Abstract
Background
The long‐term association between the status of the false lumen and poor patient outcomes in acute aortic dissection (AAD) remains unclear. This systematic review and meta‐analysis investigated whether the status of the false lumen was a predictor of poor long‐term survival in AAD.
Methods and Results
Eleven cohort studies (2924 participants) exploring the association between the false lumen status and long‐term outcomes (>1 year) in AAD were included. All studies reported multivariate‐adjusted hazard ratios (HRs) with 95% CIs for long‐term outcomes, according to false lumen status. Pooled HRs for mortality and aortic events were computed and weighted using generic inverse‐variance and random‐effect modeling. Residual patent false lumen was an independent predictor of long‐term mortality in AAD type A (HR, 1.71; 95% CI, 1.16–2.52; P=0.007) and type B (HR, 2.79; 95% CI, 1.80–4.32; P<0.001). AAD patients with residual patent false lumen exhibited an increased risk of aortic events (HR, 5.43; 95% CI, 2.95–9.99; P<0.001). Partial false lumen thrombosis was independently associated with long‐term mortality in type B AAD (HR, 2.24; 95% CI, 1.37–3.65; P=0.001). This association was not observed in AAD type A patients (HR, 1.75; 95% CI, 0.88–3.45; P=0.211).
Conclusions
The false lumen status influences late outcomes in AAD. Residual patent false lumen is independently associated with poor long‐term survival in AAD. However, only type B AAD patients with partial false lumen thrombosis had an increased late mortality risk.
Keywords: aortic dissection, false lumen, meta‐analysis, thrombosis
Subject Categories: Aortic Dissection, Thrombosis, Risk Factors, Computerized Tomography (CT), Meta Analysis
Introduction
Acute aortic dissection (AAD) is a life‐threatening vascular emergency, with high in‐hospital and follow‐up morbidity and mortality rates. Approximately 48.6% of patients with AAD die before admission.1 Dissections confined to the ascending aorta (type A) exhibit worse in‐hospital survival rates than those involving the descending aorta (type B).2 A report from the International Registry of Acute Aortic Dissection (IRAD) identified an in‐hospital surgical mortality rate of 30% in AAD type A patients and 13% in type B.2, 3 However, long‐term outcomes for AAD patients with type B dissection are not necessarily better than those having type A. Long‐term survival of surgically treated patients with type B AAD after discharge ranges from 56% to 96% at 1 year and from 48% to 83% at 5 years.4, 5, 6, 7, 8 In contrast, for surgically treated type A patients who survive until hospital discharge, survival rates range from 52% to 96% at 1 year and from 37% to 91% at 5 years.8, 9, 10, 11, 12 Therefore, given the variable prognosis of AAD with current management strategies, it is necessary to establish predictors of poor outcomes for targeted therapy.
Earlier studies have identified long‐term predictors of morbidity and mortality in AAD, including old age,13, 14 female sex,15 a history of atherosclerosis,4 and impaired renal function,16 all of which may be more representative of a patient's high‐risk clinical background than the severity and nature of the AAD itself. Indeed, as of yet, there may be no effective and simple marker available for evaluating severity of AAD, predicting long‐term clinical outcomes, and optimizing surgical management. Several reports have indicated that the status of the false lumen is associated with risk of poor outcomes. Residual patency of the false lumen in AAD has been associated with aortic expansion and death, whereas patients with complete thrombosis of the false lumen have shown improved outcomes.17, 18, 19 In addition, the IRAD data have shown that partial thrombosis, defined as the concurrent presence of both flow and thrombus, is associated with a higher 3‐year mortality rate than a completely patent false lumen in patients with type B AAD.20 This conclusion supports the concept that a wide surgical extent during initial repair could decrease late mortality rates for AAD type A patients. However, some studies have shown that preoperative residual patency of the false lumen is not a predictor of 5‐year mortality in AAD,21 and that partial thrombosis is not associated with long‐term mortality, reintervention rates, or aortic growth in type A cases after a 5‐year follow‐up.22 Therefore, conclusions regarding the association between the status of the false lumen and poor outcomes in AAD are conflicting. Given that a single study may lack the power to provide comprehensive and reliable conclusions, a systematic review and meta‐analysis of all eligible studies was performed. In this review, we aimed to determine the influence of the false lumen status on long‐term outcomes in patients presenting with AAD.
Methods
This systematic review and meta‐analysis was conducted in accord with the Meta‐Analysis of Observational Studies in Epidemiology guidelines.23
Search Strategy
An electronic search of the PubMed, Embase, and Cochrane Library databases was performed from inception to December 2015 with no language restrictions. To identify studies investigating the status of the false lumen and risk of long‐term outcomes in patients presenting with AAD, the following search terms were applied: (thrombus OR thrombosis OR thrombi OR clot OR false lumen) AND (aortic dissection OR dissection of aorta). Two independent researchers conducted the search; in cases of disagreement, the study reviewers met to resolve issues. A manual search of additional articles was conducted using references from relevant articles and review papers.
Study Selection
The inclusion criteria were: (1) studies on AAD reporting the prognostic impact of the false lumen status; (2) availability of a multiple adjusted hazard ratio (HR) with 95% CI for overall survival, or data regarding aortic events from which it could be calculated; and (3) studies with a follow‐up time of >1 year. Studies were excluded if the study was designed as a review, case‐controlled study, or an animal study, or if the HR value reported was unadjusted. If more than 1 study was published by the same authors using the same case series or overlapping case series, the study with the largest sample size was included, except for those cases where a different subgroup analysis could be performed.
Data Extraction and Quality Assessment
Data were collected using predesigned abstraction forms. Two main reviewers (D.L. and L.Y.) independently extracted the data and reached a consensus on all items. The following items were extracted from each study if available: name of the first author, publication year, study period, region, study design, number of AAD cases, age of patients, proportion of male patients, Stanford classification, treatment therapy, outcomes, follow‐up duration, adjusted HR and 95% CI, and statistical adjustments for confounding factors. A quality assessment of each selected study was conducted by 2 investigators (D.L. and L.Y.) using the Newcastle–Ottawa Scale (NOS).24 A third reviewer was consulted in cases of uncertainty. The NOS is an assessment tool for cohort studies and consists of 3 parameters of quality: selection, comparability, and exposure/outcome assessment. The NOS includes a “star system” (range, 0–9) for assessment, of which a maximum of 4 points is allocated to the selection parameter, 2 points for comparability, and 3 points for exposure/outcome assessment. A total score of 7 or greater indicated a high‐quality study, whereas a total score of 6 or lower was taken to indicate a low‐quality study.
Statistical Analyses
Meta‐analysis and statistical analyses were performed using Stata software (version 12.0; StataCorp, College Station, TX). A meta‐analysis of time to event data was performed. Reported multivariate adjusted HRs and 95% CIs were extracted from included studies. Pooled estimates, together with 95% CIs, were calculated using a random‐effects model, chosen because of expected heterogeneity among the studies. Cochran's Q test and Higgins' I2 statistic were calculated for the detection of heterogeneity. We defined an I2 less than 25%, 25% to 50%, and greater than 50% as low, moderate, and high heterogeneity, respectively.25 P<0.1 and an I2<50% were considered to be of no significant heterogeneity. Sensitivity analysis was performed to investigate the influence of a single study on overall risk estimate and was carried out by sequentially omitting 1 study at a time. Subgroup analyses were performed according to a priori groupings related to study design, region, duration of follow‐up (≤5 vs >5 years) and number of participants (≤200 vs >200). Potential publication bias was assessed using Egger's test. All hypothesis tests were 2‐sided. P<0.10 was considered indicative of statistically significant heterogeneity, and all other P values were considered statistically significant at <0.05.
Results
Study Selection and Characteristics
A total of 1408 abstracts were identified through the literature search, with 9 studies identified through manual searching of reference lists from these articles. After removal of duplicates, a review of the titles and abstracts of 908 articles was performed. A total of 24 articles were obtained and read in full. Of these, a further 13 studies were excluded. Ultimately, 11 studies, including 2924 patients with AAD, were included (Figure 1).
Figure 1.
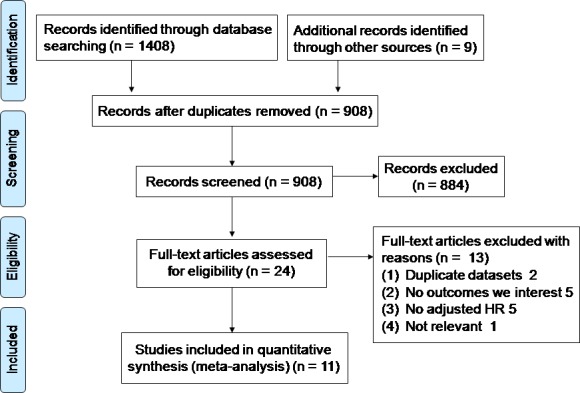
Flow diagram for study identification and inclusion. HR indicates hazard ratio.
Basic characteristics of the studies are shown in Tables 1 and 2. Four studies17, 22, 26, 27 examined the association between the false lumen status and AAD outcomes in type A patients, and another 6 studies18, 19, 20, 28, 29, 30 examined associations in type B patients. One study21 included both type A (73%) and type B (27%) AAD; in subgroup analysis based on the Stanford classification, this study was placed in the type A group. Mean patient age was 63 years old, and 53% of patients were men. Seven studies were prospective cohort studies, and 4 studies were retrospective cohort studies. All of the included studies were of high quality, with an average NOS score of 8.8.
Table 1.
Summary of Included Studies
| Study | Study Period | Region | Design | Sample Size | Male, % | Age, y | Stanford | Assay Method |
|---|---|---|---|---|---|---|---|---|
| Kimura, 201517 | 1990–2003 | Japan | Prospective cohort | 534 | 52 | 63 | A | CT |
| Akutsu, 200418 | 1981–2000 | Japan | Retrospective cohort | 138 | 65 | 64 | B | CT |
| Marui, 200719 | 1988–2004 | Japan | Retrospective cohort | 141 | 97 | 68 | B | CT |
| Tsai, 200720 | 1996–2003 | Multi centers | IRAD registry | 201 | 69 | 61 | B | CT/MRI/TEE |
| Bernard, 200121 | 1984–1996 | France | Retrospective cohort | 109 | 74 | 61 | A/B | CT/MRI/TEE |
| Larsen, 201322 | 1996–2011 | Multi centers | IRAD registry | 522 | 75 | 58 | A | CT/MRI/TEE |
| Song, 201026 | 1997–2007 | Korea | Prospective cohort | 118 | 55 | 60 | A | CT |
| Song, 201127 | 1997–2007 | Korea | Retrospective cohort | 136 | 56 | 60 | A | CT |
| Miyahara, 201128 | 2000–2009 | Japan | Prospective cohort | 160 | 63 | 66 | B | CT |
| Tanaka, 201429 | 2002–2011 | Japan | Prospective cohort | 103 | 67 | 67 | B | CT |
| Ueki, 201430 | 2003–2012 | Japan | Prospective cohort | 228 | 67 | 70 | B | CT |
CT indicates computer tomography; IRAD, International Registry of Acute Aortic Dissection; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography.
Table 2.
Summary of Included Studies
| Study | False Lumen Status | Treatment | Outcomes | Follow‐up, y | NOS |
|---|---|---|---|---|---|
| Kimura, 201517 | Patent/thrombosed | EVAR and OAR | Mortality/aortic events | 6.8 | 9 |
| Akutsu, 200418 | Patent/thrombosed | EVAR, OAR, and medication | Mortality | 10 | 9 |
| Marui, 200719 | Patent/thrombosed | Medication | Mortality/aortic events | 5.4 | 9 |
| Tsai, 200720 | Paten/partial/complete | EVAR, OAR, and medication | Mortality | 2.8 | 9 |
| Bernard, 200121 | Patent/thrombosed | OAR and medication | Mortality | 5 | 8 |
| Larsen, 201322 | Paten/partial/complete | OAR | Mortality/aortic events | 5 | 9 |
| Song, 201026 | Paten/partial/complete | OAR | Mortality/aortic events | 3.5 | 9 |
| Song, 201127 | Paten/partial/complete | OAR | Mortality | 4.3 | 9 |
| Miyahara, 201128 | Patent/thrombosed | OAR and medication | Mortality/aortic events | 3.6 | 9 |
| Tanaka, 201429 | Paten/partial/complete | Medication | Mortality | 3.1 | 8 |
| Ueki, 201430 | Paten/partial/complete | EVAR and OAR | Mortality | 3.2 | 9 |
EVAR indicates endovascular aneurysm repair; NOS, Newcastlee–Ottawa Scale; OAR, open acute aortic dissection repair.
Meta‐Analysis
Long‐term mortality
All 11 studies reported outcomes related to long‐term mortality. Four studies17, 18, 19, 21 observed that a residual patent false lumen increases risk of long‐term mortality, compared with complete thrombosis of the false lumen (n=1082; HR, 2.12; 95% CI, 1.58–2.83; P<0.001) with no significant heterogeneity (I2=12.6%; P=0.330). Among studies in the type A category,17, 21 the pooled HR for long‐term mortality with a residual patent false lumen was 1.71 (95% CI, 1.16–2.52; P=0.007; Figure 2) without significant heterogeneity (I2=0.0%; P=0.992). One study21 reported the HR and 95% CI with preoperative and postoperative residual patent false lumen for long‐term mortality. Subgroup analysis showed that the pooled HR for long‐term mortality with postoperative residual patent false lumen was 2.02 (95% CI, 1.13–3.61; P=0.017) with no significant heterogeneity (I2=29.6%; P=0.233). For studies in the type B category,18, 19, 28 the pooled HR for long‐term mortality with preoperative residual patent false lumen was 2.79 (95% CI, 1.80–4.32; P<0.001; Figure 2); no statistically significant heterogeneity was observed (I2=0.0%; P=0.386). Because of the small number of studies, sensitivity analysis and Egger's test were not performed.
Figure 2.
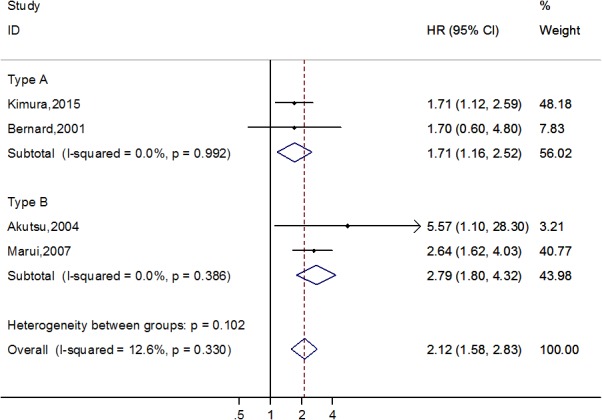
Forest plot demonstrating the association between residual patent false lumen and long‐term mortality in acute aortic dissection patients. HR indicates hazard ratio.
Six studies7, 20, 26, 27, 29, 30 observed that partial thrombosis of the false lumen, when compared to completely patent false lumen, was an independent predictor of long‐term mortality (n=1308; HR, 2.06; 95% CI, 1.38–3.06; P=0.012) without significant heterogeneity (I2=41.7%; P=0.127). For type A studies,22, 26, 27 the pooled HR for long‐term mortality with partial thrombosis of the false lumen was 1.75 (95% CI, 0.88–3.45; P=0.211; Figure 3) with moderate heterogeneity (I2=68.1%; P=0.044). Among type B studies,20, 29, 30 the pooled HR for long‐term mortality with partial thrombosis of the false lumen was 2.24 (95% CI, 1.37–3.65; P=0.001; Figure 3) with no heterogeneity (I2=0.0%; P=0.371; Figure 3). Egger's test suggested no evidence of publication bias (P=0.795).
Figure 3.
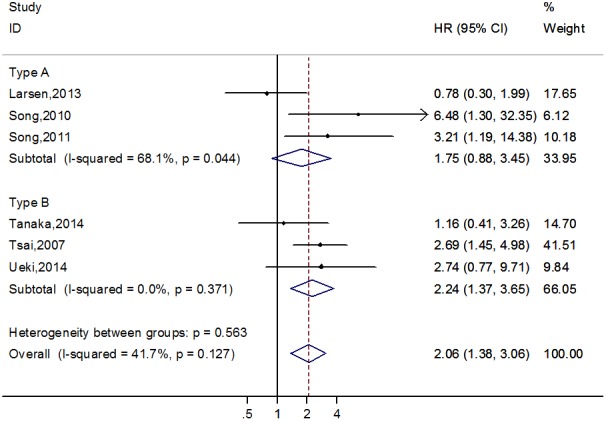
Forest plot demonstrating the association between partial thrombosis of false lumen and long‐term mortality in acute aortic dissection patients.
Long‐term aortic events
Three studies17, 18, 28 reported that a residual patent false lumen was significantly associated with an increased risk of long‐term aortic events (n=832; HR, 5.43; 95% CI, 2.95–9.99; P<0.001) without significant heterogeneity (I2=0.0%; P=0.566). One study reported an HR for long‐term aortic events with residual patent false lumen in AAD type A cases of 4.11 (95% CI, 1.85–9.16; P=0.001), and 2 studies reported that a residual patent false lumen was a predictor of aortic events in type B cases (HR, 7.97; 95% CI, 3.11–20.41; P<0.001) with no significant heterogeneity (I2=0.0%; P=0.852).
Subgroup Analysis
Subgroup analysis showed that in AAD type A, partial thrombosis of the false lumen was associated with an increased risk of long‐term mortality in the subgroup of studies examining Asian populations, with a retrospective cohort study design, sample size <200, and follow‐up <5 years. For AAD type B, partial thrombosis of the false lumen was not an independent predictor of long‐term mortality in the Asian subgroup with a sample size <200 (Table 3).
Table 3.
Subgroup Analyses of Partial Thrombosis of False Lumen and Risk of Long‐Term Mortality in Acute Aortic Dissection
| Factors | No. | Type A Acute Aortic Dissection | No. | Type B Acute Aortic Dissection | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | I2 | Heterogeneity, P Value | HR (95% CI) | P Value | I2 | Heterogeneity, P Value | |||
| Region | ||||||||||
| Asia | 2 | 4.18 (1.56–11.19) | 0.004 | 0.0 | 0.498 | 2 | 1.65 (0.72–3.76) | 0.238 | 5.5 | 0.304 |
| Europe | 0 | — | — | — | — | 1 | 2.69 (1.45–4.98) | 0.002 | — | — |
| Multicenters | 1 | 0.78 (0.30–1.99) | 0.600 | — | — | 0 | — | — | — | — |
| Design | ||||||||||
| Prospective | 2 | 2.02 (0.26–15.98) | 0.503 | 79.8 | 0.026 | 3 | 2.24 (1.37–3.65) | 0.001 | 0.0 | 0.371 |
| Retrospective | 1 | 3.21 (1.19–14.38) | 0.035 | — | — | 0 | — | — | — | — |
| Sample size | ||||||||||
| More than 200 | 1 | 0.78 (0.3–1.99) | 0.600 | — | — | 2 | 2.70 (1.55–4.70) | <0.001 | 0.0 | 0.980 |
| Less than 200 | 2 | 4.18 (1.56–11.19) | 0.004 | 0.0 | 0.498 | 1 | 1.16 (0.41–3.26) | 0.776 | — | — |
| Follow‐up, y | ||||||||||
| More than 5 | 1 | 0.78 (0.3–1.99) | 0.600 | — | — | 0 | — | — | — | — |
| Less than 5 | 2 | 4.18 (1.56–11.19) | 0.004 | 0.0 | 0.498 | 3 | 2.24 (1.37–3.65) | 0.001 | 0.0 | 0.371 |
HR indicates hazard ratio.
Discussion
There is rapidly growing interest in the association between the status of the false lumen and risk of poor outcomes in AAD. Our meta‐analysis of 11 cohort studies provides evidence that a residual patent false lumen is significantly and independently associated with an increased risk of long‐term mortality and aortic events in both type A and type B AAD, as compared with complete thrombosis of the false lumen. In addition, partial thrombosis of the false lumen appears to be an independent predictor of long‐term mortality in type B AAD, when compared with a completely patent false lumen; this association was not observed in type A cases (Figure 4).
Figure 4.
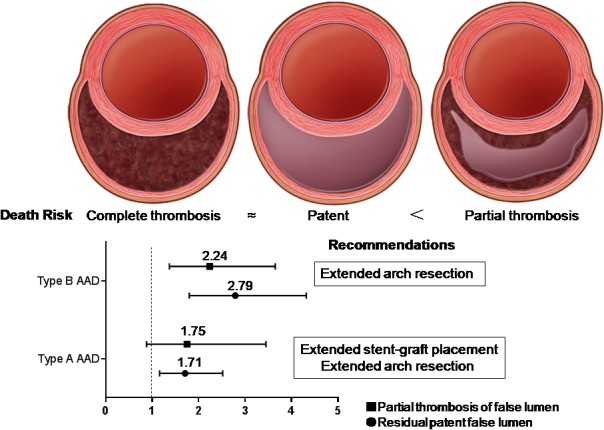
Summary of recommendations based on the meta‐analysis. AAD indicates acute aortic dissection. The figure of aortic false lumen with complete thrombosis, patent and partial thrombosis from Tsai et al.20
Several studies have investigated the association between a patent false lumen and long‐term outcomes in AAD. A large prospective cohort study indicated that preoperative false lumen patency influenced outcomes over a 6.8‐year follow‐up in AAD type A, with decreased survival rates (HR, 1.70; P=0.012) and an increase in the number of distal aortic events (HR, 4.11; P=0.001), controlling for conventional risk factors.17 Another 3 small sample‐size observational studies showed an independent association between the presence of a patent false lumen and long‐term mortality and aortic events in AAD type B.18, 19, 28 However, 1 retrospective cohort study indicated that postoperative false lumen patency was independently related to 5‐year mortality for type A cases, whereas preoperative false lumen patency was not a predictor of mortality.21 Postoperative false lumen patency may be a relatively better predictor of long‐term outcomes for AAD type A patients.
In AAD type A, the IRAD data reported that the rates for overall 5‐year survival and major adverse events were not influenced by false lumen thrombosis (using the log‐rank test).22 Conversely, another 2 studies, both having relatively small sample sizes, showed that the presence of a partial false lumen was an independent predictor of long‐term mortality, compared with a completely patent false lumen, after adjusting for confounding factors.26, 27 Our meta‐analysis indicated that partial false lumen thrombosis is not predictive of a greater risk of long‐term death in AAD type A; however, in the subgroup of Asian studies having a sample size <200, and a follow‐up <5 years, partial false lumen thrombosis was an independent predictor of poor long‐term survival in type A cases. For type B AAD, the IRAD data and our meta‐analysis have both shown that partial thrombosis of the false lumen, as compared with complete patency, is a significant independent predictor of long‐term postdischarge mortality in type B patients.20 On the contrary, 2 other studies29, 30 have suggested that the false lumen status does not influence long‐term mortality in type B AAD. The statistical power of these tests was limited because of the relatively small number of included studies. Further well‐designed trials are warranted to confirm this association.
The underlying mechanisms involved in the association between the false lumen status and poor survival remain uncertain. Several possible contributing factors have been suggested. Complete thrombosis of the false lumen has been thought to be a prerequisite for healing of the aorta postdissection, given that flow and pressurization of the false lumen are thought to contribute to late dilation and rupture.20 Therefore, a patient with complete thrombosis of the false lumen could be expected to have an improved survival rate during follow‐up. A patent false lumen may be perfused by a proximal entry tear and decompressed through distal reentry tears. Subsequently, partial thrombosis can occlude these distal tears, impeding outflow and resulting in a blind sac, in the most extreme of situations.20, 31 An increase in pressure in the false lumen will result in an increase in wall tension and elevates the risk of aneurysm expansion, redissection, and rupture; thus, mortality is expected to be increased in these patients.20, 32 Additionally, altered hemodynamics have been correlated with AAD progression. False lumen thrombus has been observed to be associated with areas of low velocity and complex flow, but does not appear to be protective from aortic expansion.33 This type of flow is known to cause stasis of the blood and endothelial activation, with initiation of thrombus formation.34 Four‐dimensional flow‐cardiac magnetic resonance is able to accurately assess blood flow at low velocities and consequently can differentiate it from false thrombosis. Furthermore, blood‐flow quantification and velocity mapping can demonstrate bidirectional flow within a false lumen that exhibits partial thrombosis. This turbulent or helical flow can induce aortic wall shear stress, which may be associated with an elevated risk of aneurysmal dilatation or tear.33, 35, 36 Therefore, partial thrombosis of the false lumen in patients with AAD may be reflective of a clinical context in which multiple risk factors exist.
The goals of open AAD repair and endovascular aneurysm repair are to prevent lethal complications, resect the entry tear, and redirect the blood flow to the true lumen.22 Blood flow through the false lumen will be altered by resection of the primary tear and aortic reconstruction, because thrombosis of the false lumen is promoted.22 Thus, the false lumen status may be altered postoperatively. After initial repair, the distal false lumen remains patent in as many as 79% of AAD patients with a type A dissection. In the present study, for type B AAD, partial false lumen thrombosis was observed to be associated with an increase in late mortality; however, this association was not observed in type A cases. Patients with type B AAD receiving stent graft therapy may exhibit partial thrombosis of the false lumen, whereby the thoracic false lumen has complete thrombosis along the length of the stent, but the abdominal false lumen remains patent. Therefore, it can be concluded that the stent graft landing zone should be left in place to protect from cases where aneurysmal dilatation or tear occurs in the distal aorta, when using stent graft therapy for treatment of type B AAD. Resection of the primary tear and aortic reconstruction will also alter flow in the false lumen and might promote thrombosis. Thus, an extended arch resection in type A repairs should be performed, such that the proximal Dacron or stent graft landing zone is left in place to account for cases of significant aneurysmal dilatation in the distal aorta. Song et al.26 found that surgical extent was the only risk factor for the presence of residual false lumen and partial thrombosis after surgery for DeBakey type I AAD. Furthermore, in their study, total arch replacement had a lower incidence of partial thrombosis at the proximal descending thoracic aortic level and showed favorable outcomes for aortic enlargement and aortic reintervention, when compared with ascending or hemiarch replacement. Although it remains unclear whether extended arch resection can improve late survival, we believe that the surgical extent during initial repair is a determinant of the natural history of the distal aorta after surgery for AAD type A (Figure 4).
Substantial heterogeneity was observed among studies investigating partial false lumen thrombosis and long‐term survival among patients with AAD type A. Subgroup analysis showed that 1 study with a relatively large sample size contributed to the heterogeneity observed. Among the patients in this study, the partial and complete false lumen thrombosis groups were small, with only 16% and 4.6% of patients having partial thrombosis or complete thrombosis of the false lumen, respectively. Because of the small number of patients, adjusted comparisons with the partial thrombosis group lacked statistical power in this study.
This study has several limitations. First, the meta‐analysis included a limited number of eligible studies, which made it more difficult to detect heterogeneity between studies related to the association between partial false lumen thrombosis and poor outcomes in AAD type A. Subgroup analysis included different groups, based on influencing factors, which lacked statistical power because of a limited number of studies. Second, a number of studies that were unadjusted for confounding factors were excluded from the meta‐analysis, which may have introduced bias. Third, some studies reported all‐cause mortality and did not provide AAD‐associated long‐term mortality. Consequently, the lack of original information may have caused an overestimation of the proportion of cardiovascular deaths in both the complete thrombosis and completely patent false lumen groups, given that the pooled HR would be negative. Fourth, our results are intrinsically related to time, and the death rate would be expected to change with varying lengths of follow‐up. Thus, the results observed in this study may be limited by the fact that the included studies did not have a uniform follow‐up duration.
Conclusions
The status of the false lumen is associated with long‐term outcomes in AAD. A residual patent false lumen is an independent predictor of long‐term mortality and aortic events in both type A and type B AAD, when compared with complete false lumen thrombosis. In addition, partial false lumen thrombosis is independently associated with long‐term mortality in type B AAD; however, this association was not observed in type A dissections. This observation supports the suggestion that the stent graft landing zone should be left in place to account for cases where aneurysmal dilatation or tear occurs in the distal aorta, when performing stent graft therapy for treatment of type B AAD. Furthermore, extended arch resection may provide an avenue for improving late survival in AAD type A patients, where significant postoperative aneurysmal dilatation is observed.
Sources of Funding
This work was supported financially by grants from the National Natural Science Foundation of China (No. 81200153).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003172 doi: 10.1161/JAHA.115.003172)
References
- 1. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. 2003;108:628–635. [DOI] [PubMed] [Google Scholar]
- 2. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 3. Tolenaar JL, Froehlich W, Jonker FH, Upchurch GR Jr, Rampoldi V, Tsai TT, Bossone E, Evangelista A, O'Gara P, Pape L, Montgomery D, Isselbacher EM, Nienaber CA, Eagle KA, Trimarchi S. Predicting in‐hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;130:S45–S50. [DOI] [PubMed] [Google Scholar]
- 4. Tsai TT, Fattori R, Trimarchi S, Isselbacher E, Myrmel T, Evangelista A, Hutchison S, Sechtem U, Cooper JV, Smith DE, Pape L, Froehlich J, Raghupathy A, Januzzi JL, Eagle KA, Nienaber CA. Long‐term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226–2231. [DOI] [PubMed] [Google Scholar]
- 5. Afifi RO, Sandhu HK, Leake SS, Boutrous ML, Kumar V III, Azizzadeh A, Charlton‐Ouw KM, Saqib NU, Nguyen TC, Miller CC III, Safi HJ, Estrera AL. Outcomes of patients with acute type B (DeBakey III) aortic dissection: a 13‐year, single‐center experience. Circulation. 2015;132:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umana JP, Lai DT, Mitchell RS, Moore KA, Rodriguez F, Robbins RC, Oyer PE, Dake MD, Shumway NE, Reitz BA, Miller DC. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg. 2002;124:896–910. [DOI] [PubMed] [Google Scholar]
- 7. Lansman SL, McCullough JN, Nguyen KH, Spielvogel D, Klein JJ, Galla JD, Ergin MA, Griepp RB. Subtypes of acute aortic dissection. Ann Thorac Surg. 1999;67:1975–1978. [DOI] [PubMed] [Google Scholar]
- 8. LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113. [DOI] [PubMed] [Google Scholar]
- 9. Tsai TT, Evangelista A, Nienaber CA, Trimarchi S, Sechtem U, Fattori R, Myrmel T, Pape L, Cooper JV, Smith DE, Fang J, Isselbacher E, Eagle KA. Long‐term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114:I350–I356. [DOI] [PubMed] [Google Scholar]
- 10. Chiappini B, Schepens M, Tan E, Dell' Amore A, Morshuis W, Dossche K, Bergonzini M, Camurri N, Reggiani LB, Marinelli G, Di Bartolomeo R. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J. 2005;26:180–186 [DOI] [PubMed] [Google Scholar]
- 11. Mehta RH, O'Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, Smith DE, Armstrong WF, Isselbacher EM, Pape LA, Eagle KA, Gilon D. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–692. [DOI] [PubMed] [Google Scholar]
- 12. Olsson C, Eriksson N, Stahle E, Thelin S. Surgical and long‐term mortality in 2634 consecutive patients operated on the proximal thoracic aorta. Eur J Cardiothorac Surg. 2007;31:963–969; discussion 969. [DOI] [PubMed] [Google Scholar]
- 13. Rylski B, Hoffmann I, Beyersdorf F, Suedkamp M, Siepe M, Nitsch B, Blettner M, Borger MA, Weigang E. Acute aortic dissection type a: age‐related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg. 2014;259:598–604. [DOI] [PubMed] [Google Scholar]
- 14. Trimarchi S, Eagle KA, Nienaber CA, Rampoldi V, Jonker FH, De Vincentiis C, Frigiola A, Menicanti L, Tsai T, Froehlich J, Evangelista A, Montgomery D, Bossone E, Cooper JV, Li J, Deeb MG, Meinhardt G, Sundt TM, Isselbacher EM. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140:784–789. [DOI] [PubMed] [Google Scholar]
- 15. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, Bossone E, Fang J, Smith DE, Isselbacher EM, Pape LA, Eagle KA. Gender‐related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. [DOI] [PubMed] [Google Scholar]
- 16. Sakakura K, Kubo N, Ako J, Fujiwara N, Funayama H, Ikeda N, Nakamura T, Sugawara Y, Yasu T, Kawakami M, Momomura S. Determinants of long‐term mortality in patients with type B acute aortic dissection. Am J Hypertens. 2009;22:371–377. [DOI] [PubMed] [Google Scholar]
- 17. Kimura N, Itoh S, Yuri K, Adachi K, Matsumoto H, Yamaguchi A, Adachi H. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2015;149:S91–S98. [DOI] [PubMed] [Google Scholar]
- 18. Akutsu K, Nejima J, Kiuchi K, Sasaki K, Ochi M, Tanaka K, Takano T. Effects of the patent false lumen on the long‐term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg. 2004;26:359–366. [DOI] [PubMed] [Google Scholar]
- 19. Marui A, Mochizuki T, Koyama T, Mitsui N. Degree of fusiform dilatation of the proximal descending aorta in type B acute aortic dissection can predict late aortic events. J Thorac Cardiovasc Surg. 2007;134:1163–1170. [DOI] [PubMed] [Google Scholar]
- 20. Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, Smith DE, Suzuki T, Fattori R, Llovet A, Froehlich J, Hutchison S, Distante A, Sundt T, Beckman J, Januzzi JL Jr, Isselbacher EM, Eagle KA. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349–359. [DOI] [PubMed] [Google Scholar]
- 21. Bernard Y, Zimmermann H, Chocron S, Litzler JF, Kastler B, Etievent JP, Meneveau N, Schiele F, Bassand JP. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001;87:1378–1382. [DOI] [PubMed] [Google Scholar]
- 22. Larsen M, Bartnes K, Tsai TT, Eagle KA, Evangelista A, Nienaber CA, Suzuki T, Fattori R, Froehlich JB, Hutchison S, Sundt TM, Januzzi JL, Isselbacher EM, Montgomery DG, Myrmel T. Extent of preoperative false lumen thrombosis does not influence long‐term survival in patients with acute type A aortic dissection. J Am Heart Assoc. 2013;2:e000112 doi: 10.1161/JAHA.113.000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 24. Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA. 1999;282:1054–1060. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song SW, Chang BC, Cho BK, Yi G, Youn YN, Lee S, Yoo KJ. Effects of partial thrombosis on distal aorta after repair of acute DeBakey type I aortic dissection. J Thorac Cardiovasc Surg. 2010;139:841–847.e841; discussion 847 [DOI] [PubMed] [Google Scholar]
- 27. Song SW, Yoo KJ, Kim DK, Cho BK, Yi G, Chang BC. Effects of early anticoagulation on the degree of thrombosis after repair of acute DeBakey type I aortic dissection. Ann Thorac Surg. 2011;92:1367–1374. [DOI] [PubMed] [Google Scholar]
- 28. Miyahara S, Mukohara N, Fukuzumi M, Morimoto N, Murakami H, Nakagiri K, Yoshida M. Long‐term follow‐up of acute type B aortic dissection: ulcer‐like projections in thrombosed false lumen play a role in late aortic events. J Thorac Cardiovasc Surg. 2011;142:e25–e31. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka A, Sakakibara M, Ishii H, Hayashida R, Jinno Y, Okumura S, Okada K, Murohara T. Influence of the false lumen status on clinical outcomes in patients with acute type B aortic dissection. J Vasc Surg. 2014;59:321–326. [DOI] [PubMed] [Google Scholar]
- 30. Ueki C, Sakaguchi G, Shimamoto T, Komiya T. Prognostic factors in patients with uncomplicated acute type B aortic dissection. Ann Thorac Surg. 2014;97:767–773; discussion 773. [DOI] [PubMed] [Google Scholar]
- 31. Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, Swedenborg J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. Ann Surg. 2003;38:1283–1292. [DOI] [PubMed] [Google Scholar]
- 32. Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, Webster MW. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. Ann Surg. 2001;34:291–299. [DOI] [PubMed] [Google Scholar]
- 33. Clough RE, Waltham M, Giese D, Taylor PR, Schaeffter T. A new imaging method for assessment of aortic dissection using four‐dimensional phase contrast magnetic resonance imaging. Ann Surg. 2012;55:914–923. [DOI] [PubMed] [Google Scholar]
- 34. Aird WC. Vascular bed‐specific thrombosis. J Thromb Haemost. 2007;5(suppl 1):283–291. [DOI] [PubMed] [Google Scholar]
- 35. Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, Higgins CB. 4D flow CMR in assessment of valve‐related ascending aortic disease. JACC Cardiovasc Imaging. 2011;4:781–787. [DOI] [PubMed] [Google Scholar]
- 36. Francois CJ, Markl M, Schiebler ML, Niespodzany E, Landgraf BR, Schlensak C, Frydrychowicz A. Four‐dimensional, flow‐sensitive magnetic resonance imaging of blood flow patterns in thoracic aortic dissections. J Thorac Cardiovasc Surg. 2013;145:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]


