Abstract
Background
The safety and long‐term outcome of systemic thrombolysis in patients receiving antiplatelet medications remain subjects of great clinical significance. The objective of this meta‐analysis was to determine how prestroke antiplatelet therapy affects the risks and benefits of intravenous thrombolysis in patients with acute ischemic stroke.
Methods and Results
A dual‐reviewer search was conducted in PubMed and EMBASE databases through November 2015, from which 19 studies involving a total of 108 588 patients with acute ischemic stroke were identified based on preset inclusion criteria. Information on study designs, patient characteristics, exposures, outcomes, and adjusting confounders was extracted, and estimates were combined by using random‐effects models. The pooled crude estimates suggested that taking long‐term antiplatelet medications was associated with higher odds of symptomatic intracranial hemorrhage (odds ratio [OR] 1.70, 95% CI 1.47–1.97) and death (OR 1.46, 95% CI 1.22–1.75) and lower odds of favorable functional outcomes (OR 0.86, 95% CI 0.80–0.93). However, the combined confounder‐adjusted results only confirmed a relatively weak positive association between prior antiplatelet therapy and symptomatic intracranial hemorrhage (OR 1.21, 95% CI 1.02–1.44) and demonstrated no significant relationship between antiplatelet therapy and the other 2 outcomes (favorable outcome OR 1.09, 95% CI 0.96–1.24; death OR 1.02, 95% CI 0.98–1.07). Subgroup analyses revealed that the associations between prestroke antiplatelet therapy and outcomes were dependent on time and antiplatelet agents.
Conclusions
Patients with acute ischemic stroke receiving long‐term antiplatelet medications were associated with greater risks of developing symptomatic intracranial hemorrhage after systemic thrombolysis. However, the overall independent association between prestroke antiplatelet therapy and unfavorable outcomes or mortality was insignificant.
Keywords: meta‐analysis, plasminogen activators, stroke
Subject Categories: Ischemic Stroke, Quality and Outcomes, Meta Analysis
Introduction
Although systemic thrombolysis remains the most effective medical treatment for acute ischemic stroke,1 many postthrombolytic patients develop intracranial hemorrhage (ICH), a dreaded complication that frequently leads to early deterioration, and have unfavorable long‐term outcomes.2, 3, 4 Numerous efforts have been made by researchers to identify factors that could cause alterations in the efficacy and safety of systemic thrombolysis,5 among which prestroke medications have always been a major area of interest.6 However, it is thus far a disturbing fact—given the large proportion of stroke patients who receive long‐term antiplatelet therapy—that no consensus has been reached on the exact risk‐benefit profile of intravenous thrombolysis in patients taking antiplatelet medications before the onset of stroke. Aside from the European guideline that merely referred to prior antiplatelet therapy as a “warning sign” of low safety,7 the latest guidelines have yet to provide a clear message as to how those patients would react differently to thrombolytic therapy and how their diseases might progress afterward.7, 8
Previous studies that sought to examine the correlations between prestroke antiplatelet therapy and postthrombolytic outcomes were mostly small, and their findings were largely inconsistent.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Meta‐analyses based on only a limited number of those studies were therefore subject to inaccuracies and biases, because they did not allow us to synthesize adjusted results or to perform comprehensive subgroup analyses.5, 6 Now with data from some of the largest registries having surfaced, our attention is once again brought to the subject of intravenous thrombolysis in patients receiving antiplatelet medications.25, 26, 29 Xian et al concluded recently that those patients had better functional outcomes despite higher risks for symptomatic intracranial hemorrhage (sICH),29 yet it needs to be pointed out that they adopted certain study designs and outcome definitions that were not completely compatible with those of many previous studies. The extent to which the newer study findings could be generalized and how they compared with prior data warrant further analysis.
We thereby conducted this meta‐analysis to determine whether preexisting antiplatelet therapy was associated with altered short‐ and long‐term outcomes in patients with acute ischemic stroke who underwent thrombolysis and attempted to identify patient and study characteristics that might have contributed to the inconsistencies of previous findings through subgroup analysis.
Methods
The study is presented in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.30
Data Sources and Search Strategy
A review protocol was established and was scrutinized and approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat‐sen University before initiation of this study. Two reviewers independently searched PubMed and EMBASE in November 2015 for the Medical Subject Headings (MeSH) term “brain infarction,” along with the following terms and their derivatives: “ischemic stroke” AND “plasminogen activator” or “alteplase” or “thrombolysis” AND “antiplatelets” or “aspirin” or “acetylsalicylate” or “dipyridamole” or “clopidogrel” or “ticlopidine” or “prasugrel” or “ticagrelor” or “cilostazol” or “GP IIb/IIIa inhibitors” or “abciximab” or “eptifibatide” or “tirofiban.” All titles and abstracts from this search were reviewed for relevance based on the inclusion/exclusion criteria described later. Full texts of appropriate abstracts were then reviewed and the final list of articles for inclusion was determined by consensus of all authors. Our selection of studies was not limited to date or language. We also searched the references of included studies to identify potentially relevant citations.
Eligibility Criteria
Studies were considered eligible if they met the following criteria: (1) studies performed on patients with acute ischemic stroke aged ≥18 years, (2) included patients who were treated with tissue plasminogen activator (tPA), (3) compared patients taking prestroke antiplatelet medications with those who were not, and (4) reported ≥1 of the outcomes defined next.
Outcomes
Major outcomes for patients with postthrombolytic acute ischemic stroke in this study include (1) sICH, (2) modified Rankin Scale score (mRS), and (3) death from all causes. Although variations in how outcomes were defined existed across studies, we considered any reported sICH within 7 days and any mRS score or mortality within 90 days from stroke onset as eligible outcomes.
Data Extraction
Two investigators individually collected data on patient demographic characteristics such as age, sex, serum glucose levels, National Institutes of Health Stroke Scale scores, thrombolysis time from onset, and specific types of prestroke antiplatelet medications by using standardized data collection forms. Study characteristics such as original study type, definitions of sICH, and time from stroke onset to outcome follow‐up were also abstracted. Both raw and adjusted outcomes and their adjusting variables were recorded. Any disagreement was resolved by consensus among all authors.
Quality of Data Assessment
The methodological quality of included studies was evaluated by using the Newcastle–Ottawa Scale (NOS) system for cohort studies,31 in which studies were judged on 3 broad perspectives: the selection of the study groups (0–4 points), the comparability of the groups (0–2 points), and the ascertainment of the outcome of interest (0–3 points). The quality scores were not used as weights in the analysis, as recommended by the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) study group,32 but rather as differentiators in subgroup analyses (score ≤7 versus >7).
Statistical Analysis
We assumed the effects being estimated in the included studies to be heterogeneous and used the random‐effects models instead of the fixed‐effects models to estimate pooled odds ratios (ORs) and 95% CIs so as to be more conservative. When multiple sets of data regarding different outcome definitions were reported within a single study, those based on definitions most frequently adopted in other included studies were used in the main analysis, and each set of these estimates were subsequently included in subgroup analyses that evaluated the influence of outcome definitions on the pooled results. For a study that reported separate adjusted ORs for patients taking single‐ and dual‐antiplatelet agents,16 combined ORs of the 2 groups were calculated by using the fixed‐effects model to provide overall estimates for the study in the main analysis. ORs and both the lower and upper limits of corresponding 95% CIs from each study were logarithmically transformed to normalize distribution before they were combined. Heterogeneity was assessed by using the χ2 test and quantified by using the I 2 statistic, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than chance.33 Subgroup analyses were carried out based on outcome definitions, onset‐to‐treatment time, specific antiplatelet agents, and the NOS scores of included studies. Funnel plot analysis and the Egger test were performed to assess publication bias if >10 studies were included in a single analysis. In case of significant funnel plot asymmetry, the trim‐and‐fill method was used to test the reliability of study results.34 All statistical analyses were conducted by using Stata 12.0 (StataCorp).
Results
Search Results
The primary search strategy produced 2243 studies for review, of which 21 satisfied all inclusion criteria. For the 2 pairs of studies that analyzed overlapped data from the same registries, we included the latest and those with the largest samples for meta‐analysis.14, 18, 22, 26 The detailed selection process is summarized in Figure 1. Additional searches of references did not provide additional studies.
Figure 1.
Selection process of studies included in the meta‐analysis. AF indicates atrial fibrillation; IV, intravenous; IA, intra‐arterial; tPA, tissue plasminogen activator.
Study Characteristics and Baseline Demographics
The final 19 studies involved a total of 108 588 patients, of whom 46 478 were receiving antiplatelet therapy before stroke onset. The characteristics of those studies are presented in Table 1 and the raw outcomes are shown in Table 2. Seven studies provided ≥1 adjusted odds estimates for the main outcomes. The characteristics of those studies are recorded in Table 3.
Table 1.
Characteristics of Studies Included for Meta‐Analysis
| First Author (Year) | Prior AP Therapy | No. of Patients, N | Median Age, y | Female, % | Mean Serum Glucose, mg/dL | Mean NIHSS | Onset to Treatment Time, min | AP Agents | sICH Definition | Outcome Follow‐up Time, d |
|---|---|---|---|---|---|---|---|---|---|---|
| Xian29 (2016) | Yes | 38 844 | 73.7 | 49.7 | 138.6 | 11.5 | 138 | A/C/AC/AD/O | NAa 0–36 h | At discharge |
| No | 46 228 | 67.2 | 51.4 | 135.6 | 11.2 | 138 | ||||
| Meseguer27 (2015) | Yes | 191 | 74.5 | 43.4 | 124 | 12 | 160 | NA | E 0–24 h | 90 |
| No | 375 | 65.8 | 46.1 | 119 | 11 | 160 | ||||
| Watson‐Fargie28 (2015) | Yes | 132 | NA | NA | NA | NA | NA | NA | E/N 0–24 h | NA |
| No | 216 | |||||||||
| Lindley26 (2015) | Yes | 775 | NA | NA | NA | NA | NA | A/C/D/O | NA 0–7 d | 7 |
| No | 740 | |||||||||
| Pan25 (2015) | Yes | 157 | 66.0 | 40.8 | 140.7 | 12 | 168 | A/C/AC/O | E/N/S24–36 h | 90 |
| No | 951 | 62.7 | 38.3 | 138.9 | 11 | 168 | ||||
| Frank23 (2013) | Yes | 727 | NA | NA | NA | NA | NA | SI/DU | E 0–4 d | NA |
| No | 1826 | |||||||||
| Meurer24 (2013) | Yes | 388 | 74 | 47.7 | 137 | 13 | 148 | NA | N 0–10 d | NA |
| No | 442 | 65 | 46.2 | 126 | 12 | 151 | ||||
| Šaňák21 (2012) | Yes | 56 | 69.8 | 50.0 | NA | 16 | 154.7 | A/C/AD | S 0–24 | NA |
| No | 90 | 65.8 | 43.3 | 15 | 157.6 | |||||
| Ibrahim20 (2010) | Yes | 95 | 71.5 | 51.3 | NA | 16.8 | NA | A/C/AC/AD | S 0–72 h | 90 |
| No | 180 | 68.3 | 33.8 | 15.8 | ||||||
| Dorado19 (2010) | Yes | 72 | 70.5 | NA | NA | NA | NA | A/C/AC/O | E 0–36 h | 90 |
| No | 163 | 66.9 | ||||||||
| Diedler18 (2010) | Yes | 3782 | 71 | 41.2 | 118 | 12 | 140 | A/C/AC/AD/O | E/N/S 0–7 d | 90 |
| No | 7954 | 66 | 35.1 | 116 | 12 | 140 | ||||
| Hermann17 (2009) | Yes | 36 | 71 | NA | NA | NA | NA | A/C/AC/AD | S 12–36 h | NA |
| No | 27 | 67 | ||||||||
| Cucchiara16 (2009) | Yes | 337 | NA | NA | NA | NA | NA | SI/AC/AD/O | NA 0–36 h | NA |
| No | 628 | |||||||||
| Bluhmki15 (2009) | Yes | 130 | NA | NA | NA | NA | NA | NA | N 22–36 h | 90 |
| No | 288 | |||||||||
| Uyttenboogaart13 (2008) | Yes | 89 | 73 | 48.4 | 115 | 12 | 165 | A/C/D/AD | S 0–36 h | 90 |
| No | 212 | 66 | 47.2 | 115 | 13 | 175 | ||||
| Bravo12 (2008) | Yes | 137 | 72.2 | 35 | 137 | 14 | 148.5 | A/C/D/O | E 24–36 h | NA |
| No | 468 | 66.6 | 43.8 | 134 | 15 | 151.1 | ||||
| Martí‐Fàbregas11 (2007) | Yes | 49 | NA | NA | NA | NA | NA | NA | NA 24–36 h | NA |
| No | 298 | |||||||||
| Schmülling10 (2003) | Yes | 95 | 66 | NA | NA | 13 | NA | A/O | N 36–48 h | 90 |
| No | 202 | 62 | 10 | |||||||
| Tanne9 (2002) | Yes | 386 | NA | NA | NA | NA | NA | A/O | N 0–36 h | NA |
| No | 813 |
AP indicates antiplatelet; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage; A, aspirin; C, clopidogrel; AC, aspirin–clopidogrel; AD, aspirin–dipyridamole; O, other antiplatelet medications; NA, not available; E, ECASS II, Second European‐Australasian Acute Stroke Study3; N, NINDS, National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group2; D, dipyridamole; S, SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study4; SI, single; DU, dual.
sICH definition was recorded as NA if it did not accord with any of the 3 definitions: ECASS II, SITS‐MOST, and NINDS.
Table 2.
Raw Outcomes
| First Author (Year) | Prior AP Therapy | No. of Patients, N | No. of Patients with sICH, N (%) | No. of Patients Followed for Outcome, N | No. of Patients With Good Outcomes, N (%) | No. of Patients Followed for Mortality, N | No. of Deaths, N, (%) |
|---|---|---|---|---|---|---|---|
| Xian29 (2016) | Yes | 38 844 | 1927 (5.0) | 15 475 | 5081 (32.8) | 38 844 | 3115 (8.0) |
| No | 46 228 | 1720 (3.7) | 17 613 | 6548 (37.2) | 46 228 | 3061 (6.6) | |
| Meseguer27 (2015) | Yes | 191 | 14 (7.3) | 191 | 113 (59.2) | 191 | 24 (12.6) |
| No | 375 | 18 (4.8) | 375 | 241 (64.2) | 375 | 43 (11.5) | |
| Watson‐Fargie28 (2015) | Yes | 132 | 14 (10.6) | NA | NA | NA | NA |
| No | 216 | 4 (1.9) | |||||
| Lindley26 (2015) | Yes | 775 | 70 (9.0) | NA | NA | 775 | 107 (13.8) |
| No | 740 | 34 (4.6) | 740 | 56 (7.5) | |||
| Pan25 (2015) | Yes | 157 | 10 (6.3) | 153 | 87 (56.9) | 154 | 22 (14.3) |
| No | 951 | 32 (3.4) | 933 | 545 (58.4) | 933 | 91 (9.8) | |
| Frank23 (2013) | Yes | 727 | 47 (6.5) | NA | NA | NA | NA |
| No | 1826 | 66 (3.6) | |||||
| Meurer24 (2013) | Yes | 388 | 35 (9.0) | NA | NA | NA | NA |
| No | 442 | 27 (6.1) | |||||
| Šaňák21 (2012) | Yes | 56 | 3 (5.4) | NA | NA | NA | NA |
| No | 90 | 2 (2.2) | |||||
| Ibrahim20 (2010) | Yes | 95 | 9 (9.5) | 84 | 33 (39.3) | NA | NA |
| No | 180 | 9 (5.0) | 160 | 85 (53.1) | |||
| Dorado19 (2010) | Yes | 72 | 8 (11.1) | 72 | 34 (47.2) | NA | NA |
| No | 163 | 8 (5.0) | 163 | 94 (57.7) | |||
| Diedler18 (2010) | Yes | 3782 | 325 (8.6) | 3782 | 1783 (47.1) | 3782 | 515 (13.6) |
| No | 7954 | 507 (6.4) | 7954 | 3922 (49.3) | 7954 | 727 (9.1) | |
| Hermann17 (2009) | Yes | 36 | 3 (8.3) | NA | NA | NA | NA |
| No | 27 | 0 (0) | |||||
| Cucchiara16 (2009) | Yes | 337 | 31 (9.2) | NA | NA | NA | NA |
| No | 628 | 23 (3.7) | |||||
| Bluhmki15 (2009) | Yes | 130 | 11 (8.5) | 130 | 68 (52.3) | 130 | 11 (8.5) |
| No | 288 | 22 (7.6) | 288 | 151 (52.4) | 288 | 21 (7.3) | |
| Uyttenboogaart13 (2008) | Yes | 89 | 12 (13.5) | 89 | 45 (50.6) | NA | NA |
| No | 212 | 6 (2.8) | 212 | 95 (44.8) | |||
| Bravo12 (2008) | Yes | 137 | 9 (6.6) | NA | NA | NA | NA |
| No | 468 | 17 (3.6) | |||||
| Martí‐Fàbregas11 (2007) | Yes | 49 | 2 (4.1) | NA | NA | NA | NA |
| No | 298 | 6 (2.0) | |||||
| Schmülling10 (2003) | Yes | 95 | 6 (6.3) | 95 | 46 (48.4) | 95 | 17 (17.9) |
| No | 202 | 5 (2.5) | 202 | 107 (53.0) | 202 | 19 (9.4) | |
| Tanne9 (2002) | Yes | 386 | 33 (8.5) | NA | NA | NA | NA |
| No | 813 | 37 (4.6) |
When multiple definitions of symptomatic intracranial hemorrhage2 (sICH) were used within 1 study, the numbers of sICH patients were recorded based on the National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group definition. When multiple definitions of favorable functional outcome were adopted within 1 study, those with modified Rankin Scale scores of 0 to 2 were considered to be patients with a good outcome. When mortality rates were recorded at multiple time points within 1 study, mortality on day 90 from stroke onset was recorded. NA indicates not available.
Table 3.
Characteristics of Studies that Presented Adjusted Odds Estimates
| First Author (Year) | No. of Patients taking AP medications, N | No. of Controls, N | Adjusted Estimates | Stratification by OTT | Stratification by AP Agents | Stratification by Outcome Definitions | Adjusting Variables |
|---|---|---|---|---|---|---|---|
| Xian29 (2016) | 22 813 | 20 221 | sICH, good functional outcome, mortality | 0–3 h | A, C, AC, AD | mRS 0–1, mRS 0–2 | G, X, OTT, APM, HC, HS |
| Pan25 (2015) | 157 | 951 | sICH, mortality | NA | A, AC | NINDS, ECASS II, SITS‐MOST | NA |
| Meurer24 (2013) | 388 | 442 | sICH | NA | NA | NA | G, S, N, OTT |
| Dorado19 (2010) | 72 | 163 | sICH | NA | NA | NA | X, E, HC, PVD, R |
| Diedler18 (2010) | 3782 | 7954 | sICH, good functional outcome, mortality | NA | A, C, AC, AD | NINDS, ECASS II, SITS‐MOST/mRS 0–1, mRS 0–2 | NA |
| Cucchiara16 (2009) | 337 | 628 | sICH, good functional outcome, | NA | Single, double | NA | G, N, SBP, DBP, HTN, SG, HC, HS, ASP |
| Uyttenboogaart13 (2008) | 89 | 212 | sICH, good functional outcome | 0–3 h | NA | NA | G, N, SG, R, SBP, HTN |
AP indicates antiplatelet; OTT, onset‐to‐treatment time; sICH, symptomatic intracranial hemorrhage; A, aspirin; C, clopidogrel, AC, aspirin‐clopidogrel; AD, aspirin‐dipyridamole; mRS, modified Rankin Scale; G, age; X, sex; APM, type of antiplatelet medication; HC, history of coronary heart disease; HS, history of stroke/ transient ischemic attack; NA, not available; NINDS, National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group2; ECASS II, Second European‐ Australasian Acute Stroke Study3; SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study4; S, tobacco smoking; N, National Institute of Health Stroke Scale; E, history of ethanol abuse; PVD, history of peripheral vascular disease; R, prehospital radiology results; SBP, systolic blood pressure; DBP, diastolic blood pressure; HTN, history of hypertension; SG, serum glucose; ASP, the Alberta Stroke Program Early CT Score (ASPECTS).
All of the studies were retrospective analyses conducted with prospectively collected data. Patients taking long‐term antiplatelet medications were significantly older and had more comorbidities than did those who were not, in most studies, which suggested that both unadjusted and adjusted estimates needed to be taken into consideration when our results were interpreted. Baseline serum glucose levels and National Institutes of Health Stroke Scale scores did not differ significantly either at the study level or between groups in each study. However, certain study‐level variations existed in terms of onset‐to‐treatment time and outcome definitions, which indicated heterogeneous study designs and patient populations, and therefore the need for subgroup analysis. Among the 19 studies, only 10 defined prestroke antiplatelet therapy, most of which considered documentation of antiplatelet medications within 7 days before stroke onset as evidence of long‐term antiplatelet therapy. Only 1 study mentioned aspirin dosage as part of its inclusion criteria.10
Quality Assessment Outcomes
The outcomes of study quality assessment are as follows: 8 studies scored 9 (Xian et al,29 Pan et al,25 Meurer et al,24 Ibrahim et al,20 Dorado et al,19 Diedler et al,18 Cucchiara et al,16 and Uyttenboogaart et al13), 3 studies scored 8 (Bluhmki et al,15 Bravo et al,12 and Tanne et al9), and 7 studies scored 7 (Meseguer et al,27 Watson‐Fargie et al,28 Lindley et al,26 Martí‐Fàbregas et al,11 Schmülling et al,10 Šaňák et al,21 and Hermann et al17). Those scores served as differentiators in a subgroup analysis that divided studies into 2 groups (NOS >7 and NOS ≤7).
Outcomes of Meta‐Analysis
Symptomatic intracranial hemorrhage
Of the 19 studies, 7 defined sICH per National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group (NINDS)2 criteria, 7 reported sICH based on the Second European‐Australasian Acute Stroke Study (ECASS II)3 definition, 5 used the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST) definition,4 and 5 studies did not define sICH according to any of these prespecified criteria. Given the number of included sICH cases diagnosed per NINDS criteria was the largest, for the 3 studies that reported multiple sets of data regarding different sICH definitions,18, 25, 28 those based on the NINDS criteria were included for the main analysis. According to the pooled unadjusted OR, preexisting antiplatelet therapy was significantly associated with postthrombolytic sICH (OR 1.70, 95% CI 1.47–1.97) (Figure 2A). Although the pooled adjusted OR was not as great, it also reached statistical significance (OR 1.21, 95% CI 1.02–1.44) (Figure 3A). Higher heterogeneity was observed in the adjusted estimates (adjusted I 2=65.3%, P=0.008 versus unadjusted I 2=40.6%, P=0.034), yet the sources of heterogeneity were able to be identified when studies were stratified by sICH definition and antiplatelet agents. Though also suggesting higher postthrombolytic sICH tendency in patients on long‐term antiplatelet therapy, certain subgroup analyses did not yield results of statistical significance likely because of insufficient sample sizes (Table 4). When stratified by antiplatelet agents, our analysis revealed that odds of postthrombolytic sICH were lower in patients receiving prestroke clopidogrel (adjusted OR 0.81, 95% CI 0.64–1.02) than in those who were receiving prior aspirin–clopidogrel dual therapy (adjusted OR 1.88, 95% CI 1.18–3.00). All subgroup analysis results are shown in Table 4.
Figure 2.
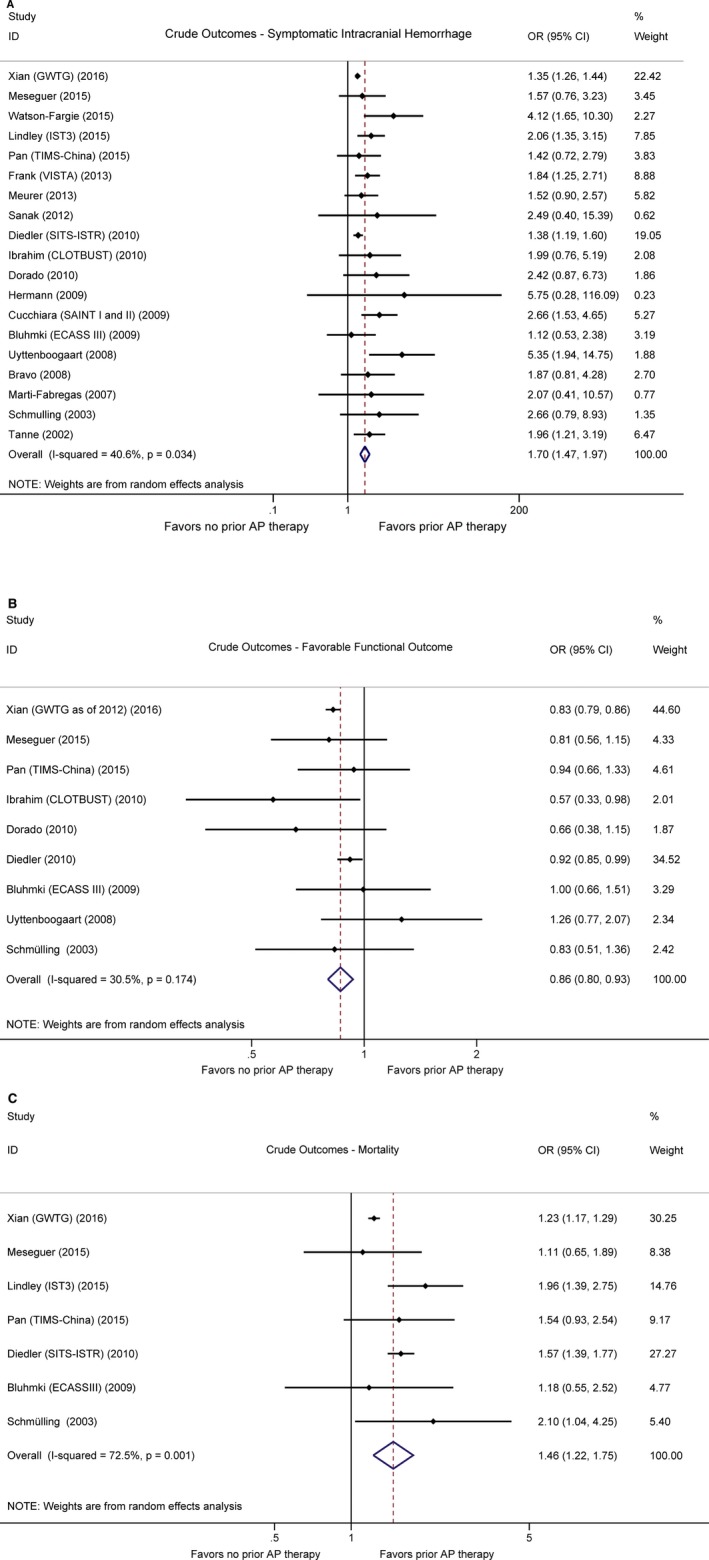
Forest plots showing crude outcomes in patients who underwent thrombolysis with or without prestroke antiplatelet therapy. A, Symptomatic intracranial haemorrhage. B, Favorable functional outcome (defined by modified Rankin Scale score ≤2). C, Mortality. AP indicates antiplatelet. Horizontal axes are placed on log scale.
Figure 3.
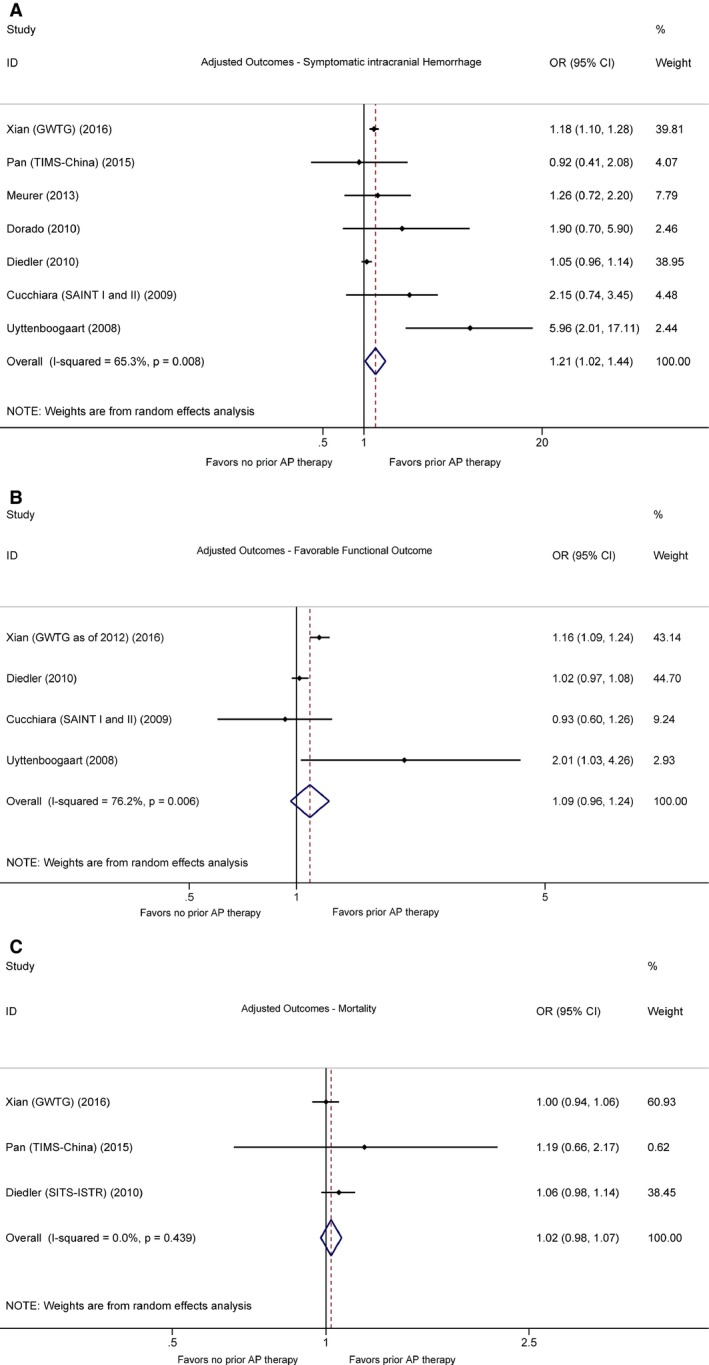
Forest plots showing adjusted outcomes in patients who underwent thrombolysis with or without prestroke antiplatelet therapy. A, Symptomatic intracranial haemorrhage. B, Favorable functional outcome (defined by modified Rankin Scale score ≤2). C, Mortality. AP indicates antiplatelet. Horizontal axes are placed on log scale.
Table 4.
Subgroup Analyses
| Outcomes | Factor | Crude OR (95% CI) | Studies, n | I 2, % (P Value) | Adjusted OR (95% CI) | Studies, n | I 2, % (P Value) |
|---|---|---|---|---|---|---|---|
| Symptomatic intracranial hemorrhage | sICH definition | ||||||
| NINDS | 1.59 (1.26–1.99) | 7 | 28.1 (0.21) | 1.05 (0.97–1.15) | 3 | 0.0 (0.78) | |
| ECASS II | 1.75 (1.42–2.16) | 7 | 16.8 (0.30) | 1.10 (0.99–1.21) | 3 | 0.0 (0.55) | |
| SITS‐MOST | 2.47 (1.92–3.17) | 5 | 0.0 (0.82) | 2.27 (0.86–5.97) | 3 | 75.6 (0.02) | |
| Onset‐to‐treatment time | |||||||
| 0–3 h | 1.59 (1.34–1.88) | 10 | 44.8 (0.06) | 3.18 (0.38–26.35) | 2 | 87.5 (0.01) | |
| >3 h | 1.26 (1.08–1.46) | 3 | 0.0 (0.54) | ||||
| Antiplatelet agents | |||||||
| Aspirin | 1.53 (1.30–1.82) | 8 | 41.0 (0.11) | 1.02 (0.75–1.38) | 3 | 68.1 (0.04) | |
| Clopidogrel | 1.25 (0.82–1.91) | 5 | 39.9 (0.16) | 0.81 (0.64–1.02) | 2 | 0.0 (0.46) | |
| Aspirin–clopidogrel | 3.32 (1.75–6.31) | 5 | 76.9 (0.02) | 1.88 (1.18–3.00) | 3 | 56.0 (0.10) | |
| Aspirin–dipyridamole | 1.02 (0.60–1.73) | 5 | 20.7 (0.29) | 0.99 (0.65–1.50) | 2 | 67.4 (0.08) | |
| NOS score | |||||||
| >7 | 1.59 (1.37–1.84) | 12 | 43.1 (0.06) | ||||
| Favorable outcome | Follow‐up time | ||||||
| 3 month | 0.91 (0.85–0.97) | 8 | 0.0 (0.46) | 1.07 (0.83–1.37) | 3 | 46.5 (0.15) | |
| Good outcome definition | |||||||
| mRS scores 0–2 | 0.86 (0.79–0.93) | 8 | 36.0 (0.14) | 1.09 (0.96–1.23) | 4 | 76.2 (0.01) | |
| mRS scores 0–1 | 0.85 (0.81–0.90) | 4 | 13.4 (0.33) | 1.06 (0.92. 1.22) | 2 | 90.4 (0.00) | |
| Onset‐to‐treatment time | |||||||
| 0–3 h | 0.84 (0.67–1.06) | 3 | 34.4 (0.22) | 1.02 (0.97–1.07) | 2 | 0.0 (0.63) | |
| Antiplatelet agents (mRS 0–2) | |||||||
| Aspirin | 0.85 (0.68–1.05) | 4 | 41.5 (0.16) | 1.11 (1.00–1.24) | 2 | 51.3 (0.15) | |
| Clopidogrel | 0.72 (0.56–0.92) | 2 | 0.0 (0.38) | 1.09 (0.95–1.25) | 2 | 0.0 (0.58) | |
| Aspirin–clopidogrel | 0.81 (0.58–1.11) | 2 | 36.4 (0.21) | 1.04 (0.79–1.36) | 2 | 54.5 (0.14) | |
| Aspirin–dipyridamole | 0.98 (0.56–1.39) | 2 | 62.2 (0.10) | ||||
| Antiplatelet agents (mRS 0–1) | |||||||
| Aspirin | 1.14 (1.06–1.23) | 2 | 0.0 (0.41) | ||||
| Clopidogrel | 1.12 (0.97–1.29) | 2 | 0.0 (0.57) | ||||
| Aspirin–clopidogrel | 1.04 (0.90–1.20) | 2 | 0.0 (0.37) | ||||
| Aspirin–dipyridamole | 0.71 (0.51–1.00) | 2 | 48.8 (0.16) | ||||
| NOS score | |||||||
| >7 | 0.87 (0.79–0.96) | 7 | 47.5 (0.08) | ||||
| Mortality | Follow‐up time | ||||||
| 90 day | 1.54 (1.38–1.73) | 5 | 0.0 (0.60) | 1.06 (0.99–1.15) | 2 | 0.0 (0.71) | |
| Antiplatelet agents | |||||||
| Aspirin | 1.52 (1.34–1.73) | 3 | 0.0 (0.46) | 0.97 (0.89–1.06) | 3 | 0.0 (0.98) | |
| Clopidogrel | 1.00 (0.83–1.23) | 2 | 0.0 (0.62) | ||||
| Aspirin–clopidogrel | 2.67 (1.82–3.90) | 2 | 0.0 (0.57) | 1.14 (0.82–1.59) | 3 | 41.1 (0.18) | |
| Aspirin–dipyridamole | 0.93 (0.63–1.38) | 2 | 25.1 (2.48) | ||||
| NOS score | |||||||
| >7 | 1.38 (1.14–1.68) | 4 | 78.2 (0.00) | ||||
OR indicates odds ratio; sICH, symptomatic intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage; ECASS II, Second European‐Australasian Acute Stroke Study3; NINDS, National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group2; SITS‐MOST, Safe Implementation of Thrombolysis in Stroke‐Monitoring Study4; NOS, Newcastle‐Ottawa Scale; mRS, modified Rankin Scale.
Favorable outcome
All studies with follow‐up patients for functional outcomes defined favorable outcome as mRS scores of either 0 to 2 or 0 to 1 on poststroke day 90, except for 1 study that recorded patient functional outcomes at hospital discharge.29 Our pooled analysis of crude ORs showed that patients taking prior antiplatelet medications tended to have lower odds of reaching favorable functional outcomes (OR 0.86, 95% CI 0.80–0.93) (Figure 2B). However, when adjusted for confounders, the independent association between pre‐existing antiplatelet therapy and unfavorable outcomes became insignificant (adjusted OR 1.09, 95% CI 0.96–1.24). Although a high level of heterogeneity was observed in the adjusted estimates (I 2=76.2%, P=0.006), we were able to locate the major sources of heterogeneity through subgroup analysis (Table 4), which included the 1 study that provided only data on outcome at hospital discharge.29 The pooled adjusted estimates remained similar after the exclusion of this study (OR 1.07, 95% CI 0.83–1.37). Of note, when stratifying for antiplatelet agents and outcome definitions, we found a positive association between prestroke aspirin and favorable functional outcomes defined by mRS 0 to 1 (adjusted OR 1.14, 95% CI 1.06–1.23) and mRS 0 to 2 (adjusted OR 1.11, 95% CI 1.00–1.24), whereas the combination of aspirin–dipyridamole was not positively associated with mRS scores of 0 to 1 (adjusted OR 0.71, 95% CI 0.51–1.00). Other subgroup analysis results are recorded in Table 4.
Mortality
Of the 7 studies that provided information on mortality, one assessed in‐hospital mortality,29 one reported both 7‐ and 90‐day data,26 and the others reported mortality on poststroke day 90. Although pooled unadjusted results indicated increased odds of all‐cause mortality among patients receiving prestroke antiplatelet medications (OR 1.46, 95% CI 1.22–1.75) (Figure 2C), pooled adjusted ORs did not reveal any significant correlations (OR 1.02, 95% CI 0.98–1.07) (Figure 3C). The robustness of such findings were proven via subgroup analyses, of which the results are shown in Table 4.
Publication Bias
The funnel plot of pooled unadjusted estimates for sICH was significantly asymmetric (Figure 4) (Egger test P<0.001), suggesting possible publication bias. To assess the impact of publication bias on our results, the trim‐and‐fill method was applied. After 9 studies were trimmed and 18 were filled, the OR of sICH was not significantly altered (OR 1.46, 95% CI 1.25–1.71). The results of the trim‐and‐fill analysis are shown in Figure 4.
Figure 4.
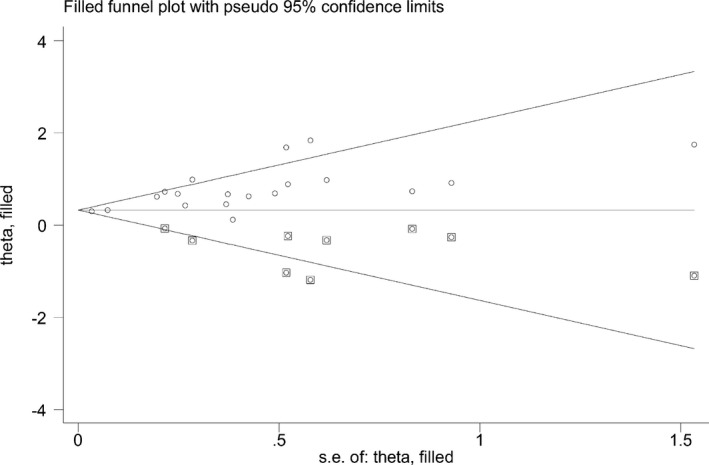
Filled funnel plots of crude estimates for symptomatic intracranial hemorrhage. Squared dots represent studies added in the trim‐and‐fill analysis.
Discussion
The present meta‐analysis provides evidence that patients with acute ischemic stroke receiving long‐term antiplatelet medications were associated with greater risks of developing sICH after systemic thrombolysis, despite a relatively small overall excess. Although those patients were frequently observed to also have higher odds of death or developing unfavorable functional outcomes as suggested by our combined crude estimates, the independent association between prestroke antiplatelet therapy and worsened long‐term outcomes proved insignificant after adjustment for confounders. Therefore, a history of antiplatelet therapy should not be considered as contraindication for systemic thrombolysis in those patients.
Because sICH is the most serious complication of thrombolytic therapy in patients with acute ischemic stroke, it remains a topic of great clinical significance whether patients taking prestroke antiplatelet medications have a substantially elevated risk of sICH. Considering both the unadjusted and adjusted outcome estimates of the present study and the findings from a previous clinical trial that demonstrated higher sICH risk in patients receiving aspirin concomitant with tPA,35 an independent and causal relationship between platelet inhibition and postthrombolytic sICH seems plausible. However, it might be difficult to fully elucidate this phenomenon at the mechanistic level, given the complexity of the development of sICH and the lack of evidence from translational research and animal experiments. It has been suggested in a previous study that recanalization was necessary for the development of sICH at the injured vasculature beyond sites of occlusions.36 Thus, one possible explanation is that it was reperfusion by tPA itself, having been facilitated by platelet inactivation, that contributed to the increased incidence of sICH.37 Given that timely recanalization is strongly associated with favorable functional outcomes,38 if these assumptions were true, the excess risk for sICH attributed to prestroke platelet inhibition might not translate into worse functional outcomes, which is indeed supported by the pooled adjusted estimates of the present study. Moreover, the existence of prior antiplatelet therapy might in some cases even improve the outcomes of postthrombolytic patients, which is again consistent with some of our subgroup analysis results, as will be discussed later.
Although it was observed in many of the included studies that patients receiving prestroke antiplatelet therapy were prone to developing unfavorable outcomes such as disability and death, the demographic contexts need to be taken into consideration. The fact that those patients were taking antiplatelet therapy already implied that they did not represent the same population as other stroke patients. According to our summary of the study subjects' demographic characteristics, most patients receiving preexisting antiplatelet agents were indeed older and had more comorbidities than did those without, and when estimates adjusted for confounders were pooled, the association between prestroke antiplatelet therapy and worse long‐term outcomes disappeared. It is important to point out, however, that even with the pooled adjusted estimate, we cannot guarantee that all possible confounders had been taken into account, as different adjustment variables were used in different studies.
We, therefore, carefully examined the characteristics of each study and conducted comprehensive subgroup analyses on more homogeneous subsets of data to further evaluate the impact of confounders on our results. Notably, those analyses also yielded some interesting practical and research implications. First, in one of the largest studies in this meta‐analysis (Xian et al29), patient functional status was not evaluated on day 90, which is generally recognized as the most appropriate time point for final functional outcome assessment after acute ischemic stroke.39 It can be observed that there existed a significant discrepancy between the findings of this study and the results of the other largest study18 that measured functional outcomes on day 90 (OR 1.16, 95% CI 1.09–1.24 versus OR 1.02, 95% CI 0.97–1.08), which suggested that the impact of prestroke antiplatelet therapy on functional outcomes after thrombolysis might possibly be time dependent. Second, there were obvious differences in the odds of sICH and favorable outcome among patients receiving different prestroke antiplatelet medications. Patients who were receiving the aspirin–clopidogrel regimen had the highest risk of developing sICH (adjusted OR 1.88, 95% CI 1.18–3.00), whereas those receiving aspirin were associated with better functional outcomes (mRS 0–2 adjusted OR 1.11 95% CI 1.00–1.24; mRS 0–1 adjusted OR 1.14, 95% CI 1.06–1.23). Taking together into consideration the fact that the combination of aspirin and clopidogrel has no absolute efficacious superiority over aspirin regarding either primary prevention of atherothrombotic events or secondary prevention of stroke,40, 41 it seems that more cautions need to be taken before such a regimen was prescribed for stroke prevention. Given those existing differences in how different agents affect the efficacy and safety of systemic thrombolysis, we might also expect distinct risk–benefit profiles and interactive characteristics of the newer antiplatelet medications (eg, ticagrelor), as well as other types of thrombolytics (eg, desmoteplase) in stroke patients. While those medications have already been proven safe and started to seek practical indications in the prevention and management of acute ischemic stroke,42, 43 it would be interesting to see how patient care could be improved when they were put into clinical practice.
The strength of the present study lies in the great sample size pooled from a large number of studies. This enabled us to combine both unadjusted and adjusted estimates on all of the 3 major postthrombolytic outcomes and to perform comprehensive subgroup analyses to thoroughly examine the relationship between prestroke antiplatelet medications and the therapeutic outcomes of tPA, which, to the best of our knowledge, no prior study has been able to achieve. However, our study has the following limitations. First, the inherent limitations of observational studies cannot be overcome by meta‐analysis. Because only retrospective studies were available for the present analysis, the overall quality of evidence of our study is low. Second, variations on the doses of prestroke antiplatelet therapy and the issue of patient compliance were not addressed in most of the included studies, which could in turn serve as a potential cause of bias in this study. It, therefore, needs to be noted that imprecise estimations might be made when our findings were applied in clinical settings, and considerations on when patients last received antiplatelet therapy and the actual dosages of their medications need to be taken. Third, the main results for sICH might be a relatively conservative estimation for sICH risk if definitions other than the NINDS criteria were used to diagnose sICH, because the odds estimates for NINDS sICH were the lowest in the 3 studies that reported separate sets of data based on different sICH definitions—which can be observed from the subgroup analysis results—and they were selected to represent those studies in the main analysis. However, when those data were replaced with the other sets of estimates, the increase in either the combined unadjusted or adjusted ORs of sICH turned out to be minimal. Fourth, the funnel plots of crude estimates for sICH were significantly asymmetric, which indicated possible publication bias. Because we limited our search to published articles only, the risk of missing data was inevitable. However, we believed that our search strategy was adequate, for additional searches of references did not provide additional studies, and further, we were able to confirm the reliability of our results by using the trim‐and‐fill method. Nevertheless, because neither the exact mechanism of publication bias nor that of the trim‐and‐fill method has been fully understood, a precise estimation of the impact of publication bias on the accuracy of our results was after all unattainable. Fifth, our study was also susceptible to outcome reporting bias. While much emphasis was laid on the association between prestroke antiplatelet therapy and post‐tPA sICH in most included studies, only a part of them reported data on long‐term outcomes, and even fewer provided adjusted estimates. As a result, there might be limitations on the generalizability of the present findings on functional outcomes and mortality.
Conclusions
Patients with acute ischemic stroke who were receiving long‐term antiplatelet medications were associated with greater risks of developing sICH after systemic thrombolysis, despite a relatively small overall excess. Although those patients were frequently observed to also have higher odds of death or of developing unfavorable functional outcomes in many studies, the overall independent association between prestroke antiplatelet therapy and worsened long‐term outcomes proved insignificant after adjustment for confounders. Nonetheless, it needs to be noticed that the impact of prestroke antiplatelet therapy on post‐thrombolytic outcomes might be dependent on time and antiplatelet agents, and further research is warranted to elucidate the mechanisms underlying those phenomena. Collectively, while a history of prior antiplatelet therapy should not be considered as an overall contraindication to systemic thrombolysis, concerns need to be raised on the efficacy and safety of tPA when it is administered to patients taking different kinds of prestroke antiplatelet medications.
Author Contributions
The authors' primary responsibilities were as follows: S.L. and J.T. conceived and designed the research; S.L. and W.Z. acquired the data; S.L, M.Z. and W.Z. analyzed and interpreted the data; S.L. drafted the contribution; J.T. handled funding, material, tools and supervision.
Sources of Funding
This work was supported by the 973 Program (2013CB531200) and National Nature Science Foundation of China (31530023, 81100201, 31370941, and 81200249). The funding sources had no role in designing, conducting, or reporting this study.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003242 doi: 10.1161/JAHA.116.003242)
References
- 1. Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta‐analysis. Lancet. 2012;379:2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez‐Tejedor E, Trouillas P. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 4. Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): an observational study. Lancet. 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 5. Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta‐analysis of 55 studies. Stroke. 2012;43:2904–2909. [DOI] [PubMed] [Google Scholar]
- 6. Pan X, Zhu Y, Zheng D, Liu Y, Yu F, Yang J. Prior antiplatelet agent use and outcomes after intravenous thrombolysis with recombinant tissue plasminogen activator in acute ischemic stroke: a meta‐analysis of cohort studies and randomized controlled trials. Int J Stroke. 2015;10:317–323. [DOI] [PubMed] [Google Scholar]
- 7. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 8. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 9. Tanne D, Kasner SE, Demchuk AM, Koren‐Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt‐PA Stroke Survey. Circulation. 2002;105:1679–1685. [DOI] [PubMed] [Google Scholar]
- 10. Schmulling S, Rudolf J, Strotmann‐Tack T, Grond M, Schneweis S, Sobesky J, Thiel A, Heiss WD. Acetylsalicylic acid pretreatment, concomitant heparin therapy and the risk of early intracranial hemorrhage following systemic thrombolysis for acute ischemic stroke. Cerebrovasc Dis. 2003;16:183–190. [DOI] [PubMed] [Google Scholar]
- 11. Marti‐Fabregas J, Bravo Y, Cocho D, Marti‐Vilalta JL, Diaz‐Manera J, San Roman L, Puig M, Blanco M, Castellanos M, Millan M, Roquer J, Obach V, Maestre J. Frequency and predictors of symptomatic intracerebral hemorrhage in patients with ischemic stroke treated with recombinant tissue plasminogen activator outside clinical trials. Cerebrovasc Dis. 2007;23:85–90. [DOI] [PubMed] [Google Scholar]
- 12. Bravo Y, Marti‐Fabregas J, Cocho D, Rodriguez‐Yanez M, Castellanos M, de la Ossa NP, Roquer J, Obach V, Maestre J, Marti‐Vilalta JL. Influence of antiplatelet pre‐treatment on the risk of symptomatic intracranial haemorrhage after intravenous thrombolysis. Cerebrovasc Dis. 2008;26:126–133. [DOI] [PubMed] [Google Scholar]
- 13. Uyttenboogaart M, Koch MW, Koopman K, Vroomen PC, De Keyser J, Luijckx GJ. Safety of antiplatelet therapy prior to intravenous thrombolysis in acute ischemic stroke. Arch Neurol. 2008;65:607–611. [DOI] [PubMed] [Google Scholar]
- 14. Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, Erila T, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kohrmann M, Larrue V, Lees KR, Machnig T, Roine RO, Toni D, Vanhooren G. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST). Stroke. 2008;39:3316–3322. [DOI] [PubMed] [Google Scholar]
- 15. Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0‐4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancel Neurol. 2009;8:1095–1102. [DOI] [PubMed] [Google Scholar]
- 16. Cucchiara B, Kasner SE, Tanne D, Levine SR, Demchuk A, Messe SR, Sansing L, Lees KR, Lyden P. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke‐Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40:3067–3072. [DOI] [PubMed] [Google Scholar]
- 17. Hermann A, Dzialowski I, Koch R, Gahn G. Combined anti‐platelet therapy with aspirin and clopidogrel: risk factor for thrombolysis‐related intracerebral hemorrhage in acute ischemic stroke? J Neurol Sci. 2009;284:155–157. [DOI] [PubMed] [Google Scholar]
- 18. Diedler J, Ahmed N, Sykora M, Uyttenboogaart M, Overgaard K, Luijckx GJ, Soinne L, Ford GA, Lees KR, Wahlgren N, Ringleb P. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke. 2010;41:288–294. [DOI] [PubMed] [Google Scholar]
- 19. Dorado L, Millan M, de la Ossa NP, Guerrero C, Gomis M, Lopez‐Cancio E, Ricciardi AC, Davalos A. Influence of antiplatelet pre‐treatment on the risk of intracranial haemorrhage in acute ischaemic stroke after intravenous thrombolysis. Eur J Neurol. 2010;17:301–306. [DOI] [PubMed] [Google Scholar]
- 20. Ibrahim MM, Sebastian J, Hussain M, Al‐Hussain F, Uchino K, Molina C, Khan K, Demchuk AM, Alexandrov AV, Saqqur M. Does current oral antiplatelet agent or subtherapeutic anticoagulation use have an effect on tissue‐plasminogen‐activator‐mediated recanalization rate in patients with acute ischemic stroke? Cerebrovasc Dis. 2010;30:508–513. [DOI] [PubMed] [Google Scholar]
- 21. Sanak D, Kuliha M, Herzig R, Roubec M, Skoloudik D, Zapletalova J, Kocher M, Kral M, Veverka T, Cechakova E, Bartkova A, Prochazka V, Kanovsky P. Prior use of antiplatelet therapy can be associated with a higher chance for early recanalization of the occluded middle cerebral artery in acute stroke patients treated with intravenous thrombolysis. Eur Neurol. 2012;67:52–56. [DOI] [PubMed] [Google Scholar]
- 22. Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, Phillips SJ, Arauz A. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST‐3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frank B, Grotta JC, Alexandrov AV, Bluhmki E, Lyden P, Meretoja A, Mishra NK, Shuaib A, Wahlgren NG, Weimar C, Lees KR. Thrombolysis in stroke despite contraindications or warnings? Stroke. 2013;44:727–733. [DOI] [PubMed] [Google Scholar]
- 24. Meurer WJ, Kwok H, Skolarus LE, Adelman EE, Kade AM, Kalbfleisch J, Frederiksen SM, Scott PA. Does preexisting antiplatelet treatment influence postthrombolysis intracranial hemorrhage in community‐treated ischemic stroke patients? An observational study Acad Emerg Med. 2013;20:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan Y, Chen Q, Liao X, Zhao X, Wang C, Liu G, Liu L, Wang C, Wang D, Wang Y, Wang Y. Preexisting dual antiplatelet treatment increases the risk of post‐thrombolysis intracranial hemorrhage in Chinese stroke patients. Neurol Res. 2015;37:64–68. [DOI] [PubMed] [Google Scholar]
- 26. Lindley RI, Wardlaw JM, Whiteley WN, Cohen G, Blackwell L, Murray GD, Sandercock PA. Alteplase for acute ischemic stroke: outcomes by clinically important subgroups in the Third International Stroke Trial. Stroke. 2015;46:746–756. [DOI] [PubMed] [Google Scholar]
- 27. Meseguer E, Labreuche J, Guidoux C, Lavallee PC, Cabrejo L, Sirimarco G, Valcarcel JG, Klein IF, Amarenco P, Mazighi M. Outcomes after stroke thrombolysis according to prior antiplatelet use. Int J Stroke. 2015;10:163–169. [DOI] [PubMed] [Google Scholar]
- 28. Watson‐Fargie T, Dai D, MacLeod MJ, Reid JM. Comparison of predictive scores of symptomatic intracerebral haemorrhage after stroke thrombolysis in a single centre. J R Coll Physicians Edinb. 2015;45:127–132. [DOI] [PubMed] [Google Scholar]
- 29. Xian Y, Federspiel JJ, Grau‐Sepulveda M, Hernandez AF, Schwamm LH, Bhatt DL, Smith EE, Reeves MJ, Thomas L, Webb L, Bettger JP, Laskowitz DT, Fonarow GC, Peterson ED. Risks and benefits associated with prestroke antiplatelet therapy among patients with acute ischemic stroke treated with intravenous tissue plasminogen activator. JAMA Neurol. 2016;73:50–59. [DOI] [PubMed] [Google Scholar]
- 30. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 31. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 02, 2016.
- 32. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 34. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 35. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380:731–737. [DOI] [PubMed] [Google Scholar]
- 36. Alsop DC, Makovetskaya E, Kumar S, Selim M, Schlaug G. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke. 2005;36:746–750. [DOI] [PubMed] [Google Scholar]
- 37. Hallevi H, Grotta JC. Antiplatelet therapy and the risk of intracranial hemorrhage after intravenous tissue plasminogen activator therapy for acute ischemic stroke. Arch Neurol. 2008;65:575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez‐Sabin J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. [DOI] [PubMed] [Google Scholar]
- 39. Savitz SI, Benatar M, Saver JL, Fisher M. Outcome analysis in clinical trial design for acute stroke: physicians' attitudes and choices. Cerebrovasc Dis. 2008;26:156–162. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry‐Ribaudo L, Booth J, Topol EJ. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 41. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias‐Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial. Lancet. 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 42. Albers GW, von Kummer R, Truelsen T, Jensen JK, Ravn GM, Gronning BA, Chabriat H, Chang KC, Davalos AE, Ford GA, Grotta J, Kaste M, Schwamm LH, Shuaib A. Safety and efficacy of desmoteplase given 3‐9 h after ischaemic stroke in patients with occlusion or high‐grade stenosis in major cerebral arteries (DIAS‐3): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancel Neurol. 2015;14:575–584. [DOI] [PubMed] [Google Scholar]
- 43. James SK, Storey RF, Khurmi NS, Husted S, Keltai M, Mahaffey KW, Maya J, Morais J, Lopes RD, Nicolau JC, Pais P, Raev D, Lopez‐Sendon JL, Stevens SR, Becker RC. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attack. Circulation. 2012;125:2914–2921. [DOI] [PubMed] [Google Scholar]


