Abstract
Background
Ivabradine is a heart rate–lowering agent approved to reduce the risk of hospitalization for worsening heart failure. This study assessed the cost‐effectiveness of adding ivabradine to background therapy in the United States from the perspective of a commercial or Medicare Advantage payer.
Methods and Results
A cost‐effectiveness, cohort‐based Markov model using a state transition approach tracked a cohort of heart failure patients with heart rate ≥70 beats per minute in sinus rhythm who were treated with ivabradine+background therapy or background therapy alone. Model inputs, including adjusted hazard ratios, rates of hospitalization and mortality, adverse events, and utility‐regression equations, were derived from a large US claims database and SHIFT (Systolic Heart failure treatment with the If inhibitor ivabradine Trial). In the commercial population, ivabradine+background therapy was associated with a cost savings of $8594 versus the cost of background therapy alone over a 10‐year time horizon, primarily because of reduced hospitalization. Ivabradine was associated with an incremental benefit of 0.24 quality‐adjusted life years over a 10‐year time horizon. In the Medicare Advantage population, the incremental cost‐effectiveness ratio for ivabradine was estimated to be $24 920/quality‐adjusted life years.
Conclusions
The cost‐effectiveness model suggests that for a commercial population, the addition of ivabradine to background therapy was associated with cost savings and improved clinical outcomes. For a Medicare Advantage population, the analysis indicates that the clinical benefit of ivabradine can be achieved at a reasonable cost.
Keywords: cost‐effectiveness, heart failure, heart rate, hospitalization
Subject Categories: Heart Failure, Cost-Effectiveness
Introduction
Heart failure (HF) is a complex clinical syndrome associated with a considerable economic burden, largely because of a high prevalence and a frequent requirement for hospitalization.1 In the United States, 5.8 million people currently suffer from HF, with the prevalence expected to rise to more than 8 million by 2030.2 About half of patients with symptomatic HF have reduced ejection fraction.3 The annual costs of HF are currently estimated at $30.7 billion.2, 3 More than two thirds of these costs can be attributed to the costs associated with hospitalization.4 For Medicare patients, 30‐day readmission rates are as high as 25% and HF is the leading cause of rehospitalization.5
HF is also a condition associated with a poor prognosis, with ≈50% of patients dying within 5 years of diagnosis.3 At particular risk are patients with a high resting heart rate (HR). HR ≥70 beats per minute (bpm) has been shown to be a risk marker in HF.6 Patients with a high HR are more likely to suffer from an exacerbation requiring hospitalization, or to have cardiovascular death.6, 7 Moreover, an analysis of the prospective, US‐based Get With the Guidelines registry indicated that patients hospitalized for HF with a HR ≥75 bpm at the time of discharge are more likely to be readmitted within 30 days.7 This result confirms that high HR is a risk factor for cardiovascular events in HF6 and highlights the need to regard HR as a target for treatment in HF.6
Ivabradine (Corlanor®; Amgen Inc., Thousand Oaks, CA) is a hyperpolarization‐activated cyclic nucleotide‐gated channel blocker that acts on the sinoatrial node to inhibit the If current in order to slow HR.8 Ivabradine is indicated for patients taking the maximally tolerated dose of β‐blockers or for those for whom β‐blockers are contraindicated. The most common side effects (ivabradine versus placebo rates) are bradycardia (10% versus 2.2%), hypertension (8.9% versus 7.8%), atrial fibrillation (8.3% versus 6.6%), and luminous phenomena (phosphenes) (2.8% versus 0.5%). Ivabradine was developed by Les Laboratoires Servier (Paris, France), and it is distributed and manufactured in the United States by Amgen Inc.
The addition of ivabradine to background therapy such as β‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists, and diuretics has been investigated in the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT).9 SHIFT was a randomized, event‐driven trial of ivabradine versus placebo added to guidelines‐driven background therapy in 6558 adult patients with New York Heart Association class II‐IV HF, left ventricular ejection failure ≤35%, and resting heart rate ≥70 bpm. The primary end point was a composite of time to cardiovascular death or hospitalization for worsening heart failure, which was significantly reduced with ivabradine+background therapy (hazard ratio: 0.82, 95% CI: 0.75, 0.90, P<0.0001). The results of SHIFT also showed that ivabradine+background therapy reduced hospitalizations for worsening HF by 26% (relative risk).9 While this indicates that ivabradine may improve patient outcomes, the economic implications of adding ivabradine to a standard HF treatment regimen in the United States have not yet been elucidated.
With the increasing cost of health care in the United States, there is a growing interest in assessing the cost‐effectiveness of novel treatments.10 When the value of an intervention is being compared with the best available alternative, as is the case with ivabradine and background therapy, an incremental cost‐effectiveness ratio (ICER) can be estimated.10 The aim of this investigation was to develop a cost‐effectiveness model to evaluate the additional value associated with adding ivabradine to background therapy, compared with background therapy alone.
Methods
Model Structure
A Markov model taking the perspective of a third‐party payer was used to track a cohort of patients with chronic HF treated with either ivabradine+background therapy or background therapy alone over a 10‐year time horizon. The study was designed by Amgen and Evidera; Evidera performed the analysis under contract to Amgen. The authors of the study interpreted the data after analysis was complete. US claims estimates were based on the analyses of the results from the Optum Research Database between January 1, 2008 and June 30, 2013. SHIFT estimates were based on the analyses of trial data (with median follow‐up of 22.9 months). The model has the flexibility to take the perspective of a commercial third‐party payer or that of Medicare Advantage. Within this model, patients with up to 3 cardiovascular hospitalizations were assigned to 10 mutually exclusive hospitalization states. The limit of 3 hospitalizations was used to reduce the complexity of the model. Using a 1‐month cycle, patients entered the model with 0 hospitalizations. With the experience of a hospitalization event (HF or non‐HF cardiovascular), they would transition to a state with a higher number of hospitalizations. Patients who did not experience a hospitalization remained in the same state. With increasing numbers of prior HF and non‐HF cardiovascular hospitalizations, the risk of future HF and non‐HF cardiovascular hospitalizations increased, and patient utility decreased. The model considered costs of treatment, hospitalization, and adverse event (AE) management. Figure 1 depicts the model structure through a state‐diagram.
Figure 1.
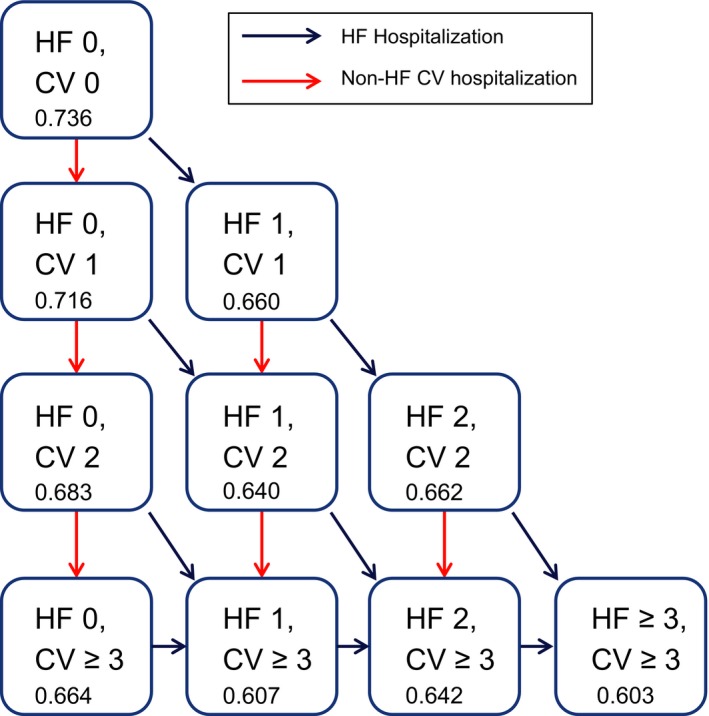
State transition diagram. CV indicates cardiovascular; HF, heart failure. The number listed at the bottom of each hospitalization state box indicates the patient utility value derived from the base‐case utility regression equation.
Data from US claims, sourced from a large US payer claims database, were used for both the commercial perspective and the Medicare Advantage perspective. Many of the inputs for the model were derived from a post hoc analysis of SHIFT data. SHIFT was a multinational, randomized, double‐blind, placebo‐controlled, parallel‐group study involving 6505 patients with symptomatic HF, with a left ventricular ejection fraction ≤35% and a resting HR ≥70 bpm in sinus rhythm. The trial was conducted at 677 centers across 37 countries. Patients were required to have been hospitalized within the previous year for HF, and to have been receiving stable background therapy.9 Patients (n=6505) were then randomly assigned to receive either ivabradine (maximum 7.5 mg twice daily) or placebo, in addition to background therapy.9 The median follow‐up during the trial was 22.9 months (interquartile range: 18–28 months). Data from this trial, including treatment efficacy, mortality, drug safety, and utility inputs, were used within this cost‐effectiveness model.
Model Inputs
Drug and clinical inputs
Hospitalization
For the base‐case analysis, reference hospitalization rates for background therapy were based on the analysis of US claims data (Table S1). Hospitalization rates for patients treated with ivabradine were calculated by adjusting the reference hospitalization rates using hazard ratios derived from SHIFT (Table S2). An alternative scenario was also run, in which hospitalization rates from SHIFT were used as inputs for the hospitalization rates for patients treated with background therapy.
Mortality
For both ivabradine and background therapy, HF and non‐HF cardiovascular mortality rates and hazard ratios were derived from a post hoc analysis of SHIFT as claims data do not comprehensively capture mortality (Table S3). Noncardiovascular mortality was calculated from the US life table,11 with cardiovascular‐associated mortality excluded.12 The baseline age and sex distribution data used for background mortality calculation were derived from US claims data for the reference scenario (ie, percent female: 42.5%; baseline age: 62.8 years).
Adverse events
Rates of AEs from SHIFT were included in the model if the rates were statistically significantly different in the ivabradine and background therapy cohorts (Table S4). Asymptomatic bradycardia, symptomatic bradycardia, atrial fibrillation, blurred vision, and phosphenes met the inclusion criteria. The data were reanalyzed to provide the number of events, rather than the number of patients experiencing an AE. Risk of an AE was assumed to remain constant across the time horizon.
Cost inputs
Drug acquisition costs
The US wholesale acquisition cost for ivabradine as of April 15, 2015, was $375 per month or $4500 per year (assuming 100% compliance). The same cost was applied to both the 5‐mg and the 7.5‐mg dose in the model.
Hospitalization
Using US claims data, HF‐related, non‐HF cardiovascular–related, and non‐cardiovascular–related hospitalizations were assigned per‐event costs (Table S5). In the model, AEs were assumed to be managed through physician visits or emergency department visits (Table S5). The unit costs of AE management for commercially insured patients were sourced from the Physicians’ Fee and Coding Guide13 and the costs for Medicare Advantage patients were sourced from the Department of Health and Human Services Centers for Medicare & Medicaid Services Medicare physician fee schedule look‐up tool and the hospital outpatient prospective payment system files (July 2014).14
Quality‐of‐life inputs
Data from SHIFT were used to inform the model utility inputs at different hospitalization states. Two regression equations were developed to estimate change from baseline in the EuroQoL 5 dimensions (EQ‐5D) index score, using 3 main independent variables including treatment, β‐blocker use, and number of hospitalizations. The number of hospitalizations was included as either a categorical variable (1 versus 0, 2 versus 0, and ≥3 versus 0) or a dichotomous variable (yes versus no) to derive the explicit equation or minimal equation, respectively (Table S6). For the base case, the explicit equation was used where the utility reduction was capped after 3 hospitalizations. Figure 1 depicts the hospitalization state utilities derived from the base‐case regression equation. The minimal equation was used as a sensitivity analysis.
Sensitivity analysis inputs
One‐way sensitivity analysis was conducted to explore the impact of model inputs and assumptions on the results. The parameters varied in the sensitivity analysis; they included the time horizon, cost of ivabradine, data source used for event rates, utility regression equations, disutility of AEs, β‐blocker use, and costs associated with AEs. A probabilistic sensitivity analysis was conducted to evaluate the impact of varying cost, utility, hospitalization rate, and treatment hazard ratio parameters simultaneously on the results.
Statistical Analysis
To derive clinical and quality‐of‐life inputs for the model, a post hoc analysis of SHIFT data was conducted. The randomized set was used. Mortality and first hospitalization rates for each type (HF and non‐HF cardiovascular) in each treatment group were calculated using the randomized set by dividing the number of first events for each patient by the total number of patient‐years (ie, the number of patient‐years from randomization until occurrence of the event or end of follow‐up, whichever came first). Rates of treatment‐emergent AEs were calculated using the safety set as the total number of emergent AEs divided by the number of patient‐years at risk (from first to last study drug intake+2 days) in accordance with the clinical study report.
Hazard ratios and 95% CIs for time to death due to an HF or a non‐HF cardiovascular reason, as well as time to HF or non‐HF cardiovascular hospitalization in the ivabradine group versus the placebo group were estimated from Cox proportional‐hazards models with adjustment for β‐blocker intake at baseline for concordance with prior publications.9
A predictive equation for change from baseline in EQ‐5D was derived using a generalized linear mixed model, which included treatment, baseline EQ‐5D score, baseline β‐blocker use, and time‐dependent variables for HF and non‐HF cardiovascular hospitalizations. Raw EQ‐5D scores were converted to utilities using UK tariffs. For this analysis, patients with a nonmissing baseline value and at least 1 nonmissing postbaseline value for the EQ‐5D index from the patient‐reported outcomes (PRO)–SHIFT substudy were used.
All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Base‐Case Results, Commercial Perspective
Overall, the results of the base‐case analysis indicated that ivabradine+background therapy was dominant over background therapy alone, with lower cost and better health outcomes (Table 1). The total costs, including hospitalization, AE, and drug acquisition costs, were estimated to be $369 762 with the addition of ivabradine, compared with $378 356 for background therapy alone over a 10‐year time horizon. Therefore, ivabradine as an add‐on treatment resulted in an estimated incremental savings of $8594. This reduction in cost occurred despite the additional $27 201 in drug acquisition costs and the additional $2572 in AE costs, because of the substantial reduction in costs associated with hospitalization. This included an incremental saving of $31 295, $34 112, and $38 366 in HF‐related, cardiovascular‐related, and all‐cause hospitalization costs, respectively (Table 1). Life years (LYs) and quality‐adjusted life years (QALYs) were also increased with the addition of ivabradine, with an incremental improvement of 0.21 and 0.24 for LYs and QALYs, respectively (Table 1).
Table 1.
Base‐Case Model Results
| Commercial | Medicare Advantage | |||||
|---|---|---|---|---|---|---|
| Ivabradine | Background Therapy | Incremental | Ivabradine | Background Therapy | Incremental | |
| Hospitalization costs | ||||||
| HF | $254 960 | $286 255 | −$31 295 | $143 394 | $159 515 | −$16 121 |
| Cardiovascular | $287 802 | $321 915 | −$34 112 | $165 010 | $182 681 | −$17 672 |
| All cause | $337 268 | $375 634 | −$38 366 | $200 032 | $220 887 | −$20 855 |
| AE costs | $5294 | $2722 | $2571 | $2581 | $1325 | $1256 |
| Drug costs | $27 201 | NA | $27 201 | $24 512 | NA | $24 512 |
| Total costs | $369 762 | $378 356 | −$8594 | $227 125 | $222 212 | $4913 |
| Health outcomes | ||||||
| LYs | 6.04 | 5.83 | 0.21 | 5.45 | 5.28 | 0.16 |
| QALYs | 4.02 | 3.78 | 0.24 | 3.60 | 3.40 | 0.20 |
AE indicates adverse event; HF, heart failure; LYs, life years; NA, not applicable; QALY, quality‐adjusted life year.
Base‐Case Results, Medicare Advantage Perspective
The base‐case results for the Medicare Advantage population showed that ivabradine+background therapy was cost‐effective compared to background therapy alone. The total costs, including hospitalization, AE, and drug acquisition costs, were estimated to be $227 125 with the addition of ivabradine, compared with $222 212 for background therapy alone over a 10‐year time horizon. The increase in drug acquisition and AE costs ($24 512 and $1256, respectively) was largely offset by the reduction in hospitalization cost (−$20 855), leading to a modest cost increase of $4913 over 10 years. A similar health benefit was predicted in the Medicare Advantage population as in the commercial population, with an incremental gain of 0.16 LYs and 0.20 QALYs (Table 1). The ICER in the Medicare Advantage population was $24 920/QALY.
Sensitivity Analysis
Results of the 1‐way sensitivity analysis are presented in Table 2 and Figure 2. The strongest drivers of the model were time horizon and treatment effect on HF hospitalization and mortality. Ivabradine was confirmed to be cost‐effective in all scenarios listed in Table 2 (ICER ≤$50 000/QALY). The results were relatively insensitive to changes in other assumptions such as drug price, treatment effect on non‐HF cardiovascular hospitalizations and noncardiovascular hospitalizations, background therapy hospitalization rates and mortality rates, and AE disutility (Figure 2).
Table 2.
Results of Sensitivity Analysis
| Parameters | Base‐Case Inputs | Sensitivity Analysis Inputs | ICER (US$) |
|---|---|---|---|
| Base case (commercial, US claims data) | NA | NA | Cost saving |
| Treatment effect only on first HF hospitalization | SHIFT analysis | Hazard ratio=1 for all hospitalizations other than first HF event | $48 571 |
| Treatment effect on first HF hospitalization | SHIFT analysis |
95% CI‐lower bound 95% CI‐upper bound |
Cost saving $16 185 |
| Treatment effect on mortality | SHIFT analysis |
95% CI‐lower bound 95% CI‐upper bound |
$21 907 Cost saving |
| Time horizon | 10 years |
Lifetime 5 years |
$30 082 Cost saving |
| Hospitalization rates | US claims | SHIFT | $11 574 |
HF indicates heart failure; ICER, incremental cost‐effectiveness ratio; NA, not applicable; SHIFT, Systolic Heart failure treatment with the If inhibitor ivabradine Trial.
Figure 2.
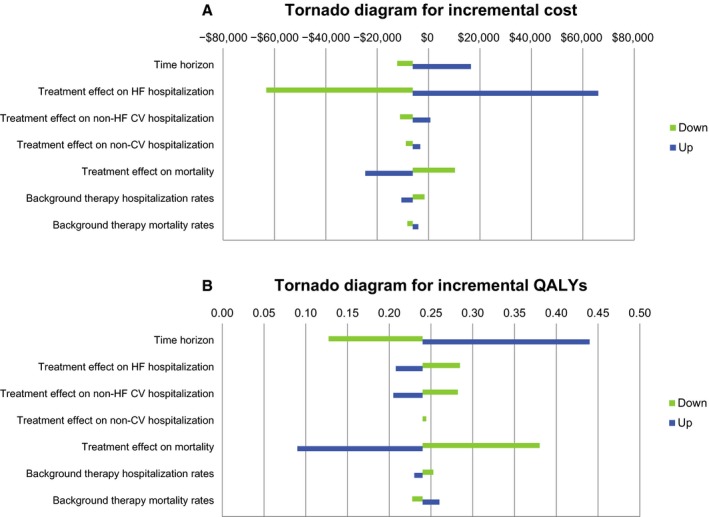
Tornado diagrams on (A) incremental cost and (B) incremental QALYs comparing the relative importance of model parameters for ivabradine vs background therapy. CV indicates cardiovascular; HF, heart failure; QALY, quality‐adjusted life year.
Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis was run for 1000 replications with appropriate distributions assigned to each selected parameter based on the confidence bounds and standard errors provided in Tables S1 through S6 (cost: Gamma distribution, utility: Normal distribution, hospitalization rates and treatments: Normal distribution). The resulting cost‐effectiveness plane and acceptability curve (Figures 3 and 4) showed that ivabradine was likely to be cost‐effective relative to background therapy for all willingness‐to‐pay thresholds (60% likely at $0 per QALY gained threshold; 85% for $150 000 per QALY gained).
Figure 3.
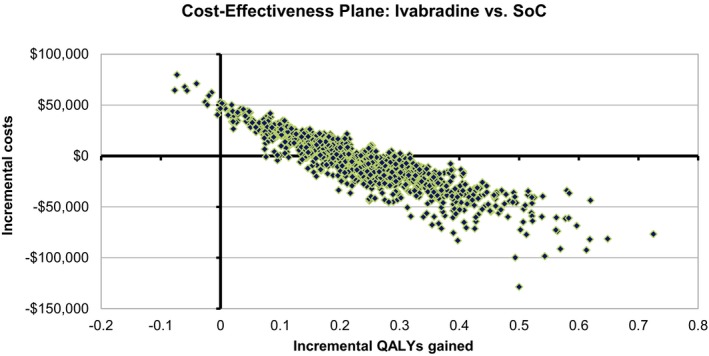
Cost‐effectiveness plane: ivabradine vs SoC. QALY indicates quality‐adjusted life year; SoC, standard of care.
Figure 4.
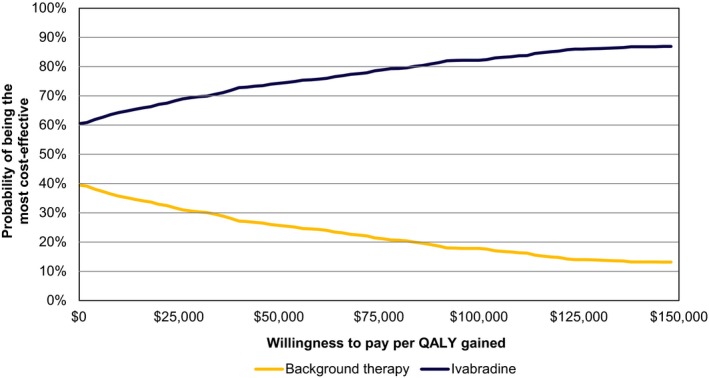
Cost‐effectiveness acceptability curve. QALY indicates quality‐adjusted life year.
Discussion
The base‐case analysis indicated that the drug costs associated with adding ivabradine to background therapy would be largely offset by the cost savings associated with reduced hospitalization. Overall, the addition of ivabradine resulted in total cost savings for the commercial population and a modest cost increase in the Medicare Advantage population, and higher LYs and QALYs compared with background therapy in both populations. The lower cost of hospitalization coupled with a high disease prevalence led to the modest cost increase in the Medicare Advantage population. The first factor reduces the economic offset caused by ivabradine, and the second results in a higher number of patients, leading to higher drug costs. An American College of Cardiology/American Heart Association Task Force recently published a statement on cost methodology where they proposed different categories of value.10 The highest category is the category of “high value,” which is defined as a therapy that provides either better outcomes at a lower cost or an ICER of <$50 000 per QALY gained. Therefore, in the base case and in all of the sensitivity analyses, ivabradine would be considered “high value” based on the American College of Cardiology/American Heart Association value guidelines.10
These results are consistent with those of several international studies investigating the cost‐effectiveness of adding ivabradine to background therapy for HF. In a study conducted in the United Kingdom, the additional £3341 associated with ivabradine drug therapy and follow‐up costs was partially offset by a reduction in hospitalization costs of £965.15 The ICER per QALY was estimated to be £8498 for patients with a HR ≥75 bpm, which is substantially lower than the £20 000 to £30 000 per QALY threshold used to determine cost‐effectiveness in the United Kingdom.15 Similar results were identified in a study conducted in Greece, in which the ICER per QALY with the addition of ivabradine to background therapy was estimated at €9986, well below the cost‐effectiveness threshold of €36 000 per QALY.16 A budget‐impact model has also demonstrated potential cost offsets associated with the use of ivabradine in both a commercial and a Medicare Advantage population from a US payer perspective (Jeffrey S. Borer, MD, Anuraag R. Kansal, PhD, Emily D. Dorman, MPH, MBA, Stanimira Krotneva, MSc, Ying Zheng, MHSA, MS, Harshali K. Patel, MS, PhD, Luigi Tavazzi, Michel Komajda, Ian Ford, Michael Böhm, Adrian Kielhorn, in press, Journal of Managed Care & Specialty Pharmacy). In the current study, cost offsets were only observed in a commercial population, whereas the Medicare Advantage population had a modest cost increase. This can be attributed to a number of differences between the 2 models. A key factor contributing to differences across the results in the models is how mortality was treated. In our cost‐effectiveness model, the effect of ivabradine on mortality was incorporated. This results in patients on ivabradine living longer, thus incurring more cost. However, for the budget‐impact model, mortality was considered at the natural rate and no mortality benefit due to ivabradine was incorporated. In our cost‐effectiveness model, therefore, ivabradine was found to be a dominant treatment over background therapy in the US commercially insured population, with a net cost saving. This is because higher cost offsets from reduced hospitalizations were realized, which can be largely attributed to a higher cost per hospitalization in the United States than in the United Kingdom or elsewhere in the European Union.17
This model has a number of strengths. First, the US claims data were derived from real‐world utilization of healthcare resources, which was used to inform the reference hospitalization rates and costs. The noncardiovascular mortality rates were taken from the US life table, and were therefore specific to the US population. The model also had the flexibility to use hospitalization rates from SHIFT. This allowed for all hospitalization, mortality, and treatment effect parameters to be informed by a consistent source.
The model does have several limitations. First, the background therapy hospitalization rates and costs derived from US claims data were based on the general chronic HF population. It was not feasible to further refine the population to match the exact eligible population for ivabradine,9 as clinical parameters such as HR and New York Heart Association classes were not collected in the claims database. Second, as the real‐world effectiveness of ivabradine in the United States is to be studied, the model extrapolates the efficacy outcome from the clinical trial (SHIFT study) to the real‐world setting. Third, efficacy inputs based on the SHIFT study do not differ between the commercial (age 18–64 years) and Medicare Advantage populations. Although the cost and background frequency of hospitalizations varied between the 2 groups of patients, we applied the same clinical benefit to both populations. Finally, the model does not include costs of background therapy; therefore, the reference case does not represent the total costs of background therapy. The model also assumed that during AE visits, the same level of care was provided to patients, regardless of treatment arm. It is expected that these cost components would have minimal impact on the model outcome as the cost outcomes were predominantly driven by hospitalization and drug costs.
In summary, the results of this cost‐effectiveness model suggest that ivabradine+background therapy for HF would be cost‐effective from a US payer perspective and would be considered “high value” based on American College of Cardiology/American Heart Association value guidelines.
Sources of Funding
This study was funded by Amgen Inc.
Disclosures
Dr Kansal, Ms Krotneva, Dr Tafazzoli, and Ms Zheng are employees of Evidera, which was engaged by Amgen as consultants on this project. Dr Cowie's salary is supported by the National Institute for Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital, London, UK. Mr Kielhorn and Dr Yurgin are employees of Amgen Inc.
Supporting information
Table S1. Annual Hospitalization Rates From US Claims
Table S2. Annual Hospitalization Rates and Treatment Effect on Hospitalization From SHIFT
Table S3. Annual Mortality Rates and Hazard Ratios Derived From SHIFT Data
Table S4. AE Rates per Year
Table S5. Hospitalization and AE Costs
Table S6. Utility Regression Equations
Acknowledgments
Medical writing support was provided by Apothecom on behalf of Amgen Inc. and editorial support was provided by Tim Peoples of Amgen Inc.
(J Am Heart Assoc. 2016;5:e003221 doi: 10.1161/JAHA.116.003221)
Accompanying Tables S1 through S6 are available at http://jaha.ahajournals.org/content/5/5/e003221/DC1/embed/inline-supplementary-material-1.pdf
References
- 1. Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure?. Europace. 2011;13(suppl 2):ii13–ii17. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG; American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 4. Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 6. Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010;376:886–894. [DOI] [PubMed] [Google Scholar]
- 7. Laskey WK, Alomari I, Cox M, Schulte PJ, Zhao X, Hernandez AF, Heidenreich PA, Eapen ZJ, Yancy C, Bhatt DL, Fonarow GC; AHA Get With The Guidelines®‐Heart Failure Program . Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. J Am Heart Assoc. 2015;4:e001626 doi: 10.1161/JAHA.114.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–446. [DOI] [PubMed] [Google Scholar]
- 9. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 10. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 11. US Social Security Administration . Actuarial life table. Available at: http://www.ssa.gov/oact/STATS/table4c6.html. Accessed April 30, 2015.
- 12. US Centers for Disease Control and Prevention, National Center for Health Statistics . National vital statistics system, mortality, 2006. Updated July 20, 2009. Available at: http://www.cdc.gov/nchs/data/dvs/MortFinal2006_-Worktable210f.pdf. Accessed April 30, 2015.
- 13. InGauge ICD‐9‐CM Expert; Physician. Atlanta, GA: InGauge Healthcare Solutions; 2014. [Google Scholar]
- 14. Physician fee schedule search. Centers for Medicare & Medicaid Services; Available at: http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed June 4, 2015. [Google Scholar]
- 15. Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. National Health Service perspective. Heart. 2014;100:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kourlaba G, Parissis J, Karavidas A, Beletsi A, Milonas C, Branscombe N, Maniadakis N. Economic evaluation of ivabradine in the treatment of chronic heart failure in Greece. BMC Health Serv Res. 2014;14:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen CL, Burton R. U.S. health care spending: comparison with other OECD countries. Congressional Research Service (CRS) Report for Congress. Available at: http://digitalcommons.ilr.cornell.edu/cgi/viewcontent.cgi?article=1316&context=key_workplace. Accessed June 1, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Annual Hospitalization Rates From US Claims
Table S2. Annual Hospitalization Rates and Treatment Effect on Hospitalization From SHIFT
Table S3. Annual Mortality Rates and Hazard Ratios Derived From SHIFT Data
Table S4. AE Rates per Year
Table S5. Hospitalization and AE Costs
Table S6. Utility Regression Equations


