Abstract
Background
Elderly patients with atrial fibrillation are at higher risk of both ischemic and bleeding events compared to younger patients. In a prespecified analysis from the ENGAGE AF‐TIMI 48 trial, we evaluate clinical outcomes with edoxaban versus warfarin according to age.
Methods and Results
Twenty‐one thousand one‐hundred and five patients enrolled in the ENGAGE AF‐TIMI 48 trial were stratified into 3 prespecified age groups: <65 (n=5497), 65 to 74 (n=7134), and ≥75 (n=8474) years. Older patients were more likely to be female, with lower body weight and reduced creatinine clearance, leading to higher rates of edoxaban dose reduction (10%, 18%, and 41% for the 3 age groups, P<0.001). Stroke or systemic embolic event (1.1%, 1.8%, and 2.3%) and major bleeding (1.8%, 3.3%, and 4.8%) rates with warfarin increased across age groups (P trend<0.001 for both). There were no interactions between age group and randomized treatment in the primary efficacy and safety outcomes. In the elderly (≥75 years), the rates of stroke/systemic embolic event were similar with edoxaban versus warfarin (hazard ratio 0.83 [0.66–1.04]), while major bleeding was significantly reduced with edoxaban (hazard ratio 0.83 [0.70–0.99]). The absolute risk difference in major bleeding (−82 events/10 000 pt‐yrs) and in intracranial hemorrhage (−73 events/10 000 pt‐yrs) both favored edoxaban over warfarin in older patients.
Conclusions
Age has a greater influence on major bleeding than thromboembolic risk in patients with atrial fibrillation. Given the higher rates of bleeding and death with increasing age, treatment of elderly patients with edoxaban provides an even greater absolute reduction in safety events over warfarin, compared to treatment with edoxaban versus warfarin in younger patients.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00781391.
Keywords: antithrombotic, bleeding, death, elderly, stroke
Subject Categories: Atrial Fibrillation, Intracranial Hemorrhage, Ischemic Stroke, Complications, Mortality/Survival
Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, and its prevalence is strongly related to age,1, 2, 3, 4 predisposing the elderly to an increased risk of embolic stroke. Although oral anticoagulant therapy with vitamin K antagonists effectively reduces the risk of ischemic stroke in patients with AF,5 there is a known increased bleeding risk with vitamin K antagonists, particularly in the elderly.6, 7, 8 Thus, older age is an overlapping factor in both the CHA2DS2‐VASc score for stroke,9 and the HAS‐BLED score for bleeding risk,10 and frequent international normalized ratio monitoring is recommended to ensure the optimal level of anticoagulation with vitamin K antagonists in the elderly. In addition, the use of vitamin K antagonists is limited by multiple drug and food interactions. As a consequence, anticoagulants are underused in the elderly, despite their high risk of ischemic events.11, 12
Edoxaban, a direct oral factor Xa inhibitor, has a linear pharmacokinetic profile and is given once daily. In the Effective aNticoaGulation with factor Xa next GEneration in Atrial Fibrillation–Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF–TIMI 48), 2 dose regimens of edoxaban were noninferior to well‐managed warfarin in preventing stroke or systemic embolic events (SEE) while reducing major bleeding and death from cardiovascular causes in patients with AF. Likewise, other non–vitamin K oral anticoagulants (NOACs) have proven to be at least as effective as warfarin in reducing stroke with lower major bleeding rates.13 However, subgroup analyses from 2 of these newer agents, namely, dabigatran and rivaroxaban, as compared to warfarin, have demonstrated a reduced safety margin with respect to bleeding in the elderly.14, 15
As previously reported in a pharmacokinetic and outcomes analysis, the risk of stroke or SEE with edoxaban decreases gradually and linearly with increasing edoxaban concentration, in contrast with the steeper increase in the risk of major bleeding.16 Thus, the therapeutic window for edoxaban is narrower for major bleeding than thromboembolism.
Given the concern over increased risk of bleeding in the elderly, we evaluated the efficacy and safety of edoxaban compared with warfarin stratified by age in the ENGAGE AF‐TIMI 48 trial.
Methods
Study Design and Study Population
The design and results of ENGAGE AF‐TIMI 48 have been described previously.17, 18 In brief, ENGAGE AF‐TIMI 48 was a randomized, double‐blind, double‐dummy trial to establish the noninferiority of edoxaban compared with warfarin. Twenty‐one thousand one‐hundred and five patients with moderate‐to‐high‐risk AF were randomized with a median follow‐up of 2.8 years. The protocol and amendments were approved by all institutional review committees and all patients provided written informed consent. Edoxaban was provided by the sponsor, Daiichi‐Sankyo (Edison, NJ), who also funded the trial. Eligible patients were 21 years of age or older with documented AF within the 12 months preceding randomization at medium or high risk of thromboembolic events (CHADS2 risk score ≥2).19 Key exclusion criteria were an estimated creatinine clearance (CrCl) <30 mL/min; a condition associated with a high risk of bleeding (eg, prior intracranial hemorrhage [ICH], gastrointestinal bleeding or active peptic ulcer in the prior year, major surgery or trauma in the preceding 10 days, uncontrolled hypertension, hemorrhagic disorder); moderate to severe mitral stenosis or mechanical heart valve, need for dual antiplatelet therapy, and an acute coronary syndrome, stroke, or revascularization procedure within 30 days prior to enrollment.17, 18 There was no upper age limit for participation. Patients were seen in follow‐up at months 1, 3, and every 3 months thereafter, at which time a complete blood count and creatinine were measured.
Randomization and Study Drugs
Patients were randomized in a 1:1:1 ratio to a higher‐dose edoxaban regimen (HDER) (60 mg once daily), a lower‐dose edoxaban regimen (LDER) (30 mg once daily), or to warfarin titrated to achieve a target international normalized ratio of 2.0 to 3.0. The dose of edoxaban was reduced by 50% if any of the following characteristics were present at the time of randomization or during the study: calculated CrCl of ≤50 mL/min using the Cockcroft‐Gault formula,20 body weight of 60 kg or less, or the concomitant use of potent P‐glycoprotein inhibitors.
Edoxaban Concentration
Plasma concentration and anti‐Factor Xa (FXa) activity were assessed using previously described methods16 and were analyzed by age group. Trough blood samples were collected 1 month after randomization. Plasma concentrations of edoxaban were measured by Quintiles Bioanalytical and ADME Laboratories using a validated turbo ion spray liquid chromatography mass spectrometry (LC‐MS)/MS method. Anti‐factor Xa activity was measured by the Rotachrome Heparin assay on the Stago STAR Evolution platform (TIMI Clinical Trials Laboratory, Boston, MA).16
Study End Points
The primary efficacy end point was a composite of all stroke or SEE. Key secondary end points included the net clinical end point of stroke, SEE, major bleeding, or death from any cause, as well as each of these end points individually. The principal safety end point was major bleeding as defined by the International Society on Thrombosis and Haemostasis.21 All primary and secondary end points were adjudicated by independent blinded central event adjudication committee members.
Statistical Analysis
The data were analyzed by prespecified age categories (<65, 65–74, and ≥75 years) and the elderly were defined as patients ≥75 years of age. We hypothesized that the efficacy and safety profile of edoxaban relative to warfarin would be consistent across age groups. Baseline characteristics were reported as frequencies and percentages for categorical variables and as medians and interquartile ranges for continuous variables. Baseline characteristics were compared using the χ2 test for categorical, and Wilcoxon rank sum test for continuous variables.
Outcomes were presented as the total number of first events and annualized event rates, and were examined by Cox proportional hazard models. Multivariate regression models were used to adjust for differences (univariate P<0.05) in baseline characteristics across age groups. Each of the continuous variables were tested for linearity before being included in the model, and as such, creatinine was included as restricted cubic spline with 4 knots to account for nonlinearity. The variables included in the model were the following: sex, body mass index, creatinine, hypertension, dyslipidemia, diabetes, smoking, prior stroke or transient ischemic attack, heart failure, type of AF, race, region, increased risk of falling, risk of neuropsychiatric disease, coronary artery disease, history of hepatic disease, history of extracranial hemorrhage, alcohol intake, and medication predisposing to bleeding.
The impact of age on outcomes was first evaluated exclusively in the warfarin group to eliminate the influence of a possible difference in age‐related treatment response. Interactions between the randomized treatment and the age categories were then tested by Cox proportional hazard modeling to address effect modification by age group. Differences between randomized treatment groups are presented both as hazard ratios (HR) and absolute risk differences (ARD) since event rates varied widely across age groups.
Additionally, primary end points were analyzed by age as a continuous variable using restricted cubic splines to evaluate for nonlinear relationships between age and outcomes and to confirm the prespecified age cut points. Furthermore, in exploratory analyses, efficacy and safety were evaluated in several post‐hoc subgroups (i.e., the very elderly populations [≥80 and ≥85 years]) and those meeting criteria for dose reduction.
All analyses were performed using the SAS software, version 9.3 (SAS Institute Inc., Cary, NC). A 2‐sided P<0.05 was considered to indicate statistical significance, without adjustment for multiple comparisons.
Due to a higher rate of ischemic stroke with LDER as compared to warfarin, LDER was not submitted for regulatory reviews. Only the HDER regimen has been approved by regulatory authorities around the world for stroke prevention in patients with AF. However, we also describe detailed results with LDER in the Supplement (Table S2b, Figures S2 and S3), given the higher risk of bleeding in the elderly and since there is strong clinical interest in understanding how the risk–benefit of lower intensity anticoagulation compares to warfarin in this more vulnerable subgroup.
Results
Patient Characteristics
Of the 21 105 patients randomized in the ENGAGE AF‐TIMI 48, the median age was 72 years (interquartile range, 64–78), and 8474 (40.2%) were ≥75 years (Figure S1). Characteristics of patients according to age category are summarized in Table. The elderly were more likely to be female, had lower CrCl and body weight, and thus were more likely to meet criteria for an edoxaban dose reduction at baseline (41% in the elderly as compared to 10% and 18% in the other 2 age groups, P<0.001). The median time in therapeutic range with warfarin was higher in the elderly compared with the younger (67.2%, 68.4%, and 69.6%, for <65, 65–74, and ≥75 years, respectively, P trend<0.001). Baseline characteristics within each age category were similar by randomized treatment group (P>0.05 for all).
Table 1.
Patient Characteristics by Age Group
| Characteristics | <65 y (N=5497, 26.0%) | 65 to 74 y (N=7134, 33.8%) | ≥75 y (N=8474, 40.2%) |
|---|---|---|---|
| Age, y | 59 [55.0–62.0] | 70 [67.0–72.0] | 79 [76.0–82.0] |
| Weight, kg | 91.0 [77.6–106.0] | 83.0 [71.0–95.7] | 76.0 [65.9–86.5] |
| Female sex | 1510 (28) | 2753 (39) | 3777 (45) |
| Hypertension | 5166 (94) | 6731 (94) | 7857 (93) |
| Dyslipidemia | 2803 (51) | 3836 (54) | 4419 (52) |
| Diabetes | 2235 (41) | 3053 (43) | 2336 (28) |
| Current smoking | 738 (13) | 490 (6.9) | 324 (3.8) |
| Prior PCI | 286 (5.2) | 500 (7.0) | 654 (7.7) |
| Prior stroke or TIA | 1520 (28) | 2334 (33) | 2119 (25) |
| Congestive heart failure | 3832 (70) | 4477 (63) | 3815 (45) |
| Type of AF | |||
| Paroxysmal | 1405 (26) | 1789 (25) | 2172 (26) |
| Persisent | 1349 (25) | 1636 (23) | 1883 (22) |
| Permanent | 2741 (50) | 3709 (52) | 4415 (52) |
| CrCl (mL/min) at randomization | 98 [79–123] | 74 [60–90] | 56 [45–69] |
| ≤50 | 145 (2.6) | 813 (11) | 3116 (37) |
| >50 to 80 | 1280 (23) | 3504 (49) | 4381 (52) |
| >80 | 4072 (74) | 2817 (40) | 977 (12) |
| Race | |||
| White | 4304 (78) | 5719 (80) | 7044 (83) |
| Asian | 872 (16) | 1048 (15) | 989 (12) |
| Black | 113 (2.1) | 95 (1.3) | 70 (0.8) |
| Other | 208 (3.8) | 272 (3.8) | 370 (4.4) |
| CHADS2 score | |||
| Mean (SD) | 2.6 (0.8) | 2.7 (0.8) | 3.2 (1.1) |
| 4 to 6 | 821 (15) | 1203 (17) | 2744 (32) |
| CHA2DS2‐VASc score | |||
| Mean (SD) | 3.2 (1.1) | 4.4 (1.1) | 5.0 (1.3) |
| 4 to 9 | 1815 (33) | 5626 (79) | 7478 (88) |
| HAS‐BLED score ≥3 | 894 (16) | 4129 (58) | 4779 (56) |
| Time in therapeutic range | 67.2 [55.5–75.9] | 68.4 [57.1–77.4] | 69.6 [57.1–78.3] |
| Dose reduction at randomization | 571 (10) | 1297 (18) | 3488 (41) |
| Prior VKA experience | 2978 (54) | 4285 (60) | 5178 (61) |
| Medication at time of randomization | |||
| Aspirin | 1647 (30) | 2073 (29) | 2460 (29) |
| Thienopyridine | 113 (2.1) | 169 (2.4) | 205 (2.4) |
Data shown are number (%) unless otherwise indicated. Data presented are median (interquartile ranges) for continuous variables and number (percentages) for categorical variables. Race was self‐reported. AF indicates atrial fibrillation; CrCl, creatinine clearance; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; VKA, vitamin K antagonists. P trend<0.001 for all comparisons except for dyslipidemia (P trend=0.33), aspirin use (P trend=0.27), and thienopyridine use (P trend=0.18).
Outcomes by Age in the Warfarin Group
The annualized rates of major efficacy and safety outcomes in the warfarin group increased significantly with age, but particularly for bleeding events. After adjustment for baseline differences, older age remained an independent risk factor for adverse outcomes. In the elderly, there were 2‐fold increases in stroke or SEE, and in ischemic stroke, a 3‐fold increase in International Society on Thrombosis and Haemostasis major bleeding and a 4‐fold increase in ICH compared to the youngest age group (P trend≤0.001 for each end point, Figure 1).
Figure 1.
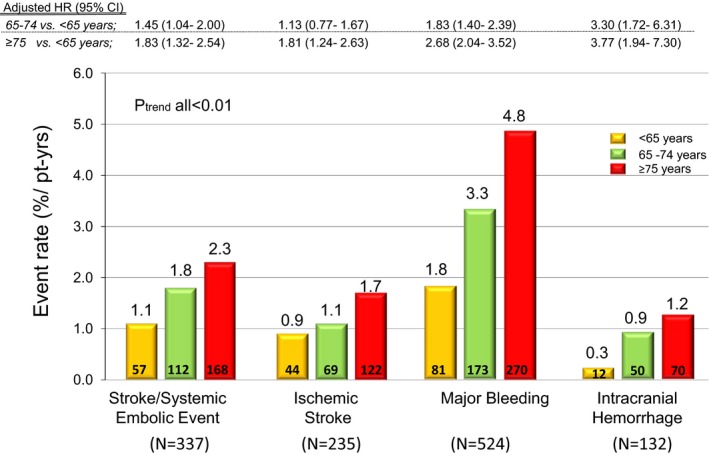
Influence of age on outcomes (warfarin group only). Multivariable model included all baseline characteristics with univariate P<0.05 (body mass index, sex, creatinine, hypertension, dyslipidemia, diabetes, smoking, prior stroke or transient ischemic attack, heart failure, type of atrial fibrillation, race, region, increased risk of falling, risk of neuropsychiatric disease, coronary artery disease, history of hepatic disease, history of nonintracranial hemorrhage, alcohol intake, and medication predisposing to bleeding). HR indicates hazard ratio.
When age was analyzed as a continuous variable, the probabilities of major bleeding exceeded that of stroke or SEE with warfarin across the range of age (Figure 2). Both risks increased as age increased, with a visually prominent increase in age ≥75 years, and with an even steeper slope for major bleeding (Figure 2).
Figure 2.
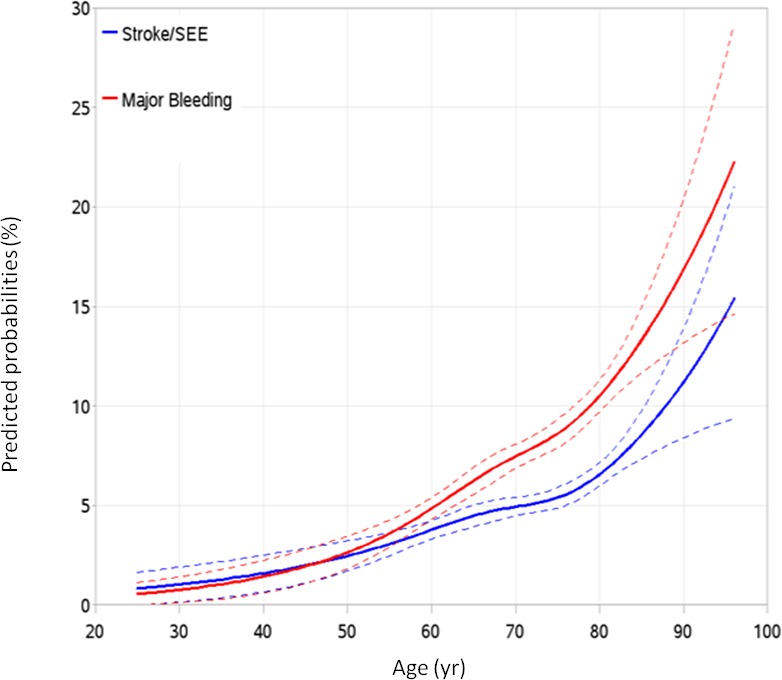
Calculated probabilities by continuous age. The outcomes were analyzed by age as a continuous variable for stroke or SEE (blue line) and major bleeding (red line). The dotted lines represent the 95% CI. SEE indicates systemic embolic event.
Outcomes by Treatment and Age
There was no significant effect modification by age group on the relative treatment effect of HDER as compared to warfarin for the primary and key secondary efficacy, safety, and net clinical outcome analyses (P interaction>0.05 for each comparison, Figure 3).
Figure 3.
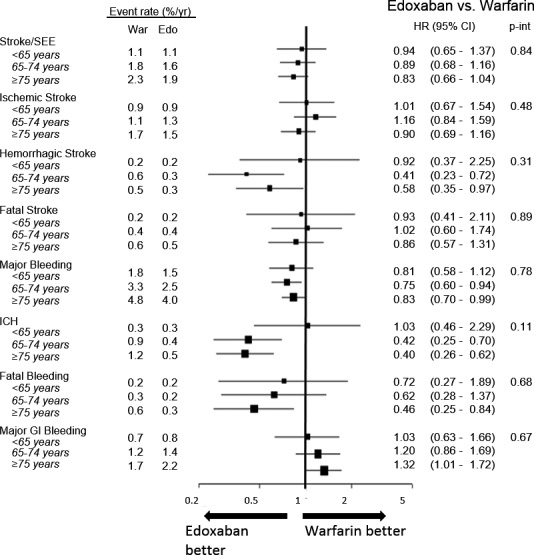
Efficacy and safety outcomes comparing the higher‐dose edoxaban regimen vs warfarin by age. Intracranial hemorrhage (ICH) and major bleeding events include primary ischemic strokes with hemorrhagic conversion. GI indicates gastrointestinal; HR indicates hazard ratio; SEE, systemic embolic event.
In the comparison of HDER with warfarin in elderly patients, the HRs and ARDs were the following: HR: 0.83 (0.66–1.04), ARD: −40 events/10 000 pt‐yrs for stroke or SEE, HR: 0.83 (0.70–0.99), ARD: −82 events/10 000 pt‐yrs for major bleeding, and HR: 0.40 (0.26–0.62), ARD: −73 events/10 000 pt‐yrs for ICH. The primary net clinical outcome in the elderly was improved (HR: 0.88 [0.79–0.97], ARD: −144/10 000 pt‐yrs), as were the secondary and tertiary net outcomes (Figure 4). Since event rates for thromboembolic events, bleeding, and death were higher in the elderly, the absolute numeric reductions in these events when edoxaban was compared to warfarin were more marked in older patients. Corresponding results with the LDER versus warfarin are shown in Figures S2 and S3.
Figure 4.
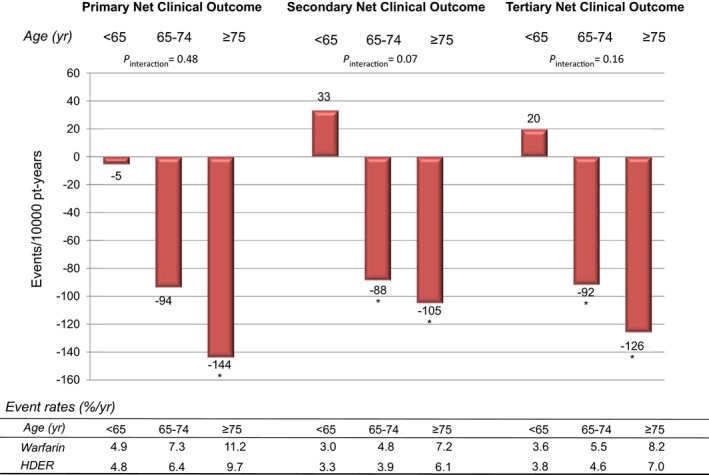
Absolute risk differences in net clinical outcomes with the higher‐dose edoxaban regimen as compared to warfarin. Primary Net Clinical Outcome: stroke, systemic embolic events, major bleeding, or death from any cause. Secondary Net Clinical Outcome: disabling stroke, life‐threatening bleeding, or death from any cause. Tertiary Net Clinical Outcome: stroke, systemic embolic events, life‐threatening bleeding, or death from any cause. Higher‐dose edoxaban regimen compared to warfarin; primary net clinical outcome; age <65 years; HR 0.99 (0.82–1.18), 65 to 74 years; 0.87 (0.76–1.00), and ≥75 years; 0.88 (0.79–0.97); P interaction=0.48, secondary net clinical outcome age <65 years; HR 1.11 (0.89–1.37), 65 to 74 years; 0.81 (0.69–0.96), and ≥75 years; 0.86 (0.76–0.97); P interaction=0.07, tertiary net clinical outcomes; age <65 years; HR 1.1 (0.85–1.28), 65 to 74 years; 0.83 (0.71–0.97), and ≥75 years; 0.85 (0.76–0.97); P interaction=0.16. *P<0.05 for comparison vs warfarin. HDER indicates higher‐dose edoxaban regimen; HR, hazard ratio; Pt‐yr, patient years; yr, years.
Special Subgroups
Dose reduction
Overall, there were 5356 (25.4%) patients who met criteria for dose reduction (10.4%, 18.2%, and 41.2% of patients age <65, 65–74, and ≥75 years, respectively). Among the 3488 elderly patients who met dose reduction criteria, 2960 (84.9%) patients were dose‐reduced due to moderate renal dysfunction (CrCl 30–50 mL/min at screening visit). Regardless of dosage, the edoxaban concentration and anti‐FXa activity were similar across age groups with broad overlap in dose groups (Table S1). Dose reduction resulted in a decrease in median exposure of 30% to 40% in all age groups and a maximum decrease in anti‐FXa activity of 40% in the elderly. Observed event rates for both stroke or SEE and for major bleeding were generally higher with increasing age both in patients who did and who did not meet dose reduction criteria (Tables S2 and S3).
The efficacy and safety profiles of edoxaban as compared to warfarin were consistent regardless of dose reduction and age group (P interaction>0.05), with the exception of major bleeding in the elderly. Dose reduction from 60 to 30 mg in elderly patients randomized to the HDER who met 1 of the 3 prespecified criteria for dose reduction at randomization decreased major bleeding as compared to warfarin to an even greater degree, than among elderly patients treated with HDER who were not dose‐reduced (HDER versus warfarin; HR 0.58 [0.43–0.77] for dose reduction, HR 1.06 [0.84–1.33] for no dose reduction, P interaction=0.0013, Table S2). There also was a similar significant interaction observed with LDER (P interaction=0.0001, Table S3).
Three‐way interactions between age group, dose reduction status, and randomized treatment were not statistically significant when HDER was compared with warfarin with regard to primary efficacy end point (P interaction=0.52) and primary safety end point (P interaction=0.09). Similarly, the 3‐way interaction was not significant for primary efficacy end point with LDER (P interaction=0.82, Table S3). However, there was a marginal significant interaction with the primary safety end point (P interaction=0.05).
Post hoc analysis of very elderly groups (age ≥80 and age ≥85)
We additionally explored the very elderly groups: 3591 (17.0%) patients aged ≥80 and 899 (4.3%) patients aged ≥85, given the aging population and increased prevalence of AF with advanced age. Baseline characteristics for the very elderly groups ≥80 years of age and ≥85 years of age are provided in Tables S4 and S5, respectively. As shown in Table S6, patients ≥80 years of age had even higher event rates compared to patients ≥75 years. Comparisons of the efficacy and safety of edoxaban and warfarin, stratified at age 80, were similar to analyses using the 3 prespecified age cut points, with no significant treatment interactions between age‐groups and treatment for all major outcomes. Similar findings were observed in patients ≥85 years of age (Table S7).
Discussion
Of 21 105 subjects enrolled in the ENGAGE AF‐TIMI 48 trial, 8474 patients were ≥75 years of age. This represents the largest number of elderly patients enrolled among the randomized controlled trials with a NOAC to date. In this prespecified analysis, we confirmed the strong relationships between age and risk of both thromboembolism and bleeding in patients with AF, even after adjustment for potential confounders. Notably, however, age had a greater impact on bleeding. As age increases, the slope for major bleeding increases more sharply than the corresponding slope for stroke or SEE, particularly above the age of 75 years. Similar findings of a greater influence of age on bleeding as compared to thromboembolic events were reported in a randomized trial of 973 patients age ≥75 years with atrial fibrillation randomized to warfarin or aspirin.22
Due to the higher thromboembolic and particularly bleeding risk in the elderly as compared to the younger patients, the absolute benefits of edoxaban over warfarin were more marked in older patients, as compared to the benefits of edoxaban versus warfarin in younger patients. Importantly, with edoxaban, the incidence of ICH, the most feared complication of anticoagulant therapy, increased only gradually with increasing age, in contrast to a steeper increase in ICH rates with warfarin as age increases. As a result, there was a more pronounced relative decline in International Society on Thrombosis and Haemostasis major bleeding with edoxaban as compared to warfarin as age increased; this was particularly apparent among the elderly who met dose reduction criteria at randomization. Furthermore, edoxaban provided superior absolute net clinical outcomes over warfarin in the elderly, including the secondary and tertiary net clinical outcomes, which were limited to more serious irreversible outcomes.
Although comparisons across the warfarin‐controlled trials of NOACs are challenging due to differences in trial entry criteria, the frequency and degree of dose reduction of NOAC, and definitions of bleeding, qualitative comparisons of results help put our results into a contemporary context. There were no interactions between treatment effect and age for the primary efficacy and safety end points in ENGAGE AF‐TIMI 48. This is in contrast with the results from RE‐LY14 and ROCKET AF15 in which there were statistically significant treatment interactions with age with respect to bleeding outcomes14, 15; bleeding rates in the elderly with dabigatran and with rivaroxaban were numerically greater than with warfarin. Our results are most consistent with those with apixaban in the ARISTOTLE trial,23 in which there was no significant effect modification of NOAC treatment by age. In ENGAGE AF‐TIMI 48 and ARISTOTLE trials, the absolute major bleeding rates were consistently lower with the NOAC than with warfarin across all age groups.
In addition, we found no significant treatment interactions with age with respect to various primary and secondary safety end points including International Society on Thrombosis and Haemostasis major bleeding, ICH, fatal bleeding, major gastrointestinal bleeding, and minor bleeding with the HDER. Directionally opposite significant interactions were reported in the primary safety events of RE‐LY and ROCKET AF.14, 15 It is noteworthy that dose reduction in the elderly was most frequent in ENGAGE AF‐TIMI 48, and the reduction in dose of 50% was greater than in the ROCKET‐AF (25%) and RE‐LY (no dose reduction) trials.
We further explored subgroups of patients aged ≥80 and ≥85 years (Tables S6 and S7), and additionally performed sensitivity analyses using the same cut points as ROCKET AF (<75 and ≥75 years) (Table S8). The treatment effects were comparable in these sensitivity analyses, thereby demonstrating the robustness of the findings with edoxaban in the elderly.
In a pharmacokinetic analysis from the RE‐LY trial,24 efficacy and safety outcomes were associated with dabigatran plasma concentration, in which age and renal function, 2 highly correlated covariates, were the major determining factors. In ENGAGE AF‐TIMI 48, while age itself was not included as 1 of the criteria for dose reduction, 41% of patients ≥75 years of age received a dose reduction, most commonly (89%) due to the presence of moderate renal dysfunction (CrCl <50 mL/min). As such, edoxaban plasma concentration and anti‐FXa activity increased only slightly with increasing age.
In the elderly, dose reduction resulted in a decrease in median edoxaban plasma concentration of 30% to 40% and a decrease in median anti‐FXa activity of 20% to 40%. However, the efficacy of edoxaban compared with warfarin in preventing stroke or SEE was preserved, while there was a significant interaction demonstrating an even greater reduction in major bleeding among elderly who received a dose reduction of edoxaban as compared to similar patients in the warfarin group (P interaction<0.01). These findings are consistent with previous analyses in the overall ENGAGE AF‐TIMI 48 population, showing that dose reduction did not alter relative treatment efficacy, but did provide a greater reduction in major bleeding with edoxaban as compared with warfarin.16, 17 As age is a strong predictor of bleeding events, and since edoxaban has a relatively wider therapeutic window for thromboembolism than major bleeding,16 our results support the dose reduction strategy incorporated in the ENGAGE AF‐TIMI 48 trial. These findings reinforce the importance of selecting the right dose for patients, particularly in elderly patients with renal dysfunction, to avoid excess bleeding. The high frequency of moderate renal insufficiency in the elderly underscores the need for close monitoring of fluctuations in renal function to ensure proper dosing. A recent practical guide on the use of NOACs suggests repeat assessment of renal function at intervals in months equal to the CrCl divided by 10 for patients with a CrCl <60 mL/min (for example, a patient with CrCl of 40 mL/min is recommended to have repeat measurements every 4 months).25
Limitations to the current analysis warrant consideration. Elderly patients in a clinical trial may be relatively more healthy and compliant than elderly in a general population. In fact, the elderly patients in ENGAGE AF‐TIMI 48 had a lower rate of diabetes, and had a higher time in therapeutic range (69.6%) compared to younger patients. In addition, the trial included a small proportion of frail elderly or elderly with neurocognitive dysfunction, since participation in a complex long‐term clinical trial would have been difficult for these more vulnerable patients. However, more than 30% of the elderly had a CHADS2 score ≥4, 35% had moderate renal dysfunction, and event rates with warfarin were similar to those reported in other NOAC trials. Minority elderly patients were underrepresented in this trial; hence extrapolation of our findings to such subgroups requires caution. In addition, the prespecified age cut points were based on CHA2D2S2‐VASc cut points,19 and although nonlinear relationships between age and primary outcomes were explored, these cut points may not be optimal for other end points. Also, since the sample size of the trial was not powered for any of the subgroup analyses and event rates were relatively low, some subgroup analyses had very few events and therefore the power was limited.
Conclusions
Age is a stronger predictor of bleeding events compared to thromboembolic events in patients with AF. The relative reduction in major bleeding with edoxaban compared to warfarin was similar across age groups. However, due to the higher absolute rates of bleeding with increasing age, an even greater absolute numeric reduction in the rates of major bleeding was observed with edoxaban versus warfarin among elderly patients than was observed with edoxaban versus warfarin in younger patients.
Sources of Funding
The ENGAGE AF‐TIMI 48 trial was supported by a research grant from Daiichi Sankyo. No funding was provided for the preparation of this article.
Disclosures
Drs Kato, Giugliano, Ruff, Nordio, Braunwald, and Antman, and Ms Murphy are members of the TIMI Study Group at the Brigham and Women's Hospital, which received research grant support from Daiichi‐Sankyo for conduct of the ENGAGE AF‐TIMI 48 trial and research grant support outside the submitted work from Merck and AstraZeneca. Dr Giugliano also reports receiving honoraria for CME lectures and/or consulting for work related to antithrombotic therapy from Boehringer Ingelheim, Bristol‐Myers‐Squibb, Daiichi Sankyo, Janssen, Merck, Pfizer, Portola, and Sanofi. Dr Ruff also reports serving as a consultant and received honoraria from Daiichi Sankyo, Boehringer Ingelheim, Bayer, and Portola, and grant support through his institution outside the submitted work from Eisai, Intarcia, and GlaxoSmithKline. Drs Koretsune, Yamashita, and Kiss were members of the ENGAGE AF‐TIMI 48 Steering Committee and received research grant support for their participation in the trial. Dr Koretsune also reports lecture fees from Daiichi‐Sankyo, Boehringer Ingelheim, Bristol‐Myers‐Squibb, Bayer Health Care, and Pfizer; and grant support through his institution from Boehringer Ingelheim outside the submitted work. Dr Yamashita reports research funding from Daiichi Sankyo, Boehringer Ingelheim, Bayer Healthcare, and Bristol‐Myers‐Squibb, and remuneration from Daiichi Sankyo, Boehringer Ingelheim, Bayer Healthcare, Pfizer, Bristol‐Myers‐Squibb, Eisai, Toa Eiyo, and Ono Pharmaceutical. Mr Kimura and Drs Jin, Lanz, and Mercuri are employees at Daiichi‐Sankyo. Dr Braunwald also reports receiving consulting fees from Sanofi, the Medicines Company, and Theravance; lecture fees from Daiichi Sankyo, Menarini, Medscape, and Bayer HealthCare; and grant support through his institution from Johnson & Johnson, GlaxoSmithKline, Bristol‐Myers‐Squibb, Merck, Novartis, and Sanofi‐Aventis. He also reports serving as an unpaid consultant for Merck.
Supporting information
Table S1. Edoxaban Trough Concentration and Anti‐FXa Activity at Trough by Age and Dose Reduction
Table S2. Primary Outcomes by Dose Reduction (Higher‐Dose Edoxaban Regimen [60/30 mg])
Table S3. Primary Outcomes by Dose Reduction (Lower‐Dose Edoxaban Regimen [30/15 mg])
Table S4. Patient Characteristics in Very Elderly Population (≥80 years)
Table S5. Patient Characteristics in Very Elderly Population (≥85 years)
Table S6. Outcomes in Very Elderly Population (≥80 years)
Table S7. Outcomes in Very Elderly Population (≥85 years)
Table S8. Outcomes by Patients Aged <75 and ≥75 years
Figure S1. Age distribution. The median age was 72 years (interquartile range, 64–78). 40.2% were aged ≥75 years including 899 (4.3%) in age group ≥85 years.
Figure S2. Efficacy and safety outcomes comparing the lower‐dose edoxaban regimen vs warfarin by age. In the elderly, the risks of International Society on Thrombosis and Haemostasis major bleeding (HR 0.47 [0.38–0.58]) and ICH (HR 0.30 [0.19–0.49]) were significantly reduced with the lower‐dose edoxaban regimen as compared to warfarin. See Figures 1 and 3 for abbreviations.
Figure S3. Net clinical outcomes of the lower edoxaban regimen compared to warfarin. The results for net clinical outcomes with the lower‐dose edoxaban regimen were directionally similar to the higher‐dose edoxaban regimen.
(J Am Heart Assoc. 2016;5:e003432 doi: 10.1161/JAHA.116.003432)
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker‐Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases I, Risk Factors S, the GBDSEG . Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 4. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 5. Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I‐III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999;30:1223–1229. [DOI] [PubMed] [Google Scholar]
- 6. Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. [DOI] [PubMed] [Google Scholar]
- 7. Scowcroft AC, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: an analysis using the general practice research database (GPRD) 2000–2009. Heart. 2013;99:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, Kothawala P, Emons M. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med. 2010;123:446–453. [DOI] [PubMed] [Google Scholar]
- 9. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 10. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 11. Hylek EM, D'Antonio J, Evans‐Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–1080. [DOI] [PubMed] [Google Scholar]
- 12. Tulner LR, Van Campen JP, Kuper IM, Gijsen GJ, Koks CH, Mac Gillavry MR, van Tinteren H, Beijnen JH, Brandjes DP. Reasons for undertreatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27:39–50. [DOI] [PubMed] [Google Scholar]
- 13. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 14. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 15. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, Breithardt G, Singer DE, Becker RC, Hacke W, Paolini JF, Nessel CC, Mahaffey KW, Califf RM, Fox KA; Committee RAS Investigators . Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;130:138–146. [DOI] [PubMed] [Google Scholar]
- 16. Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, Deenadayalu N, Jarolim P, Betcher J, Shi M, Brown K, Patel I, Mercuri M, Antman EM. Association between edoxaban dose, concentration, anti‐factor Xa activity, and outcomes: an analysis of data from the randomised, double‐blind ENGAGE AF‐TIMI 48 trial. Lancet. 2015;385:2288–2295. [DOI] [PubMed] [Google Scholar]
- 17. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; Investigators EA‐T . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 18. Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the effective anticoagulation with factor Xa next generation in atrial fibrillation‐thrombolysis in myocardial infarction study 48 (ENGAGE AF‐TIMI 48). Am Heart J. 2010;160:635–641. [DOI] [PubMed] [Google Scholar]
- 19. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 20. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 21. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. [DOI] [PubMed] [Google Scholar]
- 22. Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E; Investigators B, Midland Research Practices N . Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 23. Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, Granger CB, Hanna M, Held C, Husted S, Hylek EM, Jansky P, Lopes RD, Ruzyllo W, Thomas L, Wallentin L. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L; Investigators R‐L . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial (randomized evaluation of long‐term anticoagulation therapy). J Am Coll Cardiol. 2014;63:321–328. [DOI] [PubMed] [Google Scholar]
- 25. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–1507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Edoxaban Trough Concentration and Anti‐FXa Activity at Trough by Age and Dose Reduction
Table S2. Primary Outcomes by Dose Reduction (Higher‐Dose Edoxaban Regimen [60/30 mg])
Table S3. Primary Outcomes by Dose Reduction (Lower‐Dose Edoxaban Regimen [30/15 mg])
Table S4. Patient Characteristics in Very Elderly Population (≥80 years)
Table S5. Patient Characteristics in Very Elderly Population (≥85 years)
Table S6. Outcomes in Very Elderly Population (≥80 years)
Table S7. Outcomes in Very Elderly Population (≥85 years)
Table S8. Outcomes by Patients Aged <75 and ≥75 years
Figure S1. Age distribution. The median age was 72 years (interquartile range, 64–78). 40.2% were aged ≥75 years including 899 (4.3%) in age group ≥85 years.
Figure S2. Efficacy and safety outcomes comparing the lower‐dose edoxaban regimen vs warfarin by age. In the elderly, the risks of International Society on Thrombosis and Haemostasis major bleeding (HR 0.47 [0.38–0.58]) and ICH (HR 0.30 [0.19–0.49]) were significantly reduced with the lower‐dose edoxaban regimen as compared to warfarin. See Figures 1 and 3 for abbreviations.
Figure S3. Net clinical outcomes of the lower edoxaban regimen compared to warfarin. The results for net clinical outcomes with the lower‐dose edoxaban regimen were directionally similar to the higher‐dose edoxaban regimen.


