Abstract
Background
The association between galectin‐3 and heart failure (HF) or death is well established for white, but not for black, adults.
Methods and Results
Galectin‐3 was measured in 1809 participants (1375 white, 434 black), enrolled in a substudy of the Atherosclerosis Risk in Communities (ARIC) observational cohort during 2004–2005. We used Cox proportional hazard models to estimate the adjusted association between galectin‐3 and outcomes. Analyses were conducted overall and by race category. Median (interquartile range) galectin‐3 levels were 13.4 (11.2–16.4) and 14.8 (12–17.6) ng/mL, in white and black participants, respectively. In the sample overall, galectin‐3 was not independently associated with HF or death over a maximum of 7.9 years. However, in race‐stratified analyses, galectin‐3 was independently associated with a composite of HF or death among whites (eg, hazard ratio 2.2, 95% CI 1.2–3.9, comparing Q4 versus Q1); but not among blacks (hazard ratio of 0.8 [0.4–1.8] for Q4 versus Q1, race interaction P=0.03). Associations between galectin‐3 and both outcomes analyzed individually also demonstrated similar racial differences. Furthermore, results were qualitatively similar with galectin‐3 modeled as a continuous exposure. In addition, galectin‐3 improved discrimination for the composite of HF or death among whites (increase in Harrell's C statistic from 0.729 to 0.735 [difference of +0.006], P=0.049), but not among blacks (0.696 to 0.695 [difference of −0.001], P=0.814).
Conclusions
In contrast to whites, galectin‐3 may have limited prognostic utility for predicting HF and death in blacks. While our results require replication, they could reflect racial differences in the processes by which galectin‐3 mediates disease.
Keywords: biomarker, death, galectin‐3, heart failure, race and ethnicity
Subject Categories: Heart Failure, Epidemiology, Risk Factors, Primary Prevention
Introduction
Galectin‐3, a member of the β‐galactoside‐binding lectins family, is thought to amplify inflammatory and fibrotic processes,1 and may contribute directly to the development of atherosclerosis,2 heart failure,3 and cancer.4 Prior research has demonstrated the prognostic value of galectin‐3 in patients with existing heart failure,3, 5, 6, 7 including in heart failure with preserved ejection fraction.8 On this basis, galectin‐3 received a class IIb endorsement in 2013 American College of Cardiology/American Heart Association heart failure guidelines for use as a myocardial fibrosis biomarker to guide “risk stratification” in both ambulatory and acute settings (of note, galectin‐3 was not reviewed in recent European heart failure guidelines).9, 10 However, emerging data have cast some doubt on the prognostic value of galectin‐3 in heart failure,11, 12 particularly relative to other fibrosis biomarkers, such as ST2.13
Despite emerging uncertainty regarding the independent value of galectin‐3 in the setting of established heart failure, there is compelling evidence that galectin‐3 may also be an effective biomarker of future risk for new‐onset heart failure or mortality in overtly asymptomatic persons. Specifically, galectin‐3 has previously been shown to be an independent predictor of all‐cause mortality in a general population study of whites from the Netherlands14; all‐cause mortality and cardiovascular disease death in the U.S. Rancho Bernardo Study15; and heart failure in a nested case–control study from the U.S. Physician's Health Study.16 Consistent with these results, a report from the Framingham Heart Offspring study also found that galectin‐3 was independently associated with left ventricular mass, risk of incident heart failure, and total mortality.17
However, while the above reports suggest a potential role for galectin‐3 as a biomarker for heart failure risk in primary prevention settings, these data were derived from almost exclusively white study populations, and further research from racially diverse populations is needed. Thus, we sought to evaluate the association between baseline galectin‐3 and heart failure and death in a substudy of 1375 white and 434 black participants from the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Population
ARIC is an ongoing prospective cohort study of 15 792 subjects, enrolled between 1987 and 1989 from four U.S. communities (Forsyth Country, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland).18 Galectin‐3 was measured in 2005 ARIC participants who attended the carotid magnetic resonance imaging (CARMRI) substudy in 2004–2005. The ARIC CARMRI substudy was designed to investigate correlates of carotid atherosclerosis imaged using high‐resolution contrast‐enhanced MRI.19 The study was approved by the institutional review boards of the participating institutions, and all participants gave informed consent.
Stratified sampling, designed to enrich the sample with carotid atherosclerosis but still allow inferences to be drawn from the entire ARIC population, was used to select CARMRI participants.19 All of the 2005 participants included had blood drawn for measurement of galectin‐3. For the purposes of our main analysis of incident heart failure and death, we excluded 122 persons with baseline heart failure and 74 persons with missing data, resulting in an analytic sample of 1809 (Table S1).
Measurement of Galectin‐3 and Other Exposure Variables
Galectin‐3 was measured in 2013 in stored serum samples using the BGM Galectin‐3 assay, an ELISA on a microtiter plate platform (BG Medicine, Waltham, MA). This Galectin‐3 assay has been characterized previously.1 For this study the testing was completed in 59 analytic runs; the low control demonstrated a mean of 19.0 ng/mL, SD 1.0 ng/mL, interassay CV 5.3%, and the high control showed a mean value of 64.1 ng/mL, SD 2.9 ng/mL, and interassay CV of 4.5%. The 2 subjects with Galectin‐3 values >94.8 ng/mL underwent sample dilution as per manufacturer instructions. Otherwise, fasting blood samples were assayed for total and high‐density lipoprotein (HDL) cholesterol, as well as fasting glucose using conventional techniques.19 Glomerular filtration rate was estimated using serum creatinine and the CKD‐EPI 2009 equation (eGFR).
The CARMRI core examination procedures were identical to those previously established for prior ARIC visits.18, 19 Standardized ARIC procedures have been previously described for the measurement of blood pressure and determination of body mass index (in kg/m2). Diabetes was defined as self‐report of a doctor diagnosis, medication use, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%. To ascertain medication use, participants were asked to bring containers of current medications to the CARMRI visit. Participants self‐reported race and smoking status.
Outcome Ascertainment: Heart Failure and Death
The methods for ascertainment of deaths and adjudication of cardiovascular events in ARIC have been previously published.20 Briefly, any hospitalization was reported annually by participants or their proxy and also identified through surveillance of hospitals in each ARIC community. Trained personnel abstracted hospital records for potential cardiovascular events. Heart failure cases were identified from hospitalization diagnosis codes (ICD‐9 code 428) and deaths by surveillance (hospital discharge records for inpatient deaths and death certificates for deaths outside the hospital).21 The primary outcome for this analysis was the composite of heart failure or death; we also report findings for each outcome individually. Participants were administratively censored for events in December 31, 2012.
Statistical Analyses
The analytic plan was submitted as a prespecified proposal to the ARIC internal review committee prior to commencing analyses. In keeping with prior studies,17 characteristics for the study population were tabulated according to quartiles of galectin‐3 and were compared using ANOVA for continuous variables or χ2 testing for proportions. To facilitate generalization to the entire ARIC cohort, all analyses were weighted by the inverse of the sample fractions in the CARMRI sampling strata using standard methods. We used Poisson regression to estimate adjusted prevalence ratios for the highest quartile of galectin‐3 based on risk factors measured at baseline. Negative binomial models were also used to estimate prevalence ratios, and the results from these models were nearly identical to those from our Poisson models, with no evidence of overdispersion (α close to zero).
Cumulative survival curves were generated using the Kaplan–Meier method. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and their 95% CIs for heart failure and death, both as a composite outcome and individually. Galectin‐3 was modeled as both a categorical (quartiles) and a continuous exposure (log‐transformed). P‐values for linear trends across galectin‐3 quartiles were obtained by assigning the median galectin‐3 value in each quartile and modeling this ordinal variable continuously. For all models, we verified the proportionality of the hazards with Schoenfeld residuals.
Models were adjusted for age (years), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), sex (male or female), smoking status (current, former, or never), diabetes status (yes or no), mean systolic blood pressure (mm Hg), mean diastolic blood pressure (mm Hg), antihypertensive medication use (yes or no), total cholesterol (mg/dL), HDL cholesterol (mg/dL), and eGFR (continuous, in mL/min per 1.73 m2). In the Poisson models reporting prevalence ratios, age was rescaled to per 10‐year increment. The above variables were chosen based on directed acyclic graphs designed to illustrate the proposed causal link between galectin‐3 and events. Model discrimination was assessed using Harrell's C‐statistic,22 and we evaluated improvement in the C‐statistic for the addition of galectin‐3 as a log‐transformed continuous variable to the fully adjusted model. In sensitivity analyses, we additionally adjusted for history of any cardiovascular disease at baseline. We tested for interactions by age, sex, and race group.
We also modeled galectin‐3 using linear splines in fully adjusted Cox models for both heart failure and all‐cause mortality. A two‐sided P<0.05 was considered statistically significant. All analyses were performed using Stata version 13.0 (StataCorp, College Station, TX).
Results
In our community‐based sample of 1809 subjects, without baseline heart failure, the mean age was 71 years, 51% were female, and 24% were black. Other demographic variables, overall and stratified by galectin‐3, are presented in Table 1. Compared to those in the lowest quartile, persons in the highest quartile of galectin‐3 were older, more likely to be female, obese, hypertensive, diabetic, and have lower HDLcholesterol and eGFR <60 mL/min per 1.73 m2 (Table 1). In addition, there were more blacks in the higher quartiles of galectin‐3. In contrast, the crude prevalence of current smoking was marginally lower in the fourth quartile of galectin‐3. Race‐stratified demographics are presented in Table 2.
Table 1.
Baseline Characteristics of the Analytic Sample; Overall and by Galectin‐3 Quartile (N=1809)
| Overall | Q1 | Q2 | Q3 | Q4 | P Value | |
|---|---|---|---|---|---|---|
| Galectin‐3, range in ng/mL | 4.2 to 184.1 | 4.2 to 11.38 | 11.4 to 13.8 | 13.8 to 16.7 | 16.7 to 184.1 | |
| Age, mean (SD), y | 71.0 (5.6) | 69.7 (5.2) | 70.4 (5.4) | 71.2 (5.3) | 72.8 (5.9) | <0.001 |
| Female, % | 51.0 | 36.6 | 54.3 | 52.5 | 60.4 | <0.001 |
| Black, % | 24.0 | 18.3 | 20.1 | 28.2 | 29.4 | <0.001 |
| Body mass index (BMI), % | 0.021 | |||||
| Normal weight (<25 kg/m2) | 23.9 | 26.0 | 24.1 | 23.1 | 22.3 | |
| Overweight (25–30 kg/m2) | 41.8 | 46.6 | 41.5 | 40.1 | 38.9 | |
| Obese (>30 kg/m2) | 34.3 | 27.4 | 34.4 | 36.8 | 38.7 | |
| Mean (SD) BMI | 28.7 (5.1) | 28.0 (4.7) | 28.7 (5.0) | 28.8 (5.0) | 29.3 (5.6) | <0.001 |
| Smoking status, % | 0.061 | |||||
| Never smoked | 45.6 | 41.3 | 45.0 | 44.3 | 51.5 | |
| Current smoker | 8.8 | 8.4 | 10.2 | 9.3 | 7.5 | |
| Former smoker | 45.6 | 50.3 | 44.8 | 46.3 | 40.9 | |
| Total cholesterol, mean (SD) in mg/dL | 193.1 (41.0) | 195.2 (41.9) | 192.0 (39.4) | 190.9 (41.3) | 194.1 (41.4) | 0.314 |
| High total cholesterol (≥200 mg/dL), % | 41.0 | 41.7 | 40.2 | 39.2 | 42.9 | 0.685 |
| HDL, mean (SD) in mg/dL | 49.2 (14.5) | 50.5 (15.5) | 49.8 (14.4) | 48.7 (14.5) | 48.0 (13.6) | 0.061 |
| Low HDL cholesterol, %a | 40.9 | 33.8 | 39.1 | 44.3 | 46.2 | <0.001 |
| Systolic blood pressure, mean (SD) in mm Hg | 127.2 (18.9) | 127.2 (17.9) | 126.2 (18.3) | 126.7 (18.9) | 128.8 (20.3) | 0.191 |
| Hypertension, %b | 74.8 | 66.2 | 70.9 | 78.9 | 83.4 | <0.001 |
| Hemoglobin A1c %, mean (SD) | 5.8 (0.8) | 5.8 (0.8) | 5.8 (0.7) | 5.9 (0.9) | 5.9 (0.8) | 0.018 |
| Diabetes, %c | 32.9 | 30.5 | 29.6 | 33.9 | 37.6 | 0.041 |
| Low eGFR (<60 mL/min per 1.73 m2), % | 19.7 | 6.8 | 10.2 | 21.3 | 40.7 | <0.001 |
| Cardiovascular disease at baseline, % | 14.7 | 12.6 | 13.9 | 16.6 | 15.7 | 0.314 |
eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol; Q, quartile.
Low HDL cholesterol is defined as <40 mg/dL for men and <50 mg/dL for women.
Hypertension is defined as diastolic blood pressure ≥90 mm Hg, systolic blood pressure ≥140 mm Hg, or blood pressure–lowering medication use.
Diabetes is defined as self‐report doctor diagnosed or medication use, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%.
Table 2.
Baseline Characteristics of the Analytic Sample; Overall and by Race (N=1809)
| Overall | Whites (N=1375) | Blacks (N=434) | P Value | |
|---|---|---|---|---|
| Galectin‐3, range in ng/mL | 4.2 to 184.1 | 4.2 to 184.1 | 6.2 to 87.5 | |
| Age, y | 71.0 (5.6) | 71.3 (5.5) | 70.1 (5.8) | <0.001 |
| Female, % | 51.0 | 48.0 | 60.4 | <0.001 |
| Black, % | 24.0 | 0.0 | 100.0 | <0.001 |
| Body mass index (BMI), % | <0.001 | |||
| Normal weight (<25 kg/m2) | 23.9 | 26.3 | 16.4 | |
| Overweight (25–30 kg/m2) | 41.8 | 42.7 | 38.9 | |
| Obese (>30 kg/m2) | 34.3 | 31.1 | 44.7 | |
| Mean (SD) BMI | 28.7 (5.1) | 28.2 (4.8) | 30.1 (5.6) | <0.001 |
| Smoking status, % | <0.001 | |||
| Never smoked | 45.6 | 43.6 | 51.6 | |
| Current smoker | 8.8 | 8.1 | 11.1 | |
| Former smoker | 45.6 | 48.2 | 37.3 | |
| Total cholesterol, mean (SD) in mg/dL | 193.1 (41.0) | 190.7 (40.7) | 200.5 (41.0) | <0.001 |
| High total cholesterol (≥200 mg/dL), % | 41.0 | 38.8 | 48.2 | <0.001 |
| HDL, mean (SD) in mg/dL | 49.2 (14.5) | 48.6 (14.6) | 51.4 (14.1) | <0.001 |
| Low HDL cholesterol, %* | 40.9 | 42.5 | 35.7 | 0.013 |
| Systolic blood pressure, mean (SD) in mm Hg | 127.2 (18.9) | 126.5 (18.4) | 129.3 (20.4) | <0.001 |
| Hypertension, %† | 74.8 | 72.1 | 83.6 | <0.001 |
| Hemoglobin A1c %, mean (SD) | 5.8 (0.8) | 5.7 (0.6) | 6.2 (1.1) | <0.001 |
| Diabetes, %‡ | 32.9 | 29.2 | 44.7 | <0.001 |
| Low eGFR (<60 mL/min per 1.73 m2), % | 19.7 | 20.0 | 18.9 | 0.614 |
| Cardiovascular disease at baseline, % | 14.7 | 15.6 | 11.8 | 0.046 |
eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol.
Low HDL cholesterol is defined as <40 mg/dL for men and <50 mg/dL for women.
Hypertension is defined as diastolic blood pressure ≥90 mm Hg, systolic blood pressure ≥140 mm Hg, or blood pressure–lowering medication use.
Diabetes is defined as self‐report doctor diagnosed or medication use, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%.
Median (interquartile range) galectin‐3 levels were 13.4 (11.2–16.4) and 14.8 (12–17.6) ng/mL, in white and black participants, respectively. The continuous distribution of galectin‐3 within each race category is demonstrated in Figure S1. In adjusted analyses evaluating cross‐sectional associations between baseline demographics and elevated galectin‐3 levels, age, female sex, black race, low HDL cholesterol, and, particularly, eGFR <60 mL/min per 1.73 m2 were all independently associated with prevalent galectin‐3 levels in the fourth quartile (Table 3). However, after adjustment, smokers did not have lower galectin‐3 levels. Findings for race‐stratified prevalence ratios were similar.
Table 3.
Adjusteda Prevalence Ratios (PRs) for the Highest Quartile of Galectin‐3 (Unweighted N=1809); Overall and Stratified by Race
| Overall | White (N=1375) | Black (N=434) | |
|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Age, per 10 y | 1.63 (1.34–1.97) b | 1.80 (1.41–2.30) b | 1.26 (0.94–1.69) |
| Female (vs male) | 1.32 (1.07–1.62) b | 1.32 (1.03–1.68) b | 1.37 (0.95–2.00) |
| Black (vs white) | 1.37 (1.11–1.70) b | — | — |
| Body mass index, kg/m2 | |||
| 25–30 (vs <25) | 1.03 (0.78–1.35) | 1.05 (0.77–1.43) | 0.96 (0.56–1.64) |
| ≥30 (vs <25) | 1.22 (0.92–1.60) | 1.16 (0.84–1.61) | 1.29 (0.77–2.17) |
| Cigarette smoking | |||
| Current smoker (vs never) | 0.90 (0.61–1.33) | 0.85 (0.51–1.41) | 0.91 (0.49–1.69) |
| Former smoker (vs never) | 0.94 (0.76–1.16) | 0.97 (0.76–1.25) | 0.84 (0.58–1.22) |
| High total cholesterol (≥200 mg/dL), yes/no | 1.19 (0.96–1.48) | 1.31 (1.01–1.69) | 0.91 (0.64–1.30) |
| Low HDL cholesterolc, yes/no | 1.31 (1.07–1.61)b | 1.28 (1.00–1.63) | 1.35 (0.94–1.94) |
| Hypertensiond, yes/no | 1.07 (0.82–1.39) | 1.11 (0.81–1.51) | 0.94 (0.60–1.48) |
| Diabetese, yes/no | 1.05 (0.86–1.29) | 1.22 (0.95–1.56) | 0.74 (0.53–1.05) |
| Low eGFRf, yes/no | 2.34 (1.92–2.86)b | 2.35 (1.85–3.00)b | 2.44 (1.75–3.39)b |
ARIC indicates Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol.
Poisson model adjusted for all variables listed above and are weighted, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design.
P<0.05.
Low HDL cholesterol is defined as <40 mg/dL for men and <50 mg/dL for women.
Hypertension is defined as diastolic blood pressure ≥90 mm Hg, systolic blood pressure ≥140 mm Hg, or antihypertensive medication use.
Diabetes is defined as self‐report doctor‐diagnosed diabetes or medication use, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%.
Low eGFR is defined as <60 mL/min per 1.73 m2.
Over a median of 6.8 years and a maximum of 7.9 years follow‐up, the composite outcome of heart failure or death occurred in 313 participants. There were 167 episodes of incident heart failure and 212 deaths in the sample overall. The incidence rate for heart failure (per 1000 person‐years) was 13.4 for whites and 18.1 for blacks overall. Incidence rate for death was 18.3 for whites and 16.4 for blacks. Persons in the highest quartile of galectin‐3 had the highest cumulative risk of the composite outcome and both heart failure and death outcomes individually (Figure 1). In contrast, survival curves for those in the first, second, and third quartiles of galectin‐3 overlapped and were similar for each outcome. After stratification by race, Kaplan–Meier curves for whites also demonstrates reduced survival in the fourth quartile with overlapping survival in the lower 3 quartiles; however, among blacks the survival appeared to overlap irrespective of galectin‐3 status (Figure 2).
Figure 1.
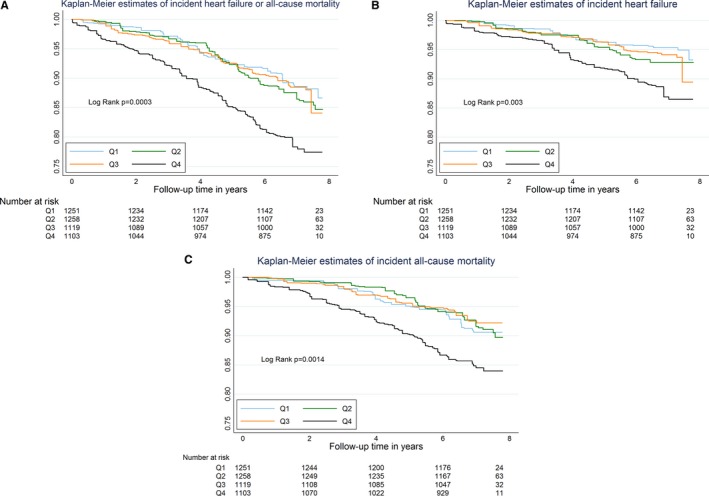
Kaplan–Meier estimates of survival free from the composite outcome of heart failure or death (A), incident heart failure (B), and death (C), according to baseline galectin‐3 quartile, in a community‐based asymptomatic sample from ARIC (unweighted N=1809). This is a weighted Kaplan–Meier, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design. Log‐rank P‐values are for differences across all 4 quartiles. ARIC indicates Atherosclerosis Risk in Communities.
Figure 2.
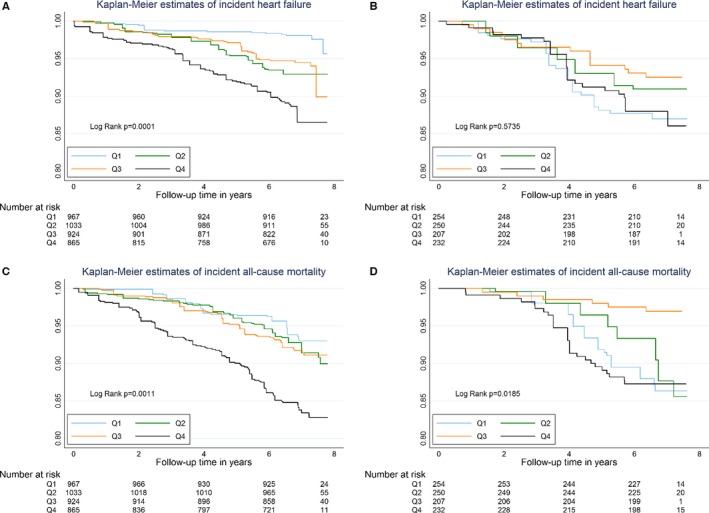
Kaplan–Meier (KM) estimates of survival free from incident heart failure or death, according to galectin‐3 quartile, stratified by race (unweighted N=1809). A=KM of incident HF in whites, B=KM of incident HF in blacks, C=KM of death in whites, D=KM of death in blacks. See Figure 1 legend.
In demographic (age‐, sex‐, and race‐adjusted) Cox proportional hazards models, there was a significant association between elevated baseline galectin‐3 and the primary composite outcome in the sample overall (eg, HR of 1.72 [1.12–2.62], for Q4 versus Q1; Table 4). However, in fully adjusted models in the sample overall (inclusive of correction for eGFR as a continuous variable), the HRs for increasing quartiles of galectin‐3 were no longer statistically significant for the composite outcome, or for heart failure or death individually (eg, HRs of 1.50 [0.93, 2.39], 1.55 [0.77, 3.09], and 1.37 [0.78, 2.41], for Q4 versus Q1, respectively). These results were not appreciably altered after further adjustment for history of cardiovascular disease at baseline (Table S2). In addition, when galectin‐3 was modeled as a continuous exposure (log‐transformed), the fully adjusted HRs for events all remained nonsignificant in the sample overall (Table 4).
Table 4.
Adjusted Hazard Ratios (HRs) for Events; by Baseline Galectin‐3 Modeled as a Categorical and Continuous Exposure (Unweighted N=1809)
| Galectin‐3, ng/mL | Events (n) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| HR | P Value | HR | P Value | ||
| Composite outcome (heart failure or death) | |||||
| Categorical exposure | |||||
| Quartile 1 (4.2–11.4) | 55 | 1 (ref) | — | 1 (ref) | — |
| Quartile 2 (>11.4–13.8) | 68 | 1.24 (0.79–1.94) | 0.351 | 1.13 (0.72–1.77) | 0.59 |
| Quartile 3 (>13.8–16.7) | 70 | 1.05 (0.66–1.68) | 0.835 | 0.97 (0.61–1.56) | 0.912 |
| Quartile 4 (>16.7–184.1) | 120 | 1.72 (1.12–2.62) | 0.013 | 1.50 (0.93–2.39) | 0.094 |
| P‐value for trend | 0.026 | 0.147 | |||
| Continuous exposure | |||||
| log(Galectin 3) | 313 | 1.52 (1.05–2.20) | 0.028 | 1.42 (0.94–2.14) | 0.097 |
| Heart failure | |||||
| Categorical exposure | |||||
| Quartile 1 (4.2–11.4) | 27 | 1 (ref) | — | 1 (ref) | — |
| Quartile 2 (>11.4–13.8) | 35 | 1.52 (0.81–2.86) | 0.189 | 1.38 (0.74–2.58) | 0.315 |
| Quartile 3 (>13.8–16.7) | 40 | 1.24 (0.64–2.40) | 0.521 | 1.14 (0.58–2.25) | 0.707 |
| Quartile 4 (>16.7–184.1) | 65 | 1.96 (1.07–3.61) | 0.03 | 1.55 (0.77–3.09) | 0.218 |
| P‐value for trend | 0.057 | 0.322 | |||
| Continuous exposure | |||||
| log(Galectin 3) | 167 | 1.69 (1.05–2.73) | 0.032 | 1.47 (0.85–2.55) | 0.166 |
| Death | |||||
| Categorical exposure | |||||
| Quartile 1 (4.2–11.4) | 37 | 1 (ref) | — | 1 (ref) | — |
| Quartile 2 (>11.4–13.8) | 43 | 0.91 (0.53–1.57) | 0.727 | 0.84 (0.48–1.47) | 0.542 |
| Quartile 3 (>13.8–16.7) | 45 | 0.80 (0.46–1.40) | 0.439 | 0.75 (0.43–1.32) | 0.321 |
| Quartile 4 (>16.7–184.1) | 87 | 1.52 (0.92–2.51) | 0.103 | 1.37 (0.78–2.41) | 0.266 |
| P‐value for trend | 0.129 | 0.301 | |||
| Continuous exposure | |||||
| log(Galectin 3) | 212 | 1.55 (0.99–2.45) | 0.058 | 1.51 (0.91–2.51) | 0.111 |
ARIC indicates Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol.
Model 1: adjusted for age (yrs), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), and sex (male or female). Model 2: adjusted for age (yrs), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), sex (male or female), smoking status (current, former, or never), mean systolic blood pressure (mm Hg), mean diastolic blood pressure (mm Hg), antihypertensive medication use (yes or no), total cholesterol (mg/dL), HDL cholesterol (mg/dL), diabetes status (self‐reported history, medication use, glucose, HbA1c), and eGFR (mL/min per 1.73 m2). Note that Cox models are weighted, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design.
While there was no evidence of statistical interaction based on age or sex, we did find interaction by race for the composite (P‐for‐interaction=0.03) and the heart failure outcome (P‐for‐interaction=0.004), but not for all‐cause mortality (P‐for‐interaction=0.17). In fully adjusted analyses stratified by race and using race‐specific quartiles, white ARIC participants had significantly elevated risk for the composite outcome of heart failure or death (eg, HR of 2.15 [1.20, 3.88], for Q4 versus Q1, Table 5). Results for this composite outcome with galectin‐3 modeled continuously were also significant among whites (Table 5 and Figure 3). In contrast, there was no association present in blacks (eg, HR of 0.83 [0.38, 1.82], for Q4 versus Q1; and HR 0.60 [0.18–2.01] for galectin‐3 modeled as a continuous exposure). Given the Kaplan–Meier findings, we conducted sensitivity analyses comparing the fourth quartile of galectin‐3 to the bottom 3 quartiles (Q4 versus Q1–3) and found a persistent association among whites (HR 1.59 [1.05, 2.41]) for the composite outcome, but again no association for blacks (HR 1.09 [0.60, 1.99]).
Table 5.
Fully Adjusteda Hazard Ratios (HRs) for Events; by Baseline Galectin‐3 Modeled as a Categorical and Continuous Exposure (Unweighted N=1809); Stratified by Race and Using Race‐Specific Quartiles
| Galectin‐3, ng/mL | White (N=1375) | Black (N=434) | ||||
|---|---|---|---|---|---|---|
| n/N | HR | P Value | n/N | HR | P Value | |
| Composite outcome (heart failure or death) | ||||||
| Categorical exposure | ||||||
| Quartile 1 | 33/344 | 1 (ref) | — | 20/109 | 1 (ref) | — |
| Quartile 2 | 54/344 | 1.43 (0.82–2.51) | 0.21 | 12/108 | 0.82 (0.34–2.00) | 0.668 |
| Quartile 3 | 57/344 | 1.48 (0.84–2.61) | 0.174 | 15/109 | 0.46 (0.20–1.08) | 0.074 |
| Quartile 4 | 97/343 | 2.15 (1.20–3.88) | 0.01 | 25/108 | 0.83 (0.38–1.82) | 0.638 |
| P‐value for trend | 0.014 | 0.409 | ||||
| Continuous exposure | ||||||
| log(Galectin 3) | 241/1375 | 1.70 (1.10–2.63) | 0.016 | 72/434 | 0.60 (0.18–2.01) | 0.408 |
| Heart failure | ||||||
| Categorical exposure | ||||||
| Quartile 1 | 14/344 | 1 (ref) | — | 14/109 | 1 (ref) | — |
| Quartile 2 | 24/344 | 2.49 (1.08–5.71) | 0.032 | 7/108 | 0.78 (0.25–2.48) | 0.677 |
| Quartile 3 | 31/344 | 2.38 (1.00–5.68) | 0.05 | 12/109 | 0.58 (0.22–1.55) | 0.276 |
| Quartile 4 | 49/343 | 2.86 (1.13–7.25) | 0.027 | 16/108 | 0.89 (0.35–2.29) | 0.813 |
| P‐value for trend | 0.095 | 0.702 | ||||
| Continuous exposure | ||||||
| log(Galectin 3) | 118/1375 | 1.74 (0.96–3.15) | 0.069 | 49/434 | 0.75 (0.18–3.04) | 0.685 |
| Death | ||||||
| Categorical exposure | ||||||
| Quartile 1 | 21/344 | 1 (ref) | — | 12/109 | 1 (ref) | — |
| Quartile 2 | 37/344 | 1.05 (0.53–2.09) | 0.889 | 8/108 | 0.90 (0.33–2.42) | 0.828 |
| Quartile 3 | 38/344 | 1.19 (0.61–2.32) | 0.609 | 6/109 | 0.23 (0.07–0.72) | 0.012 |
| Quartile 4 | 70/343 | 1.91 (0.94–3.87) | 0.073 | 20/108 | 1.14 (0.48–2.71) | 0.767 |
| P‐value for trend | 0.054 | 0.782 | ||||
| Continuous exposure | ||||||
| log(Galectin 3) | 166/1375 | 1.79 (1.05–3.03) | 0.032 | 46/434 | 0.79 (0.19–3.24) | 0.740 |
ARIC indicates Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol.
White galectin‐3 Quartiles (ng/mL): 4.2 to 11.2, >11.2 to 13.4, >13.4 to 16.4, >16.4 to 184.1. Black galectin‐3 Quartiles (ng/mL): 6.2 to 12, >12 to 14.8, >14.8 to 17.6, >17.6 to 87.5
Adjusted for age (years), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), sex (male or female), smoking status (current, former, or never), mean systolic blood pressure (mm Hg), mean diastolic blood pressure (mm Hg), antihypertensive medication use (yes or no), total cholesterol (mg/dL), HDL cholesterol (mg/dL), diabetes status (self‐reported history, medication use, glucose, HbA1c), and eGFR (mL/min per 1.73 m2). Note that Cox models are weighted, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design.
Figure 3.
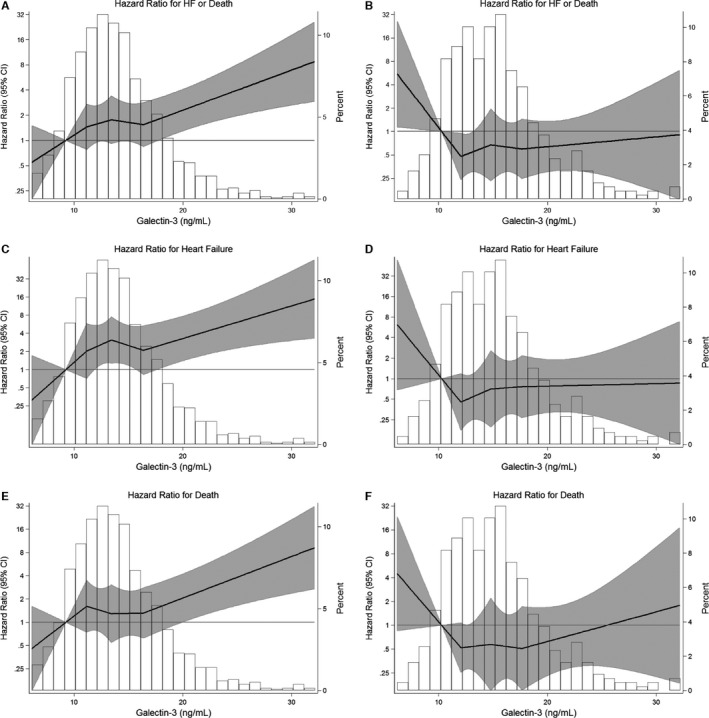
Adjusted hazard ratio (95% CI) for events, according to baseline galectin‐3 (ng/mL) modeled as linear splines (knots at quartiles and centered at the 10th percentile), with background histogram of galectin‐3 values in the sample; stratified by race (A=HF or death among whites, B=HF or death among blacks, C=HF among whites, D=HF among blacks, E=death among whites, F=death among blacks). Adjusted for age (years), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), sex (male or female), smoking status (current, former, or never), mean systolic blood pressure (mm Hg), mean diastolic blood pressure (mm Hg), antihypertensive medication use (yes or no), total cholesterol (mg/dL), HDL cholesterol (mg/dL), diabetes status (self‐reported history, medication use, glucose, HbA1c), and eGFR (mL/min per 1.73 m2). Weighted Cox models, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design. Linear splines of hazard for events with background distributional histogram of baseline galectin‐3 levels, truncated at the 1st and 99th percentile before model fitting. Note that the “Percent” axis label identifies the percentage of the ARIC analytic sample at each point on this background histogram. The shaded area around the regression line represents the 95% CI. ARIC indicates Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure.
Racial differences in the association between galectin‐3 were also present for each of the individual outcomes. Parsimonious demographic adjusted models demonstrated increased risk for both heart failure and death among whites, but no association between galectin‐3 and events among blacks (Table 6). Furthermore, in the fully adjusted model, there was also increased risk for heart failure (2.86 [1.13, 7.25]) and nominally increased risk for all‐cause mortality (1.91 [0.94, 3.87]), comparing the fourth quartile of galectin‐3 to the first among whites (Table 5). Race‐stratified results with galectin‐3 modeled as a continuous exposure demonstrated a significant association between galectin‐3 and death in whites (1.79 [1.05–3.03]). These HRs among whites contrast with HRs of 0.89 (0.35–2.29) for heart failure and 1.14 (0.48–2.71) for death, in the fourth versus first quartiles among blacks, with similar null associations when galectin‐3 was modeled as a continuous exposure in blacks (Table 5, Figure 3).
Table 6.
Demographic Adjusteda Hazard Ratios (HRs) for Events; by Baseline Galectin‐3 Modeled as a Categorical and Continuous Exposure (Unweighted N=1809); Stratified by Race and Using Race‐Specific Quartiles
| Galectin‐3, ng/mL | White (N=1375) | Black (N=434) | ||||
|---|---|---|---|---|---|---|
| n/N | HR | P Value | n/N | HR | P Value | |
| Heart failure | ||||||
| Categorical exposure | ||||||
| Quartile 1 | 14/344 | 1 (ref) | — | 14/109 | 1 (ref) | — |
| Quartile 2 | 24/344 | 3.09 (1.31–7.32) | 0.01 | 7/108 | 0.71 (0.23–2.19) | 0.551 |
| Quartile 3 | 31/344 | 2.75 (1.16–6.54) | 0.022 | 12/109 | 0.53 (0.20–1.42) | 0.204 |
| Quartile 4 | 49/343 | 4.32 (1.87–9.99) | <0.001 | 16/108 | 0.81 (0.34–1.95) | 0.638 |
| P‐value for trend | 0.002 | 0.581 | ||||
| Continuous exposure | ||||||
| log(Galectin 3) | 118/1375 | 1.99 (1.24–3.19) | 0.005 | 49/434 | 0.76 (0.18–3.22) | 0.705 |
| Death | ||||||
| Categorical exposure | ||||||
| Quartile 1 | 21/344 | 1 (ref) | — | 12/109 | 1 (ref) | — |
| Quartile 2 | 37/344 | 1.20 (0.60–2.38) | 0.604 | 8/108 | 0.79 (0.28–2.25) | 0.662 |
| Quartile 3 | 38/344 | 1.30 (0.66–2.57) | 0.446 | 6/109 | 0.21 (0.07–0.65) | 0.007 |
| Quartile 4 | 70/343 | 2.18 (1.14–4.15) | 0.018 | 20/108 | 0.90 (0.37–2.23) | 0.826 |
| P‐value for trend | 0.013 | 0.548 | ||||
| Continuous exposure | ||||||
| log(Galectin 3) | 166/1375 | 1.74 (1.10–2.74) | 0.017 | 46/434 | 0.70 (0.15–3.29) | 0.646 |
ARIC indicates Atherosclerosis Risk in Communities.
White galectin‐3 Quartiles (ng/mL): 4.2 to 11.2, >11.2 to 13.4, >13.4 to 16.4, >16.4 to 184.1. Black galectin‐3 Quartiles (ng/mL): 6.2 to 12, >12 to 14.8, >14.8 to 17.6, >17.6 to 87.5
Adjusted for age (yrs), race‐center (whites–Washington County; whites–Minneapolis; blacks–Jackson; blacks–Forsyth County, whites–Forsyth County), and sex (male or female). Note that Cox models are weighted, where the number observed to be at risk (N=1809) is reweighted to reflect the entire available ARIC cohort (N=4731) using a stratified sampling design.
Finally, the addition of galectin‐3 to the fully adjusted model increased the C‐statistic for the composite outcome of heart failure or death in the sample overall (0.713–0.718 [+0.005], P=0.037). Consistent with the above findings, this was driven by a significant increase in discrimination among whites (0.729–0.735 [+0.006], P=0.049), but no change among blacks (0.696–0.695 [−0.001], P=0.814).
Discussion
In this biracial sample of community‐based adults, we found that, overall, galectin‐3 was not independently associated with incident heart failure or all‐cause mortality over a median follow‐up of 6.8 years. However, there was significant interaction based on race, and, after stratification, galectin‐3 was revealed as a significant risk factor for the composite of heart failure or mortality in white participants, but not in black subjects. Similar racial differences were also present for heart failure and mortality as individual outcomes. Indeed, the HR point estimates for elevated galectin‐3 were consistently less than 1 among blacks. Therefore, the null association between galectin‐3 and outcomes in the sample overall appears to have been driven by null associations in blacks.
Our results extend the literature in this field, particularly as prior reports of galectin‐3 as a risk factor for incident outcomes were all derived from cohorts comprised almost exclusively of white participants.14, 15, 17 A major question for the interpretation of our race‐stratified results is whether the lack of association between galectin‐3 and our outcomes of interest is due to biological heterogeneity for galectin‐3 among different racial categories (eg, no causal effect in blacks) or whether the stratified analysis by race in this sample was underpowered. We believe our results provide evidence for the former, as HRs for heart failure and death with galectin‐3, analyzed both by quartiles and continuously, were consistently higher than 1 (positive association) in whites and less than 1 (suggesting a negative association) in blacks (Table 5), even in minimally adjusted demographic models (Table 6).
Similarly, while galectin‐3 discriminated events among whites, it did not do so among blacks. Furthermore, post‐hoc power analyses demonstrate that this analysis had sufficient power (with an α of 0.05 and a β of 0.20), requiring only 290 black participants to demonstrate the same relative risk that was found in whites (e.g., HR of 1.7 for the composite outcome, per SD increase in log‐transformed galectin‐3). While there is a dearth of published data evaluating the impact of galectin‐3 among black subjects, it is worth noting that one of the few reports suggesting a null association for galectin‐3 and outcomes was the HF‐Action study, which had a high proportion of black participants (31%).11
We believe that our results highlight the importance of evaluating novel biomarkers in racially and ethnically diverse samples in order to ensure sufficient generalizability of the findings from biomarker research to the clinic. This is particularly important for heart failure biomarkers, given the increased risk and burden of heart failure in blacks compared to whites.23 Specifically, while estimates differ by heart failure definition, geography, and secular trends, heart failure incidence rates are typically higher in blacks than in whites.24 For example, in ARIC, the age‐standardized incidence of acute decompensated heart failure among adults over 55 years differs significantly by race (10.9 in whites versus 14.3 in blacks, per 1000 person‐years, P<0.05).25 Relevant to these racial differences, our results suggest that, rather than just simply representing a noncausal marker of heart‐failure physiology (galectin‐3 is in fact thought to mediate heart failure through the development of fibrosis3, 26), the biologic processes by which galectin‐3 mediates heart failure may differ by race. While, for example, racial differences are also known to exist in heart‐failure phenotype and response to therapy,27 we are not aware of any research evaluating racial differences in galectin‐3‐mediated cardiac fibrosis and remodeling. Further research is warranted to address this question.
Our analysis has some limitations to consider. Our observational results are hypothesis generating and, due to limitations in power, we cannot rule out weaker adverse associations in blacks. Thus, larger studies are needed to validate our results. Similarly, overfitting of the fully adjusted model is a potential concern among the black subsample. However, in parsimonious demographic models, inclusive of just 3 adjusted covariates (Table 6), the associations between galectin‐3 and events were similarly null (all HRs <1) among blacks. Furthermore, ICD diagnosis codes may not capture the true impact of galectin‐3 on heart failure and we cannot discern between heart failure subtypes. In addition, while CARMRI preferentially selected subjects with carotid atherosclerosis, we used sampling weights to adjust our analyses, allowing generalization to the entire ARIC study sample. Finally, we do not have brain natriuretic peptide, N‐terminal pro‐brain natriuretic peptide, or other biomarker data available from the ARIC CARMRI study visit (so we cannot adjust for these additional markers in our analysis of galectin‐3 drawn at this time point) and we also only have only 1 measurement of galectin‐3 (repeat testing may better capture the utility of this biomarker28). Strengths include the rigorous collection of covariate data and active surveillance in ARIC, a diverse multicenter community‐based sample.
In conclusion, galectin‐3 is associated with heart failure and death in whites, but does not appear to be associated with either outcome in blacks. There is evidence of statistical interaction based on race status and, given that galectin‐3 is thought to directly mediate heart failure (rather than just representing a noncausal biomarker), our results suggest that the processes by which galectin‐3 mediates disease may differ by race. Further research is necessary to confirm this important finding.
Sources of Funding
This work was supported by a grant from the American Heart Association to Dr Selvin. The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The ARIC carotid MRI examination was funded by U01HL075572‐01.
Disclosures
Dr R. H. Christenson reports being a paid consultant for BG Medicine and that BG Medicine donated reagents for the galectin‐3 measurements used in this analysis. All other coauthors report no relevant conflicts.
Supporting information
Table S1. Study Population for Incident Heart Failure and All‐Cause Mortality (Main Analysis)
Table S2. Sensitivity Analysis—Fully Adjusted Hazard Ratios for Heart Failure and All‐Cause Mortality by Galectin‐3 Quartiles at Baseline (N=1809), With and Without Further Adjustment for Baseline Cardiovascular Disease Status
Figure S1. Untransformed and log‐transformed distributions of galectin‐3, by race status (Kernel density plots).
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Dr McEvoy is supported by Johns Hopkins Division of Cardiology Magic the Matters Award and the Schafer fund for early career investigators.
(J Am Heart Assoc. 2016;5:e003079 doi: 10.1161/JAHA.115.003079)
References
- 1. Christenson RH, Duh SH, Wu AH, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P. Multi‐center determination of galectin‐3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43:683–690. [DOI] [PubMed] [Google Scholar]
- 2. Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, Greaves DR. Galectin‐3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28:433–440. [DOI] [PubMed] [Google Scholar]
- 3. de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin‐3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. [DOI] [PubMed] [Google Scholar]
- 4. Radosavljevic G, Volarevic V, Jovanovic I, Milovanovic M, Pejnovic N, Arsenijevic N, Hsu DK, Lukic ML. The roles of galectin‐3 in autoimmunity and tumor progression. Immunol Res. 2012;52:100–110. [DOI] [PubMed] [Google Scholar]
- 5. McCullough PA, Olobatoke A, Vanhecke TE. Galectin‐3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200–210. [DOI] [PubMed] [Google Scholar]
- 6. van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ, de Boer RA. Prognostic value of changes in galectin‐3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. 2013;6:219–226. [DOI] [PubMed] [Google Scholar]
- 7. van Kimmenade RR, Januzzi JL Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino‐terminal pro‐brain natriuretic peptide, galectin‐3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. [DOI] [PubMed] [Google Scholar]
- 8. Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher‐Krainer E, Duvinage A, Unkelbach I, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Stough WG, Pieske BM. Galectin‐3 in patients with heart failure with preserved ejection fraction: results from the Aldo‐DHF trial. Eur J Heart Fail. 2015;17:214–223. [DOI] [PubMed] [Google Scholar]
- 9. Writing Committee M , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice G . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; Guidelines ESCCfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 11. Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O'Connor CM. Galectin‐3 in ambulatory patients with heart failure: results from the HF‐ACTION study. Circ Heart Fail. 2012;5:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, McMurray JJ, Wikstrand J, Aukrust P. The predictive value of galectin‐3 for mortality and cardiovascular events in the controlled rosuvastatin multinational trial in heart failure (CORONA). Am Heart J. 2012;164:878–883. [DOI] [PubMed] [Google Scholar]
- 13. Bayes‐Genis A, de Antonio M, Vila J, Penafiel J, Galan A, Barallat J, Zamora E, Urrutia A, Lupon J. Head‐to‐head comparison of 2 myocardial fibrosis biomarkers for long‐term heart failure risk stratification: ST2 versus galectin‐3. J Am Coll Cardiol. 2014;63:158–166. [DOI] [PubMed] [Google Scholar]
- 14. de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P. The fibrosis marker galectin‐3 and outcome in the general population. J Intern Med. 2012;272:55–64. [DOI] [PubMed] [Google Scholar]
- 15. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett‐Connor E. Galectin‐3 is independently associated with cardiovascular mortality in community‐dwelling older adults without known cardiovascular disease: the Rancho Bernardo Study. Am Heart J. 2014;167:674–682.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Djousse L, Matsumoto C, Petrone A, Weir NL, Tsai MY, Gaziano JM. Plasma galectin 3 and heart failure risk in the Physicians’ Health Study. Eur J Heart Fail. 2014;16:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin‐3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19. Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, Ballantyne C, Sharrett R, Boerwinkle E. Correlates of carotid plaque presence and composition as measured by MRI: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2009;2:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 21. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 23. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, Wruck LM, Rosamond WD. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez‐Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, Lopez‐Andres N. Galectin‐3 mediates aldosterone‐induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. [DOI] [PubMed] [Google Scholar]
- 27. Sharma K, Hill T, Grams M, Daya NR, Hays AG, Fine D, Thiemann DR, Weiss RG, Tedford RJ, Kass DA, Schulman SP, Russell SD. Outcomes and worsening renal function in patients hospitalized with heart failure with preserved ejection fraction. Am J Cardiol. 2015;116:1534–1540. [DOI] [PubMed] [Google Scholar]
- 28. Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin‐3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val‐HeFT. Eur J Heart Fail. 2013;15:511–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study Population for Incident Heart Failure and All‐Cause Mortality (Main Analysis)
Table S2. Sensitivity Analysis—Fully Adjusted Hazard Ratios for Heart Failure and All‐Cause Mortality by Galectin‐3 Quartiles at Baseline (N=1809), With and Without Further Adjustment for Baseline Cardiovascular Disease Status
Figure S1. Untransformed and log‐transformed distributions of galectin‐3, by race status (Kernel density plots).


