Abstract
Background
Studies have reported increasing incidence of ischemic stroke in adults younger than 50 to 55 years. Information on temporal trends of other stroke subtypes and transient ischemic attack (TIA) is sparse. The aim of this study was to investigate temporal trends of the incidence of hospitalizations for TIA and stroke including sex‐ and subtype‐specific trends in young adults aged 15 to 30 years.
Methods and Results
From the Danish National Patient Register, we identified all cases of first‐ever stroke and TIA (age 15–30 years) in Denmark, who were hospitalized during the study period of 1994 to 2012. Incidence rates and estimated annual percentage changes (EAPCs) were estimated by using Poisson regression. During the study period, 4156 cases of first‐ever hospitalization for stroke/TIA were identified. The age‐standardized incidence rates of hospitalizations for stroke increased significantly (EAPC 1.83% [95% CI 1.11–2.55%]) from 11.97/100 000 person‐years (PY) in 1994 to 16.77/100 000 PY in 2012. TIA hospitalizations increased from 1.93/100 000 PY in 1994 to 5.81/100 000 PY in 2012 and after 2006 more markedly in men than in women (EAPC 16.61% [95% CI 10.45–23.12%]). The incidence of hospitalizations for ischemic stroke was markedly lower among men, but increased significantly from 2006 (EAPC 14.60% [95% CI 6.22–23.63%]). The incidences of hospitalizations for intracerebral hemorrhage and subarachnoid hemorrhage remained stable during the study period.
Conclusions
The incidence rates of first‐time hospitalizations for ischemic stroke and TIA in young Danish adults have increased substantially since the mid 1990s. The increase was particularly prominent in the most recent years.
Keywords: epidemiology, incidence, sex‐specific, stroke in young adults, transient ischemic attack
Subject Categories: Intracranial Hemorrhage, Ischemic Stroke, Transient Ischemic Attack (TIA), Epidemiology
Introduction
During the past decades, the incidence of stroke in developed countries has stabilized or even declined despite an aging population.1, 2, 3, 4 Stroke and transient ischemic attacks (TIAs) rarely occur in the young but may have a profound and long‐lasting impact for the individual when they do occur. Compared with elderly individuals, stroke in the young may have greater economic impact, because it impairs the ability to work during the most productive years of life. Further, stroke in the young is associated with life‐long cognitive impairment, increased risk of suicide, depression and anxiety, and increased mortality.5, 6 The consequences of TIA are less severe, but as many as 50% have from long‐term cognitive impairments.5, 6, 7
Recent studies have reported a trend toward increasing incidence of ischemic stroke in the young.4, 8, 9, 10 Data on temporal trends of the incidence of intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and TIA remain sparse. The objectives of this study were (1) to determine the incidence rates (IRs) of hospitalizations for stroke, stroke subtypes, and TIA in young adults aged 15 to 30 years in Denmark and (2) to identify sex‐specific temporal trends in IRs for hospitalizations in stroke subtypes since the 1994.
Methods
Data Sources
This study was based on the Danish National Patient Register (NPR),11 which contains information on all hospital contacts in Denmark since 1977 and includes all outpatient consultations since 1995. Acute in‐hospital treatment and rehabilitation of patients with cerebrovascular diseases are exclusively performed at public hospitals in Denmark. From 1994 on, patient contacts were classified according to the International Classification of Diseases, 10 th Revision (ICD‐10). All inhabitants of Denmark have a unique personal identification number12 that enables unambiguous tracing of all individuals living in Denmark through nationwide registers. Information on fatal cases was obtained from the Danish Registry of Causes of Death.13
Outcome
The outcome of this study was first‐ever discharge diagnosis of stroke (all types) or TIA. Only inpatients and emergency department patients were considered. Stroke was further stratified according to subtype: ischemic stroke, ICH, SAH, and unspecified stroke. TIA was considered separately (Table 1). Overall stroke included all subtypes.
Table 1.
International Classification of Disease, Tenth Revision (ICD‐10) Codes Included
| Diagnosis | ICD‐10 Codes |
|---|---|
| Stroke | |
| Ischemic stroke | DI63 |
| Intracerebral hemorrhage | DI61 |
| Unspecified stroke | DI64, DI679, DI67–DI68 (‐DI674), DG46 |
| Subarachnoid hemorrhage | DI60 |
| Transient ischemic attack | DI45 |
Previous reports have indicated that the category unspecified stroke to a large extent (60%) consisted of ischemic stroke.14 Therefore, we defined a new stroke subtype termed nonhemorrhagic stroke that consisted of ischemic and unspecified stroke combined, to give a more accurate picture of the incidence of ischemic stroke.
The study was approved by the Danish Data Protection Agency. The study did not include direct contact with patients and therefore patient consents and ethical approval were not required according to Danish law.
Statistical Analysis
We estimated age‐specific IRs of first‐time hospitalization for stroke and TIA as well as for subtypes of stroke and reported it as numbers of new cases per 100 000 person‐years. The IRs were calculated annually for the study period of January 1, 1994, to December 31, 2012. The IRs were standardized to the age distribution of the Danish population in 2012 obtained from Statistics Denmark,15 and the approximate bootstrap method was used for calculating 95% CIs.
The temporal trends in incidence were analyzed by using Poisson regression on the age‐standardized IRs. We analyzed overall stroke and subtypes of stroke for both males and females together and separately. Estimated annual percentage change (EAPC) and corresponding 95% CIs were calculated in piecewise linear models.16 Deviations from the piecewise linear models were tested by means of restricted cubic splines.17 A positive EAPC represents an increasing trend, and a negative EAPC relates to a decreasing trend. If the trends did not differ significantly over time during the study period, tested by a likelihood ratio test, we present a single EAPC for the entire study period. However, if the best fit of a trend for males and females together was a two‐segment model, this was also applied in the sex‐specific analyses. Sex‐specific differences (P sex) in temporal trends were tested by likelihood ratio tests comparing models with and without an interaction term between calendar year and sex.
When computing the IRs, we did not take into account stroke or TIA diagnoses recorded before 1994 and thus may have overestimated the IRs of the first‐time events. To address this issue, we repeated the analyses of the trends, omitting 1994–1996 from the analyses as a sensitivity analysis. In addition, a sensitivity analysis omitting emergency department cases was performed.
The statistical software program R version 3.0.218 was used for all analyses, and level of significance was set to 5%.
Results
Demographics
The study covered >20 million person‐years corresponding to ≈1 million persons per calendar year (Table 2). A total of 4156 incident cases of hospitalizations for stroke or TIA were identified, including 3431 cases of stroke and 725 cases of TIA.
Table 2.
Characteristics of Study Population 1994–2012
| Calendar Year | Population Size | No. of First‐Ever Stroke | Age‐Standardized Incidence Rates for Stroke/100 000 Person‐Years (PY) | No. of First‐Ever Transient Ischemic Attack (TIA) | Age‐Standardized Incidence Rates for TIA/100 000 PY |
|---|---|---|---|---|---|
| 1994 | 1 203 942 | 154 | 11.97 | 25 | 1.93 |
| 1995 | 1 185 596 | 192 | 15.26 | 33 | 2.52 |
| 1996 | 1 165 948 | 165 | 13.44 | 33 | 2.54 |
| 1997 | 1 138 317 | 167 | 13.71 | 42 | 3.45 |
| 1998 | 1 107 762 | 187 | 15.69 | 47 | 3.97 |
| 1999 | 1 086 047 | 177 | 15.28 | 27 | 2.37 |
| 2000 | 1 070 066 | 152 | 12.90 | 26 | 2.27 |
| 2001 | 1 057 344 | 176 | 15.90 | 37 | 3.21 |
| 2002 | 1 045 331 | 190 | 17.06 | 29 | 2.42 |
| 2003 | 1 029 217 | 191 | 17.85 | 28 | 2.61 |
| 2004 | 1 018 778 | 194 | 18.08 | 42 | 3.82 |
| 2005 | 1 013 540 | 172 | 16.13 | 37 | 3.48 |
| 2006 | 1 011 326 | 176 | 16.92 | 36 | 3.23 |
| 2007 | 1 015 314 | 180 | 17.34 | 45 | 4.31 |
| 2008 | 1 030 426 | 187 | 17.86 | 33 | 3.11 |
| 2009 | 1 044 899 | 177 | 16.81 | 41 | 3.76 |
| 2010 | 1 055 245 | 219 | 20.72 | 51 | 4.79 |
| 2011 | 1 072 801 | 193 | 17.94 | 50 | 4.61 |
| 2012 | 1 085 001 | 182 | 16.77 | 63 | 5.81 |
| Total | 20 436 900 | 3431 | — | 725 | — |
Incidence of Hospitalizations for Stroke and TIA
The age‐standardized IRs for hospitalizations for overall stroke and TIA are presented in Figure 1 and Table 2. The corresponding EAPCs are given in Table 3. The age‐standardized overall stroke hospitalization IR increased significantly (EAPC 1.83% [95% CI 1.11–2.55%]) from 11.98/100 000 person‐years in 1994 to 16.74/100 000 person‐years in 2012. The age‐standardized IR of hospitalizations for TIA increased from 1.93/100 000 person‐years in 1994 to 5.81/100 000 person‐years in 2012, corresponding to an even stronger positive trend (EAPC 4.12% [95% CI 2.44–5.83%]).
Figure 1.
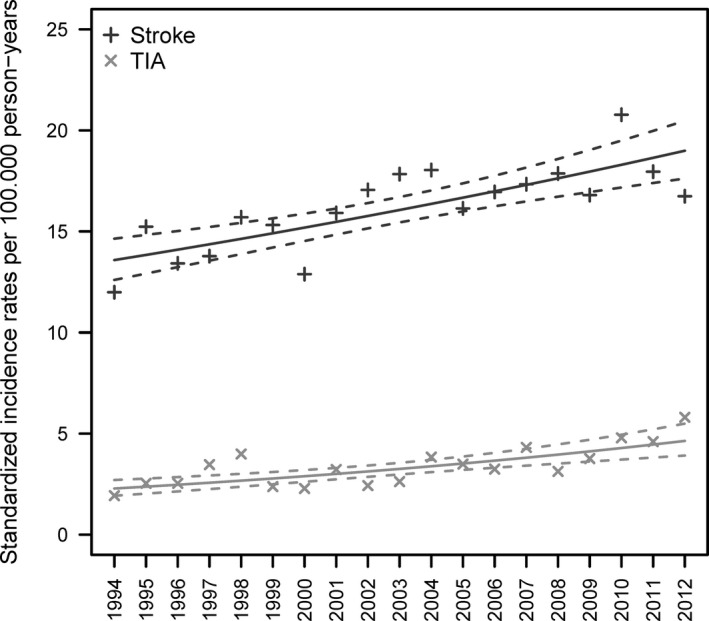
Age‐standardized incidence rates per 100 000 person‐years for hospitalizations for overall stroke (ie, all subtypes combined) and transient ischemic attack (TIA) separately. Points are the age‐standardized incidence rates, whereas the solid lines are the estimated trends of the standardized incidence rates and the dashed lines represent the corresponding 95% CIs.
Table 3.
Estimated Annual Percentage Change (EAPC) for Total and Sex Specific
| Type | Total | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | EAPC | 95% CI | P Value | P sex a | Period | EAPC | 95% CI | P Value | Period | EAPC | 95% CI | P Value | |
| Transient ischemic attack | 1994–2012 | 4.12 | 2.44 to 5.83 | <0.001 | 0.03 | 1994–2006 | 0.77 | −1.84 to 3.45 | 0.566 | 1994–2012 | 3.45 | 1.62 to 5.31 | <0.001 |
| — | — | — | — | — | 2006–2012 | 16.61 | 10.45 to 23.12 | <0.001 | — | — | — | — | |
| Stroke | 1994–2012 | 1.83 | 1.11 to 2.55 | <0.001 | 0.94 | 1994–2012 | 1.80 | 0.82 to 2.78 | <0.001 | 1994–2012 | 1.85 | 0.68 to 3.04 | 0.002 |
| Subarachnoid hemorrhage | 1994–2012 | −0.38 | −1.62 to 0.89 | 0.559 | 0.02 | 1994–2012 | 0.91 | −0.36 to 2.20 | 0.162 | 1994–2012 | −1.93 | −3.89 to 0.08 | 0.060 |
| Intracerebral hemorrhage | 1994–2012 | 0.22 | −1.85 to 2.32 | 0.838 | 0.58 | 1994–2012 | 0.59 | −1.82 to 3.07 | 0.632 | 1994–2012 | −0.44 | −2.94 to 2.12 | 0.731 |
| Ischemic | 1994–2006 | 2.56 | 0.60 to 4.56 | 0.010 | 0.58 | 1994–2006 | 0.98 | −2.60 to 4.69 | 0.597 | 1994–2012 | 3.72 | 0.13 to 7.43 | 0.042 |
| 2006–2012 | 11.80 | 7.40 to 16.38 | <0.001 | — | 2006–2012 | 14.60 | 6.22 to 23.63 | <0.001 | 2006–2012 | 9.87 | 2.16 to 18.15 | 0.011 | |
| Unspecied | 1994–2006 | 5.09 | 3.15 to 7.08 | <0.001 | 0.53 | 1994–2012 | 2.63 | 0.64 to 4.66 | 0.010 | 1994–2012 | 3.56 | 1.72 to 5.42 | <0.001 |
| 2006–2012 | −1.82 | −5.96 to 2.49 | 0.402 | — | — | — | — | — | — | — | — | — | |
| Nonhemorrhagic stroke | 1994–2012 | 3.92 | 2.98 to 4.88 | <0.001 | 0.51 | 1994–2012 | 3.47 | 1.96 to 4.99 | <0.001 | 1994–2012 | 4.19 | 2.80 to 5.61 | <0.001 |
Test for sex‐differences in EAPC.
Temporal Changes of Stroke Subtypes
Age‐standardized IRs and corresponding estimated trends of hospitalizations for ischemic stroke, unspecified stroke, nonhemorrhagic stroke (ischemic and unspecified stroke combined), SAH, and ICH are presented in Figure 2. The IR of hospitalizations for ischemic stroke doubled from 1994 to 2012 as a consequence of a small but significant increase until 2006 (EAPC 2.56% [95% CI 0.60–4.56%]) followed by a much larger increase (EAPC 11.80% [95% CI 7.40–16.38%]) in the subsequent years. The IR for hospitalizations for unspecified stroke increased significantly (EAPC 5.09% [95% CI 3.15–7.08%]) from 1994 to 2006 but was followed by a small nonsignificant negative trend (EAPC −1.82% [95% CI −5.96% to 2.49%]). The IR for hospitalizations for nonhemorrhagic stroke increased significantly (EAPC 3.92% [95% CI 2.98–4.88%]) from 1994 to 2012, whereas the IRs for hospitalizations for ICH and SAH showed only small variations and no significant trends over time.
Figure 2.
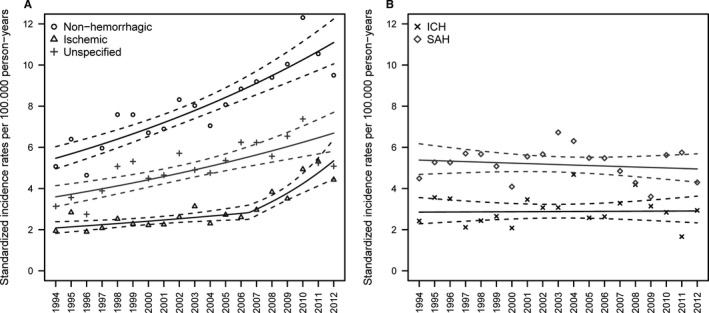
Age‐standardized incidence rates per 100 000 person‐years for hospitalizations for subtypes of stroke: (A) ischemic, unspecified, and nonhemorrhagic stroke and (B) intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH), separately. Points are the age‐standardized incidence rates, whereas the solid lines are the estimated trends in the standardized incidence rates and the dashed lines represent the corresponding 95% CIs.
Temporal Changes in Sex‐Specific Incidence of Hospitalizations According to Stroke Subtype
Table 3 shows the overall and sex‐specific EAPCs for stroke, TIA, and stroke subtypes, as well as tests for differences in sex‐specific temporal trends. The age‐standardized IRs and estimated trends for hospitalizations of TIA, ischemic stroke, unspecified stroke, and nonhemorrhagic stroke for men and women are given in Figure 3. Sex‐specific EAPCs for men (EAPC 1.80% [95% CI 0.82–2.78%]) and women (EAPC 1.85% [95% CI 0.68–3.04%]) were similar (P sex=0.94) for overall stroke. For hospitalizations for TIA, significant differences were seen between men and women (P sex=0.028); in women, the IR increased slightly throughout the study period (EAPC 3.45% [95% CI 1.62–5.31%]), whereas in men, the IR appeared stable until 2006 (EAPC 0.77% [95% CI −1.84% to 3.45%]), followed by a significant increase thereafter (EAPC 16.61% [95% CI 10.45–23.12%]). In men, the IR for hospitalizations for ischemic stroke increased insignificantly until 2006 (EAPC 0.98% [95% CI −2.60% to 4.69%]), and from 2006 to 2012, it increased steeply (EAPC 14.60% [95% CI 6.22–23.63%]). A similar pattern was seen in women from 2006 to 2012 but with a slightly lower, although not significantly lower (P sex=0.58), increase compared with men (EAPC 9.87% [95% CI 2.16–18.15%]).
Figure 3.
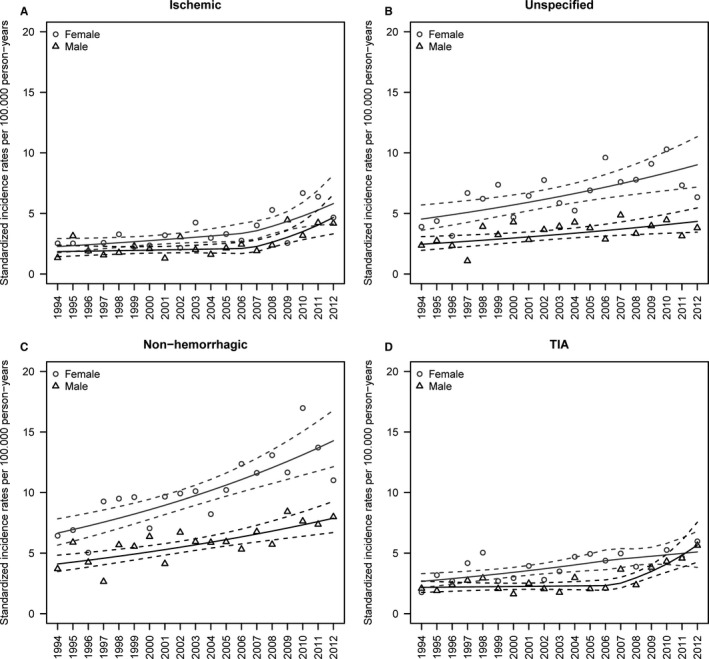
Age‐standardized incidence rates per 100 000 person‐years for men and women for hospitalizations for (A) ischemic stroke, (B) unspecified stroke, (C) nonhemorrhagic stroke, and (D) transient ischemic attack (TIA). Points are the age‐standardized incidence rates, whereas the solid lines are the estimated trends in the standardized incidence rates and the dashed lines represent the corresponding 95% CIs.
The trends for hospitalizations for unspecified stroke were similar in men (EAPC 2.63% [95% CI 0.64–4.66%]) and women (3.56% [95% CI 1.72–5.42%]) (P sex=0.53). Hospitalizations for nonhemorrhagic stroke IR increased significantly throughout the entire study period in both men (EAPC 3.47% [95% CI 1.96–4.99%]) and women (EAPC 4.19% [95% CI 2.80–5.61%]). Although the IR was higher among women compared with that in men, there was no difference with regard to trend (P sex=0.51).
The sensitivity analysis of 1997–2012 did not alter the main conclusions (Table 4). The main difference was that the trend for hospitalizations for SAH among women changed from near significant to significant with a faster decrease (EAPC −3.45% [95% CI −5.89% to −0.95%]) during the period compared with 1994–2012 (EAPC −1.93% [95% CI −3.89% to 0.08%]). Analysis excluding emergency department cases did not change the trends (data not shown).
Table 4.
Sensitivity Analysis of the Sex‐Specific Trends Omitting the Period 1994–1996
| Type | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Period | Estimated Annual Percentage Change (EAPC) | 95% CI | P Value | Period | EAPC | 95% CI | P Value | |
| Transient ischemic attack | 1997–2006 | 0.01 | −3.92 to 4.10 | 0.995 | 1997–2012 | 2.47 | 0.31 to 4.68 | 0.025 |
| 2006–2012 | 17.22 | 10.21 to 24.67 | <0.001 | |||||
| Stroke | 1997–2012 | 2.10 | 0.88 to 3.35 | <0.001 | 1997–2012 | 2.88 | 1.36 to 4.43 | <0.001 |
| Subarachnoid hemorrahge | 1997–2012 | 1.03 | −0.69 to 2.79 | 0.241 | 1997–2012 | −3.45 | −5.89 to −0.95 | 0.007 |
| Intracerebral hemorrhage | 1997–2012 | 1.10 | −2.31 to 4.62 | 0.533 | 1997–2012 | 0.37 | −2.82 to 3.66 | 0.824 |
| Ischemic stroke | 1997–2006 | 2.34 | −2.38 to 7.30 | 0.337 | 1997–2012 | 5.94 | 2.92 to 9.05 | <0.001 |
| 2006–2012 | 13.69 | 5.80 to 22.17 | <0.001 | |||||
| Unspecified stroke | 1997–2006 | 4.24 | −0.44 to 9.15 | 0.076 | 1997–2006 | 3.23 | −0.44 to 7.04 | 0.085 |
| 2006–2012 | −2.51 | −10.10 to 5.73 | 0.540 | 2006–2012 | −0.08 | −6.20 to 6.43 | 0.979 | |
| Nonhemorrhagic stroke | 1997–2012 | 3.77 | 1.87 to 5.71 | <0.001 | 1997–2012 | 3.38 | 1.73 to 5.05 | <0.001 |
Discussion
This nationwide study focuses on the youngest adults (15–30 years old) with stroke and TIA. It is, to our knowledge, one of the first studies to investigate temporal trends of incidence of stroke, including major subtypes, and TIA for this specific age group. We found increasing incidence of first‐time hospitalization for both stroke and TIA among young Danish adults. Further, the IR of hospitalizations for ischemic stroke and TIA increased markedly from 2006 to 2012. The IRs of hospitalizations for hemorrhage of any type did not change. Some sex‐specific differences were also detected; the IRs for hospitalizations for ischemic stroke and TIA among women appeared higher compared with those for men, while the incidence among men increased more steeply during the last part of the study period.
Direct comparison of our results to previous studies is difficult primarily for 2 reasons. First, previously studies analyze temporal trends in a broader age range (ie, individuals up to 50 to 55 years old). The incidence of stroke increases steeply with age.14, 19 This makes it difficult to examine trends among the very young, as this group is outnumbered by individuals older than 30 years. Incidence trends in the very young thus might be concealed by the larger incidence of cerebrovascular diseases among older persons. Second, different statistical methods have been used (ie, IR ratio and annual percentage change); however, these are not directly comparable. While the IR ratio only expresses the increase or decrease, the EAPC enables us to quantify trends from 1 year to another. In this study, we focused entirely on young adult stroke and TIA, whereas temporal trends of the older part of the Danish population have been described elsewhere.3
The present IR for hospitalizations for ischemic stroke in the young was slightly lower than that previously reported.8, 9, 10, 20, 21 A Swedish study found that IRs of hospitalizations for ischemic stroke among adults between 18 and 44 years showed a continuous annual percentage increase of 1.3% for men and 1.6% for women during 1984–2010,10 which were lower rates than those observed in the present study. Other studies have also reported increasing IRs, although results were given as IR ratios.8, 9, 22 In addition, in individuals younger than age 30, ischemic strokes were reported more frequently in women than in men.9, 21 This was also the case in our study. However, as the hospitalization rate among men increased, the difference between men and women disappeared in the last part of the study period. Studies of TIA incidence from Greater Cincinnati and Spain reported IRs between 1.0 and 3.2/100 000 PY.22, 23 However, these studies reported temporal trends confined to the years 2006 and 1993–1994. Nevertheless, our results from 1994–2006 were similar to these findings, whereas the IRs from the last part of our study period were considerably higher. The IR of SAH has been reported to vary between 0.1 and 7.7/100 000 PY with no apparent change over time reported so far.24, 25, 26, 27, 28 Our IRs were equivalent to the highest previously reported rates and also showed no apparent trend. The present results for ICH with a relative constant incidence over time are comparable to those of previous studies.8, 20
In the past, stroke in the young was considered to be caused by rare conditions. However, recent reports argue to some extent against rare risk factors as the most common causes of stroke. Instead diabetes, hypertension, smoking, low aerobic fitness, and obesity are believed to be the prominent risk factors for stroke and TIA in the young.8, 29, 30, 31, 32, 33 Interestingly, a family history and illicit drug abuse also seem to be important risk factors.34 While the prevalence of most of the traditional risk factors have declined slightly during the study period,35, 36 the prevalence and incidence of diabetes have increased markedly among young adults in Denmark.37 This is perhaps one likely explanation for the increased incidence of stroke and TIA seen in this study.
Strengths and Limitations
The use of high‐quality nationwide health registries with accurate linkage enables near‐complete ascertainment of all hospitalized stroke and TIA cases in a specific age group in an entire country during an 18‐year period. Further, the use of a nationwide cohort enables analysis of cerebrovascular disease in young adults, which is a rare outcome. Nonetheless, the study is limited by the register‐based design that includes only hospitalized cases of stroke and TIA. For stroke diagnoses in NPR, misclassification may have occurred in up to 20% of the patients38; however, other studies report that ischemic stroke in particular may be underestimated.14 Although validation of stroke diagnoses in NPR has led to varying conclusions, we find it less likely that the underestimation should have changed during the study period. Therefore, although our data may underreport IRs, this would most likely not affect temporal trends. The quality of neuroimaging improved significantly, leading to detection of smaller brain lesions during the study period. Further, imaging became more widespread and available in the Danish hospitals. Together, these issues would potentially flaw our results by leading to more accurate diagnosis of stroke subtypes, which in turn would alter the “true” IRs for, in particular, unspecified stroke and ischemic stroke. Thus, improved imaging quality would in theory lead to increased detection of ischemic stroke incidence and, to some extent, explain the decrease of registered unspecified stroke seen in this study as well as in previous studies.3 Nevertheless, we do not believe that this change in case ascertainment influenced our results to a large extent, because even though subtypes were not determined on imaging in the 1990s, the patients were still diagnosed as having “unspecified” stroke. In an attempt to adjust for neuroimaging availability, we constructed the category of nonhemorrhagic stroke (ischemic and unspecified collapsed into 1 category). We found that hospitalization of patients with nonhemorrhagic stroke increased at a constant rate throughout the study period, indicating that admissions of nonhemorrhagic stroke increased regardless of changes in diagnostic practice. In addition, a study of the older part of the Danish population showed increased rate of hospitalization for ischemic stroke.3 This suggests that the increase of incidence over time found in this study represents a real increase in incidence. For TIA, the improved detection of smaller ischemic lesions may have caused a decrease in incidence and a corresponding increase in ischemic stroke, because some patients may have had visible lesions on imaging, regardless of clinical TIA symptoms. Nonetheless, we saw an increased incidence of hospitalizations for TIA over time despite these improvements. We therefore believe that the changes in diagnostic routines did not have a major impact on our findings. Finally, it must be noted that we found increased incidences only for ischemic type and not for SAH and ICH. This indicates that the increases for ischemic stroke and TIA are not caused by a general increase in hospitalization for stroke but represent a real increase in incidence.
Summary/Conclusion
We found increased rates of hospital admissions for stroke and TIA. This was particularly prominent in the later period of the study. Ischemic stroke and TIA showed similar patterns, with steep increases among men from 2006 to 2012. In contrast, the hospitalization rates for SAH and ICH appeared stable. These results may suggest that the increase in both TIA and ischemic stroke is caused by risk factors, which should be explored in future research.
Sources of Funding
The study was funded by grants from the Danish Ministry of Health and the Capital Region of Denmark. None of the institutions had any roles in planning, designing, and/or conducting of the study; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003158 doi: 10.1161/JAHA.115.003158)
Parts of the results (ie, overall stroke and ischemic stroke) were presented at the European Stroke Organization Conference 2015, April 17–19, 2015, in Glasgow, UK.
References
- 1. Bejot Y, Benatru I, Rouaud O, Fromont A, Besancenot JP, Moreau T, Giroud M. Epidemiology of stroke in Europe: geographic and environmental differences. J Neurol Sci. 2007;262:85–88. [DOI] [PubMed] [Google Scholar]
- 2. Vaartjes I, O'Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44:591–597. [DOI] [PubMed] [Google Scholar]
- 3. Demant MN, Andersson C, Ahlehoff O, Charlot M, Olesen JB, Gjesing A, Hansen PR, Gislason GH, Truelsen T, Torp‐Pedersen C. Temporal trends in stroke admissions in Denmark 1997–2009. BMC Neurol. 2013;13:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker‐Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moran GM, Fletcher B, Feltham MG, Calvert M, Sackley C, Marshall T. Fatigue, psychological and cognitive impairment following transient ischaemic attack and minor stroke: a systematic review. Eur J Neurol. 2014;21:1258–1267. [DOI] [PubMed] [Google Scholar]
- 6. Volonghi I, Pendlebury ST, Welch SJ, Mehta Z, Rothwell PM. Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart. 2013;99:1509–1514. [DOI] [PubMed] [Google Scholar]
- 7. van Rooij FG, Schaapsmeerders P, Maaijwee NA, van Duijnhoven DA, de Leeuw FE, Kessels RP, van Dijk EJ. Persistent cognitive impairment after transient ischemic attack. Stroke. 2014;45:2270–2274. [DOI] [PubMed] [Google Scholar]
- 8. Bejot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. 2014;85:509–513. [DOI] [PubMed] [Google Scholar]
- 9. Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first‐ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40:1195–1203. [DOI] [PubMed] [Google Scholar]
- 10. Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty‐four‐year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44:2388–2393. [DOI] [PubMed] [Google Scholar]
- 11. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 12. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- 13. Helweg‐Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. [DOI] [PubMed] [Google Scholar]
- 14. Krarup LH, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. [DOI] [PubMed] [Google Scholar]
- 15. Statistics Denmark . 2016. Available at: StatBank.dk. Accessed June 27, 2015.
- 16. Harell FE Jr. Regression Modeling Strategies With Application to Linear Models, Logistic Regression and Survival Analysis. New York: Springer‐Verlag New York Inc.; 2001. [Google Scholar]
- 17. Devlin TF, Weeks BJ. Spline functions for logistic regression modeling. Cary, NC:1986:646–651. [Google Scholar]
- 18. R Development Core Team . R: a language and environment for statistical computing. 2013. Available at: http://www.r-project.org. Accessed May 2, 2014.
- 19. Putaala J, Yesilot N, Waje‐Andreassen U, Pitkaniemi J, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, Kloss M, Ferrari J, Lemmens R, Coban O, Haapaniemi E, Maaijwee N, Rutten‐Jacobs L, Bersano A, Cereda C, Baron P, Borellini L, Valcarenghi C, Thomassen L, Grau AJ, Palm F, Urbanek C, Tuncay R, Durukan‐Tolvanen A, van Dijk EJ, de Leeuw FE, Thijs V, Greisenegger S, Vemmos K, Lichy C, Bereczki D, Csiba L, Michel P, Leys D, Spengos K, Naess H, Bahar SZ, Tatlisumak T. Demographic and geographic vascular risk factor differences in European young adults with ischemic stroke: the 15 Cities Young Stroke Study. Stroke. 2012;43:2624–2630. [DOI] [PubMed] [Google Scholar]
- 20. Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naess H, Nyland HI, Thomassen L, Aarseth J, Nyland G, Myhr KM. Incidence and short‐term outcome of cerebral infarction in young adults in western Norway. Stroke. 2002;33:2105–2108. [DOI] [PubMed] [Google Scholar]
- 22. Diaz‐Guzman J, Egido JA, Gabriel‐Sanchez R, Barbera‐Comes G, Fuentes‐Gimeno B, Fernandez‐Perez C. Stroke and transient ischemic attack incidence rate in Spain: the IBERICTUS study. Cerebrovasc Dis. 2012;34:272–281. [DOI] [PubMed] [Google Scholar]
- 23. Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short‐term prognosis of transient ischemic attack in a population‐based study. Stroke. 2005;36:720–723. [DOI] [PubMed] [Google Scholar]
- 24. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon JW, Lee HJ, Hyun MK, Choi JE, Kim JH, Lee NR, Hwang JS, Lee EJ. Trends in the incidence of subarachnoid hemorrhage in South Korea from 2006–2009: an ecological study. World Neurosurg. 2013;79:499–503. [DOI] [PubMed] [Google Scholar]
- 26. Sandvei MS, Mathiesen EB, Vatten LJ, Muller TB, Lindekleiv H, Ingebrigtsen T, Njolstad I, Wilsgaard T, Lochen ML, Vik A, Romundstad PR. Incidence and mortality of aneurysmal subarachnoid hemorrhage in two Norwegian cohorts, 1984–2007. Neurology. 2011;77:1833–1839. [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Liu G, Arima H, Li Y, Cheng G, Shiue I, Lv L, Wang H, Zhang C, Zhao J, Anderson CS. Incidence and risks of subarachnoid hemorrhage in China. Stroke. 2013;44:2891–2893. [DOI] [PubMed] [Google Scholar]
- 28. Ziemba‐Davis M, Bohnstedt BN, Payner TD, Leipzig TJ, Palmer E, Cohen‐Gadol AA. Incidence, epidemiology, and treatment of aneurysmal subarachnoid hemorrhage in 12 midwest communities. J Stroke Cerebrovasc Dis. 2014;23:1073–1082. [DOI] [PubMed] [Google Scholar]
- 29. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–721. [DOI] [PubMed] [Google Scholar]
- 30. Hogstrom G, Nordstrom A, Eriksson M, Nordstrom P. Risk factors assessed in adolescence and the later risk of stroke in men: a 33‐year follow‐up study. Cerebrovasc Dis. 2015;39:63–71. [DOI] [PubMed] [Google Scholar]
- 31. Maaijwee NA, Rutten‐Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw FE. Ischaemic stroke in young adults: risk factors and long‐term consequences. Nat Rev Neurol. 2014;10:315–325. [DOI] [PubMed] [Google Scholar]
- 32. Putaala J, Curtze S, Hiltunen S, Tolppanen H, Kaste M, Tatlisumak T. Causes of death and predictors of 5‐year mortality in young adults after first‐ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40:2698–2703. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Rudd AG, Wolfe CD. Age and ethnic disparities in incidence of stroke over time: the South London Stroke Register. Stroke. 2013;44:3298–3304. [DOI] [PubMed] [Google Scholar]
- 34. de los Ríos RF, Kleindorfer DO, Khoury J, Broderick JP, Moomaw CJ, Adeoye O, Flaherty ML, Khatri P, Woo D, Alwell K, Eilerman J, Ferioli S, Kissela BM. Trends in substance abuse preceding stroke among young adults: a population‐based study. Stroke. 2012;43:3179–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen AI, Davidsen M, Ekholm O, Hansen SE, Holst M, Juel K. The National Health Profile 2010—How are you? (den Nationale sundhedsprofil 2010—Hvordan har du det?). The Danish National Board of Health; 2011. [Google Scholar]
- 36. Ekholm O, Juel K. Youth National Health profile—2011. The Danish National Board of Health; 2011. [Google Scholar]
- 37. Carstensen B, Kristensen JK, Ottosen P, Borch‐Johnsen K. The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia. 2008;51:2187–2196. [DOI] [PubMed] [Google Scholar]
- 38. Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, Johnsen SP. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2014;6:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


