Abstract
Background
The goal of this study was to compare the safety and effectiveness of individual antiembolic interventions in nonvalvular atrial fibrillation (AF): novel oral anticoagulants (NOACs) (apixaban, dabigatran, edoxaban, and rivaroxaban); vitamin K antagonists (VKA); aspirin; and the Watchman device.
Methods and Results
A network meta‐analysis of randomized, clinical trials (RCTs) was performed. RCTs that included patients with prosthetic cardiac valves or mitral stenosis, mean or median follow‐up <6 months, <200 participants, without published report in English language, and NOAC phase II studies were excluded. The placebo/control arm received either placebo or no treatment. The primary efficacy outcome was the combination of stroke (of any type) and systemic embolism. All‐cause mortality served as a secondary efficacy outcome. The primary safety outcome was the combination of major extracranial bleeding and intracranial hemorrhage. A total of 21 RCTs (96 017 nonvalvular AF patients; median age, 72 years; 65% males; median follow‐up, 1.7 years) were included. In comparison to placebo/control, use of aspirin (odds ratio [OR], 0.75 [95% CI, 0.60–0.95]), VKA (0.38 [0.29–0.49]), apixaban (0.31 [0.22–0.45]), dabigatran (0.29 [0.20–0.43]), edoxaban (0.38 [0.26–0.54]), rivaroxaban (0.27 [0.18–0.42]), and the Watchman device (0.36 [0.16–0.80]) significantly reduced the risk of any stroke or systemic embolism in nonvalvular AF patients, as well as all‐cause mortality (aspirin: OR, 0.82 [0.68–0.99]; VKA: 0.69 [0.57–0.85]; apixaban: 0.62 [0.50–0.78]; dabigatran: 0.62 [0.50–0.78]; edoxaban: 0.62 [0.50–0.77]; rivaroxaban: 0.58 [0.44–0.77]; and the Watchman device: 0.47 [0.25–0.88]). Apixaban (0.89 [0.80–0.99]), dabigatran (0.90 [0.82–0.99]), and edoxaban (0.89 [0.82–0.96]) reduced risk of all‐cause death as compared to VKA.
Conclusions
The entire spectrum of therapy to prevent thromboembolism in nonvalvular AF significantly reduced stroke/systemic embolism events and mortality.
Keywords: anticoagulation, atrial fibrillation, comparative effectiveness, left atrial appendage, nonvalvular, oral anticoagulants, stroke, vitamin K antagonists, watchman
Subject Categories: Atrial Fibrillation, Meta Analysis, Cerebrovascular Disease/Stroke, Anticoagulants, Treatment
Introduction
Atrial fibrillation (AF) significantly increases the risk of stroke and system thromboembolism1 and is associated with substantial stroke‐related morbidity and mortality.2 Antithrombotic therapy is a standard of care for stroke prevention in AF3 in selected patients, stratified by the risk scores (CHADS2, CHA2DS2‐VASc, and HAS‐BLED).3 Recently, several nonvitamin K oral anticoagulants (NOACs) demonstrated equivalent or superior efficacy and safety, with greater convenience, as compared to vitamin K antagonists (VKA) treatment (eg, warfarin)4 and shifted the paradigm of stroke prevention in AF.5 The NOACs are represented by two drug classes: the oral direct thrombin inhibitors (eg, dabigatran) and the oral Factor Xa inhibitors (eg, apixaban, edoxaban, and rivaroxaban). In addition, a mechanical left atrial appendage (LAA) occlusion device is now available for stroke prevention in AF.6, 7 With 4 currently approved NOACs among other pharmacological and nonpharmacologic options, it is challenging to compare the safety and efficacy of individual agents in order to identify the optimal stroke prevention strategy or provide data for clinicians, patients, and policy makers to make informed decisions. Notably, the comparative effectiveness of available NOACs and the LAA occlusion device, as well as aspirin and VKA, is unknown, because direct comparisons among the many alternatives have not been performed in randomized, clinical trials (RCTs).
In the absence of RCTs, several recent meta‐analyses8, 9, 10, 11 compared the effectiveness of NOACs (as a group) and VKAs. At the same time, very few indirect comparisons12, 13, 14, 15, 16 of individual NOAC agents with the LAA occlusion device was performed. Previously conducted indirect comparison analyses did not rank the interventions, and adjusted for RCT population characteristics comparison with LAA occlusion device was not performed. The goal of this study was to compare, by way of a network meta‐analysis, the relative effectiveness of several antithrombotic drug therapies as well as the LAA occlusion device for stroke prevention in nonvalvular AF.
Methods
The study conformed to principles outlined in the Declaration of Helsinki and The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta‐analyses of Health Care Interventions.17
Eligibility Criteria
A study was considered eligible if it was an RCT that enrolled patients with nonvalvular AF and presented efficacy and safety outcomes data. RCTs that included patients with prosthetic cardiac valves or mitral stenosis, mean or median follow‐up <6 months, <200 participants, and NOAC phase II studies were not considered. We included RCTs that tested the following antithrombotic interventions: aspirin, VKA, 4 NOACs (apixaban, dabigatran, edoxaban, and rivaroxaban), and the Watchman LAA occlusion device. We excluded RCTs with a high probability of bias and RCTs without published reports in the English language. No treatment (control) arm or placebo arm were considered together as a single placebo/control comparator.
Study Outcomes
The combined outcome of stroke (both embolic and hemorrhagic) and systemic embolism served as a primary efficacy outcome. Transient ischemic attacks were not included. All‐cause mortality served as a secondary efficacy outcome. The primary safety outcome was a combined outcome of major extracranial bleeding and intracranial hemorrhage (including epidural, subdural, and subarachnoid hemorrhage) or major Watchman device implantation–related complications.
Selection of Studies, Extraction of Data, and Assessment of Data Quality
We searched Medline from 1966 to August 2015, as well as screened and cross‐checked relevant systematic reviews and meta‐analyses. Two physician‐reviewers (L.T. and C.H.) identified eligible studies and abstracted key features of included RCTs. Quality of the data was analyzed using the Cochrane Collaboration Risk of Bias Tool. We evaluated the quality of RCTs focusing on selection bias (method of randomization, allocation concealment), information bias (masking of outcome adjudicators), and bias in the analysis (intention to treat analysis, completeness of follow‐up). The overall risk of bias was determined as low (all analyzed items were appropriate, or at least 5 items were appropriate and the remaining 2 unclear), unclear (>2 items were not reported), and high (≥1 quality dimension suggested possible bias). Two studies (ESPS II18 and LASAF19) were excluded because of high risk of bias.
Statistical Analysis
A multiple treatment comparison network meta‐analysis (NMA) was conducted that included both the direct RCT comparisons and also indirect comparisons of treatments. Both direct and indirect RCT comparisons were performed using STATA software (version 14; StataCorp LP, College Station, TX) mvmeta with NMA graphical tools by Chaimani et al.20 Multivariate random‐effect meta‐analysis and multivariate random‐effect meta‐regression was performed on a data set of point estimates, variances, and covariances. The unadjusted and adjusted analysis was performed. Meta‐regression was adjusted for RCT population characteristics (mean/median CHADS2 score, time in therapeutic range [TTR], and duration of follow‐up) and properly accounted for correlations between effect sizes from multiarm studies. For the contribution assessment, the direct estimates were derived using a comparison‐specific random‐effects model. If a comparison was informed by less than 2 studies, a fixed‐effects model was used.
In order to evaluate inconsistency between direct and indirect effect estimates for the same comparison, we evaluated each closed loop in the network. Only triangular (formed by 3 treatments all compared with one another) loops were considered. There was no quadratic loop in our network. In each loop, we estimated the inconsistency factor (IF) as the absolute difference (with 95% CI and a z‐test21) between direct and indirect estimates for each paired comparison in the loop. IF is the logarithm of the ratio of two odds ratios (RoR) from direct and indirect evidence in the loop; RoR values close to 1 indicate that the 2 sources are in agreement. A comparison‐adjusted funnel plot was used to assess the presence of small‐study effect.22 Tau‐squared (an estimate of the between‐study variance in a random‐effects meta‐analysis) estimated SD of underlying effects across studies. The empirical Bayes method was used for estimation of loop‐specific heterogeneity.
In addition, to address potential heterogeneity attributed to a wide range of years in which the studies were conducted (1990s–2010s), we evaluated the comparison‐adjusted funnel plots. Funnel plot is a scatterplot of the study effect size versus a measure of its precision (inverted SE). To ensure appropriate comparison in the funnel plot, we ordered treatments in the data set from the oldest to newest.
Mean summary effects were presented together with their predictive intervals (PrI) to facilitate interpretation of the results in the light of the magnitude of heterogeneity. PrI provide an interval within which the estimate of a future study is expected to be.
Ranking of evaluated antithrombotic interventions was performed. The surface under the cumulative ranking curves (SUCRA) was used to provide a hierarchy of the treatments. SUCRA is a relative ranking measure that accounts for the uncertainty in treatment order, that is, accounts both for the location and the variance of all relative treatment effects.23 The larger the SUCRA value, the better the rank of the treatment.
In order to account for both efficacy and safety, we used multivariate methods to account for dependency between outcomes. Clustering methods and 2‐dimensional plots were used to produce clusters of treatments for all 3 outcomes. A hierarchical agglomerative clustering method evaluated different metrics (Euclidean, squared Euclidean, and absolute‐value distance) and linkage methods (single, average, weighted, complete, Ward, centroid, and median). The choice of the appropriate metric and linkage criterion was driven from the Cophenetic correlation coefficient, which measures how faithfully the output dendrogram represents the dissimilarities among observations.24 To choose the optimal level of dendrogram and define the optimal number of resulting partitions, an internal cluster validation measure was used, which is based on a value of “clustering gain.” Clustering gain has been designed to have a maximum value when intracluster similarity is maximized and the intercluster similarity is minimized.25
Sensitivity analysis was conducted excluding RCTs with a combination of anticoagulants (aspirin with a small, stable dose of VKA; aspirin with clopidogrel) and with control instead of placebo.
Results
Evidence Base
A total of 21 RCTs with 29 study arms were included in this NMA. These studies included 96 017 nonvalvular AF patients with a median age of 71.5 years; 65% were males. Median length of follow‐up was 1.7 years. Clinical characteristics of the included RCT populations are reported in Table 1.6, 7, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Quality indicators of the included studies are described in Table 2.
Table 1.
Baseline Characteristics of the Intention‐to‐Treat Populations of the Included Trials
| Study, Year | Intervention | Comparator | F/U, y | Age, Int/Comp, y | Men, Int/Comp, % | CHADS2; Int/Comp, mean | VKA‐Naïve; Int/Comp, % | 2° Prev; Int/Comp, % | TTR | Participants, Int/Comp, n/n | SSE, Int/Comp, n/n | Mortality, Int/Comp, n/n | Major Bleeding, Int/Comp, n/n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARISTOTLE,26 2011 | Apixaban 2.5 to 5 mg |
VKA INR 2 to 3 |
1.8 | 70/70 | 64.5/65.0 | 2.1/2.1 | 42.9/42.8 | 19.2/19.7 | 66 | 9120/9081 | 214/267 | 603/669 | 327/462 |
| AVERROES,27 2011 | Apixaban 2.5 to 5 mg | ASA | 1.1 | 70/70 | 59/58 | 2.0/2.1 | 85.7/84.7 | 13.8/13.4 | n/a | 2808/2791 | 51/113 | 111/140 | 44/39 |
| ENGAGE AF‐TIMI,28 2013 | Edoxaban 60 mg |
VKA INR 2 to 3 |
2.8 | 72/72 | 61.1/62.5 | 2.8/2.8 | 41.2/41.2 | 28.1/28.3 | 68.4 | 7035/7036 | 296/340 | 773/839 | 418/524 |
| Edoxaban 30 mg |
VKA INR 2 to 3 |
2.8 | 72/72 | 61.2/62.5 | 2.8/2.8 | 40.8/41.2 | 28.5/28.3 | 68.4 | 7034/7036 | 389/340 | 737/839 | 254/524 | |
| JROCKET,29 2012 | Rivaroxaban 15 mg |
VKA INR 2 to 2.6 or 3 |
1.3 | 71.0/71.2 | 82.9/78.2 | 3.27/3.22 | 9.7/10.3 | 63.8/63.4 | 65 | 640/640 | 11/22 | 7/5 | 26/33 |
| ROCKET AF,30 2011 | Rivaroxaban 20 mg |
VKA INR 2 to 3 |
1.62 | 73/73 | 60.3/60.3 | 3.48/3.46 | 37.7/37.5 | 54.9/54.6 | 58 | 7131/7133 | 188/241 | 208/250 | 395/386 |
| RE‐LY,31 2009 | Dabigatran 150 mg |
VKA INR 2 to 3 |
2.0 | 71.5/71.6 | 63.2/63.3 | 2.2/2.1 | 49.8/51.4 | 20.3/19.8 | 64 | 6076/6022 | 134/199 | 438/487 | 375/397 |
| Dabigatran 110 mg |
VKA, INR 2 to 3 |
2.0 | 71.4/71.6 | 64.3/63.3 | 2.1/2.1 | 49.9/51.4 | 19.9/19.8 | 64 | 6015/6022 | 182/199 | 446/487 | 322/397 | |
| AFASAK I,32, 33 1989, 1990; 3 arms |
VKA 2.4 to 4.2 |
ASA 75 mg/day, Placebo | 1.2 | 72.8/75.1/74.6 | 53/55/54 | 1.5/1.7/1.6a | 100/100/100 | 6/5/6 | 73 | 335/336/336 | 10/19/22 | 3/14/16b | 13/4/3 |
| BAATAF,34 1990 |
VKA INR 1.5 to 2.7 |
Control | 2.2 | 68.5/67.5 | 75/70 | 1.4/1.5a | 100/100 | 3/3 | 83 | 212/208 | 3/13 | 11/26 | 2/1 |
|
SPAF I,35 1991 3 arms |
VKA INR 2 to 4.5 |
ASA 325 mg/day, Placebo | 1.3 | 65/67/67 | 74/71/70 | 1.1/1.3/1.4a | 100/100/100 | 8/6/7 | 71 | 210/552/568 | 6/26/46 | 6/39/50 | 4/10/14 |
| CAFA,36 1991 |
VKA INR 2 to 3 |
Placebo | 1.3 | 68/67.4 | 75.9/73.3 | 1.15/1.0a | 100/100 | 3.2/4.2 | 44 | 187/191 | 6/11 | 10/8 | 5/2 |
| SPINAF,371992 |
VKA INR 1.4 to 2.8 |
Placebo | 1.7 | 67/67 | 100/100 | 1.13/1.24a | 100/100 | 18/21 | 71 | 281/290 | 8/24 | 20/26 | 6/4 |
|
EAFT,38 1993 3 arms |
VKA INR 2.5 to 4 |
ASA 300 mg/day, Placebo | 2.3 | 71/73/73 | 55/59/53 | 3.26/3.4/3.41a | 100/100/100 | 100/100/100 | 62 | 225/404/378 | 21/94/99 | 41/102/99 | 13/6/4 |
| SPAF II,39, 40 1994 |
VKA INR 2 to 4.5 |
ASA 325 mg/day | 2.3 | 72.5/72 | 67/67 | 1.35/1.3 | 90/90 | 7.5/8 | 73.5 | 555/545 | 28/39 | 62/65 | 32/13 |
| SPAF III,41 1996 |
VKA INR 2 to 3 |
ASA 325 mg/dayc | 1.1 | 71/72 | 59/62 | 2.27/2.62a | 56/56 | 36/40 | 61 | 523/521 | 11/44 | 35/42 | 12/13 |
| AFASAK II,42 1998 |
VKA INR 2 to 3 |
ASA 300 mg/dayc | 2.2 | 73.2/72.9 | 57/62 | 2.47/2.45a | 100/100 | 8/10.5 | 73 | 170/340 | 12/22 | 17/23 | 4/6 |
| PATAF,43 1999 |
VKA, INR 2.5 to 3.5 |
ASA 150 mg/dayc | 2.7 | 70/75.2 | 44/45 | 0.5/1.67a | 100/100 | 0/0 | 48 | 131/598 | 4/41 | 12/107 | 2/21 |
| SAFT,44 2003 | ASA 75 mg/dayc | Control | 2.75 | 72/73 | 64/61 | 1/1a | 100/100 | 0/0 | n/a | 334/334 | 37/46 | 31/36 | 19/4 |
| JAST,45 2006 | ASA 150 to 200 mg/day | Control | 2.1 | 65.5/64.8 | 71.1/69.7 | 1.9/2.14a | 93/91.5 | 2.6/2.5 | n/a | 426/445 | 20/21 | 10/9 | 7/2 |
| ACTIVE‐W,46 2006 |
VKA, INR 2 to 3 |
ASA 75 to 100 mg/day+clopidogrel 75 mg/day | 1.3 | 70.2/70.2 | 66/67 | 2.0/2.0 | 22/24 | 15/15 | 63.8 | 3371/3335 | 63/118 | 158/159 | 93/101 |
| PROTECT‐AF,6 2009 | Watchman | VKA | 1.5 | 71.7/72.7 | 70.4/70.1 | 2.17/2.34 | 0/0 | 17.7/20.1 | 66 | 463/244 | 18/12 | 21/18 | 49/16d |
| PREVAIL,7 2014 | Watchman | VKA | 1 | 74.0/74.9 | 67.7/74.6 | 2.6/2.6 | 0/0 | 27.5/28.3 | 68 | 269/138 | 7/1 | 7/3 | 29/7d |
Mean or median values (percentages) are reported. ASA indicates aspirin; Comp, comparator; INR, international normalized ratio therapeutic range; Int, intervention; qod, every other day; TTR, time in therapeutic range; VKA, vitamin K antagonists.
Estimated based on reported baseline characteristics.
Only vascular or unknown death.
Aspirin plus low‐dose unadjusted warfarin was considered as aspirin‐only intervention, because VKA dose was inefficacious reported.
Major bleeding or procedure‐related complications.
Table 2.
Risk of Bias in the Included Trials
| Study, Year | Intervention | Comparator | Selection Bias | Information Bias | Analysis Bias | Sum Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adequate Sequence Generation | Allocation Concealment | Masking (SSE Outcome | Masking (All‐Cause Mortality) | Masking (Major Bleeding) | Intention to Treat Analysis | Loss of Follow‐Up | ||||
| ARISTOTLE,26 2011 | Apixaban 2.5‐5mg | VKA | Low | Low | Low | Low | Low | Low | Unclear | Low |
| AVERROES,27 2011 | Apixaban 2.5‐5mg | ASA | Low | Low | Low | Low | Low | Low | Unclear | Low |
| ENGAGE AF‐TIMI,28 2013 | Edoxaban 30‐60mg | VKA | Low | Low | Low | Low | Low | Low | Low | Low |
| JROCKET,29 2012 | Rivaroxaban 15mg | VKA | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| ROCKET AF,30 2011 | Rivaroxaban 20mg | VKA | Low | Low | Low | Low | Low | Low | Low | Low |
| RE‐LY,31 2009 | Dabigatran110‐150 mg | VKA | Low | Low | Low | Low | Low | Low | Low | Low |
| AFASAK I,32,33 1989, 1990 | VKA | ASA, placebo | Low | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| BAATAF,34 1990 | VKA | control | Low | Unclear | Low | Low | Low | Low | Unclear | Low |
| SPAF I,35 1991 | VKA | ASA, placebo | Low | Unclear | Low | Low | Low | Low | Low | Low |
| CAFA,36 1991 | VKA | Placebo | Low | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| SPINAF,371992 | VKA | Placebo | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| EAFT,38 1993 | VKA | ASA, placebo | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| SPAF II,39 1994 | VKA | ASA | Low | Low | Low | Low | Low | Low | Low | Low |
| SPAF III,41 1996 | VKA | ASA | Low | Low | Low | Low | Low | Low | Low | Low |
| AFASAK II,42 1998 | VKA | ASA | Low | Unclear | Low | Low | Low | Low | Low | Low |
| PATAF,43 1999 | VKA | ASA | Low | Low | Low | Low | Low | Low | Low | Low |
| SAFT,44 2003 | ASA/VKA | Control | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| JAST,45 2006 | ASA | Control | Low | Low | Low | Low | Low | Low | Unclear | Low |
| ACTIVE‐W,46 2006 | VKA | ASA+clop | Low | Unclear | Low | Low | Low | Low | Low | Low |
| PROTECT‐AF,6 2009 | Watchman | VKA | Low | Low | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| PREVAIL,7 2014 | Watchman | VKA | Low | Low | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
ASA indicates aspirin; clop, clopidogrel; SSE, stroke or systemic embolism; VKA, vitamin K antagonists.
Figure 1 shows a network of stroke‐preventive interventions in patients with nonvalvular AF. VKA, aspirin, and placebo/control were more frequently compared directly. VKA is the most frequent comparator across the studies. The included NMA studies had low bias overall, with only 3 comparisons at unclear risk of bias.
Figure 1.
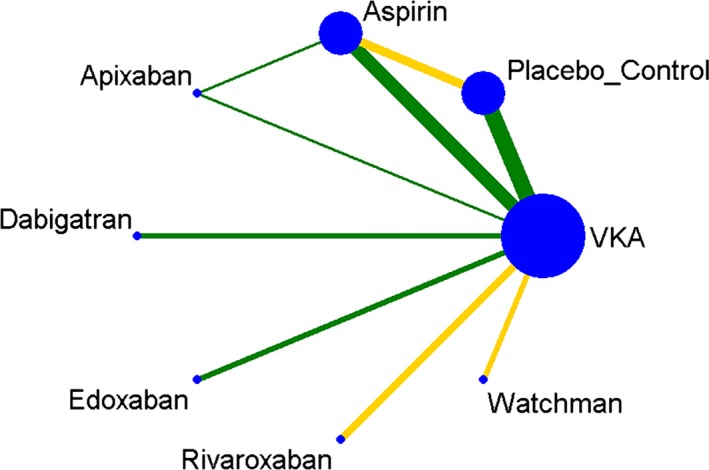
Plot of the study network. Nodes show interventions being compared, and edges represent an available direct comparison between pairs of interventions. Edges are according to the level of bias in the majority of included studies in each comparison (green=low; yellow=unclear) and are weighted according to the number of studies in each comparison. VKA indicates vitamin K antagonists.
The contribution of each direct comparison to the estimation of the network summary effects is shown in Figure 2. Four comparisons (dabigatran vs VKA, edoxaban vs VKA, rivaroxaban vs VKA, and the Watchman vs VKA) were informed by direct evidence alone. Five comparisons (apixaban vs aspirin, apixaban vs VKA, aspirin vs placebo/control, aspirin vs VKA, and placebo/control vs VKA) were informed by mixed (both direct and indirect) evidence. Nineteen comparisons were informed by indirect evidence alone. Overall, the contribution of all 9 direct comparisons in the network was balanced and comparable with an average of 11%, which assures valid and appropriate data synthesis.
Figure 2.
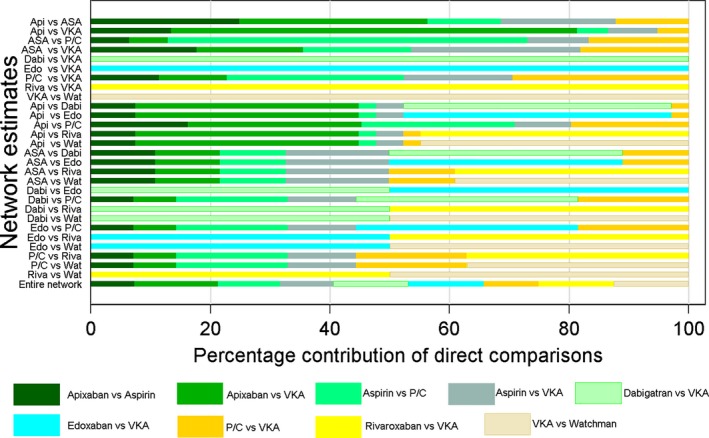
Contribution plot for each direct comparison in the network. Percentage contribution of each direct comparison to the network summary estimates and in the entire network. A bar graph shows the percentage of information in each network estimate that corresponds to the different levels of the characteristic. Bars are colored according to bias level (shades of green=low; shades of yellow=unclear), and their length is proportional to the percentage contribution of each direct comparison to the network estimates. Api indicates apixaban; ASA, aspirin; Dabi, dabigatran; Edo, edoxaban; P/C, placebo/control; Riva, rivaroxaban; VKA, vitamin K antagonists; Wat; watchman.
Assumptions of the Network Meta‐Analysis
There was no inconsistency between direct and indirect point estimates. In our network, there were 2 closed loops (Figure 3). All confidence intervals for RoRs were compatible with zero inconsistency (RoR=1) for all study outcomes.
Figure 3.

Inconsistency plot for the primary efficacy outcome stroke and systemic embolism (A and B), primary safety outcome major bleedings (C and D), and secondary efficacy outcome all‐cause mortality (E and F) for unadjusted (A, C, and E) and adjusted (B, D, and F) analyses. Forest plot shows the ratio of two odds ratios (RoR) from direct and indirect evidence in the loop. Confidence intervals are truncated at zero given that the direction of the inconsistency factor (IF) is unimportant. VKA indicates vitamin K antagonists.
Overall in our network, study size did not influence effect size (absence of a significant small study effects). Comparison‐adjusted funnel plots (Figure 4) for all study outcomes were symmetrical around the zero line. Adjustment for studies population characteristics (Figure 4B, 4D, and 4F) harmonized comparisons and decreased inconsistencies.
Figure 4.

Comparison‐adjusted funnel plots for the primary efficacy outcome stroke and systemic embolism (A and B), primary safety outcome major bleedings (C and D), and secondary efficacy outcome all‐cause mortality (E and F). Unadjusted (A, C, and E) and adjusted (B, D, and F) network meta‐analyses. The horizontal axis shows the difference of each i‐study's estimate y_iXY from the summary effect for the respective comparison (y_iXY‐mu_XY). The vertical axis presents a measure of dispersion of y_iXY. The red line represents the null hypothesis that the study‐specific effect sizes do not differ from the respective comparison‐specific pooled effect estimates. The green line is the regression line. Different colors correspond to different comparisons. VKA indicates vitamin K antagonists.
Comparative Effectiveness of Interventions to Prevent Any Stroke or Systemic Embolism
Estimated pair‐wise summary effects are presented in Figure 5. All interventions significantly reduced the risk of any stroke and systemic embolism as compared to placebo/control. Importantly, in 6 of 7 interventions (VKA, all 4 NOACs, and the Watchman), not only 95% CI, but also 95% PrI indicated the significant benefit of interventions. This suggests that any future RCTs would likely simply confirm the efficacy of these 6 interventions. However, 95% PrI for aspirin crossed the identity line, which suggests that future results of RCTs (if ever conducted) comparing aspirin with placebo/control remain uncertain. After adjustment for RCT population characteristics (mean/median CHADS2 score, TTR, and duration of follow‐up), VKA and the 4 NOACs confirmed significant (27% to 77%) reduction in stroke or systemic embolism in comparison to placebo/control, whereas the effect of aspirin and the Watchman device lost statistical significance (Figure 6).
Figure 5.
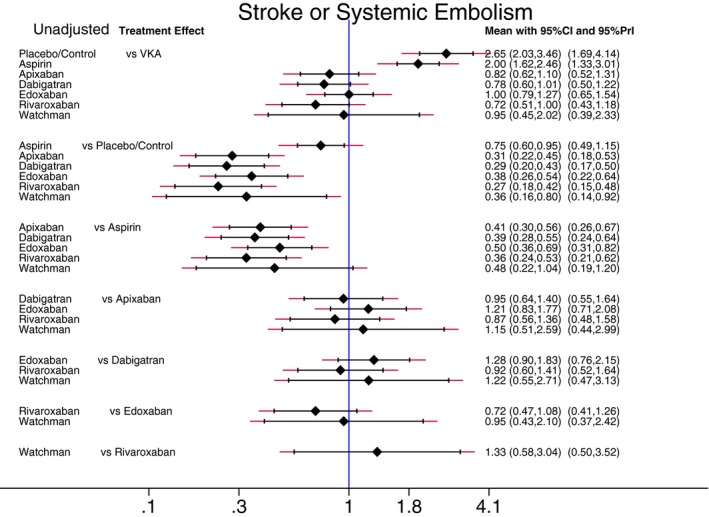
Reduction of stroke and systemic embolism. Unadjusted predictive interval plot for the primary efficacy outcome stroke and systemic embolism, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective predictive intervals (PrI). The blue line is the line of no effect (odds ratio equal to 1). VKA indicates vitamin K antagonists.
Figure 6.
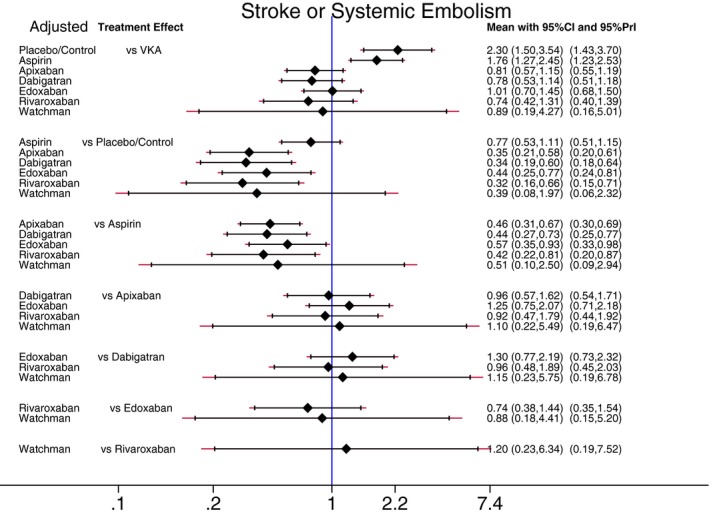
Adjusted predictive interval plot for the primary efficacy outcome stroke and systemic embolism, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective PrI. The blue line is the line of no effect (odds ratio equal to 1). PrI indicates predictive intervals; VKA, vitamin K antagonists.
Compared with aspirin, VKA and NOACs reduced the risk of stroke or systemic embolism by around 50% to 60%, both in unadjusted and adjusted analyses. There was no statistically significant difference in effects of aspirin and the Watchman device.
No antithrombotic intervention was significantly better than VKA (Figures 5 and 6). Aspirin was significantly worse than VKA: The risk of stroke or systemic embolism was twice as high for patients taking aspirin as compared to patients taking VKA. There were no statistically significant differences in effectiveness for each of the 4 NOACs in comparison to one another (Figures 5 and 6).
Comparative Safety of Interventions
Figures 7 and 8 report estimated pair‐wise summary effects for the primary safety outcome. In unadjusted analysis, use of aspirin, dabigatran, rivaroxaban, and VKA was associated with the significantly increased rate of major bleeding by around 2‐fold, whereas there was no statistically significant difference in the rate of major bleeding between the placebo/control group and apixaban and edoxaban (Figure 7). In comparison to placebo/control, use of the Watchman device was associated with the significantly increased rate of major bleeding by 4‐fold, although procedure‐related complications (rather than bleeding) were responsible for the high rate of the primary safety endpoint in the Watchman LAA occlusion device group. After adjustment for RCT population characteristics, risk of major bleeding in all groups of antiembolic interventions, including risk of procedure ‐related complications in the Watchman device group (Figure 8), did not differ significantly from risk of major bleeding in the placebo/control group.
Figure 7.
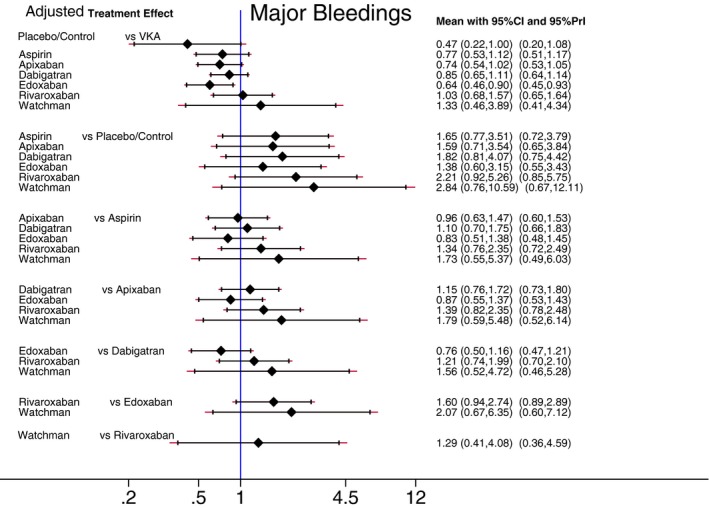
Reduction of major bleeding. Unadjusted predictive interval plot for the primary safety outcome major bleedings, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective PrI. The blue line is the line of no effect (odds ratio equal to 1). PrI indicates predictive intervals; VKA, vitamin K antagonists.
Figure 8.
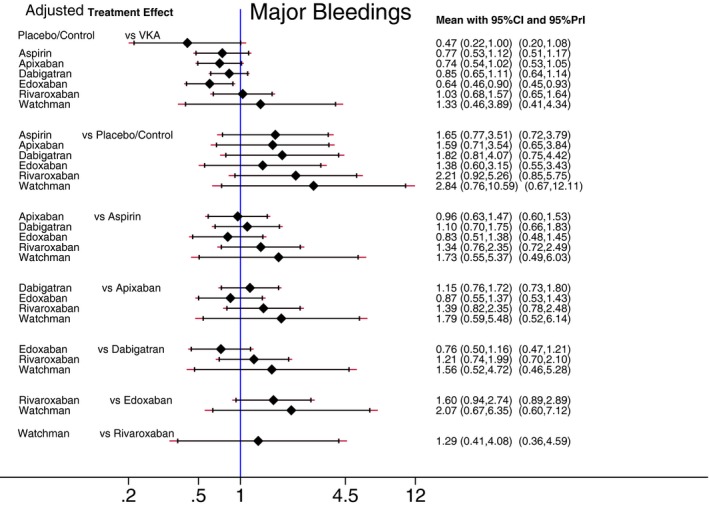
Adjusted predictive interval plot for the primary safety outcome major bleedings, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective PrI. The blue line is the line of no effect (odds ratio equal to 1). PrI indicates predictive intervals; VKA, vitamin K antagonists.
In unadjusted analysis, rates of major bleeding in patients receiving VKA and NOACs did not differ. However, after adjustment for RCT population characteristics, edoxaban stood out as the safest NOAC, demonstrating the significantly lower rate of major bleeding, as compared to VKA.
Comparative Effectiveness of Interventions on All‐Cause Mortality
Figures 9 and 10 show estimated pair‐wise summary effects for the all‐cause mortality. In unadjusted analyses (Figure 9), all 7 interventions (as compared to placebo/control) robustly reduced all‐cause mortality by 18% to 53%, which was confirmed for the Watchman device and for NOACs not only by 95% Cis, but also, importantly, by 95% PrIs. This is important evidence of a strong life‐saving effect of antistroke therapy in patients with nonvalvular AF. However, 95% PrI for aspirin crossed the “no effect” line, indicating that the life‐saving effect of aspirin might not be confirmed in future RCTs, if ever conducted. Aspirin reduced all‐cause mortality by 18%. NOACs and VKA reduced all‐cause mortality further than aspirin by an additional 24% to 29%. Three NOACs (apixaban, dabigatran, and edoxaban) significantly improved survival by an additional 10% above VKA effect, whereas the effect of rivaroxaban was only borderline. However, after adjustment for RCT population characteristics (Figure 10), no antiembolic intervention was statistically significantly life saving.
Figure 9.

Reduction of all‐cause mortality. Unadjusted predictive interval plot for the secondary efficacy outcome all‐cause mortality, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective PrI. The blue line is the line of no effect (odds ratio equal to 1). PrI indicates predictive intervals; VKA, vitamin K antagonists.
Figure 10.
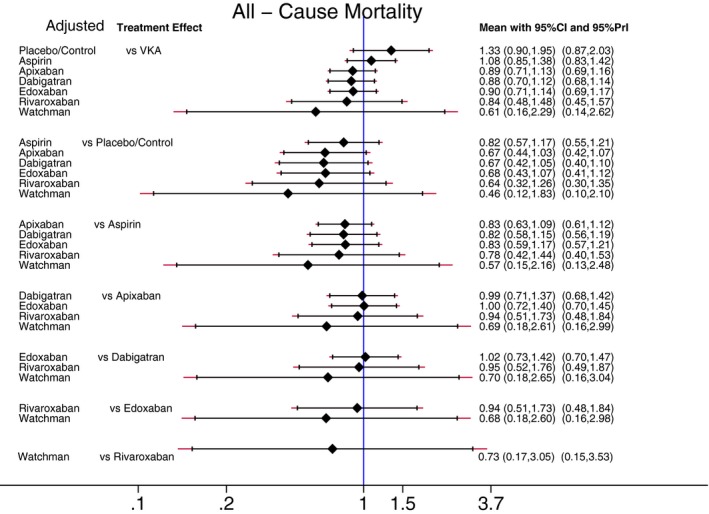
Adjusted predictive interval plot for the secondary efficacy outcome all‐cause mortality, on a logarithmic scale. Solid black lines represent the confidence intervals (CI) for summary odds ratios for each comparison and the red dashed lines the respective PrI. The blue line is the line of no effect (odds ratio equal to 1). PrI indicates predictive intervals; VKA, vitamin K antagonists.
Ranking of the Interventions on a Single Outcome
Table 3 reports ranking of the antithrombotic interventions separately for each outcome. There was no single winner for the primary efficacy outcome: The probability of being the best intervention to prevent stroke and systemic embolism did not exceed 50% (ie, pure chance) for any of the treatment options. Rivaroxaban was ranked as the best, followed by dabigatran and apixaban. Adjustment did not change the ranking. As expected, placebo/control clearly was the safest “intervention,” with edoxaban being the second safest, both in unadjusted and adjusted analyses.The Watchman device was the best life‐saving intervention in nonvalvular AF, with a probability of around 72%.
Table 3.
Ranking of the Antithrombotic Interventions
| Treatment | 1° Efficacy: Stroke or Systemic Embolism | 1° Safety: Major Bleedings | 2° Efficacy: All‐Cause Mortality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUCRA | Pr. Best | Rank | SUCRA | Pr. Best | Rank | SUCRA | Pr. Best | Rank | ||||||||||
| U | A | U | A | U | A | U | A | U | A | U | A | U | A | U | A | U | A | |
| VKA | 44.3 | 47.5 | 0 | 0 | 4.9 | 4.7 | 27 | 21.9 | 0 | 0 | 6.1 | 6.5 | 30.7 | 38.3 | 0 | 0 | 5.8 | 5.3 |
| Placebo/control | 0.2 | 2.9 | 0 | 0 | 8 | 7.8 | 96.4 | 90.8 | 81.4 | 72.2 | 1.2 | 1.6 | 0.4 | 8 | 0 | 0.2 | 8 | 7.4 |
| Aspirin | 14.6 | 16.3 | 0 | 0 | 7 | 6.9 | 61.2 | 57.3 | 0.5 | 2.4 | 3.7 | 4 | 14.7 | 27 | 0 | 0.3 | 7 | 6.1 |
| Apixaban | 72 | 72.5 | 13.2 | 13.9 | 3 | 2.9 | 57.4 | 63.1 | 3.6 | 3.9 | 4 | 3.6 | 63 | 62.8 | 3.6 | 6.1 | 3.6 | 3.6 |
| Dabigatran | 78.9 | 75.7 | 21.1 | 19.5 | 2.5 | 2.7 | 44.3 | 46.1 | 1.1 | 0.7 | 4.9 | 4.8 | 62.2 | 64.4 | 2.8 | 7.4 | 3.6 | 3.5 |
| Edoxaban | 46.4 | 49.5 | 0.6 | 2 | 4.8 | 4.5 | 74.7 | 78.1 | 12.6 | 16.8 | 2.8 | 2.5 | 65.4 | 61.1 | 3.1 | 5.9 | 3.4 | 3.7 |
| Rivaroxaban | 86.5 | 77 | 46.1 | 30.4 | 1.9 | 2.6 | 36.5 | 23.7 | 0.8 | 0.4 | 5.4 | 6.3 | 77.1 | 63.7 | 18.3 | 19.8 | 2.6 | 3.5 |
| Watchman | 57 | 58.6 | 19 | 34.2 | 4 | 3.9 | 2.5 | 19 | 0 | 3.6 | 7.8 | 6.7 | 86.5 | 74.7 | 72.2 | 60.4 | 1.9 | 2.8 |
A indicates adjusted; Pr. Best, probability of being the best; SUCRA, the surface under the cumulative ranking curve; U, unadjusted; VKA, vitamin K antagonists.
Simultaneous Ranking of the Interventions for Two Primary Outcomes
Clustered ranking plots of the network for each pair of outcomes are shown in Figure 11. The upper right corner in Figure 11A and 11C is empty, which means that a treatment that is both the most effective and the safest does not exist. Clustered ranking for both primary outcomes (efficacy and safety) revealed 5 separate clusters (Figure 11A). Apixaban, dabigatran, and rivaroxaban formed a cluster of “the most effective and reasonably safe” interventions. The Watchman device was the single representative of a cluster of “the most effective and the most dangerous.” VKA and edoxaban formed a cluster of “reasonably effective and reasonably safe.” Aspirin formed a separate cluster of “low effectiveness and moderate safety,” whereas placebo/control represented “ineffective, but the safest” cluster. Interestingly, after adjustment, antiembolic interventions formed only 4 clusters (Figure 11C). The Watchman device comprised an “effective and safe” cluster together with VKA and edoxaban.
Figure 11.
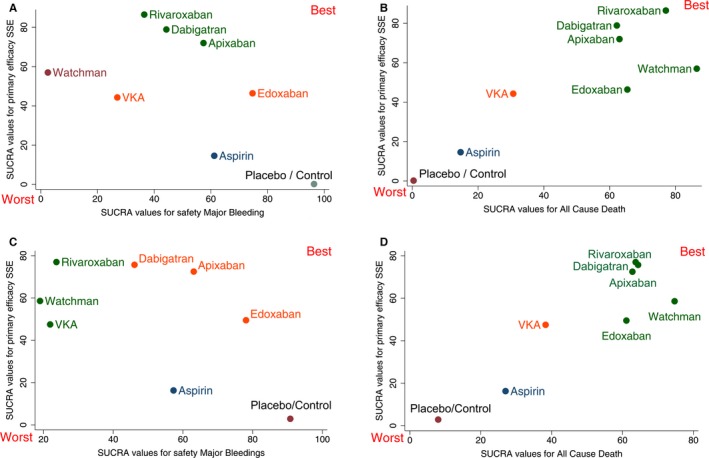
Clustered ranking plot of the network. The plot is based on cluster analysis of surface under the cumulative ranking curves (SUCRA) values, derived from unadjusted (A and B) or adjusted (C and D) analyses. Each plot shows SUCRA values for two outcomes: primary efficacy (stroke or systemic embolism; SSE), secondary efficacy (all‐cause mortality), and safety (major bleeding). Each color represents a group of treatments that belong to the same cluster. Treatments lying in the upper right corner are more effective and safe than the other treatments. P/C indicates placebo/control; VKA, vitamin K antagonists.
Simultaneous Ranking of the Interventions for Reduction of Stroke, Systemic Embolism, and Mortality
Simultaneous ranking of antithrombotic interventions on 2 efficacy outcomes (stroke or systemic embolism and all‐cause mortality) revealed a desired development axis from the worst to the best intervention. In unadjusted and adjusted analysis (Figure 11B and 11D), all 4 NOACs and the Watchman device formed a single cluster of “the most effective and life‐saving” interventions. VKA alone formed a cluster of “moderately effective” treatment. Aspirin was the single representative of a “low effectiveness and low safety” cluster. Placebo/control occupied the lower left corner as completely ineffective.
Sensitivity Analyses
Conducted sensitivity analyses confirmed the robustness of the reported findings. We repeated analyses after removal of the (1) studies that used a combination of antithrombotic interventions (aspirin with stable, low dose of VKA; aspirin with clopidogrel) and (2) studies that used “no treatment” comparator instead of placebo. Exclusions did not change estimated effects in the network.
Discussion
Despite the numerous therapeutic interventions evaluated in previous RCTs to prevent stroke or systemic embolism in the setting of nonvalvular AF, the majority has not been analyzed in head‐to‐head comparisons, with limited NMA.14, 15 This void of comparative data is sorely felt in the medical community, where clinicians must regularly decide among several therapeutic options for stroke prevention in at‐risk patients with nonvalvular AF. In an attempt to address this void, we conducted an NMA of the 7 available antistroke interventions and placebo/control. These detailed and comprehensive analyses derived from nearly 100 000 patients enrolled in RCTs found moderate‐to‐high quality evidence to support the efficacy of all tested interventions (including aspirin, VKA, 4 NOACs, and the Watchman device) for prevention of stroke or systemic embolism and reduction in all‐cause mortality in the setting of nonvalvular AF. These observations strongly support the 2014 American Heart Association/American College of cardiology/Heart Rhythm Society AF guidelines3 that underscored individualized therapy for stroke prevention, based on shared decision making. The NMA indicates that AF patients at risk of stroke have a choice of 7 antiembolic interventions (aspirin, VKA, apixaban, dabigatran, edoxaban, rivaroxaban, and the Watchman LAA occlusion device) that have measurable, but nonequivalent, efficacy and safety. Selection of therapy can be matched to individual patient risks of thromboembolic and bleeding events and aligned with the specific pharmacokinetic, pharmacodynamic, and device characteristics of antiembolic treatments.
Our study confirmed the notion that there is a trade‐off between efficacy and safety of the tested interventions and that a single most effective and safest intervention does not exist. In this NMA, rivaroxaban had the highest probability of being the most effective for prevention of stroke or systemic embolism. The Watchman device demonstrated solid probability (72%) of being ranked the most effective life‐saving intervention. Edoxaban showed the highest probability of being the safest antithrombotic intervention (after placebo/control). Thus, NMA did not reveal obvious winners and confirmed the substantial overlap in the efficacy and safety of individual treatments. The most meaningful clustered ranking by 2 efficacy outcomes (stroke or systemic embolism and all‐cause mortality), after adjustment for RCT population characteristics (CHADS2 score, TTR, and duration of follow‐up), revealed that the most effective and safe cluster included all 4 NOACs and the Watchman device. This group may represent treatments that have the broadest applicability, but our study does not include any financial considerations or cost‐efficacy analyses.
Aspirin reduced the risk of stroke or systemic embolism by 25% and the risk of death by 18%, but, at the same time, increased the risk of major bleeding by around 80%. VKA further (over and above aspirin) reduced the risk of a thromboembolic event by 50% and risk of all‐cause death by 18%. NOACs provided an additional (50% to 60%) reduction of stroke or systemic embolism risk above aspirin, and around a 25% additional reduction of all‐cause mortality, without increasing risk of major bleeding. The Watchman device reduced risk of stroke or systemic embolism by around 60% and risk of death by 54%, as compared to control/placebo, at the price of a greater risk of postprocedural complications or major bleeding. An LAA occlusion device is clearly a viable alternative to anticoagulants, but further technical or procedural advancement is desirable to decrease the rate of postprocedural complications. Similar to traditional meta‐analysis,8 this NMA finds significant differences in primary efficacy (rivaroxaban) and safety (edoxaban) outcomes between VKA and individual NOACs. Importantly, additional survival benefit (10% above VKA) was demonstrated by 3 NOACs (apixaban, dabigatran, and edoxaban).
The Watchman device is the most recent addition to the “antiembolic armamentarium.” Indirect unadjusted pair‐wise estimates obtained for the Watchman device in our study are consistent with a recent NMA.15 We showed that the Watchman device is significantly more effective than placebo/control, but there was no evidence to prove that the Watchman device is more effective than aspirin. The fact that, after adjustment for RCT population characteristics, the effect of the Watchman device did not differ from any other comparator (including placebo/control) is likely an indicator of an insufficient statistical power of the knowledge base. Importantly, the Watchman device was ranked as the best life‐saving antiembolic intervention. Moreover, simultaneous clustered ranking for 2 of the most important outcomes (primary efficacy and all‐cause mortality) included the Watchman device together with 4 NOACs in the cluster of most effective and safe interventions. Clearly, further development of LAA occlusion devices and techniques is needed. The EWOLUTION registry recently showed that rate of periprocedural strokes and bleedings could be further decreased.47 Future RCTs of LAA occlusion devices are needed to prove the effectiveness of the LAA occlusion approach given that it remains unclear whether LAA is a mechanistically essential structure for stroke development in AF.
Strengths and Limitations
Different RCT population characteristics (especially CHADS2 risk score and TTR) are always of concern when considering results of a traditional meta‐analysis. In this study, we, for the first time, adjusted for RCT population characteristics, which improved consistency and homogeneity of the knowledge base. Unlike in traditional meta‐analysis (reporting pair‐wise comparisons as main results), the main results in this NMA are presented by simultaneous clustered ranking for 2 outcomes. Our study, for the first time, showed that across all spectrum of stroke risk and regardless of TTR, all 4 NOACs and the Watchman device are significantly more effective and life saving.
There are several limitations to the present NMA. The first is the inclusion of RCTs that tested different doses of medications. In a previous meta‐analysis of NOACs, only high doses were considered.8 However, we did not observe heterogeneity of effects associated with the different dosage of drugs across comparators. There are 2 major reasons for this finding. First, dosages of all comparators in our study varied (aspirin, 75–325 mg; VKA target international normalized ratio [INR], 1.4–4.5; apixaban, 2.5–5.0 mg; dabigatran, 110–150 mg; edoxaban, 30–60 mg; rivaroxaban, 15–20 mg). Second, it must be emphasized that the objectives of NMA differ from conventional meta‐analyses. NMA compared the effectiveness of individual antithrombotic agents, whereas traditional meta‐analysis considered all NOACs as a group and conducted group comparisons. Whereas conventional meta‐analyses summarize evidence from RCTs for a particular therapeutic intervention, NMA summarizes evidence from multiple competing interventions simultaneously, informs clinical practice, and suggests a direction for future research. Also, the methods applied in this study for adjustment by RTC population characteristics are novel and therefore should be interpreted with caution. This NMA did not adjust for rates of antiplatelet use, which could affect rate of bleeding. Several included studies were conducted more than 20 years ago (CHADS2 score was not reported), which added uncertainty in ability to adjust for study population characteristics, as well as in applicability and generalizability of the findings. The wide range of years in which the studies were conducted (the 1990s–2010s) might introduce heterogeneity. To address this issue, we evaluated the comparison‐adjusted funnel plot with treatments ordered from the oldest to newest (Figure 4). The study estimates were lying symmetrically around the line of the meta‐analysis summary effect, which suggested no evidence of earlier‐conducted, older‐study effects. Of note, adjustment for RCT population characteristics further improved the consistency of the network.
Conclusions
In conclusion, the present NMA found that use of all antiembolic intervention (aspirin, VKA, apixaban, dabigatran, edoxaban, rivaroxaban, and Watchman device) significantly reduced all‐cause mortality and risk of any stroke or systemic embolism in nonvalvular AF patients, although to different degrees. After adjustment for RCT population characteristics, the highest probability of being the most effective, life‐saving antiembolic intervention cluster included the 4 NOACs and the Watchman device.
Disclosures
Steinberg reports receiving consulting fees from Janssen, Pfizer, and Boston Scientific and speaking fees from Bristol‐Myers Squibb and Pfizer.
(J Am Heart Assoc. 2016;5:e003206 doi: 10.1161/JAHA.116.003206)
References
- 1. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44:3103–3108. [DOI] [PubMed] [Google Scholar]
- 2. Lip GYH. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why?. Eur Heart J. 2013;34:1041–1049. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; Members AATF . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potpara TS, Lip GY, Apostolakis S. New anticoagulant treatments to protect against stroke in atrial fibrillation. Heart. 2012;98:1341–1347. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950–1962. [DOI] [PubMed] [Google Scholar]
- 6. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; Investigators PA . Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 7. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 8. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 9. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 10. Caldeira D, Rodrigues FB, Barra M, Santos AT, de Abreu D, Goncalves N, Pinto FJ, Ferreira JJ, Costa J. Non‐vitamin K antagonist oral anticoagulants and major bleeding‐related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta‐analysis. Heart. 2015;101:1204–1211. [DOI] [PubMed] [Google Scholar]
- 11. Briceno DF, Villablanca P, Cyrille N, Massera D, Bader E, Manheimer E, Aagaard P, Ferrick K, Gross J, Kim SG, Krumerman A, Palma E, Guttenplan N, Romero J, Fisher J, Garcia M, Natale A, Di Biase L. Left atrial appendage occlusion device and novel oral anticoagulants versus warfarin for stroke prevention in nonvalvular atrial fibrillation: systematic review and meta‐analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2015;8:1057–1064. [DOI] [PubMed] [Google Scholar]
- 12. Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta‐analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466–474. [DOI] [PubMed] [Google Scholar]
- 13. Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012;108:476–484. [DOI] [PubMed] [Google Scholar]
- 14. Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, Carrier M, Coyle K, Bai A, Moulton K, Clifford T, Wells G. Systematic review and network meta‐analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4:e004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panikker S, Lord J, Jarman JW, Armstrong S, Jones DG, Haldar S, Butcher C, Khan H, Mantziari L, Nicol E, Hussain W, Clague JR, Foran JP, Markides V, Wong T. Outcomes and costs of left atrial appendage closure from randomized controlled trial and real‐world experience relative to oral anticoagulation. Eur Heart J. 2016; pii: ehw048. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koifman E, Lipinski MJ, Escarcega RO, Didier R, Kiramijyan S, Torguson R, Waksman R. Comparison of Watchman device with new oral anti‐coagulants in patients with atrial fibrillation: a network meta‐analysis. Int J Cardiol. 2016;205:17–22. [DOI] [PubMed] [Google Scholar]
- 17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá‐López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations PRISMA extension for network meta‐analysis. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 18. Diener CH, Lowenthal A. Correspondence Reply to Dr G. Hart and Dr O. Benavente (University of Texas). J Neurol Sci. 1997;153:112. [PubMed] [Google Scholar]
- 19. Posada IS, Barriales V. Alternate‐day dosing of aspirin in atrial fibrillation. LASAF Pilot Study Group. Am Heart J. 1999;138:137–143. [DOI] [PubMed] [Google Scholar]
- 20. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One. 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol. 2008;61:455–463. [DOI] [PubMed] [Google Scholar]
- 22. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 23. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 24. Handl J, Knowles J, Kell DB. Computational cluster validation in post‐genomic data analysis. Bioinformatics. 2005;21:3201–3212. [DOI] [PubMed] [Google Scholar]
- 25. Jung Y, Park H, Du D‐Z, Drake B. A decision criterion for the optimal number of clusters in hierarchical clustering. J Glob Optim. 2003;25:91–111. [Google Scholar]
- 26. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas‐Zanetti F, Gonzalez‐Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; Committee AS and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 28. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 29. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, Izumi T, Koretsune Y, Kajikawa M, Kato M, Ueda H, Iwamoto K, Tajiri M; Investigators JRAs . Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J‐ROCKET AF study. Circ J. 2012;76:2104–2111. [DOI] [PubMed] [Google Scholar]
- 30. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 31. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Committee R‐LS and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 32. Petersen P, Godtfredsen J, Boysen G, Andersen E, Andersen B. Placebo‐controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK Study. Lancet. 1989;333:175–179. [DOI] [PubMed] [Google Scholar]
- 33. Prevention of stroke in atrial fibrillation. N Engl J Med. 1990;323:481–484. [DOI] [PubMed] [Google Scholar]
- 34. The effect of low‐dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. N Engl J Med. 1990;323:1505–1511. [DOI] [PubMed] [Google Scholar]
- 35. Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84:527–539. [DOI] [PubMed] [Google Scholar]
- 36. Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian atrial fibrillation anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18:349–355. [DOI] [PubMed] [Google Scholar]
- 37. Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, Krause‐Steinrauf H, Kurtzke JF, Nazarian SM, Radford MJ. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327:1406–1412. [DOI] [PubMed] [Google Scholar]
- 38. Secondary prevention in non‐rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255–1262. [PubMed] [Google Scholar]
- 39. Stroke Prevention in Atrial Fibrillation I . Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 40. Warfarin compared to aspirin for prevention of arterial thromboembolism in atrial fibrillation. Cerebrovasc Dis. 1992;2:332–341. [Google Scholar]
- 41. Stroke Prevention in Atrial Fibrillation I . Adjusted‐dose warfarin versus low‐intensity, fixed‐dose warfarin plus aspirin for high‐risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–638. [PubMed] [Google Scholar]
- 42. Gullov AL, Koefoed BG, Petersen P, Pedersen TS, Andersen ED, Godtfredsen J, Boysen G. Fixed minidose warfarin and aspirin alone and in combination vs adjusted‐dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med. 1998;158:1513–1521. [DOI] [PubMed] [Google Scholar]
- 43. Hellemons BS, Langenberg M, Lodder J, Vermeer F, Schouten HJ, Lemmens T, van Ree JW, Knottnerus JA. Primary prevention of arterial thromboembolism in non‐rheumatic atrial fibrillation in primary care: randomised controlled trial comparing two intensities of coumarin with aspirin. BMJ. 1999;319:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edvardsson N, Juul‐Möller S, Ömblus R, Pehrsson K. Effects of low‐dose warfarin and aspirin versus no treatment on stroke in a medium‐risk patient population with atrial fibrillation. J Intern Med. 2003;254:95–101. [DOI] [PubMed] [Google Scholar]
- 45. Sato H, Ishikawa K, Kitabatake A, Ogawa S, Maruyama Y, Yokota Y, Fukuyama T, Doi Y, Mochizuki S, Izumi T, Takekoshi N, Yoshida K, Hiramori K, Origasa H, Uchiyama S, Matsumoto M, Yamaguchi T, Hori M. Low‐dose aspirin for prevention of stroke in low‐risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37:447–451. [DOI] [PubMed] [Google Scholar]
- 46. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 47. Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM, Bergmann MW; Investigators E . Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri‐procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016; pii: ehv730. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


