Abstract
Background
A growing body of evidence suggests that atrial fibrillation (AF) is associated with myocardial infarction (MI). However, incidence and management of MI in AF is still undefined.
Methods and Results
We searched MEDLINE via PubMed and Cochrane database between 1965 and 2015. All observational clinical studies and interventional trials reporting 1‐year incidence of MI in AF were included. We also discussed pathophysiological mechanisms, predictors, and therapeutic approaches to reduce the risk of MI in AF. Twenty‐one observational studies and 10 clinical trials were included. The annual rate of MI in observational studies including AF patients ranged from 0.4% to 2.5%. Higher rates of MI were reported in AF patients with stable coronary artery disease (11.5%/year), vascular disease (4.47%/year), heart failure (2.9%/year), and in those undergoing coronary artery interventions (6.3%/year). However, lower annual rates have been described in AF patients from Eastern countries (0.2–0.3%/year), and in those enrolled in clinical trials (from 0.4 to 1.3%/year).
Conclusions
AF patients had a significant residual risk of MI despite anticoagulant treatment. Coexistence of atherosclerotic risk factors and platelet activation account for the increased risk of MI in AF. Identification of high‐risk AF patients is a needed first step to develop cost‐effective approaches for prevention. A new score, the 2MACE score, has been recently developed to stratify MI risk in AF, and may help not only in allocating resources to high‐risk groups, but also in design of studies examining novel therapies for prevention of MI in AF.
Keywords: atherosclerosis, atrial fibrillation, myocardial infarction
Subject Categories: Atrial Fibrillation, Cardiovascular Disease, Risk Factors
Introduction
Atrial fibrillation (AF) is the most frequent sustained cardiac arrhythmia encountered in Western countries, with millions of individuals expected to suffer from it in the next decades. Progressive increase in the life expectancy is the major drive for the high prevalence of AF in Western countries.1 Aging has a negative impact not only for the occurrence of AF but also for ischemic stroke, which sharply increases in the elderly population as well.1 Consequently, the clinical history of most AF patients is often complicated by thromboembolic events; for this reason AF patients are treated with oral anticoagulants such as vitamin K antagonists (VKAs) to prevent the risk of ischemic stroke.2 In the last decade a new anticoagulant category, non–vitamin K oral anticoagulants (NOACs), has been introduced in the market. This includes a direct thrombin inhibitor such as dabigatran, and factor Xa inhibitors, namely, rivaroxaban, apixaban, and edoxaban. Warfarin and NOACs are equivalent in terms of cerebrovascular events reduction, but NOACs have been associated with less risk of bleeding.3
In addition to thromboembolism, AF is characterized by a constellation of atherosclerotic risk factors including hypertension, diabetes, metabolic syndrome, and dyslipidemia and by systemic signs of atherosclerosis such as aortic plaque or peripheral artery disease, which can increase the risk of stroke. It is for this reason that vascular disease has been included in the new score for stroke risk stratification, ie, the CHA2DS2‐VASc score.
The association between systemic atherosclerosis and AF can explain, at least partially, the complex clinical picture complicating the disease course in AF patients. Thus, atherosclerosis of coronary arteries with ensuing development of acute coronary syndromes, such as myocardial infarction (MI), is another typical feature of AF clinical history. Epidemiologic studies on the occurrence of MI provided divergent results4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24; also unclear are the clinical and laboratory predictors, which may help to identify AF patients at high risk of MI. The occurrence of MI in AF patients yields serious problems of management. For example, the coexistence of major adverse cardiovascular events (MACE) and thromboembolism could require a more complex antithrombotic treatment.
This systematic review will focus on incidence of MI in clinical trials and in observational studies, mechanisms of disease, predictors of MI, and potential therapeutic approaches to reduce the risk MI in AF.
Methods
Eligibility Criteria
We included all original clinical research articles in English language with full text available. In particular, observational clinical studies and interventional trials that reported data on the incidence of MI in nonvalvular AF patients, with at least 1 year of follow‐up and with no limitations regarding the type of antithrombotic treatment, were selected. We did not include the following: (1) case reports, editorials/comments, letters; (2) subgroup analysis from the same clinical trial; (3) studies reporting only cerebrovascular events as primary outcome, and/or combined end point of ischemic events, or other cardiovascular outcomes than MI (ie, mortality or incident heart failure); and (4) studies investigating postacute MI/cardiac revascularization AF.
Information Sources and Search Strategy
We performed a systematic review of the literature searching MEDLINE via PubMed and Cochrane database for a combination of the following keywords: “atrial fibrillation,” “myocardial infarction,” and “cardiovascular events.” The research strategy included only journal articles in English language between 1965 and 2015, and was performed according to PRISMA guidelines.25
Study Selection
The study selection was performed in multiple phases. In the first phase, potentially relevant studies were obtained by combined searches of electronic databases using the selected above‐mentioned keywords. Then, studies not in English language, with no abstract/full text available or not involving humans, were excluded. In the second phase, studies were reviewed and excluded by study typology; thus, letters, editorials, case reports, and comments were excluded. The third phase consisted of a detailed analysis of full‐text articles to assess whether they addressed the specific study question (Figure 1).
Figure 1.
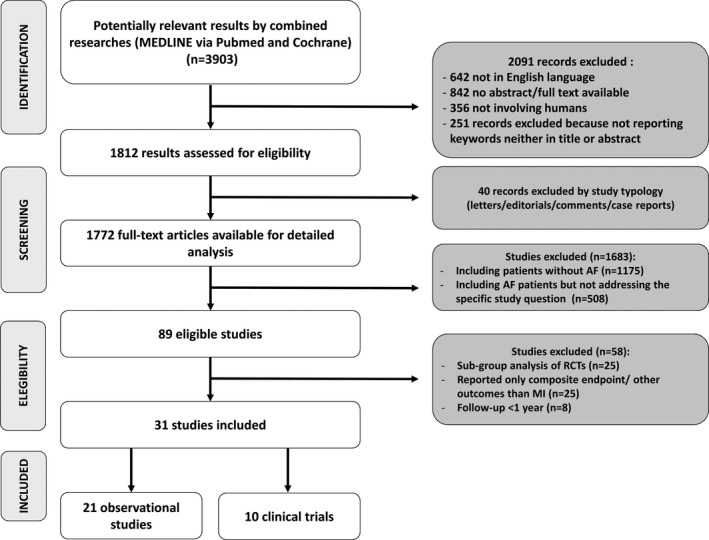
Flow diagram of search strategy. AF indicates atrial fibrillation; MI, myocardial infarction; RCTs, randomized controlled trials.
Data Collection Process and Data Items
Two physicians (D.P., P.P.) independently screened the titles and abstracts of manuscripts identified through the database searches to identify studies potentially eligible for further assessment. Controversies were resolved by a third investigator (F.V.).
For each study we collected the following information: authors, year of publication, study typology, antithrombotic treatment, number of participants included, percentage of women, age, CHADS2/CHA2DS2‐VASc scores, follow‐up (year), number (n/%), and rate of MI (%/year).
Ethical Review
Given the study type (review article), an ethical approval was not necessary.
Results
Study Selection
Our systematic review of literature yielded 3903 results (Figure 1). Two‐thousand ninety‐one records were excluded as 642 were not in English language, 842 had no abstract/full text available, 356 were not involving humans, and 251 because they did not report keywords either in the title or the abstract. Moreover, 40 were excluded by study typology (letters/editorials/comments/case reports). Of the 1772 remaining full‐text articles, 1175 did not include patients with AF, and 508 included AF patients but did not address the specific study question. Thus, 89 were eligible studies; of these, we excluded 25 subgroup analysis of randomized clinical trials, 25 studies reporting only composite end point/other outcomes than MI, and 8 with a follow‐up <1 year. Thirty‐one studies were included in the systematic review: 21 observational studies and 10 clinical trials.
Study Characteristics and Results of Individual Studies
Observational studies reported divergent data on the incidence of MI in the AF population4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 (Table 1). The first consolidated evidence suggesting that AF per se is an independent predictor of MI has been provided by Soliman et al,9 who analyzed the incidence of MI in a population‐based cohort study that included 1631 participants with and 22 297 without AF from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. The study demonstrated that AF increases the risk of MI by 2‐fold during a median follow‐up of 4.5 years.
Table 1.
Incidence of Myocardial Infarction in Observational Studies Including Atrial Fibrillation Patients
| Author/Study (y) | Antithrombotic Agent | Participants (n) | Female (%) | Age, y | CHADS2/CHA2DS2‐VASc Scores | Number of MI, n (%) | Follow‐Up (y) | Rate of MI (%/y) | |
|---|---|---|---|---|---|---|---|---|---|
| LIFE study (2005)4 | Not reported | 342 | 43.3 | 70.3 | Not reported | 19 (5.6%) | 4 | 1.13 | |
| Goto et al/REACH Registry (2008)5 | Warfarin (53.1%) | 6814 | 35.6 | 72.8 | CHADS2 0 to 1=17.6%; CHADS2 ≥2=82.4% | Not reported | 1 | 1.36 | |
| Winkel et al/REACH Registrya (2010)6 | Warfarin (60.7%) | 392 | 25.6 | 71.6 | Not reported | 18 (4.6%) | 2 | 2.30 | |
| Goto et al/J‐TRACE (2011)7 | Warfarin (70.0%) | 2056 | 31.3 | 70.0 | Not reported | Not reported | 1 | 0.20 | |
| ADHERE Registryb (2012)8 | Warfarin | 537 | 45.1 | 78.1 | CHADS2 ≥2=93.3 | 15 (2.8) | 1 | 2.80 | |
| No warfarin | 2049 | 46.3 | 80.0 | CHADS2 ≥2=96.6 | 60 (2.9) | 2.90 | |||
| Soliman et al/REGARDS study (2014)9 |
Warfarin (19.9%) Aspirin (41.2%) |
1631 | 61.6 | 66.5 |
CHADS2 ≤1=983 CHADS2 >1=648 |
78 (4.8) CHADS2 ≤1=30 (3.0) CHADS2 >1=48 (7.4) |
4.5 | 1.20 | |
| Andersson et al (2014)10 | Not reported | 9519 | 31.0 | Women: 67.7 |
CHA2DS2‐VASc: <55 years and 55 to 64=1 65 to 74=2 75 to 85=3 |
Not reported | >10 |
<55: 0.20%, 55 to 64: 0.40%, 65 to 74: 0.80% 75 to 85: 1.40% |
|
| Men: 54.9 |
CHA2DS2‐VASc: <55 years and 55 to 64=0 65 to 74=1 75 to 85=2 |
<55: 0.20%, 55 to 64: 0.50%, 65 to 74: 1.20% 75 to 85: 1.90% |
|||||||
| Chao et al (2014)11 | Not reported | 12 114 | 40.0 | — |
Men: CHA2DS2‐VASc=0 Women: CHA2DS2‐VASc=1 |
258 | 5.7 | 0.29 | |
| Senoo et al (2014)12 |
Warfarin (47.2%) Aspirin (39%) |
1835 | 24.6 | 63.2 | CHADS2 0 to 1=69.4%; CHADS2 ≥2=30.6% | 51c (2.78) | 1.46 | 1.90 (3.60 for CHADS2 ≥2) | |
| Shore et al (2014)13 | Dabigatran | 5376 | 1.7 | 71.3 |
CHADS2=2.38 CHA2DS2‐VASc=3.22 |
20 (0.4) | 1 | 0.40 | |
| AFCAS Registryd (2014)14 | DAPT+warfarin | 679 | 29.0 | 73.0 | CHADS2=2.3 | 43 (6.3) | 1 | 6.30 | |
| DAPT | 162 | 35.0 | 73.0 | CHADS2=2.1 | 4 (2.5) | 2.50 | |||
| Warfarin+clopidogrel | 73 | 29.0 | 74.0 | CHADS2=2.4 | 4 (5.5) | 5.50 | |||
| Lamberts et ale (2014)15 |
Prevalent AF: warfarin (20.7%) antiplatelet (29.2%) both (6%) |
7804 | 35.7 | 76.8 | CHA2DS2‐VASc=5.3 | Not reported | 3 | 4.47h | |
|
Incident AF: warfarin (25.0%) antiplatelet (30.3%) both (8.0%) |
6432 | 34.9 | CHA2DS2‐VASc=5.2 | Not reported | 4.37h | ||||
| Martinez et al (2014)16 | Not reported | 5555 | 38.4 | 70.9 | CHA2DS2‐VASc=2.5 | Not reported | 3 | 0.90 | |
| Larsen et al (2014)17 | Naive | Dabigatran 110 | 2124 | 55.5 | 82.0 |
CHADS2=1.90 CHA2DS2‐VASc=3.70 |
29/97f | 16 months | 1.20/4.10f |
| Dabigatran 150 | 2694 | 37.6 | 68.0 |
CHADS2=0.98 CHA2DS2‐VASc=2.19 |
30/134f | 0.91/4.15f | |||
| Warfarin | 8133 | 42.2 | 72.0 |
CHADS2=1.29 CHA2DS2‐VASc=2.74 |
142/481f | 1.37/4.75f | |||
| Warfarin experienced | Dabigatran 110 | 1554 | 54.6 | 82.0 |
CHADS2=2.07 CHA2DS2‐VASc=3.89 |
26/73f | 1.33/3.82f | ||
| Dabigatran 150 | 1825 | 35.9 | 69.0 |
CHADS2=1.30 CHA2DS2‐VASc=2.63 |
21/85f | 0.81/3.34f | |||
| Warfarin | 49 868 | 37.9 | 75.0 |
CHADS2=1.58 CHA2DS2‐VASc=3.05 |
2564/8968f | 0.72/2.55f | |||
| Piccini et al/Medicare (2014)18 | Not reported | 186 461 | 55.4 | 79.5 |
CHADS2=2.8 CHA2DS2‐VASc=5.0 |
2272 (1.2) | 1 | 1.20 | |
| O'Neal et al (2014)19 | Aspirin (31%) | 434 | 55.0 |
65 to 70 y=35% 71 to 74 y=25% ≥75=40% |
Not reported | Overall (AF+non‐AF) 797 (17.3) | 12.2 | 2.55 | |
| Lamberts et alg (2014)20 |
Aspirin: 3273 Clopidogrel: 417 DAPT: 1767 |
5457 | 37 to 46 | 73.0 to 76.1 | CHA2DS2‐VASc 0=1% to 2%; CHA2DS2‐VASc 1=4% to 8%; CHA2DS2‐VASc ≥2=89% to 95% |
Aspirin: 1048 (32) Clopidogrel: 55 (13.2) DAPT: 119 (6.7) |
3.3 |
Aspirin: 8.40 Clopidogrel: 9.0 DAPT: 11.50 |
|
|
VKAs:950 VKA+aspirin:1471 VKA+Clopidogrel:322 VKA+DAPT: 500 |
3243 | 20 to 38 | 71.0 to 73.6 | CHA2DS2‐VASc 0=0% to 1%; CHA2DS2‐VASc 1=4% to 8%; CHA2DS2‐VASc ≥2=91% to 95% |
VKAs: 188 (14.5) VKA+Aspirin: 339 (23) VKA+Clopidogrel: 17 (5.3) VKA+DAPT: 16 (3.2) |
VKAs: 4.70 VKA+aspirin: 4.70 VKA+Clopidogrel: 4.40 VKA+DAPT: 7.80 |
|||
| Pastori et al (2015)21 | Warfarin | 1019 | 43.8 | 73.2 | CHA2DS2‐VASc=4.0 | 29 (0.9) | 2.8 | 0.90 | |
| XANTUS (2015)22 | Rivaroxaban | 6784 | 41.0 | 71.5 |
CHADS2=2.0 CHA2DS2‐VASc=3.4 |
27 (0.4) | 1 | 0.40 | |
| Soliman et al/ARIC cohort (2015)23 |
Warfarin (1.3%) Aspirin (48.4%) |
1545 | 49.9 | 56.6 | Not reported | 107 (6.9) | 21.6 | 1.16 | |
| Vermond et al (2015)24 | Not reported | 265 | 30.0 | 62.0 | Not reported | 2.06 | |||
ADHERE indicates acute decompensated heart failure national registry; AFCAS, atrial fibrillation undergoing coronary artery stenting; ARIC, Atherosclerosis Risk in Communities; DAPT, dual antiplatelet therapy; J‐TRACE, Japan Thrombosis Registry for Atrial Fibrillation, Coronary, or Cerebrovascular Events; LIFE, Losartan Intervention for End Point Reduction in Hypertension; MI, myocardial infarction; REACH, REduction of Atherothrombosis for Continued Health; REGARDS, REasons for Geographic and Racial Differences in Stroke; VKA, vitamin K antagonists; XANTUS, Xarelto (®) for Prevention of Stroke in Patients with Atrial Fibrillation.
AF in patients with symptomatic peripheral arterial disease.
AF patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device.
Coronary events.
AF patients undergoing coronary artery stenting.
Heart failure and vascular disease Plus AF.
Considering all myocardial ischemic events.
Patients with AF and stable coronary artery disease.
Refers to all group.
Further support to this finding was provided by other reports indicating that the annual rate of MI in AF patients is close to or over 1%/year, with the exception of the Xantus study where MI incidence was 0.4%.22 Thus, as reported in Table 1, the annual rate of MI in observational studies of AF patients ranges from 0.4% to 2.5%.
Higher rates of MI were found in AF patients with stable coronary artery disease,20 peripheral vascular disease,8 heart failure,10 or in those undergoing coronary artery intervention15 (Table 1).
A lower annual rate has been described in patients from Eastern countries. For example, in the J‐TRACE study, which included 2056 Japanese AF patients, the rate of MI was 0.2%/year.7 Similarly, a large nationwide cohort on 12 114 subjects from Taiwan found a rate of MI of 0.29%/year.11
Clinical trials reported a lower annual rate of MI in AF26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 (Table 2). Overall, 46 923 AF patients were treated with NOACs, 34 800 with VKAs, and 14 725 with antiplatelets. In particular, MI incidence in AF patients treated with NOACs ranged from 0.53%/year for apixaban to 1.1%/year for ximelagatran, which was similar to that in patients treated with VKAs (from 0.44%/year in the SIFA study to 1.1%/year of ROCKET trial). Finally, for the group of patients treated with antiplatelets, the rate of MI ranged from 0.43%/year in the SIFA study to 1.3%/year in the CHARISMA (Table 2).
Table 2.
Incidence of Myocardial Infarction in Clinical Trials Including Patients With Atrial Fibrillation
| Author/Study | Antithrombotic Agent | Participants (n) | Female (%) | Age, y | CHADS2/CHA2DS2‐VASc Scores | Number of MI (n/%) | Follow‐Up (y) | Rate of MI (%/y) |
|---|---|---|---|---|---|---|---|---|
| SIFA (1997)26 | Indobufen | 462 | 54.5 | 72.8 | Not reported | 2 (0.43) | 1.0 | 0.43 |
| Warfarin | 454 | 51.5 | 72.2 | 68% with ≥2 risk factors | 2 (0.44) | 0.44 | ||
| SPORTIF III (2003)27 | Ximelagatran | 1704 | 32.0 | 70.3 | 70% with ≥2 risk factors | 24 | 1.4 | 1.10 |
| Warfarin | 1703 | 30.0 | 70.1 | 68% with ≥2 risk factors | 13 | 0.60 | ||
| ACTIVE W (2006)28 | Clopidogrel+aspirin | 3335 | 33.0 | 70.2 | CHADS2=2.0 | 36 (1.1) | 1.28 | 0.86 |
| Warfarin | 3371 | 34.0 | 23 (0.7) | 0.55 | ||||
| CHARISMA (2008)29 | Clopidogrel+aspirin | 298 | 19.0 | 70.0 | Not reported | 9 (3.0) | 2.3 | 1.30 |
| Aspirin | 285 | 24.0 | 6 (2.1) | 0.91 | ||||
| ACTIVE (2009)30 | Clopidogrel+aspirin | 3772 | 41.4 | 70.9 | CHADS2=2.0 | 90 (2.4) | 3.6 median | 0.70 |
| Aspirin | 3782 | 42.4 | 71.1 | 115 (3.0) | 0.90 | |||
| RE‐LY (2009)31 | Dabigatran 150 mg | 6076 | 36.8 | 71.5 | CHADS2 0 to 1=32.2%; CHADS2 2=35.2%; CHADS2 >2=32.6% | 89 (1.5) | 2.0 | 0.74 |
| Dabigatran 110 mg | 6015 | 35.7 | CHADS2 0 to 1=32.6%; CHADS2 2=34.7%; CHADS2 >2=32.7% | 86 (1.4) | 0.72 | |||
| Warfarin | 6022 | 36.7 | CHADS2 0 to 1=30.9%; CHADS2 2=37.0%; CHADS2 >2=32.1% | 63 (1.0) | 0.53 | |||
| ARISTOTLE (2011)32 | Apixaban | 9120 | 35.5 | 70.0 | CHADS2=2.1 | 90 (1.0) | 1.8 | 0.53 |
| Warfarin | 9081 | 35.0 | 102 (1.1) | 0.61 | ||||
| AVERROES (2011)33 | Apixaban | 2808 | 41.0 | 70.0 | CHADS2=2.0 | 24 | 1.1 | 0.80 |
| Aspirin | 2791 | 42.0 | 70.0 | CHADS2=2.1 | 28 | 0.90 | ||
| ROCKET AF (2011)34 | Rivaroxaban | 7131 | 39.7 | 73.0 | CHADS2=3.48 | 101 (1.4) | 1.9 | 0.90 (overall) (0.57 AF without prior MI; 2.77 AF with prior MI)36 |
| Warfarin | 7133 | 39.7 | CHADS2=3.46 | 126 (1.8) | 1.10 (overall) (0.77 AF without prior MI; 2.80 AF with prior MI)36 | |||
| ENGAGE AF TIMI 48 (2013)35 | Edoxaban 60 mg | 7035 | 37.9 | 72.0 | CHADS2 ≤3=77.1%; CHADS2 4 to 6=22.9%. | 133 (1.9) | 2.8 | 0.70 |
| Edoxaban 30 mg | 7034 | 38.8 | CHADS2 ≤3=77.8%; CHADS2 4 to 6=22.2% | 169 (2.4) | 0.89 | |||
| Warfarin | 7036 | 37.5 | CHADS2 ≤3=77.4%; CHADS2 4 to 6=22.6% | 141 (2.0) | 0.65 |
ACTIVE indicates Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events; AF, atrial fibrillation; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; ENGAGE AF‐TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48; MI, myocardial infarction; RE‐LY, Randomized Evaluation of Long‐Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SIFA, studio italiano fibrillazione atriale; SPORTIF III, Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation.
The reason for these differences is unclear. Differences in the populations and the distribution of atherosclerotic risk factors and comorbidities are possible explanations for some of these differences. Moreover, another aspect to be considered is that patients included in clinical trials may have been monitored more closely than patients in observational studies, resulting in an improved treatment of comorbid conditions and atherosclerotic risk factors, and, thereby, in lower rates of MI.
Finally, many patients included in the observational cohorts were not treated with oral anticoagulants, suggesting that in the real world of AF, a significant proportion of patients do not receive an adequate prevention for thromboembolism. This is in keeping with findings from recent surveys reporting that at least 15% to 30% of AF patients are treated only with aspirin, or do not receive any antithrombotic treatment.37, 38 MI rate is also influenced by sex, with females being at higher risk of MI compared to men, as shown in the REGARDS study.9 Of note, the percentage of females included in the interventional trials is typically lower than that in the observational studies (30–40% versus about 50%; Tables 1 and 2).
Synthesis of Results
The annual rate of MI in observational studies including AF patients ranges from 0.4% to 2.5%. Higher rates of MI were reported in AF patients with stable coronary artery disease (11.5%/year), vascular disease (4.47%/year), heart failure (2.9%/year), or in those undergoing coronary artery intervention (6.3%/year). A low annual rate has been described in AF patients from Eastern countries (0.2–0.3%/year). Overall, a lower rate has been described in clinical trials (from 0.4% to 1.3%/year).
Discussion
Mechanisms of Cardiovascular Disease in AF
AF is associated with systemic signs of inflammation that could promote a pro‐thrombotic state and eventually MI.39 Systemic inflammation may depend on AF per se or on the concomitant presence of the classic atherosclerotic risk factors, which are typically associated with AF.40 In this context, it is worth noting that hypertension, diabetes, and dyslipidemia are associated with platelet activation,41 a key step in the pathogenesis of MI. This has been evidenced by studies showing that aspirin, which irreversibly acetylates COX1, thus inhibiting the formation of the pro‐aggregating molecule thromboxane (Tx) A2,42 significantly reduces the risk of cardiovascular outcomes in patients at risk of or with MI.43 Several biomarkers of platelet activation such as plasma levels of P‐selectin, β‐thromboglobulin, and soluble CD40L (sCD40L)44 have been found to be elevated in AF, suggesting a role for platelets in precipitating vascular disease. This hypothesis is supported by an observational prospective study performed in 231 patients with AF, where high plasma levels of sCD40L discriminated patients who experienced MI during a follow‐up of 28 months.45 Further support to the role of platelets in favoring MACE is provided by a prospective single‐center study in which the predictive value of 11‐dehydro‐TxB2, a marker of in vivo platelet activation, was examined in 837 AF patients followed up to 30 months. During the follow‐up, 99 patients experienced fatal and nonfatal MACE. Patients who were in the top tertile of 11‐dehydro‐TxB2 showed the highest risk of experiencing ischemic events, including cardiac complications. The relationship between platelet activation and cardiovascular disease has been also confirmed by another prospective observational study in which the predictive value of prostaglandin PGF2alpha was analyzed in 1002 AF patients on treatment with VKAs and followed up for 25.7 months.46 Prostaglandin PGF2alpha is a chemically stable eicosanoid stemming from arachidonic acid oxidation by superoxide anion, which activates platelets via TxA2 receptors.47 During the follow‐up, 125 patients experienced a MACE including 44 (35.2%) cardiac complications, such as fatal and nonfatal MI (n=31) and cardiac revascularization (n=13). Patients in the highest tertile of prostaglandin PGF2alpha were at higher risk of experiencing MACE, suggesting again a role for platelets in precipitating cardiac complications.
In addition to atherosclerosis, MI may occur in AF by other mechanisms. For example, episodes of AF with high ventricular rates may yield type 2 MI, which is characterized by an imbalance between demand and blood supply, and is usually associated with non‐ST elevation MI.48 Another possibility is direct thromboembolization from left atrium into the coronary arteries.49 In 1776 patients with new‐onset MI, Shibata et al50 showed that 52 were due to coronary embolism and most of them (n=38, 73%) were observed in patients with AF, suggesting that AF may cause thromboembolism not only to the cerebral but also to the coronary circulation.
Together these findings suggest that at least 3 mechanisms may account for MI in AF: (1) atherosclerosis and its associated inflammatory process, yielding a pro‐thrombotic state; (2) direct coronary thromboembolism from left atrial appendage; and (3) tachyarrhythmia episodes resulting in supply–demand mismatch (Figure 2).
Figure 2.
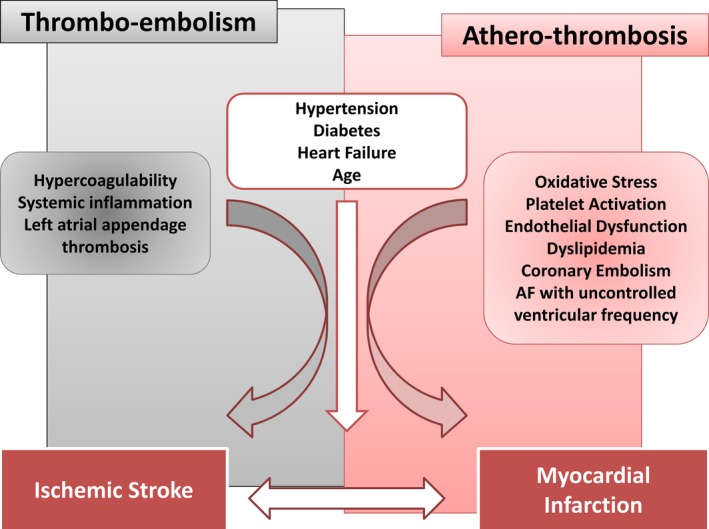
Mechanisms of thromboembolism and atherothrombosis in atrial fibrillation (AF).
Predictors of MI in AF
Instrumental and clinical variables have been investigated as predictors of MI in AF patients. Ankle‐brachial index (ABI) and brachial flow‐mediated dilation (FMD), two markers of systemic atherosclerosis,51 have been prospectively studied to assess their predictive value against cardiovascular disease in AF.52, 53
ABI is a simple, inexpensive, and noninvasive marker of peripheral artery disease, which is recommended as a first‐line screening tool to assess the presence of a peripheral artery disease in asymptomatic adults at moderate risk.54 Low ABI (ie, <0.90) indicates ≥50% stenosis between the aorta and the distal leg arteries and is associated with an increased risk of cardiovascular events.55 Prior studies have reported that AF patients had a low ABI compared to controls,56 and that an ABI ≤0.90 is detectable in about 20% of the AF population.57 Low ABI is associated with ischemic events also in AF patients, as shown by the “Ankle‐brachial index Prevalence Assessment‐Collaborative Italian Study” (ARAPACIS) registry, which followed 2027 AF patients for a median of 35 months. The study showed that a low ABI ≤0.90 was more prevalent in patients with, compared to those without, a vascular event (32.2% versus 20.2%, P<0.05). During follow‐up, 176 vascular events occurred (3.81%/year) and, at multivariate analysis, low ABI was an independent predictor of MI.53
An impaired FMD has been described in many cardiovascular diseases.58, 59 An increased risk of cardiovascular events seems to be present for a value of FMD <10%, further rising for a value <5%.60 A meta‐analysis by Inaba et al showed how 1% increase of FMD is associated with 13% reduction of risk of cardiovascular event.61
In a cohort of 514 AF patients, a reduced value of FMD was detected (median 4.6%), with an increase of cardiovascular events in patients with FMD below the median value (3.06% [interquartile range 0.00–6.00] versus 4.67% [interquartile range 1.58–8.22], P=0.027).62
In addition to ABI and FMD, other atherosclerotic risk factors have been studied in AF. Metabolic syndrome (MetS) represents a worldwide challenge, with a widespread prevalence that appears to be increasing in parallel with the prevalence of obesity.63 MetS encloses a cluster of cardiometabolic alterations including visceral adiposity and atherogenic dyslipidemia. It is estimated that about 40% of the population aged 60 years old have MetS in the United States.64 Subjects with MetS have an excess risk for subsequent cardiovascular events and death, as confirmed by a recent meta‐analysis including 951 083 patients.65 Moreover, a higher risk to develop AF has been well recognized in patients with MetS,66 and the prevalence of MetS in AF ranges from 18.8% to 52.1%.67
In accordance with data from the general population without AF, the association between MetS and cardiovascular events was also evident in the AF population. For example, in a cohort of 1019 AF patients followed for a median of 33.7 months, the prevalence of MetS was 52.1%, conferring an increased risk of MACE (hazard ratio, 1.663; 95% CI, 1.107–2.499; P=0.014).21
In an attempt to improve cardiovascular risk stratification, a new simple score to identify AF patients at higher risk to experience MACE has been proposed.68 The 2MACE score (Figure 3) assigning 2 points for Metabolic Syndrome and Age ≥75, 1 point for MI/revascularization, Congestive heart failure (ejection fraction ≤40%), thromboembolism (stroke/transient ischemic attack) was developed and validated in >2000 AF patients in a multicenter study; a 2MACE score ≥3 identified patients at highest risk for MACE.68
Figure 3.
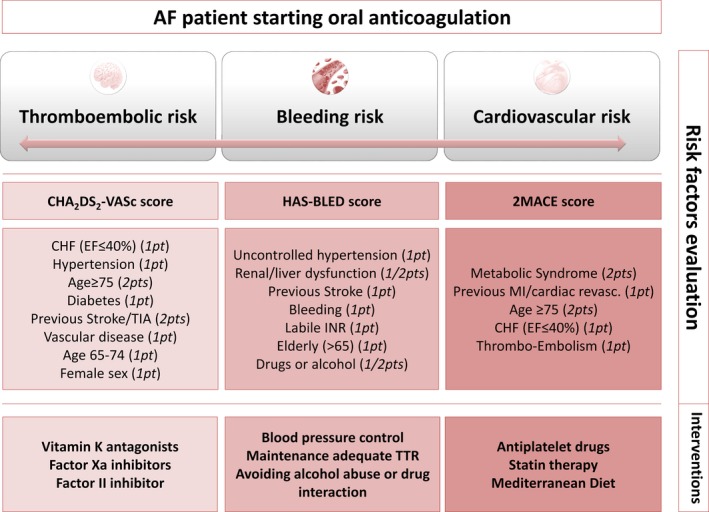
Holistic antithrombotic approach for the management of AF patients starting oral anticoagulation. AF indicates atrial fibrillation; CHF, congestive heart failure; EF, ejection fraction; INR, international normalized ratio; MI, myocardial infarction; TIA, transient ischemic attack; TTR, time in therapeutic range.
Therapeutic Approach to Cardiovascular Risk in AF Patients
Management of AF patients at risk or with previous MI is becoming a hot topic, because of the complexity of antithrombotic treatment in this setting. MI may precede AF, and about one quarter of the AF population has a history of MI; thus, MI is considered a risk factor for AF.69 This likely explains the reason for the frequent association of oral anticoagulants with aspirin in the AF population, as evidenced by the recent trials with NOACs in which 29% to 41% of patients included have been treated with such a combination. Alternatively, the addition of aspirin to oral anticoagulants may depend on the physician's perception of a high risk of vascular disease. However, this combination therapy is associated with a significant increase of bleeding, while the clinical benefit is dubious.20 The use of aspirin in combination with oral anticoagulants in patients with or without a history of MI is a critical issue that should be addressed. Previous studies consistently demonstrated that warfarin alone lowers the risk of MI recurrence in patients with previous acute coronary syndromes.70 In accordance with this hypothesis, a retrospective analysis of the SPORTIF III trial27 showed that good‐quality anticoagulation treatment had a positive impact on MACE occurrence. Also, in the warfarin arm of the RE‐LY study including >6000 AF patients, a time in therapeutic range >65% was associated with lower MI risk versus patients with time in therapeutic range <65%. This finding was confirmed in a prospective observational study including 627 AF patients who were followed up for 30 months.71 The study showed that higher time in therapeutic range (>70%) was associated with lower risk of MI, reinforcing the concept that a good quality of anticoagulation treatment is necessary not only to reduce stroke but also MI. A matter of concern is, however, that the rate of MACE was still elevated with a rate >2%/year, suggesting that oral anticoagulants alone are not enough to lower the risk of MI in AF. In this context, it may be of potential interest to see whether, differently from warfarin, a combination of aspirin with NOACS could be clinically effective. The association between rivaroxaban and aspirin has been tested for patients with recent acute coronary syndrome in the ATLAS ACS 2–TIMI 51 study72; 15 526 patients were randomly assigned to twice‐daily administration of either 2.5 or 5.0 mg of rivaroxaban or placebo, in addition to standard medical therapy including low dose of aspirin. Rivaroxaban, at both dosages, significantly reduced the rate of death from cardiovascular causes, MI, or stroke, as compared with placebo, but increased major bleeding with the twice‐daily 5‐mg dose. Furthermore, in the ENGAGE AF‐TIMI 48 study,35 about 30% of patients were treated with aspirin in association with VKAs, edoxaban 60 mg, or edoxaban 30 mg; a positive effect of low‐dose edoxaban in association with aspirin in reducing the rate of cardiovascular events was observed.
Due to the complexity of treating AF patients with MI, much effort should be made to come up with cost‐effective prevention strategies. Scores identifying AF patients at higher risk, such as the 2MACE score, should be further validated, as a score ≥3 seems to be particularly useful in identifying AF patients at risk of cardiac events.68 In this context, it would be interesting to investigate whether AF patients with 2MACE ≥3 would benefit from the combination of old or new anticoagulants with aspirin. Another treatment option may be represented by statins, as they have been shown to reduce the risk of MI in primary and secondary prevention trials.73 Furthermore, statins possess antithrombotic activity including antiplatelet and anticoagulant properties, which may be useful in AF patients, who are characterized by a systemic and local ongoing prothrombotic state. Preliminary data from an observational study demonstrated that statins reduce cardiovascular death in AF patients.74 In the meantime, correction of atherosclerotic risk factors with a pharmacological approach, or a dietary intervention, could be a reasonable strategy to reduce the atherosclerotic complications. AF patients, for instance, could be advised to adhere to Mediterranean diet, which is known to possess antioxidant property, does not interfere with time in therapeutic range in warfarin‐treated patients,75 and is associated with lower risk of MI and stroke.76
Based on these considerations, a holistic approach including management of thromboembolism and MI is proposed (Figure 3). Thus, in addition to the CHA2DS2‐VASc and HAS‐BLED scores to stratify stroke and bleeding risk, respectively, inclusion of a score for MACE risk should be taken into account. This approach could prove useful not only to assess the stroke and bleeding risks, but also to assess the MACE risk, and to drive therapeutic choices. For instance, future clinical trials are needed to examine the usefulness of addition of aspirin or statins to oral anticoagulants in reducing the risk of both MI and stroke in AF patients.
Limitation
A limitation of this systematic review may be represented by the different definitions used to diagnose MI, as these changed over time, and may be responsible for some of the differences in the annual rate of MI observed among studies. In particular, since the first universal definition of MI,77 which indicated cardiac troponin over creatine kinase as a more specific diagnostic biomarker for MI, the inclusion of electrocardiographic changes other than ST‐segment modifications or Q waves (ie, new‐onset left bundle branch block), and availability of new cardiac imaging techniques have improved the diagnosis of MI.78
Conclusions
In conclusion, MI and AF are closely related; MI may precede or complicate the clinical course of AF. The presence of MI in AF patients creates a challenge in clinical management, because of the complexity of using anticoagulants and antiplatelet drugs, with the ensuing high risk of bleeding. Reducing the risk of MI in AF should be an objective for future studies.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003347 doi: 10.1161/JAHA.116.003347)
References
- 1. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 4. Wachtell K, Hornestam B, Lehto M, Slotwiner DJ, Gerdts E, Olsen MH, Aurup P, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Rokkedal J, Devereux RB. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:705–711. [DOI] [PubMed] [Google Scholar]
- 5. Goto S, Bhatt DL, Rother J, Alberts M, Hill MD, Ikeda Y, Uchiyama S, D'Agostino R, Ohman EM, Liau CS, Hirsch AT, Mas JL, Wilson PW, Corbalan R, Aichner F, Steg PG; Investigators RR . Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863, 863.e852. [DOI] [PubMed] [Google Scholar]
- 6. Winkel TA, Hoeks SE, Schouten O, Zeymer U, Limbourg T, Baumgartner I, Bhatt DL, Steg PG, Goto S, Rother J, Cacoub PP, Verhagen HJ, Bax JJ, Poldermans D. Prognosis of atrial fibrillation in patients with symptomatic peripheral arterial disease: data from the reduction of atherothrombosis for continued health (REACH) registry. Eur J Vasc Endovasc Surg. 2010;40:9–16. [DOI] [PubMed] [Google Scholar]
- 7. Goto S, Ikeda Y, Shimada K, Uchiyama S, Origasa H, Kobayashi H; Investigators JT . One‐year cardiovascular event rates in Japanese outpatients with myocardial infarction, stroke, and atrial fibrillation. Results from the Japan Thrombosis Registry for Atrial Fibrillation, Coronary, or Cerebrovascular Events (J‐TRACE). Circ J. 2011;75:2598–2604. [DOI] [PubMed] [Google Scholar]
- 8. Hess PL, Greiner MA, Fonarow GC, Klaskala W, Mills RM, Setoguchi S, Al‐Khatib SM, Hernandez AF, Curtis LH. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2012;35:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D. Gender‐related differences in risk of cardiovascular morbidity and all‐cause mortality in patients hospitalized with incident atrial fibrillation without concomitant diseases: a nationwide cohort study of 9519 patients. Int J Cardiol. 2014;177:91–99. [DOI] [PubMed] [Google Scholar]
- 11. Chao TF, Huang YC, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Hsieh MH, Lip GY, Chen SA. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2‐VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm. 2014;11:1941–1947. [DOI] [PubMed] [Google Scholar]
- 12. Senoo K, Suzuki S, Sagara K, Otsuka T, Matsuno S, Uejima T, Oikawa Y, Yajima J, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Lip GY, Yamashita T. Coronary artery diseases in Japanese patients with nonvalvular atrial fibrillation. J Cardiol. 2014;63:123–127. [DOI] [PubMed] [Google Scholar]
- 13. Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, Bradley SM, Maddox TM, Grunwald GK, Baron AE, Rumsfeld JS, Varosy PD, Schneider PM, Marzec LN, Ho PM. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubboli A, Schlitt A, Kiviniemi T, Biancari F, Karjalainen PP, Valencia J, Laine M, Kirchhof P, Niemela M, Vikman S, Lip GY, Airaksinen KE; Group AS . One‐year outcome of patients with atrial fibrillation undergoing coronary artery stenting: an analysis of the AFCAS Registry. Clin Cardiol. 2014;37:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamberts M, Lip GY, Ruwald MH, Hansen ML, Ozcan C, Kristensen SL, Kober L, Torp‐Pedersen C, Gislason GH. Antithrombotic treatment in patients with heart failure and associated atrial fibrillation and vascular disease: a nationwide cohort study. J Am Coll Cardiol. 2014;63:2689–2698. [DOI] [PubMed] [Google Scholar]
- 16. Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost. 2014;112:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsen TB, Rasmussen LH, Gorst‐Rasmussen A, Skjoth F, Rosenzweig M, Lane DA, Lip GY. Myocardial ischemic events in ‘real world’ patients with atrial fibrillation treated with dabigatran or warfarin. Am J Med. 2014;127:329–336.e324. [DOI] [PubMed] [Google Scholar]
- 18. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neal WT, Sangal K, Zhang ZM, Soliman EZ. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol. 2014;37:750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sorensen R, Kober L, Torp‐Pedersen C, Hansen ML. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. [DOI] [PubMed] [Google Scholar]
- 21. Pastori D, Pignatelli P, Angelico F, Farcomeni A, Del Ben M, Vicario T, Bucci T, Raparelli V, Cangemi R, Tanzilli G, Lip GY, Violi F. Incidence of myocardial infarction and vascular death in elderly patients with atrial fibrillation taking anticoagulants: relation to atherosclerotic risk factors. Chest. 2015;147:1644–1650. [DOI] [PubMed] [Google Scholar]
- 22. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG; Investigators X : XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2015;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soliman EZ, Lopez F, O'Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M, Alonso A. Atrial fibrillation and risk of ST‐segment‐elevation versus non‐ST‐segment‐elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community‐based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. [DOI] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, Cataldo G, Milanesi G, Lavezzari M, Pamparana F, Coccheri S. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (studio italiano fibrillazione atriale) Investigators. Stroke. 1997;28:1015–1021. [DOI] [PubMed] [Google Scholar]
- 27. Olsson SB; Executive Steering Committee of the SIIII . Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non‐valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–1698. [DOI] [PubMed] [Google Scholar]
- 28. Investigators AWGotA , Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 29. Hart RG, Bhatt DL, Hacke W, Fox KA, Hankey GJ, Berger PB, Hu T, Topol EJ; Investigators C . Clopidogrel and aspirin versus aspirin alone for the prevention of stroke in patients with a history of atrial fibrillation: subgroup analysis of the CHARISMA randomized trial. Cerebrovasc Dis. 2008;25:344–347. [DOI] [PubMed] [Google Scholar]
- 30. Investigators A , Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078.19336502 [Google Scholar]
- 31. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Committee R‐LS, Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 32. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A, Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 33. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas‐Zanetti F, Gonzalez‐Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; Committee AS, Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 34. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 35. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 36. Mahaffey KW, Stevens SR, White HD, Nessel CC, Goodman SG, Piccini JP, Patel MR, Becker RC, Halperin JL, Hacke W, Singer DE, Hankey GJ, Califf RM, Fox KA, Breithardt G; Investigators RA . Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur Heart J. 2014;35:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. ‘Real‐world’ antithrombotic treatment in atrial fibrillation: the EORP‐AF pilot survey. Am J Med. 2014;127:519–529.e511. [DOI] [PubMed] [Google Scholar]
- 38. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the Prevention of Thromboemolic Events–European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 40. O'Neal WT, Soliman EZ, Howard G, Howard VJ, Safford MM, Cushman M, Zakai NA. Inflammation and hemostasis in atrial fibrillation and coronary heart disease: the reasons for geographic and racial differences in stroke study. Atherosclerosis. 2015;243:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davi G, Gresele P, Violi F, Basili S, Catalano M, Giammarresi C, Volpato R, Nenci GG, Ciabattoni G, Patrono C. Diabetes mellitus, hypercholesterolemia, and hypertension but not vascular disease per se are associated with persistent platelet activation in vivo. Evidence derived from the study of peripheral arterial disease. Circulation. 1997;96:69–75. [DOI] [PubMed] [Google Scholar]
- 42. Catella F, Healy D, Lawson JA, FitzGerald GA. 11‐Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA. 1986;83:5861–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antithrombotic Trialists C . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duygu H, Barisik V, Kurt H, Turk U, Ercan E, Kose S. Prognostic value of plasma soluble CD40 ligand in patients with chronic non‐valvular atrial fibrillation. Europace. 2008;10:210–214. [DOI] [PubMed] [Google Scholar]
- 45. Ferro D, Loffredo L, Polimeni L, Fimognari F, Villari P, Pignatelli P, Fuster V, Violi F. Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol. 2007;27:2763–2768. [DOI] [PubMed] [Google Scholar]
- 46. Pignatelli P, Pastori D, Carnevale R, Farcomeni A, Cangemi R, Nocella C, Bartimoccia S, Vicario T, Saliola M, Lip GY, Violi F. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb Haemost. 2015;113:617–624. [DOI] [PubMed] [Google Scholar]
- 47. Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation—a review. Curr Med Chem. 2003;10:1723–1740. [DOI] [PubMed] [Google Scholar]
- 48. Sandoval Y, Smith SW, Thordsen SE, Apple FS. Supply/demand type 2 myocardial infarction: should we be paying more attention? J Am Coll Cardiol. 2014;63:2079–2087. [DOI] [PubMed] [Google Scholar]
- 49. Kolodgie FD, Virmani R, Finn AV, Romero ME. Embolic myocardial infarction as a consequence of atrial fibrillation: a prevailing disease of the future. Circulation. 2015;132:223–226. [DOI] [PubMed] [Google Scholar]
- 50. Shibata T, Kawakami S, Noguchi T, Tanaka T, Asaumi Y, Kanaya T, Nagai T, Nakao K, Fujino M, Nagatsuka K, Ishibashi‐Ueda H, Nishimura K, Miyamoto Y, Kusano K, Anzai T, Goto Y, Ogawa H, Yasuda S. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132:241–250. [DOI] [PubMed] [Google Scholar]
- 51. Feinstein SB, Voci P, Pizzuto F. Noninvasive surrogate markers of atherosclerosis. Am J Cardiol. 2002;89:31C–43C; discussion 43C‐44C. [DOI] [PubMed] [Google Scholar]
- 52. Perri L, Pastori D, Pignatelli P, Violi F, Loffredo L. Flow‐mediated dilation is associated with cardiovascular events in non‐valvular atrial fibrillation patients. Int J Cardiol. 2015;179:139–143. [DOI] [PubMed] [Google Scholar]
- 53. Violi F, Davi G, Proietti M, Pastori D, Hiatt WR, Corazza GR, Perticone F, Pignatelli P, Farcomeni A, Vestri AR, Lip GY, Basili S; Investigators AS . Ankle‐brachial index and cardiovascular events in atrial fibrillation. The ARAPACIS Study. Thromb Haemost. 2016;115:856–863. [DOI] [PubMed] [Google Scholar]
- 54. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular P, Rehabilitation, Guidelines ESCCfP , European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 55. Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD; Aspirin for Asymptomatic Atherosclerosis T . Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848. [DOI] [PubMed] [Google Scholar]
- 56. Masanauskiene E, Naudziunas A. Comparison of ankle‐brachial index in patients with and without atrial fibrillation. Medicina. 2011;47:641–645. [PubMed] [Google Scholar]
- 57. Violi F, Pastori D, Perticone F, Hiatt WR, Sciacqua A, Basili S, Proietti M, Corazza GR, Lip GY, Pignatelli P; Group AS . Relationship between low ankle‐brachial index and rapid renal function decline in patients with atrial fibrillation: a prospective multicentre cohort study. BMJ Open. 2015;5:e008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Poisa P, Porteri E, Agabiti‐Rosei C, Paderno V, Belotti E, Rizzoni D, Castellano M, Agabiti‐Rosei E. Prognostic role of flow‐mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 59. Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. [DOI] [PubMed] [Google Scholar]
- 60. Matsushima Y, Takase B, Uehata A, Kawano H, Yano K, Ohsuzu F, Ishihara M, Kurita A. Comparative predictive and diagnostic value of flow‐mediated vasodilation in the brachial artery and intima media thickness of the carotid artery for assessment of coronary artery disease severity. Int J Cardiol. 2007;117:165–172. [DOI] [PubMed] [Google Scholar]
- 61. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 62. Perri L, Pastori D, Pignatelli P, Violi F, Loffredo L. Flow‐mediated dilation is associated with cardiovascular events in non‐valvular atrial fibrillation patients. Int J Cardiol. 2014;179C:139–143. [DOI] [PubMed] [Google Scholar]
- 63. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. [DOI] [PubMed] [Google Scholar]
- 64. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 65. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 66. Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang SL, Tuan TC, Tai CT, Lin YJ, Lo LW, Hu YF, Tsao HM, Chang CJ, Tsai WC, Chen SA. Comparison of outcome in catheter ablation of atrial fibrillation in patients with versus without the metabolic syndrome. Am J Cardiol. 2009;103:67–72. [DOI] [PubMed] [Google Scholar]
- 68. Pastori D, Farcomeni A, Poli D, Antonucci E, Angelico F, Del Ben M, Cangemi R, Tanzilli G, Lip GY, Pignatelli P, Violi F. Cardiovascular risk stratification in patients with non‐valvular atrial fibrillation: the 2MACE score. Intern Emerg Med. 2016;11:199–204. [DOI] [PubMed] [Google Scholar]
- 69. Stamboul K, Fauchier L, Gudjoncik A, Buffet P, Garnier F, Lorgis L, Beer JC, Touzery C, Cottin Y. New insights into symptomatic or silent atrial fibrillation complicating acute myocardial infarction. Arch Cardiovasc Dis. 2015;108:598–605. [DOI] [PubMed] [Google Scholar]
- 70. Cohen M, Adams PC, Parry G, Xiong J, Chamberlain D, Wieczorek I, Fox KA, Chesebro JH, Strain J, Keller C. Combination antithrombotic therapy in unstable rest angina and non‐Q‐wave infarction in nonprior aspirin users. Primary end points analysis from the ATACS trial. Antithrombotic Therapy in Acute Coronary Syndromes Research Group. Circulation. 1994;89:81–88. [DOI] [PubMed] [Google Scholar]
- 71. Pastori D, Pignatelli P, Saliola M, Carnevale R, Vicario T, Del Ben M, Cangemi R, Barilla F, Lip GY, Violi F. Inadequate anticoagulation by vitamin K antagonists is associated with major adverse cardiovascular events in patients with atrial fibrillation. Int J Cardiol. 2015;201:513–516. [DOI] [PubMed] [Google Scholar]
- 72. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook‐Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM; Investigators AAT . Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 73. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pastori D, Pignatelli P, Farcomeni A, Cangemi R, Hiatt WR, Bartimoccia S, Nocella C, Vicario T, Bucci T, Carnevale R, Lip GY, Violi F. Urinary 11‐dehydro‐thromboxane B2 is associated with cardiovascular events and mortality in patients with atrial fibrillation. Am Heart J. 2015;170:490–497.e491. [DOI] [PubMed] [Google Scholar]
- 75. Pignatelli P, Pastori D, Vicario T, Bucci T, Del Ben M, Russo R, Tanzilli A, Nardoni ML, Bartimoccia S, Nocella C, Ferro D, Saliola M, Cangemi R, Lip GY, Violi F. Relationship between Mediterranean diet and time in therapeutic range in atrial fibrillation patients taking vitamin K antagonists. Europace. 2015;17:1223–1228. [DOI] [PubMed] [Google Scholar]
- 76. Pastori D, Carnevale R, Bartimoccia S, Nocella C, Tanzilli G, Cangemi R, Vicario T, Catena M, Violi F, Pignatelli P. Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxid Redox Signal. 2015;23:682–687. [DOI] [PubMed] [Google Scholar]
- 77. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 78. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C , Thygesen K, Alpert JS, White HD; Biomarker S , Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA; Subcommittee ECG , Chaitman BR, Clemmensen PM, Johanson P, Hod H; Imaging S , Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ; Classification S , Fox KA, Atar D, Newby LK, Galvani M, Hamm CW; Intervention S , Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J; Trials, Registries S , Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML; Trials, Registries S , Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G; Trials, Registries S , Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D; Trials, Registries S , Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Guidelines ESCCfP , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Document R , Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598.22958960 [Google Scholar]


