Abstract
Background
The aim of this study was to evaluate the relationship between aortic valve area (AVA) obtained by Doppler echocardiography and outcome in patients with severe asymptomatic aortic stenosis and to define a specific threshold of AVA for identifying asymptomatic patients at very high risk based on their clinical outcome.
Methods and Results
We included 199 patients with asymptomatic severe aortic stenosis (AVA ≤1.0 cm2). The risk of events (death or need for aortic valve replacement) increased linearly on the scale of log hazard with decreased AVA (adjusted hazard ratio 1.17; 95% CI 1.06–1.29 per 0.1 cm2 AVA decrement; P=0.002). Event‐free survival at 12, 24, and 48 months was 63±6%, 51±6%, and 34±6%, respectively, for AVA 0.8 to 1 cm2; 49±6%, 36±6%, and 26±6%, respectively, for AVA 0.6 to 0.8 cm2; and 33±8%, 20±7%, and 11±5%, respectively, for AVA ≤0.6 cm2 (P trend=0.002). Patients with AVA ≤0.6 cm2 had a significantly increased risk of events compared with patients with AVA 0.8 to 1 cm2 (adjusted hazard ratio 2.22; 95% CI 1.41–3.52; P=0.001), whereas patients with AVA 0.6 to 0.8 cm2 had an increased risk of events compared with those with AVA 0.8 to 1 cm2, but the difference was not statistically significant (adjusted hazard ratio 1.38; 95% CI 0.93–2.05; P=0.11). After adjustment for covariates and aortic valve replacement as a time‐dependent variable, patients with AVA ≤0.6 cm2 had a significantly greater risk of all‐cause mortality than patients with AVA >0.6 cm2 (hazard ratio 3.39; 95% CI 1.80–6.40; P<0.0001).
Conclusions
Patients with severe asymptomatic aortic stenosis and AVA ≤0.6 cm2 displayed an important increase in the risk of adverse events during short‐term follow‐up. Further studies are needed to determine whether elective aortic valve replacement improves outcome in this high‐risk subgroup of patients.
Keywords: aortic stenosis, Doppler echocardiography, outcome, surgery
Subject Categories: Echocardiography, Prognosis
Introduction
Severe aortic stenosis (AS) is defined as an aortic valve area (AVA) of <1.0 cm2 and/or a mean transaortic pressure gradient (MPG) of >40 mm Hg and/or a peak aortic jet velocity (Vmax) of >4 m/s.1, 2 According to European Society of Cardiology (ESC) guidelines and American Heart Association (AHA) and American College of Cardiology (ACC) guidelines, only patients having severe AS associated with either symptoms by history or on exercise testing or left ventricular ejection fraction (LVEF) <50% have a class 1 indication for aortic valve replacement (AVR).1, 2 Although symptoms secondary to AS seldom develop before the orifice area is reduced to <1 cm2, some patients with very severe narrowing of the aortic valve (<0.5 cm2) may remain asymptomatic.3, 4 The management of asymptomatic patients with severe AS remains controversial.5 Indeed, a “wait for symptoms” strategy is proposed in clinical practice because the surgical risk exceeds the spontaneous risk in most patients with severe asymptomatic AS.6 In contrast, it has been advocated that some patients with severe asymptomatic AS may be operated too late in the course of the disease, at a stage at which myocardial impairment is, at least in part, irreversible7, 8 and thus resulting in a higher risk of mortality and heart failure.9 In this regard, simple echocardiographic parameters such as Vmax have been proposed to identify patients at higher risk of adverse events. Previous reports demonstrated that asymptomatic patients with very severe AS defined by Vmax >5.50 m/s have a very high risk of adverse events during a short‐term follow‐up.10 Consistently, current ESC and AHA/ACC guidelines consider elective AVR reasonable in asymptomatic patients with a low surgical risk (class 2a recommendation) when Vmax is >5 m/s (AHA/ACC) or >5.50 m/s (ESC) or when MPG is >60 mm Hg (AHA/ACC).1, 2 Nevertheless, the ability of AVA obtained by Doppler echocardiography to identify a subgroup of asymptomatic patients with severe AS who are at very high risk of events during short‐term follow‐up has not been investigated. The present study was designed (1) to evaluate the relationship between AVA and outcome and (2) to define, on the basis of clinical outcome, a specific threshold of AVA that identifies a very high‐risk subgroup of patients with asymptomatic severe AS.
Methods
Study Population
Patients aged >18 years diagnosed with severe AS (AVA ≤1.0 cm2) and LVEF ≥50% at the echocardiography laboratories of 2 French tertiary centers (Amiens and Lille) between 2000 and 2012 were identified prospectively and included in an electronic database. We excluded patients (1) with more than mild aortic and/or mitral regurgitation; (2) with prosthetic valves, congenital heart disease (with the exception of bicuspid aortic valves), supravalvular or subvalvular AS, or dynamic left ventricular outflow tract (LVOT) obstruction; (3) with symptoms by history or on exercise testing including angina, syncope, or dyspnea; (4) who denied authorization for research participation. A referent group of nonsevere AS was constituted including the patients with mild to moderate asymptomatic AS (AVA 1–1.5 cm2) examined at the echocardiography laboratories during the study period. Exclusion criteria were identical to those in patients with severe AS.
A comorbidity index summating each patient's individual comorbidities was calculated.11 Coronary artery disease was defined by the presence of documented history of acute coronary syndromes, coronary artery disease previously confirmed by coronary angiography (reduction of the normal diameter ≥50% in the left main coronary artery and ≥70% in the right coronary, left anterior descending, and circumflex arteries), or history of coronary revascularization.
We obtained institutional review board authorizations prior to conducting the study, and patients involved in the study gave informed consent. The study was conducted in accordance with institutional policies, national legal requirements, and the revised Declaration of Helsinki.
Echocardiography
Doppler echocardiographic measurements
All patients underwent a comprehensive Doppler echocardiographic study using commercially available ultrasound systems. Peak aortic velocity was recorded using continuous‐wave Doppler in several acoustic windows (apical 5‐chamber, right parasternal, suprasternal, epigastric views).12 The highest aortic velocity was used to calculate aortic time–velocity integral, MPG, and Vmax. Pressure gradients were calculated using the simplified Bernoulli equation.13 AVA was calculated by the continuity equation.14, 15 The stroke volume was calculated by multiplying the area of the LVOT by the outflow tract time–velocity integral and indexed to the body surface area. If patients were in sinus rhythm, 3 cardiac cycles were averaged for all measures. For patients in atrial fibrillation, 5 cardiac cycles were averaged. LVEF was calculated using Simpson's biplane method. Left ventricular mass was calculated by the corrected formula of the American Society of Echocardiography and indexed for body surface area.16 Valvuloarterial impedance was calculated as described previously.17, 18 Tricuspid regurgitation peak velocity was estimated using continuous‐wave Doppler.
Clinical decision and follow‐up
After initial medical management, clinical follow‐up was planned every 6 months associated with an echocardiography, and an exercise test was planned every 6 to 12 months (for patients able to perform an exercise test). Information on follow‐up was obtained retrospectively by direct patient interview and clinical examination and/or by repeated follow‐up letters, questionnaires, and telephone calls to physicians, patients, and (if necessary) next of kin. The outcome end points were time to occurrence of all‐cause death or need for AVR and all‐cause death regardless of whether or not there was AVR, respectively. Clinical decisions regarding medical management and referral for surgery were made by the heart team with the approval of the patient's cardiologist based on ESC guidelines.19, 20 Indications for AVR were occurrence during follow‐up of symptoms, left ventricular dysfunction (LVEF <50%), or symptoms during an exercise test.
Statistical Analysis
Continuous variables were expressed as mean±SD. The Shapiro‐Wilk test was used to verify whether the residuals obtained on analysis of variance approximated a normal distribution. If this test failed, nonparametric analysis of variance (Kruskal–Wallis test) was used, and these variables were expressed as median (interquartile range). Post hoc comparisons were performed using either Scheffe's comparison or Mann–Whitney U tests with Bonferroni correction for multiple comparisons, as appropriate. Comparisons between 2 groups of patients were performed using the Student t test of the Mann–Whitney U test. Categorical data are expressed as a percentage and compared with a chi‐square test. Follow‐up time was quantified using the median of the Kaplan–Meier estimate of potential follow‐up (also called the reverse Kaplan–Meier method). Cumulative survival probability was estimated with the Kaplan–Meier method and compared between groups using a log‐rank test. Univariable followed by multivariable analysis of occurrence of death or need for AVR was assessed by using Cox proportional hazard models. We did not use model‐building techniques and entered covariates considered of potential prognostic impact on an epidemiological basis in the models. These covariates were age, sex, comorbidity index, coronary artery disease, hypertension, history of atrial fibrillation, and LVEF. For continuous variables, the assumption of linearity was assessed by plotting martingale residuals against independent variables. The proportional hazards assumption was confirmed using statistics and graphs based on the Schoenfeld residuals. We also analyzed all‐cause death in Cox proportional hazards multivariable models regardless of whether or not there was AVR. The effect of surgery on outcome was analyzed as a time‐dependent covariate using the entire follow‐up period. A P<0.05 was considered statistically significant. In case of Bonferroni correction for multiple comparisons, statistical significance was considered if the P value was <0.05 divided by the number of comparisons for each variable. Statistical analyses and figures were obtained using PASW 18.0 (IBM, Inc) and R 3.0.3 (R Foundation for Statistical Computing).
Results
The study population consisted of 199 patients (Table 1). Overall, 110 (55%) were men and 89 (45%) were women, with a mean age of 69±14 years; 118 (59%) had a history of hypertension, 87 (44%) were dyslipidemic, 54 (27%) were diabetic, 57 (29%) were smokers, and 75 (38%) had a history of coronary artery disease.
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Patients According to Aortic Valve Area
| Overall | AVA ≤0.6 cm2 | AVA 0.6–0.8 cm2 | AVA 0.8–1.0 cm2 | P Value | |
|---|---|---|---|---|---|
| Number of patients | 199 | 39 | 80 | 80 | |
| Demographic and baseline data | |||||
| Age, y | 69±14 | 71±12 | 68±15 | 70±13 | 0.62 |
| Male | 110 (55) | 16 (41) | 46 (58) | 48 (60) | 0.13 |
| BMI, kg/m2 | 26 (23–30) | 25 (21–30) | 26 (23–31) | 27 (24–29) | 0.23 |
| SBP, mm Hg | 130 (120–150) | 130 (120–140) | 130 (120–150) | 138 (125–153) | 0.22 |
| DBP, mm Hg | 71 (69–80) | 70 (63–80) | 70 (68–80) | 74 (70–83) | 0.53 |
| Medical history and risk factors | |||||
| Diabetes mellitus | 54 (27) | 14 (36) | 18 (23) | 22 (28) | 0.30 |
| Dyslipidemia | 87 (44) | 18 (46) | 31 (39) | 38 (48) | 0.53 |
| Smoking | 57 (29) | 12 (31) | 26 (33) | 19 (24) | 0.45 |
| Hypertension | 118 (59) | 24 (62) | 42 (53) | 52 (65) | 0.27 |
| Coronary artery disease | 75 (38) | 14 (36) | 34 (43) | 27 (34) | 0.50 |
| History of atrial fibrillation | 45 (23) | 12 (31) | 15 (19) | 18 (23) | 0.30 |
| Charlson comorbidity index | 1 (1–2) | 1 (1–3) | 1 (1–2) | 1 (1–3) | 0.70 |
Statistical significance was considered for a P value of ≤0.025 according to the Bonferroni correction for 2 comparisons (AVA ≤0.6 cm2 vs 0.8–1.0 cm2 and 0.6–0.8 cm2 vs 0.8–1.0 cm2) for continuous variables expressed as median (interquartile range). AVA indicates aortic valve area; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Eighty patients (40%) had an AVA between 0.8 and 1 cm2, 80 patients (40%) had an AVA between 0.6 and 0.8 cm2, and 39 (20%) an AVA ≤0.6 cm2. Table 1 shows the baseline clinical data according to AVA.
As shown in Table 1, the study subgroups (defined by AVA values) showed no difference in age, sex, body mass index, comorbidity index, systolic and diastolic blood pressures, frequency of diabetes, hypertension, dyslipidemia, smoking, and history of atrial fibrillation or coronary artery disease (Table 1). As shown in Table 2, lower AVA was associated with higher Vmax, MPG, and valvuloarterial impedance. Indexed stroke volume was smaller in patients with AVA ≤0.6 cm2. Other echocardiographic parameters were not significantly different across the AVA subgroups.
Table 2.
Echocardiographic Parameters of the Study Patients According to Aortic Valve Area
| Overall | AVA ≤0.6 cm2 | AVA 0.6–0.8 cm2 | AVA 0.8–1.0 cm2 | P Value | |
|---|---|---|---|---|---|
| Number of patients | 199 | 39 | 80 | 80 | |
| Aortic valve | |||||
| AVA, cm2 | 0.79 (0.65–0.90) | 0.50 (0.46–0.58)a | 0.73 (0.69–0.79)a | 0.90 (0.88–0.99) | <0.0001 |
| Indexed AVA, cm2/m2 | 0.40 (0.33–0.47) | 0.28 (0.23–0.31)a | 0.37 (0.34–0.42)a | 0.47 (0.44–0.53) | <0.0001 |
| MPG, mm Hg | 45 (36–56) | 57 (44–74)a | 50 (42–59)a | 38 (33–47) | <0.0001 |
| Vmax, m/s | 4.2 (3.7–4.7) | 4.7 (4.1–5.4)a | 4.3 (4.1–4.9)a | 4.0 (3.2–4.3) | <0.0001 |
| Aortic TVI, cm | 100 (81–119) | 119 (96–130)a | 100 (86–124)b | 90 (72–109) | <0.0001 |
| Valvuloarterial impedance, mm Hg/mL per mm2 | 4.6 (3.8–5.7) | 6.0 (5.0–6.4)a | 4.7 (3.9–5.7)b | 4.1 (3.5–4.8) | <0.0001 |
| Stroke volume index, mL | 38 (31–46) | 32 (24–37)a | 38 (31–44)b | 42 (36–50) | <0.0001 |
| LV function | |||||
| LVEF (%) | 65 (58–71) | 65 (57–71) | 65 (57–71) | 65 (60–70) | 0.52 |
| LV mass index, g/m2 | 107 (87–133) | 107 (90–145) | 106 (87–133) | 111 (82–130) | 0.71 |
| Others | |||||
| LA area, cm2 | 23±7 | 24±7 | 23±6 | 23±6 | 0.45 |
| sPAP, mm Hg | 30 (23–37) | 35 (24–40) | 30 (24–36) | 30 (25–35) | 0.48 |
Statistical significance was considered for a P value of ≤0.025 according to the Bonferroni correction for 2 comparisons (AVA ≤0.6 cm2 vs 0.8–1.0 cm2 and 0.6–0.8 cm2 vs 0.8–1.0 cm2) for continuous variables expressed as median (interquartile range). AVA indicates aortic valve area; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MPG, mean pressure gradient; sPAP, systolic pulmonary arterial pressure; Vmax, peak aortic jet velocity; TVI, time‐velocity integral.
P<0.0001, individual category vs 0.8–1.0 cm2.
P<0.01, individual category vs 0.8–1.0 cm2.
Analysis of Outcome
Follow‐up was completed for all patients. The estimated median follow‐up time, as calculated by the reverse Kaplan–Meier method, was 48 months. Among patients who underwent surgery, 13 (7%) had at least 1 associated coronary artery bypass graft at the time of surgery.
In total, 137 patients (69%) reached an end point during follow‐up: 112 underwent an AVR and 36 died. Among the 25 patients who died without AVR (13% of the study population), 5 patients had an AVA ≤0.6 cm2 (20%), 8 patients (32%) had an AVA between 0.6 and 0.8 cm2, and 12 (48%) had an AVA between 0.8 and 1.0 cm2 (overall P=0.28). Twenty‐nine of the 39 patients with an AVA ≤0.6 cm2 (74%), 46 of the 80 patients with an AVA 0.6 to 0.8 cm2 (58%), and 37 of the 80 (46%) with an AVA 0.8 to 1.0 cm2 experienced AVR (overall P=0.014). In the overall population, event‐free survival (all‐cause death or AVR) at 12, 24, and 48 months was 52±4%, 39±4%, and 26±4%, respectively. Event‐free survival at 12, 24, and 48 months was 63±6%, 51±6%, and 34±6%, respectively, in patients with an AVA between 0.8 and 1 cm2; 49±6%, 36±6%, and 26±6%, respectively, in patients with an AVA between 0.6 and 0.8 cm2; and 33±8%, 20±7%, and 11±5%, respectively, in patients with an AVA ≤0.6 cm2 (P trend=0.002) (Figure 1). On univariate Cox analysis, the risk of events increased linearly (on the scale of log hazard), whereas AVA decreased (hazard ratio [HR] 1.16; 95% CI 1.06–1.28 per 0.1 cm2 AVA decrement; P=0.002) (Table 3). The relationship remained unchanged after adjustment for covariates of prognostic importance (HR 1.17; 95% CI 1.06–1.29 per 0.1 cm2 AVA decrement; P=0.002) (Table 3).
Figure 1.
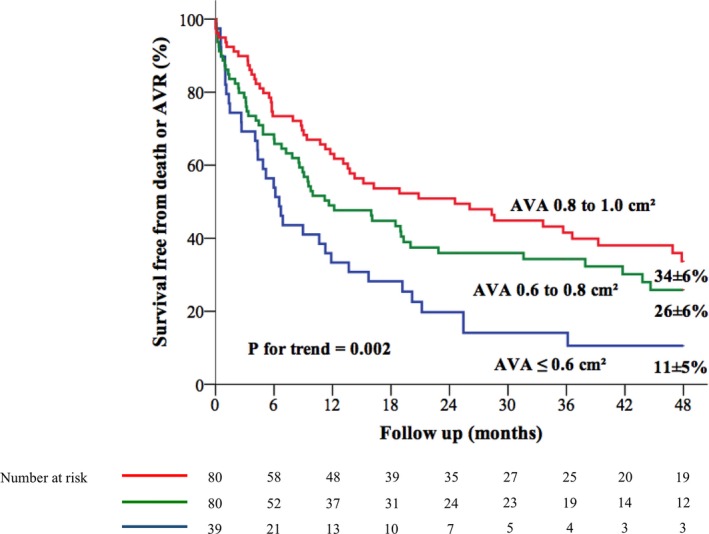
Kaplan–Meier event‐free survival curves according to AVA categories. AVA indicates aortic valve area; AVR, aortic valve replacement.
Table 3.
Relative Risk of Events (All‐Cause Death or Aortic Valve Replacement Surgery) During Follow‐up Associated With Aortic Valve Area
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| AVA categories | |||
| Unadjusted | |||
| AVA ≤0.6 cm2 | 2.19 | 1.40–3.40 | 0.001 |
| AVA >0.6 to ≤0.8 cm2 | 1.35 | 0.91–1.99 | 0.13 |
| AVA >0.8 to ≤1.0 cm2 | Referent | ||
| Adjusteda | |||
| AVA ≤0.6 cm2 | 2.22 | 1.41–3.52 | 0.001 |
| AVA >0.6 to ≤0.8 cm2 | 1.38 | 0.93–2.05 | 0.11 |
| AVA >0.8 to ≤1.0 cm2 | Referent | ||
| Per 0.1 cm2 AVA decrement | |||
| Unadjusted | 1.16 | 1.06–1.28 | 0.002 |
| Adjusteda | 1.17 | 1.06–1.29 | 0.002 |
AVA indicates aortic valve area.
Adjustment for age, sex, hypertension, coronary artery disease, history of atrial fibrillation, Charlson comorbidity index, and left ventricular ejection fraction.
Compared with patients with an AVA between 0.8 and 1.0 cm2, those with an AVA ≤0.6 cm2 displayed an increased risk of events (HR 2.19; 95% CI 1.40–3.40; P=0.001), whereas no significant increase in the risk of events was observed in those with an AVA between 0.6 and 0.8 cm2 (P=0.13) (Table 3). After adjustment for covariates, the increased risk of events in patients with an AVA ≤0.6 cm2 remained significant compared with patients with an AVR between 0.8 and 1 cm2 (adjusted HR 2.22; 95% CI 1.41–3.52; P=0.001) (Table 3, Figure 2). Conversely, the risk was increased in patients with an AVA between 0.8 and 1 cm2 compared with those with an AVA between 0.6 and 0.8 cm2, but the difference was not statistically significant (P=0.11) (Table 3, Figure 2).
Figure 2.

Adjusted curves of event‐free survival according to AVA categories. Curves are adjusted for age, sex, comorbidity index, coronary artery disease, hypertension, atrial fibrillation, and ejection fraction. AVA indicates aortic valve area; AVR, aortic valve replacement.
The cumulative overall mortality was higher in patients with an AVA ≤0.6 cm2 compared with patients with severe AS and an AVA >0.6 cm2 (cumulative mortality at 12, 24, and 48 months of 17±6%, 20±7%, and 36±9% versus 5±2%, 12±3%, and 19±4% respectively; P=0.037) (Figure 3). On multivariable analysis, patients with an AVA ≤0.6 cm2 had a significantly greater risk of all‐cause mortality compared with patients with an AVA >0.6 cm2 (adjusted HR 2.52; 95% CI 1.20–5.29; P=0.015). There was no difference in terms of mortality between patients with an AVA between 0.6 and 0.8 cm2 and those with an AVA between 0.8 and 1.0 cm2 (P=0.53). After further adjustment on AVR as a time‐dependent variable, the relationship between AVA ≤0.6 cm2 and mortality was strengthened (adjusted HR 3.39; 95% CI 1.80–6.40; P<0.0001) (Figure 4).
Figure 3.
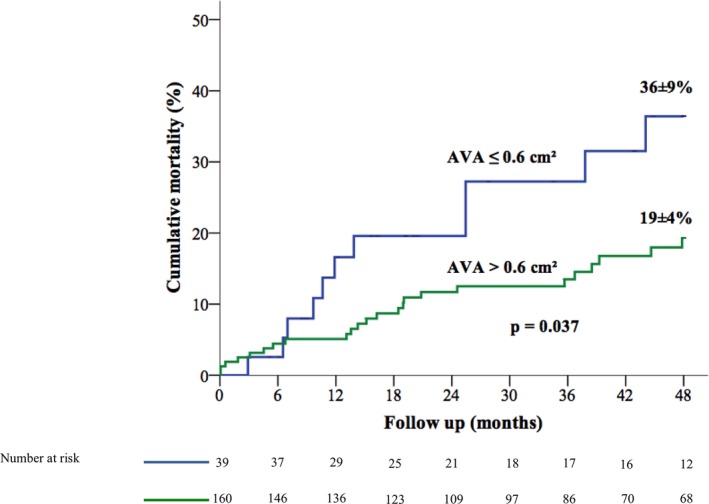
Kaplan–Meier curves of cumulative mortality according to AVA ≤0.6 and >0.6 cm2. AVA indicates aortic valve area.
Figure 4.

Cumulative hazard of death according to AVA ≤0.6 and >0.6 cm2. Curves are adjusted for age, sex, comorbidity index, coronary artery disease, hypertension, atrial fibrillation, left ventricular ejection fraction and aortic valve replacement as a time‐dependent variable. AVA indicates aortic valve area.
The demographic, clinical, and echocardiographic characteristics of the referent group of 90 patients with mild to moderate AS are detailed in Table S1. Compared with these patients with mild to moderate AS, patients in each category of AVA (AVA ≤0.6 cm2, 0.6 to 0.8 cm2, and 0.8 to 1.0 cm2) experienced a stepwise increase in the risk of death and/or AVR (Table 4, Figure 5A). In addition, only patients with an AVA ≤0.6 cm2 experienced a significant increase in the risk of mortality during follow‐up compared with patients with mild to moderate AS (Table 4, Figure 5B). Indeed, no excess mortality was observed in patients with an AVA 0.6 to 0.8 cm2 or 0.8 to 1.0 cm2 in comparison with patients with mild to moderate asymptomatic AS (Table 4).
Table 4.
Relative Risk of Events (All‐Cause Death or Aortic Valve Replacement Surgery and All‐Cause Death) During Follow‐up in Each Aortic Valve Area Category Compared With Patients With Mild to Moderate Asymptomatic Aortic Stenosis (1.0–1.5 cm2) as the Referent Group
| End Points | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| All‐cause death or aortic valve replacement surgery | |||
| Unadjusted | |||
| AVA ≤0.6 cm2 | 5.80 | 3.51–9.58 | <0.0001 |
| AVA >0.6 to ≤0.8 cm2 | 3.48 | 2.21–5.47 | <0.0001 |
| AVA >0.8 to ≤1.0 cm2 | 2.55 | 1.60–4.04 | <0.0001 |
| Mild to moderate AS (>1.0 to ≤1.5 cm2) | Referent | ||
| Adjusteda | |||
| AVA ≤0.6 cm2 | 6.74 | 3.98–11.40 | <0.0001 |
| AVA >0.6 to ≤0.8 cm2 | 3.89 | 2.44–6.22 | <0.0001 |
| AVA >0.8 to ≤1.0 cm2 | 2.78 | 1.73–4.45 | <0.0001 |
| Mild to moderate AS (1.0–1.5 cm2) | Referent | ||
| All‐cause death | |||
| Unadjusted | |||
| AVA ≤0.6 cm2 | 2.95 | 1.28–6.82 | 0.011 |
| AVA >0.6 to ≤0.8 cm2 | 1.17 | 0.50–2.76 | 0.72 |
| AVA >0.8 to ≤1.0 cm2 | 1.57 | 0.71–3.45 | 0.27 |
| Mild to moderate AS (1.0–1.5 cm2) | Referent | ||
| Adjusteda | |||
| AVA ≤0.6 cm2 | 3.25 | 1.37–7.73 | 0.008 |
| AVA >0.6 to ≤0.8 cm2 | 1.11 | 0.45–2.75 | 0.82 |
| AVA >0.8 to ≤1.0 cm2 | 1.50 | 0.67–3.36 | 0.33 |
| Mild to moderate AS (1.0–1.5 cm2) | Referent | ||
AS indicates aortic stenosis; AVA, aortic valve area.
Adjustment for age, sex, hypertension, coronary artery disease, history of atrial fibrillation, Charlson comorbidity index, and left ventricular ejection fraction.
Figure 5.
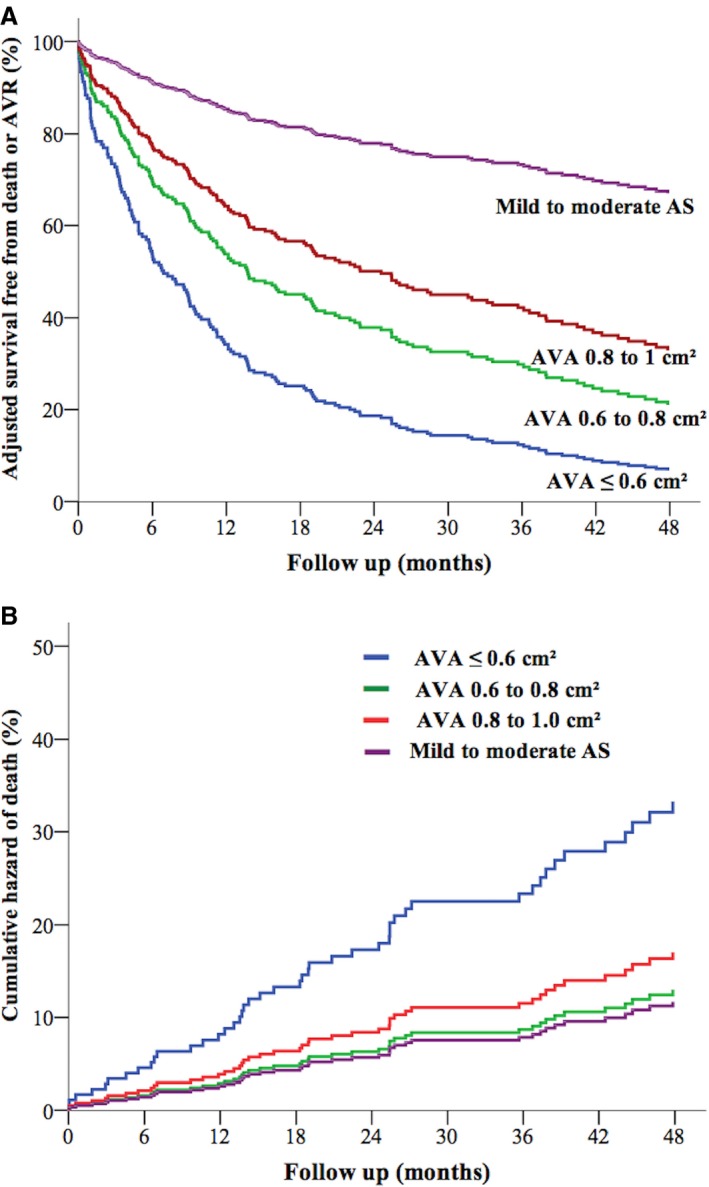
A, Adjusted curves of event‐free survival according to AVA categories with patients with mild to moderate AS as the referent group. B, Cumulative hazard of death according to AVA ≤0.6, 0.6–0.8, and 0.8–1 cm2, with patients with mild to moderate AS as the referent group. Curves are adjusted for age, sex, comorbidity index, coronary artery disease, hypertension, atrial fibrillation, and left ventricular ejection fraction. AS indicates aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement.
Discussion
The present study, performed in a cohort of patients with asymptomatic severe AS, established the relationship between AVA obtained by Doppler echocardiography and outcome. Overall, 20% of patients with asymptomatic severe AS had an AVA ≤0.6 cm2 in the present report. Importantly, these patients with severe asymptomatic AS and an AVA ≤0.6 cm2 displayed an important increase in the risk of events during short‐term follow‐up. Almost two‐thirds of patients with an AVA ≤0.6 cm2 experienced an event during 1 year of follow‐up, and 80% experienced an event over 2 years. In addition, patients with an AVA ≤0.6 cm2 had an important increase in the risk of death during the short follow‐up.
In 1968, Ross and Braunwald reported a dramatic increase in mortality after symptom onset in patients with AS.21 Following these findings, only patients presenting with symptoms were classically referred for surgery because “operative treatment was deemed to be the most common cause of sudden death in asymptomatic AS patients.”3 This landmark report was performed before the widespread use of echocardiography, during a time when rheumatic AS was highly prevalent. The risk of mortality and sudden death in asymptomatic patients with AS has been studied extensively since the late 1990s. The annualized rate of sudden death is deemed to be ≈0.8% per year in asymptomatic patients5 and must be compared with operative mortality of AVR (>1% for AVR without coronary artery bypass graft in patients aged >80 years).22 It has been suggested, however, that when using a “wait for symptoms” strategy, some patients may be operated too late in the course of the disease, at a stage at which myocardial impairment is, at least in part, irreversible.7, 8 Recent studies suggested that the outcome of asymptomatic patients with severe AS was improved by surgical management compared with conservative management.9, 23 These findings have prompted several investigators to recommend early AVR in asymptomatic patients with severe AS6; however, when performing an AVR, the native valve disease is generally traded for prosthetic valve disease because prosthetic valves are associated with complications that may affect patients’ outcome. Consequently, the decision to operate on asymptomatic patients requires careful weighing of the risk–benefit ratio. Early elective surgery, at the asymptomatic stage, should be considered only for selected patients who are at low operative risk and at high risk on medical management or for those who have no or limited access to medical facilities, precluding close follow‐up of disease progression. Transarterial valvular replacement has not been evaluated in severe asymptomatic AS and is not currently recommended.1, 2
Vmax and MPG are the cornerstones of AS severity assessment.24, 25, 26 The detection of Vmax >5 or >5.50 m/s and a MPG ≥60 mm Hg identifies patients with very severe AS.1, 2 Recently, a peak aortic velocity >5.5 m/s was found to be associated with a high risk of events in asymptomatic patients, with rates of death or surgery of 56% at 1 year, 75% at 2 years, and 89% at 3 years.10 Given these data, current ESC guidelines consider elective AVR reasonable in cases of very severe AS, defined as Vmax >5.50 m/s if the surgical risk is low (class 2a recommendation).1, 2 In contrast, previous studies suggested that an AVA ≤0.6 cm2 was not predictive of outcome.10
In the present study, patients with an AVA ≤0.6 cm2 had a very high rate of clinical events during a short‐term follow‐up; 67% and 80% of these patients had experienced death or AVR at 1 year and 2 years, respectively. In addition, these patients had an increased risk of all‐cause mortality compared with patients with severe AS and an AVA between 0.6 and 1 cm2. Similarly, compared with patients with mild to moderate AS, the risk of all‐cause mortality was significantly increased in patients with an AVA ≤0.6 cm2 but not in patients with an AVA 0.6 to 0.8 cm2 or 0.8 to 1 cm2. Interestingly, median Vmax and MPG values were 4.7 m/s (interquartile range 4.1–5.4 m/s) and 57 mm Hg (interquartile range 44–74 mm Hg) in patients with an AVA ≤0.6 cm2, indicating that a substantial proportion of our patients with an AVA ≤0.6 cm2 did not reach the criteria of very severe AS according to recent guidelines (ie, peak aortic velocity >5.5 m/s and MPG >60 mm Hg), despite having high transvalvular gradients (ie, peak aortic velocity >4 m/s and MPG >40 mm Hg). Vmax and MPG are strongly influenced by volume flow rate. In this study, patients with an AVA ≤0.6 cm2 had lower flow states, indicated by a lower stroke volume index (median 32 mL/m2) compared with higher AVA categories. These data suggest that the cutoff value of AVA ≤0.6 cm2 may identify a very high‐risk group of asymptomatic patients with severe AS that cannot always be identified by “very high” transaortic velocities and gradients. Consistently, Dumesnil and colleagues found in patients with severe AS that those with low flow and high transvalvular gradients had very poor outcomes, especially if medically treated.27 Whether these patients may benefit from elective AVR in the context of severe asymptomatic AS deserves consideration.
Limitations
Whereas echocardiographic data were prospectively collected, clinical and outcome data were obtained by review of medical records. Consequently, our study has the inherent limitations of retrospective analyses. An objective measure of symptom status is of major importance in the setting of asymptomatic severe AS.28 Exercise testing (treadmill, cardiopulmonary exercise testing, or exercise stress echocardiography) was performed systematically during the inclusion period in patients with severe asymptomatic AS whenever the patient was able to perform an exercise test (>80% of cases) to ensure that patients were truly asymptomatic.29, 30, 31 In routine practice, some patients are unable to perform an exercise test because of extracardiac conditions (eg, orthopedic, vascular, respiratory, obese). Plasma B‐type natriuretic peptides were not available in the present study and may refine the predictive value of AVA.32 Echo readings were not specifically reviewed by a core laboratory; however, echocardiograms were performed by experienced echocardiographers with extensive experience in the field of valvular heart diseases. The calculation of the AVA by the continuity equation is prone to errors because of the notoriously difficult measure of the LVOT cross‐sectional area owing to noncircular geometry of LVOT33 or massive calcifications of the aortic annulus in some patients.34 Consequently, assessment of stenosis severity in asymptomatic patients with AS should not rely on a single parameter but on a multiparametric approach incorporating data derived from transaortic gradients and velocities in addition to less flow‐dependent indices such as AVA. AVR may be a biased end point because the physician referring the patient to AVR knew the results of the AVA and gradient; however, the surgical decisions were made by the heart teams of the 2 tertiary centers in accordance with practice guidelines,19 and we observed a comparable delay between inclusion and AVR in patients with an AVA ≤0.6 cm2, 0.6 to 0.8 cm2, and 0.8 to 1.0 cm2. Moreover, we found excess mortality in patients with an AVA ≤0.6 cm2 compared with patients with mild to moderate AS, whereas no excess mortality was noted in patients with an AVA 0.6 to 0.8 cm2 or 0.8 to 1.0 cm2, reinforcing the prognostic importance of AVA in the setting of patients with asymptomatic severe AS.
Conclusions
Patients with severe asymptomatic AS and an AVA ≤0.6 cm2 displayed an important increased risk of adverse events and mortality during short‐term follow‐up. Further prospective and randomized studies are needed to determine whether elective AVR improves outcomes in this high‐risk subgroup of asymptomatic patients.
Disclosures
None.
Supporting information
Table S1. Baseline Demographic, Clinical, and Echocardiographic Parameters of the Group of Patients With Mild to Moderate AS (>1.0 to ≤1.5 cm2) Compared With Patients With Severe Aortic Stenosis (AVA ≤1.0 cm2). AS indicates aortic stenosis; AVA, aortic valve area; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MPG, mean pressure gradient; sPAP, systolic pulmonary arterial pressure; VTI, velocity–time integral.
(J Am Heart Assoc. 2016;5:003146 doi: 10.1161/JAHA.115.003146)
References
- 1. Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio‐Thoracic S , Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; Members AATF . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 3. Braunwald E. On the natural history of severe aortic stenosis. J Am Coll Cardiol. 1990;15:1018–1020. [DOI] [PubMed] [Google Scholar]
- 4. Lombard JT, Selzer A. Valvular aortic stenosis. A clinical and hemodynamic profile of patients. Ann Intern Med. 1987;106:292–298. [DOI] [PubMed] [Google Scholar]
- 5. Iung B. Management of asymptomatic aortic stenosis. Heart. 2011;97:253–259. [DOI] [PubMed] [Google Scholar]
- 6. Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J. 2002;23:1417–1421. [DOI] [PubMed] [Google Scholar]
- 7. Lund O, Larsen KE. Cardiac pathology after isolated valve replacement for aortic stenosis in relation to preoperative patient status. Early and late autopsy findings. Scand J Thorac Cardiovasc Surg. 1989;23:263–270. [DOI] [PubMed] [Google Scholar]
- 8. Lund O. Preoperative risk evaluation and stratification of long‐term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation. 1990;82:124–139. [DOI] [PubMed] [Google Scholar]
- 9. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino‐Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T; Investigators CAr . Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–2838. doi: 10.1016/j.jacc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 10. Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler‐Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 12. Tribouilloy C, Rusinaru D, Marechaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Levy F. Low‐gradient, low‐flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. [DOI] [PubMed] [Google Scholar]
- 13. Currie PJ, Seward JB, Reeder GS, Vlietstra RE, Bresnahan DR, Bresnahan JF, Smith HC, Hagler DJ, Tajik AJ. Continuous‐wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler‐catheter correlative study in 100 adult patients. Circulation. 1985;71:1162–1169. [DOI] [PubMed] [Google Scholar]
- 14. Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two‐dimensional echocardiography. Circulation. 1985;72:810–818. [DOI] [PubMed] [Google Scholar]
- 15. Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol. 1986;7:509–517. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 17. Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–1011. [DOI] [PubMed] [Google Scholar]
- 18. Marechaux S, Carpentier E, Six‐Carpentier M, Asseman P, LeJemtel TH, Jude B, Pibarot P, Ennezat PV. Impact of valvuloarterial impedance on left ventricular longitudinal deformation in patients with aortic valve stenosis and preserved ejection fraction. Arch Cardiovasc Dis. 2010;103:227–235. [DOI] [PubMed] [Google Scholar]
- 19. Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. [DOI] [PubMed] [Google Scholar]
- 20. Iung B, Gohlke‐Barwolf C, Tornos P, Tribouilloy C, Hall R, Butchart E, Vahanian A; Working Group on Valvular Heart D . Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J. 2002;23:1253–1266. [DOI] [PubMed] [Google Scholar]
- 21. Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. [DOI] [PubMed] [Google Scholar]
- 22. Elayda MA, Hall RJ, Reul RM, Alonzo DM, Gillette N, Reul GJ Jr, Cooley DA. Aortic valve replacement in patients 80 years and older. Operative risks and long‐term results. Circulation. 1993;88:II11–II16. [PubMed] [Google Scholar]
- 23. Pai R, Kapoor N, Bansal R, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–2122. [DOI] [PubMed] [Google Scholar]
- 24. Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake‐Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. [DOI] [PubMed] [Google Scholar]
- 25. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 26. Castel AL, Marechaux S, Laaouaj J, Rusinaru D, Levy F, Tribouilloy C. Relationship between cutoff values of peak aortic valve velocity and those of other Doppler echocardiographic parameters of severity in patients with aortic stenosis and normal flow. Echocardiography. 2012;29:1150–1156. [DOI] [PubMed] [Google Scholar]
- 27. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ennezat PV, Marechaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis. Heart. 2009;95:877–884. [DOI] [PubMed] [Google Scholar]
- 29. Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV, Pibarot P. Usefulness of exercise‐stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maréchaux S, Ennezat P, LeJemtel T, Polge A, de Groote P, Asseman P, Nevière R, Le Tourneau T, Deklunder G. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography. 2007;24:955–959. [DOI] [PubMed] [Google Scholar]
- 31. Levy F, Fayad N, Jeu A, Choquet D, Szymanski C, Malaquin D, Peltier M, Tribouilloy C. The value of cardiopulmonary exercise testing in individuals with apparently asymptomatic severe aortic stenosis: a pilot study. Arch Cardiovasc Dis. 2014;107:519–528. [DOI] [PubMed] [Google Scholar]
- 32. Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Pierard L, Gueret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. [DOI] [PubMed] [Google Scholar]
- 33. Jabbour A, Ismail TF, Moat N, Gulati A, Roussin I, Alpendurada F, Park B, Okoroafor F, Asgar A, Barker S, Davies S, Prasad SK, Rubens M, Mohiaddin RH. Multimodality imaging in transcatheter aortic valve implantation and post‐procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. 2011;58:2165–2173. [DOI] [PubMed] [Google Scholar]
- 34. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M; EAE/ASE . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Demographic, Clinical, and Echocardiographic Parameters of the Group of Patients With Mild to Moderate AS (>1.0 to ≤1.5 cm2) Compared With Patients With Severe Aortic Stenosis (AVA ≤1.0 cm2). AS indicates aortic stenosis; AVA, aortic valve area; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MPG, mean pressure gradient; sPAP, systolic pulmonary arterial pressure; VTI, velocity–time integral.


