detected in all AAA aortic wall tissue, confirming localized expression of these cytokines. Data represented as means and 1 SD. AAA indicates abdominal aortic aneurysm; CCL, chemokine (C‐C motif) ligand; MIP‐1b, macrophage inflammatory protein 1 beta; PCR, polymerase chain reaction; RANTES, regulated on activation, normal T‐cell expressed and secreted.
Discussion
Several inflammation‐associated proteins have previously been implicated in the pathogenesis of AAA, including hsCRP, HDL‐cholesterol, and IL‐6.8, 9, 10, 13, 14, 15 Perhaps the most comprehensive study examining the relationship between inflammatory cytokines and AAA to date was performed by Middleton et al., who examined 42 cytokines in 10 AAA and 9 control infrarenal aortic biopsies.16 Notably, they observed increased AAA expression of IL‐1b, IL‐6, granulocyte colony‐stimulating factor (G‐CSF), monocyte chemoattractant protein 1 (MCP‐1), and RANTES, which was, at least partially, consistent with our current aortic wall (or sublayer) tissue observations.
In this current study, we significantly advanced the investigation of inflammation‐associated biomarkers in AAA by including a comparison between tissue and plasma inflammatory cytokine profiles. A particular strength of the circulating marker analysis was its ability to model for confounding interactions between a large number of inflammatory cytokines and other demographic and clinical confounders. In so doing, we identified elevated plasma eotaxin and decreased plasma RANTES, as independent risk markers in AAA. In addition, hsCRP and HDL were also validated as circulating biomarkers for AAA, with hsCRP also having a modest correlation with aneurysm size.
When the plasma markers were analyzed by vPCA, eotaxin, RANTES, hsCRP, and HDL were more‐remote members of the network, which may, in part, explain why these markers remained significant in the adjusted regression models. Inflammation has long been broadly implicated in atherosclerosis17 and with cardiovascular disease risk factors, such as smoking.18 In the search for disease‐specific biomarkers, it is important that candidate circulating cytokines are not significantly confounded by concurrent disease or “conventional” risk factors. These issues were exemplified by IL‐6, which, while replicating previous univariate associations with AAA, lost its significance in the multivariate logistic regression models that included concurrent heart disease, smoking, and other AAA‐associated inflammatory cytokines. Whereas we were able to replicate previous reports suggesting a strong association between plasma CRP and AAA, its broad association with other inflammatory disorders, as well as cardiovascular risk factors, such as hypertension, and concurrent atherosclerotic disease,19 may limit this protein's utility as an AAA‐specific biomarker.
Probably the most interesting associations observed in this study were those with eotaxin and RANTES, which showed differential expression in both aortic tissue and plasma. RANTES, is a CC chemokine family T‐cell mitogen that has been implicated in the release of proinflammatory cytokines.20, 21 The release of RANTES from CD8 T cells has been associated with a wide range of tissue‐inflammatory reactions, including allergic reactions, cancers, and gastritis.22 Notably, RANTES can be released by multiple cell types relevant to AAA, including not only T cells, but also smooth muscle and platelets and has been shown to be associated with CAD risk.12 In addition, pharmacological inhibition of platelet degranulation reduced the inflammatory cytokine burden (including RANTES) in intramural thrombi and was associated with reduced rupture rates in AAA patients.23 Consistent with our current study, levels of RANTES mRNA24 and protein16 have been previously reported to be elevated in AAA aortic wall tissue. In contrast to tissue expression, we observed significantly lower plasma RANTES in AAA patients than controls. Although serum RANTES has been reported as elevated in CAD,12 there are also contradictory reports25 regarding this relationship. It is also worth noting that, because RANTES is released from platelets during blood clotting, serum measures should be viewed cautiously and direct plasma/serum comparisons avoided altogether. Nevertheless, in our plasma analysis, we observed a strong association between elevated RANTES and a history of ischemic heart disease (IHD) in our nonaneurysmal control group (Table 11). This association was strikingly absent within the AAA group, the reasons for which are unclear; however, we postulate that this may be attributed to the aforementioned relationship between RANTES and platelets. AAA has been previously linked to reduced circulating platelet counts26 and increased platelet activation,27 both of which may, in part, explain increased localized (AAA wall) and decreased circulating levels of platelet‐associated factors such as RANTES.
Eotaxin‐1 (or CCL11), another member of the CC chemokine family, is known to be a key mediator in asthma.28 Eotaxin was included in our analysis solely on the basis that it formed part of a broad (relatively unbiased) cytokine multiplex panel, and we had no a priori suggestion of a link with AAA. Intriguingly, however, acute asthma has recently been linked to AAA susceptibility and rupture,29 an observation that appears to indirectly validate our observed eotaxin‐AAA association.
The antibody used in protein bioplex array could not distinguish between eotaxin ‐1, ‐2, and ‐3; however, our mRNA studies suggested that eotaxin 1/CCL11 was the dominantly expressed variant. Haley et al.30 demonstrated that eotaxin expression could be induced in cultured aortic smooth muscle cells by treatment with TNF‐α, and there have been conflicting reports regarding a possible association with atherosclerosis.31, 32 More‐recent studies have also suggested an association with cardiovascular risk factors and the soluble form of the receptor for advanced glycation endproducts.33 B cells form a significant (≈40%) proportion of the adventitial lymphocyte population in AAA,15, 34 but are rare within the adventitia of aortic occlusive and normal aortae.35 B cells, from patients with inflammatory bowel disease, have been shown to release eotaxin‐1, in a disease‐associated fashion, a process that has been suggested as a mediating factor in immune cell activation.36 Whether eotaxin acts in a similar fashion in AAA is unclear; however, the elevated levels of eotaxin observed within AAA adventitial tissue certainly warrant further investigation.
In conclusion, contrary to some previous reports suggesting a distinct T helper 2–associated inflammatory (IL‐4, ‐5, and ‐10) profile in AAA,37 this current study is more consistent with those studies that have suggested a more‐generalized pattern of inflammation.14 Whereas the plasma inflammatory cytokine profile of AAA patients contained numerous cytokines with differential expression compared to nonaneurysmal controls, 3 markers (eotaxin, hsCRP, and RANTES) were notable as potentially useful AAA biomarkers.
Sources of Funding
The study was funded by grants from the Health Research Council of New Zealand (08‐75, 14‐155) and the Lottery Health Research (New Zealand).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002993 doi: 10.1161/JAHA.115.002993)
References
- 1. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. [DOI] [PubMed] [Google Scholar]
- 2. Johnsen SH, Forsdahl SH, Singh K, Jacobsen BK. Atherosclerosis in abdominal aortic aneurysms: a causal event or a process running in parallel? The Tromso study. Arterioscler Thromb Vasc Biol. 2010;30:1263–1268. [DOI] [PubMed] [Google Scholar]
- 3. Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. [DOI] [PubMed] [Google Scholar]
- 4. Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–1430. [DOI] [PubMed] [Google Scholar]
- 5. Golledge J, Norman PE. Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors? Arterioscler Thromb Vasc Biol. 2010;30:1075–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones GT. The pathohistology of abdominal aortic aneurysm In: Grundmann RT, ed. Diagnosis, Screening and Treatment of Abdominal, Thoracoabdominal and Thoracic Aortic Aneurysms. Rijeka, Croatia: InTech; 2011:414. [Google Scholar]
- 7. Hurks R, Vink A, Hoefer IE, de Vries JP, Schoneveld AH, Schermerhorn ML, den Ruijter HM, Pasterkamp G, Moll FL. Atherosclerotic risk factors and atherosclerotic postoperative events are associated with low inflammation in abdominal aortic aneurysms. Atherosclerosis. 2014;235:632–641. [DOI] [PubMed] [Google Scholar]
- 8. Folsom AR, Yao L, Alonso A, Lutsey PL, Missov E, Lederle FA, Ballantyne CM, Tang W. Circulating biomarkers and abdominal aortic aneurysm incidence: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;132:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parry DJ, Al‐Barjas HS, Chappell L, Rashid ST, Ariens RA, Scott DJ. Markers of inflammation in men with small abdominal aortic aneurysm. J Vasc Surg. 2010;52:145–151. [DOI] [PubMed] [Google Scholar]
- 10. Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6:543–552. [DOI] [PubMed] [Google Scholar]
- 11. Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. [DOI] [PubMed] [Google Scholar]
- 12. Koh SJ, Kim JY, Hyun YJ, Park SH, Chae JS, Park S, Kim JS, Youn JC, Jang Y, Lee JH. Association of serum RANTES concentrations with established cardiovascular risk markers in middle‐aged subjects. Int J Cardiol. 2009;132:102–108. [DOI] [PubMed] [Google Scholar]
- 13. Burillo E, Lindholt JS, Molina‐Sanchez P, Jorge I, Martinez‐Pinna R, Blanco‐Colio LM, Tarin C, Torres‐Fonseca MM, Esteban M, Laustsen J, Ramos‐Mozo P, Calvo E, Lopez JA, Vega de Ceniga M, Michel JB, Egido J, Andres V, Vazquez J, Meilhac O, Martin‐Ventura JL. ApoA‐I/HDL‐C levels are inversely associated with abdominal aortic aneurysm progression. Thromb Haemost. 2015;113:1335–1346. [DOI] [PubMed] [Google Scholar]
- 14. Lindeman JH, Abdul‐Hussien H, Schaapherder AF, Van Bockel JH, Von der Thusen JH, Roelen DL, Kleemann R. Enhanced expression and activation of pro‐inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL‐6‐ and IL‐8‐dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond). 2008;114:687–697. [DOI] [PubMed] [Google Scholar]
- 15. Abdul‐Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg. 2010;51:1479–1487. [DOI] [PubMed] [Google Scholar]
- 16. Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro‐inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–580. [DOI] [PubMed] [Google Scholar]
- 17. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 18. Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J265. [DOI] [PubMed] [Google Scholar]
- 19. Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high‐sensitivity C‐reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. [DOI] [PubMed] [Google Scholar]
- 20. Appay V, Dunbar PR, Cerundolo V, McMichael A, Czaplewski L, Rowland‐Jones S. RANTES activates antigen‐specific cytotoxic T lymphocytes in a mitogen‐like manner through cell surface aggregation. Int Immunol. 2000;12:1173–1182. [DOI] [PubMed] [Google Scholar]
- 21. Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP‐70 in human T cells. J Exp Med. 1996;184:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohtani N, Ohtani H, Nakayama T, Naganuma H, Sato E, Imai T, Nagura H, Yoshie O. Infiltration of CD8+ T cells containing RANTES/CCL5+ cytoplasmic granules in actively inflammatory lesions of human chronic gastritis. Lab Invest. 2004;84:368–375. [DOI] [PubMed] [Google Scholar]
- 23. Owens AP, Edwards TL, Antoniak S, Geddings JE, Jahangir E, Wei WQ, Denny JC, Boulaftali Y, Bergmeier W, Daugherty A, Sampson UK, Mackman N. Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:2032–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tung WS, Lee JK, Thompson RW. Simultaneous analysis of 1176 gene products in normal human aorta and abdominal aortic aneurysms using a membrane‐based complementary DNA expression array. J Vasc Surg. 2001;34:143–150. [DOI] [PubMed] [Google Scholar]
- 25. Herder C, Peeters W, Illig T, Baumert J, de Kleijn DP, Moll FL, Poschen U, Klopp N, Muller‐Nurasyid M, Roden M, Preuss M; Consortium CA , Karakas M, Meisinger C, Thorand B, Pasterkamp G, Koenig W, Assimes TL, Deloukas P, Erdmann J, Holm H, Kathiresan S, Konig IR, McPherson R, Reilly MP, Roberts R, Samani NJ, Schunkert H, Stewart AF. RANTES/CCL5 and risk for coronary events: results from the MONICA/KORA Augsburg case‐cohort, Athero‐Express and CARDIoGRAM studies. PLoS One. 2011;6:e25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milne AA, Adam DJ, Murphy WG, Ruckley CV. Effects of asymptomatic abdominal aortic aneurysm on the soluble coagulation system, platelet count and platelet activation. Eur J Vasc Endovasc Surg. 1999;17:434–437. [DOI] [PubMed] [Google Scholar]
- 27. Wallinder J, Bergqvist D, Henriksson AE. Haemostatic markers in patients with abdominal aortic aneurysm and the impact of aneurysm size. Thromb Res. 2009;124:423–426. [DOI] [PubMed] [Google Scholar]
- 28. Williams TJ, Jose PJ. Role of eotaxin and related CC chemokines in allergy and asthma. Chem Immunol. 2000;78:166–177. [DOI] [PubMed] [Google Scholar]
- 29. Liu CL, Wemmelund H, Wang Y, Liao M, Lindholt JS, Johnsen SP, Vestergaard H, Fernandes C, Sukhova GK, Cheng X, Zhang JY, Yang C, Huang X, Daugherty A, Levy BD, Libby P, Shi GP. Asthma associates with human abdominal aortic aneurysm and rupture. Arterioscler Thromb Vasc Biol. 2016;36:570–578. doi: 10.1161/ATVBAHA.115.306497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, Thompson JF, Sukhova GH, Libby P, Lee RT. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. [DOI] [PubMed] [Google Scholar]
- 31. Mosedale DE, Smith DJ, Aitken S, Schofield PM, Clarke SC, McNab D, Goddard H, Gale CR, Martyn CN, Bethell HW, Barnard C, Hayns S, Nugent C, Panicker A, Grainger DJ. Circulating levels of MCP‐1 and eotaxin are not associated with presence of atherosclerosis or previous myocardial infarction. Atherosclerosis. 2005;183:268–274. [DOI] [PubMed] [Google Scholar]
- 32. Falcone C, Minoretti P, D'Angelo A, Buzzi MP, Coen E, Emanuele E, Aldeghi A, Olivieri V, Geroldi D. Markers of eosinophilic inflammation and risk prediction in patients with coronary artery disease. Eur J Clin Invest. 2006;36:211–217. [DOI] [PubMed] [Google Scholar]
- 33. Falcone C, Buzzi MP, Bozzini S, Boiocchi C, D'Angelo A, Schirinzi S, Choi J, Ochan Kilama M, Esposito C, Torreggiani M, Mancia G. Relationship between sRAGE and eotaxin‐3 with CRP in hypertensive patients at high cardiovascular risk. J Nephrol. 2013;26:144–151. [DOI] [PubMed] [Google Scholar]
- 34. Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune‐mediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 36. Rehman MQ, Beal D, Liang Y, Noronha A, Winter H, Farraye FA, Ganley‐Leal L. B cells secrete eotaxin‐1 in human inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:922–933. [DOI] [PubMed] [Google Scholar]
- 37. Schonbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


