Abstract
Similarities in neocortical circuit organization across areas and species suggest a common strategy to process diverse types of information, including sensation from diverse modalities, motor control, and higher cognitive processes. Cortical neurons belong to a small number of major classes. The properties of these classes are remarkably similar between areas, including their local and long-range connectivity, developmental history, gene expression, intrinsic physiology, and in vivo activity patterns. Each class contains multiple subclasses; for a rapidly growing number of these, conserved patterns of input and output connections are also becoming evident. The ensemble of circuit connections constitutes a basic circuit pattern that appears to be repeated across neocortical areas, with area- and species-specific modifications. Such “serially homologous” organization may adapt individual neocortical regions to the type of information each must process.
Introduction
The neocortex is the brain structure most commonly believed to give us our unique cognitive abilities. Yet, the cellular organization of the neocortex is broadly similar not only between species, but also between cortical areas. This similarity has led to the idea of a common “canonical microcircuit”, employing a similar computational strategy to process multiple types of information 1–4. If correct, this principle is very powerful for brain research, as understanding the organization of more tractable cortices such as the primary regions of experimentally accessible organisms, would provide insight into the circuits responsible for our most complex cognitive abilities.
The type of relationship found between different regions of the neocortex is also seen elsewhere in the body. Different cortical areas are similar in the same way that hands are similar to feet, or different bones are similar within the vertebral column. This type of relationship is termed serial homology: a similarity in the organization of different structures within a single organism*. Serially homologous structures consist of variations on a theme, containing similar classes of cells, organized in the same basic pattern. Differences between serially homologous structures are typically quantitative: the sizes and relative positions of different substructures, or the number and precise physiological parameters of cells of a given class. Quantitative differences, however, can allow serially homologous structures to serve very different functions, such as how the sizes and mechanical properties of bones and muscles in human hands and feet adapt them for grasping and walking.
In order to understand the serially homologous organization of the neocortex, it is necessary to characterize the classes of cortical neurons consistently across multiple levels. Neurons can be classified according to different criteria: their morphology; their patterns of local and long-range connectivity; their developmental history and gene expression profile; their intrinsic physiology; and the strategies they use to encode information in vivo. Finding a classification of cortical neurons that is consistent across these levels, and that applies to neurons of multiple cortical regions, would constitute a detailed understanding of the serially homologous neocortical circuit. As we shall see below, this goal has now been partly achieved in the form of a “top level” classification of excitatory cells and interneurons (Tables 1 and 2). Nevertheless, these top-level classes contain distinct subclasses, for which the correspondence between the different classification criteria is not yet clear, and the relationships between areas are not yet fully known.
Table 1. Properties of top-level excitatory classes.
See main text for references. The table entries are not intended to be comprehensive, but rather to provide key examples. Recurrent connections exist within each class (e.g. IT to IT,), which for simplicity are not listed.
| Top-level class | IT | PT | CT | |
|---|---|---|---|---|
| Major subclass | IT-L4 | IT - Other layers | ||
| Characteristic genes (subset) | Rorb, Satb2 | Satb2 | Fezf2, Ctip2 | Tbr1 |
| Inputs from other local excitatory classes | Few | Many, including L4-IT and other IT. | Many, mainly from IT | Few, mainly deep-layer (L5B/6) IT |
| Outputs to other local cell classes | Mainly IT, especially in L3. In at least some cases, also PT. | IT (but not L4-IT), PT, CT | Few | Some interconnectivity with IT, possibly PT |
| Long-range inputs | Thalamus, lower-order cortex | Thalamus, higher and lower-order cortex | Thalamus, higher and lower-order cortex | Higher order cortex |
| Long-range outputs | Few | Many, but only within telencephalon (neocortex, striatum); the only ECs sending callosal/commisural projections | Many, to multiple subcortical and subcerebral regions (brainstem, tectum, spinal cord, thalamus, basal ganglia) | Thalamus. The only ECs to excite reticular nucleus. The only ECs without longer-range corticocortical axons. |
| Morphology/layer | L4 pyramidal/stellate | L2/3, 5A, 5B, 6; pyramidal | L5B, thick tufted pyramidal | L6, pyramidal |
| Intrinsic physiology | Regular spiking or bursting | Hyperpolarized (L2/3), little h-current, spike train adaption | Depolarized, strong h-current, little adaptation, bursting (subset) | Regular spiking |
| In vivo activity | Rapid sensory response | Sparse code | Dense code | Very sparse |
Table 2. Major classes of interneurons and their properties.
See main text for references
| Top-level class: | Htr3a | Pvalb | Sst | |||
|---|---|---|---|---|---|---|
| Subclass: | Vip | Neurogliaform | Basket | Chandelier | Martinotti | L4 Sst |
| Local outputs | Descending axon, inhibit Sst and Pvalb | Nonsynaptic GABA release | Inhibit ECs (soma), other Pvalb | Inhibit axon initial segment of ECs | Inhibit Pvalb, EC dendrites including tufts | Inhibit L4 Pvalb |
| Local inputs | Excited by ECs | Excited by ECs | Excited by ECs, inhibited by Pvalb, Sst, Vip | Excited by ECs | Excited by ECs, inhibited by Vip | Excited by ECs |
| Long-range input | Higher order cortex | ? | Thalamus, lower-order cortex | ? | ? | ? |
| Intrinsic physiology | Irregular spiking | Late spiking | Fast spiking | Fast spiking | Low threshold spiking | Intermediate FS/LTS |
| In vivo activity | Driven by behavior | ? | Dense code, weakly tuned | ? | Modulated by motor activity; wide visual receptive fields | ? |
Neocortical neurons are extensively interconnected, but in a highly specific manner. Indeed, a 1 mm3 volume spanning the layers of rodent neocortex – corresponding, for example, to a barrel-related column in S1 – contains ~105 neurons, ~4 km of axon, and ~0.4 km of dendrite 5, 6. In contrast to the 1010 potential connections these cells could form, there are “only” ~109 actual synapses 5. Moreover, a substantial fraction of these synapses come from extrinsic axons (>50% in one estimate) 7, and presynaptic axons typically connect to postsynaptic neurons via multiple (e.g. 4–5) synapses 8. Consistent with these calculations, paired recordings show that connectivity rates between excitatory neurons are low in general, rising to 10–20% or higher only for specific pre- and postsynaptic cells, such as functionally co-tuned neurons in primary visual cortex 9. While the vast majority of neuronal pairs in the local circuit are thus either unconnected or only weakly connected, the connections that do occur follow systematic patterns.
The last few years have seen a tremendous growth in knowledge of neocortical organization and function, accruing especially from newer experimental methods available primarily in rodents. The present article presents an updated view of cortical circuitry based on these recent results. Space and citation limits preclude comprehensive coverage and adequate acknowledgement of a vast literature, and we primarily review data on excitatory neurons of rodent sensory and motor cortex. We review the major classes of neurons and consider their local circuit and long-range connections. Drawing on examples from different cortical areas in rodent, we speculate on how quantitative differences in homologous circuitry may allow different functional specialization in different areas, for example regarding how sensory processing is modulated by behavior. We suggest that a change of emphasis is now required to understand homologies between different cortical regions. In this view, lamination is not the sole or even primary organizing principle of neocortex. Instead, what different regions share is their hodology: the patterns of connection between different genetically defined cell classes. These connectivity “rules” can be highly conserved, whether the cell classes are intermingled or segregated into layers and sublayers. These rules are of course not immutable laws: the concept of serial homology means that circuit features (e.g. cell classes and their connections) will generally be repurposed, rather than discarded or invented de novo between areas and species. The evolutionary success of mammals suggests that the conservation of a homologous neocortical circuit across species, and its serialization across areas, has provided an advantageous substrate for the evolution of diverse mammalian behaviors.
Excitatory circuits
Neocortical excitatory cells (ECs) constitute ~80% or more of cortical neurons. ECs have been divided into three major classes based on their axonal projection patterns (Figure 1; Table 1), with each class containing multiple subclasses whose classification is a topic of active research 10–13. The first major class comprises the intratelencephalic (IT) neurons, which are found in layers (L) 2–6, and project axons only within the telencephalon (neocortex, striatum, and corticoid structures such as the amygdala and claustrum). IT neurons are the only ECs that project to contralateral cortex, their axons extensively inter-connecting the two hemispheres via the corpus callosum and anterior commissure. IT neurons are numerous and diverse, with hodologically distinct subclasses such as L4 ITs. The second major class, the pyramidal tract (PT) neurons, also known as subcerebral projection neurons, are large pyramidal neurons of L5B; indeed L5B is traditionally defined as the layer containing these neurons 14, a convention we follow here. PT neurons project to multiple subcerebral destinations including brainstem, spinal cord, and midbrain, and also have axonal branches in the ipsilateral cortex, striatum, and thalamus. Finally, corticothalamic (CT) neurons, found in L6, project primarily to ipsilateral thalamus. Each projection class has a characteristic laminar distribution, but these overlap, such that two layers have mixed composition: IT and PT neurons are intermingled in L5B, and IT and CT neurons in L6.
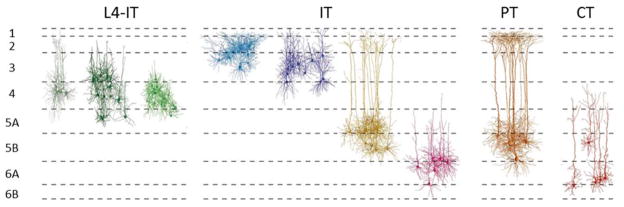
Figure 1.
Dendritic morphology of excitatory neurons in S1 barrel cortex. Modified with permission from Ref. 6. L4-IT: the 3 morphological classes of L4 intratelencephalic (IT) neurons: pyramidal, star pyramidal, and spiny stellate cells. IT: intratelencephalic neurons of layers 2, 3, 5A/B, and 6. PT: pyramidal tract neurons of L5B. CT: corticothalamic neurons of L6.
The hodology of cortical ECs is complex, but appears governed by some basic principles that are conserved across areas. All classes of EC form recurrent connections with local neurons of the same class (Box 1). Connectivity across EC classes is asymmetric, and research in frontal, visual, barrel, and motor cortex is consistent with the hypothesis of a common sequential organization within the local circuit (Figure 2) 2, 3, 8, 15–19. We emphasize, however, that this sequential organization does not constrain the flow of information to a single linear, feedforward pathway: because all EC classes receive external inputs, there are multiple entry points into this circuit; and, because ECs are usually projection neurons, there are also multiple exit points – a key principle of cortical circuit organization 2, 20, 21 often lost in simplified schematics. The remainder of this section will review the organization of the major excitatory circuits, following the sequential hodology from thalamus through L4 ITs, IT neurons of other layers, and then PT neurons. Finally, we discuss the connectivity of CT neurons, whose role in the cortical circuit is still largely uncertain.
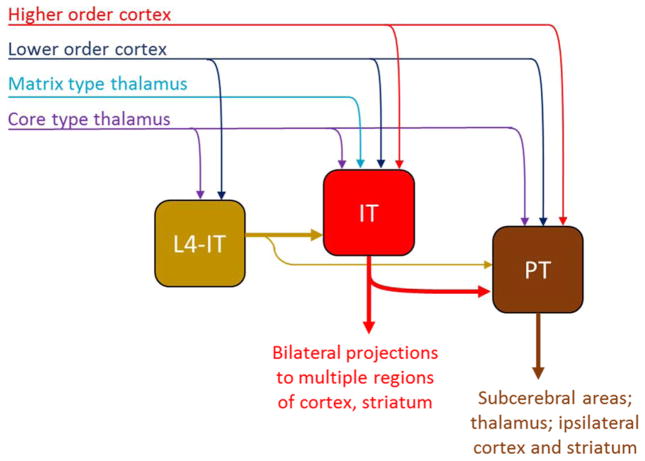
Figure 2.
Excitatory hodology of ECs in layers 2–5, including intratelencephalic neurons in layer 4 (L4-IT), IT neurons of other layers (L2/3, L5A, L5B; grouped as “IT” here), and pyramidal tract (PT) neurons. L4-IT neurons project mostly unidirectionally to other IT neurons, which in turn project mostly unidirectionally to PT neurons. Each class receives extrinsic inputs, but information flows across classes in a largely directional manner due to asymmetric inter-class connectivity. All classes have recurrent connections with other members of their own class (not shown). The relationship of CT neurons and IT neurons in L6 to this stratified hodology is not yet established.
Thalamocortical axons innervate multiple cell types
Most subcortical inputs to neocortex come from the thalamus. The rules governing the projections from the brain’s many thalamic nuclei to its many cortical areas are complex and still not fully understood. Nevertheless, a useful working model has been described, based on a division of thalamocortical (TC) projections into distinct patterns, arising from types of relay cell known as “core” and “matrix” 22, 23 (Figure 3).
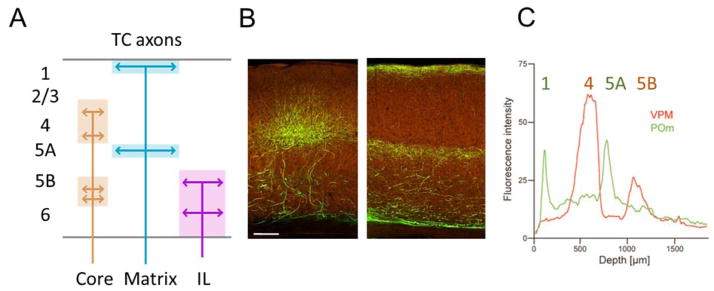
Figure 3.
Thalamocortical (TC) input streams. A, Illustration of the layers of termination of matrix-, core-, and intralaminar (IL)-type TC projections. B, projections to V1 from dorsal lateral geniculate (left) and from lateral posterior and adjoining nuclei (right). Images from Allen connectivity atlas (http://connectivity.brain-map.org/, experiments 293914766 and 267608343). Scale bar: 140 μm. C, Interdigitating laminar profiles in barrel cortex of matrix-type TC axons (green; from POm in thalamus) and core-type TC axons (red; from VPM in thalamus). Modified, with permission, from Ref. 25.
Core-type relay neurons are believed to be carriers of rapid sensory/motor information, and are located mainly in the primary (first-order) relay nuclei. Their axons project in a topographic manner to primary sensory cortices, chiefly to L4 and also (with areal and species variability) to L3 and L5B/6. “Matrix-type” relay neurons, occurring predominantly in higher-order thalamic nuclei, project to L1 but avoid L4 of primary sensory cortex, and have been further divided into subclasses according to whether they target a single cortical area or project more broadly 23. The information conveyed by matrix-type afferents is poorly understood. In addition to these two main classes of TC neuron, a third proposed class of “IL-type” relay cells, primarily found in the intralaminar nuclei, innervate striatum and other subcortical structures but also project to L5/6 of mainly motor and frontal cortex 23, 24.
Most TC projections to primary sensory cortex can be classified as core or matrix type. In barrel cortex, core-type VPM axons project primarily to L4 barrels and to L5B, and matrix-type POm axons project to L1, L5A, and (in rats) L4 septa 8, 25, with excitatory synaptic connections forming onto postsynaptic dendrites located in those layers 16 (Fig. 3c). In primary auditory cortex (A1), core-type ventral MGN axons project to L4 and the L5/6 border, while matrix-type afferents from dorsal and medial MGN project to L1 and subgranular layers but avoid L4 26, 27. In primary visual cortex (V1), core-type projections from dorsal LGN heavily project to L4, and in some species also L6 2, 28, whereas matrix-type projections from LP and LD project to L1 and L5A 29 (Figure 3). Not all projections from primary relay nuclei are of core type: in primates, matrix-type cells of LGN project to heavily to L1 and L2/3 28; in mice, a subdivision of dorsal LGN conveys information from directionally selective retinal ganglion cells to L1/2 but avoids L4 30.
The applicability of the core/matrix scheme beyond primary sensory cortex is not yet fully clear. Thalamic projections to primary motor cortex (M1) appear at least in rodents to follow a rough core/matrix organization: TC axons relaying cerebellar information project to the L3/L5A border, while TC axons relaying basal ganglia information project more heavily to L1, consistent with core and matrix-type patterns respectively 31; upper-layer neurons in M1 can also receive matrix-type inputs from POm 32. Thalamocortical inputs to secondary sensory and association cortex come from nuclei containing chiefly matrix type neurons 23. However, at least in secondary somatosensory and auditory cortex, these inputs terminate heavily in L4, suggesting they strongly drive secondary cortical areas, in contrast to their modulatory effects on primary areas 26, 33, 34. Thus, the concepts of core- and matrix-type projections may need to be extended to deal with the full complexity of thalamic projections to higher order cortex.
L4 neurons process extrinsic input in an area-specific manner
Excitatory neurons in L4 can be considered a special class of IT neurons. Due to their predominantly asymmetric local connectivity with other ECs, L4 neurons appear to be situated upstream in the local excitatory network. They project heavily to L2/3, and also to L5A/B, but receive little excitatory input in return (e.g. Ref. 8). Excitatory connectivity between L4 and L6 appears to be common in some species and areas but scarce in others 2, 35, 36: in rodent, L6→4 excitatory projections often appear weak compared to other interlaminar projections 19, 37–40 and are proposed to have modulatory or inhibitory functions 36, 40–42.
L4 IT neurons comprise multiple morphological subclasses (pyramidal, star-pyramidal, spiny stellate; Figure 1) but these appear to have broadly similar circuit properties, at least in barrel cortex 8, 39, 43. Although spiny stellate cells have traditionally been considered the prototypical L4 excitatory neuron, their occurrence varies markedly between areas and species: they are found in V1 of cat and monkey but not rodent 2, 28, 44, and are generally absent in A1 45.
Layer 4 can be greatly expanded in sensory cortices, with dramatic architectural differences between cortical areas and species. In primary sensory regions, L4 receives massive core-type thalamic input, but very few inputs from other thalamic or cortical areas. It thus appears that L4 circuits are specialized for sensory processing, structured in a manner appropriate to each modality 2, 8, 28. Consistent with their predominantly sensory-related inputs, L4 neurons in A1 are not modulated by behavior (unlike their L2/3 counterparts) 46, while in primate V1, L4 neurons show less of the trial-to-trial variability and noise correlation that might reflect common modulation by non-sensory inputs 47, 48.
Consistent with a modality-specific role, functional studies have yielded different perspectives on L4 processing in different modalities and species. In cat V1, for example, the spatial structure of visual receptive fields arises from the arrangement of TC inputs onto L4 ITs. These cells respond to stimuli of a particular orientation, which are derived by integrating inputs from a subset of LGN neurons with spatially aligned circular receptive fields of both on- and off-center polarity 49. In tree shrews, however, L4 contains sublayers that receive inputs of different polarity, which are only integrated to form orientation selective responses further downstream in L2/3 50.
A complementary example comes from rodent S1 and A1, where L4 processing of TC inputs appears to depend critically on the precise timing of excitation and inhibition. TC inputs excite not only L4 ITs but also interneurons, resulting in rapid and powerful feedforward inhibition of ECs (e.g. 51, 52). Because this inhibition arrives with a slight delay relative to excitation, it creates a narrow “window of opportunity” for ECs to fire an accurately timed spike; furthermore, stimulus-dependent differences in the timing of excitatory input allow L4 ITs to respond selectively to specific stimuli 53, 54. These examples suggest that the spatial and temporal properties of TC and intracortical circuits are tuned to produce receptive fields in L4 neurons that are appropriate for particular sensory modalities and species.
L4 neurons have received less attention outside of primary sensory areas. Although “agranular” areas such as motor cortex lack spiny stellate cells, they may nevertheless possess a rudimentary L4, based on the expression of L4-associated molecular markers at the expected laminar zone 31, 38, 55–58. L4 neurons in higher-order sensory areas receive long-range inputs from different thalamic sources as well as corticocortical inputs from lower-order areas 59–61, a pattern which is used to define hierarchical relationships between areas (discussed further below).
In summary, IT neurons in L4 appear to be specialized for receiving external input from thalamus or lower order cortex and processing it in a manner adapted to the specific input types each area receives.
IT neurons interconnect and project widely within the cerebral hemispheres
Whereas L4 ITs appear specialized for processing of extrinsic inputs, IT neurons of other layers integrate signals from L4 with multiple TC and cortical inputs. These neurons thus constitute the second stage of the local excitatory circuit. In contrast to the largely unidirectional projections out of L4, connections among IT neurons in other layers tend to be bi-directional. Their outputs go to distant neocortex and striatum, as well as locally to PT and probably CT neurons. IT neurons are a diverse cell class whose connectivity and physiology differs between and within layers.
L2/3 ITs send a major descending interlaminar axonal projection that branches extensively and densely in L5A and L5B, but not in L4. This L2/3→5A/B pathway appears to be a particularly prominent and consistent feature of cortical circuits across areas and species 2, 3, 37, 38, 62, and is mirrored at a functional level: optogenetic stimulation of L2/3 ITs has been shown to generate strong oscillatory activity in L2/3 and L5A/B, but not L4 or L6 63.
It is increasingly evident that L2/3 ITs comprise multiple subclasses based on gene expression, projection target, and firing patterns 11, 64–66. Although the supragranular layers are often studied as a single entity, hodological distinctions between sublayers are an important aspect of inter-areal connectivity in primate neocortex 61, and recent work in rodent is also revealing increasing distinctions between L2 and L3 (e.g. Refs. 8, 67, 68).
L2 ITs receive matrix-type TC input both monosynaptically and indirectly via ascending projections. In mouse barrel cortex, L2 ITs receive matrix-type input from POm 16, but receive little core-type input as their basal dendrites overlap little with these TC axons (Figure 4a, 3b) 32. L2 ITs also receive substantial input from L5A as well as L4 19, 38, particularly (in rat) for L2 ITs located above septa 8, 67–69. The main local interlaminar target of L2 ITs is IT neurons of lower L5A and PT neurons of upper L5B (at least in motor cortex 70).
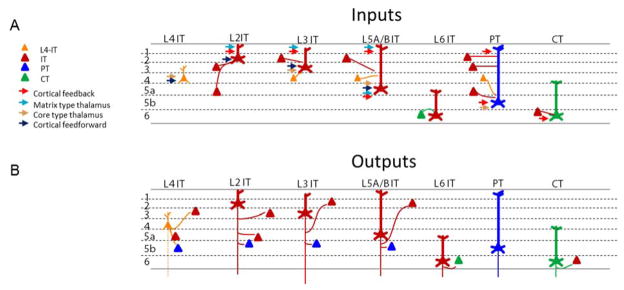
Figure 4.
Hypothesized excitatory hodology of the major EC classes. For clarity, intra-class (recurrent) connections are omitted, but exist for all cell types. This connectivity scheme is derived primarily from rodent barrel and motor cortex (see main text), and the existence of many of these connections in other regions remains to be tested. L5A ITs and L5B ITs have been represented together for simplicity. Additional connections for which only limited and/or conflicting evidence is available, or whose connectivity rates have often been found to be low (e.g. L4 to L6-IT and CT), have been omitted. A, Hypothesized patterns of excitatory input to each major cortical excitatory class. For clarity, many connections are drawn as going to the dendrites of the postsynaptic neurons within the same layer as the presynaptic neurons; however, available evidence suggests that inputs tend to go mostly to the perisomatic dendrites 16. B, Hypothesized patterns of output from each excitatory class onto other excitatory classes.
L3 ITs, in contrast, receive core-type TC input on their basal dendrites, matrix-type and higher-order cortical input on their apical dendrites 16, many inputs from local L4 ITs, and relatively weaker input from local L5A ITs 19, 38, 67 (Figure 4a). Locally, L3 ITs project primarily within the superficial layers as well to L5A/B, where they preferentially innervate PT rather than IT neurons (at least in motor cortex 70) (Figure 4b).
Despite receiving extensive excitatory input from L4, L2/3 ITs fire sparsely in vivo (i.e. at low rates), with L2 ITs exhibiting sparser firing than L3 71–75. This sparse firing may arise from their hyperpolarized resting potentials 19, as well as strong activation of L2/3 interneurons 76. Theoretical considerations suggest that sparse firing is advantageous for efficient learning in neural networks, and L2/3 is indeed a site of high plasticity in sensory cortex 77, 78.
IT neurons of L5A/B are generally interconnected with those of L2/3 19, 38, 79 (Figure 4a, b). L5A/B ITs are smaller than PT neurons, with “thin-tufted” apical dendrites stretching to L1. In vivo, they are more active than L2/3 ITs, but less so than PT neurons 71, 72, 75. Their long-range projections broadly resemble those of L2/3 ITs, but with more extensive connections to striatum 10, 12.
L6 IT neurons are the least studied IT type. Their inputs arise mainly from local deep layer neurons, at least in V1 80. L6 ITs make extensive long-range horizontal connections within neocortex, and some project to claustrum 36. L6 ITs contain multiple neurochemically distinct subclasses, whose sublamination patterns can vary between areas 81, 82. A distinct class of neurons found in the deepest stratum of neocortex (referred to as L6B, L7, or subgrisial neurons) are the surviving members of the subplate, a structure that is essential for cortical development, but whose neurons largely die before adulthood 83. This sublayer contains excitatory neurons but also long-range GABAergic projection neurons, and its neuronal composition and function remains to be fully elucidated 83, 84.
In summary, IT neurons are a highly diverse class, whose local and long-range connectivity forms the backbone of communication within and between cortical areas and hemispheres. There is as yet no clear evidence for areal differences in the local-circuit hodology of IT neurons. However, much remains to be learned about the connectivity and function of different subclasses of IT neurons, and it is possible that such investigations will reveal differences in not only the long-range but also local connectivity of IT subtypes between cortical areas.
PTs integrate cortical and TC inputs and broadcast to subcerebral structures
PT neurons represent the third and final stage of the local excitatory circuit. These large neurons receive extensive inputs from IT neurons of multiple layers but give little back locally, a result that has been seen in several cortical regions 15, 17, 18 (Figure 4a, b). They receive direct TC inputs, which are strong enough to drive them even without inputs from local L2/3 ITs 85. Their TC inputs appear to be primarily core-type, at least in barrel cortex: although PT tuft dendrites in L1 receive corticocortical inputs from motor cortex, they receive very little matrix-type TC input from POm 16.
PT neurons are found across the neocortex. PT neurons in any particular area often project to a characteristic subcerebral target, for example corticospinal neurons in motor cortex and corticotectal neurons in visual cortex. Nevertheless, PT axons tend to be multi-projectional, branching to multiple subcerebral destinations as well as cortex, striatum, and thalamus. Their specific projection targets vary considerably from cell to cell, even for PT neurons within the same cortical area (e.g. Ref. 86; reviewed in Ref. 10). The intracortical axons of PT neurons are only ipsilateral, and contribute together with IT neurons to inter-areal corticocortical projections between areas. Cortical PT collaterals have to date mostly been observed only in “feedback” type projections 87–89; their laminar termination differs from those of IT axons, at least for projections from motor cortex to S1 89. The extent to which these patterns generalize for all intracortical branches of PT axons remains to be assessed.
The physiology of PT neurons is broadly consistent across areas, and distinct from that of neighboring IT neurons. PT neurons have relatively depolarized resting membrane potentials, nonadapting spike trains, relatively narrow spikes, strong expression of hyperpolarization-activated currents, and distinct neuromodulatory properties 10, 90–92. These properties have been observed in multiple regions, although some properties are area-specific such as expression of Kv1 currents 93. Subclasses of PT neurons have been distinguished within an area based on electrophysiological properties such as action potential conduction velocity 94, 95. Although PTs have been associated with intrinsic bursting firing patterns, they do not always display this property 96, and are not the only cells that can burst in vivo 85, 97. Notably, PTs have the highest in vivo firing rates of all EC classes 71–73, 75, 95, 98, 99; such “dense coding” may provide an information-theoretic advantage to the broadcast of cortical output through a relatively small number of long-range projection fibers 4.
Data so far thus suggest that PTs in multiple regions act as downstream elements in the local circuit: integrating the results of local computations with direct thalamic inputs, and efficiently broadcasting the results mainly to distant subcortical structures. PTs of different regions send these outputs to different places; furthermore, PT neurons with diverse projection patterns may exist even within a single area, integrating particular combinations of local and long-range input and routing them to distinct sets of subcortical targets.
CT neurons: mysterious creatures of the deep
CT neurons are a distinct class of L6 cell, with unique developmental history and molecular characteristics 11, 36. They are distinct from L6 IT neurons, which they are intermingled with, and also from PT neurons that send axonal branches to the thalamus. Anatomically they are positioned to receive inputs from the many axon classes crossing their dendritic span, including local L4, IT, and PT neurons; matrix-, IL-, and core-type TC inputs; and long-range projections from multiple cortical regions and claustrum 31, 36. Nevertheless, CT neurons appear to receive a general paucity of thalamic and local input 8, 36, 80, 100. Studies in several cortical areas have indicated that they are instead innervated by high-order cortical areas 32, 80, 101.
CT neurons in sensory areas generally project back mostly to the thalamic relay nucleus providing their own cortical area with core-type TC input. However, a subclass of CT neurons found in lower L6 of multiple sensory areas project also to higher-order thalamic nuclei 102. The thalamic nuclei targeted by CT neurons in motor and associative areas are poorly understood. CT projections can be extremely slow, with delays of up to 30 ms reported in rabbit S1 99. Their synapses on thalamic relay cells are small, distal, and relatively weak with a major metabotropic component, leading to their classification as “modulators” as opposed to the “drivers” conveying input to thalamus from the sense organs and from cortical PTs 103.
The intracortical axons of CT neurons are limited to sparse, locally ascending branches 36, 104. In rats, the upper and lower CT subclasses give off local branches in L4 and L5A respectively 104. Similarly, in tree shrew V1, the axons of different CT subclasses target different sublayers in L4 35. In mouse S1, CT neurons innervate IT neurons in L5A but not those in L4, which they instead indirectly inhibit 40, whereas in V1 they exert a predominantly inhibitory effect on all other layers, via a subclass of Pvalb-positive interneurons 42, 105.
The functional role of CT neurons remains enigmatic 36. While CT connections have been proposed to close excitatory thalamocortical “loops”, the dominance of corticocortical over local excitatory inputs suggests a specialization for integrating long-range signals to modulate thalamocortical activity. Indeed, optogenetic experiments suggest that CT neurons, through their strong inhibitory influences on neurons in other cortical layers and in thalamus, can provide gain control in perceptual processing 42, 106. Puzzlingly, but consistent with their paucity of local inputs, most CT neurons in vivo are remarkably silent, even during various behaviors 75, 80, 98, 107. Thus, even though L6 occupies a substantial fraction of cortical volume, the role of CT neurons remains largely a mystery.
IT subclasses may explain inter-areal connection patterns
The neocortex’s many areas are elaborately interconnected through the axons of primarily IT neurons. Inter-areal connectivity is complex, and large-scale studies of primate and rodent have suggested the existence of subnetworks showing elevated interconnection, linked together by diversely connected “hub” regions 61, 108–110. The connectivity of N cortical areas can be summarized by a matrix of N2 numbers; but a more intuitive understanding of these connections could be gained if there exist simpler organizing principles governing connections of multiple regions. One such principle is the “cortical hierarchy,” which arose from primate studies showing that the layers of origin and termination of inter-areal projections differ between “feedforward” (FF) and “feedback” (FB) projections 60, 61 (Figure 5a). In rodents, evidence for hierarchical organization also exists, but its relation to lamination is less clear, with the primary laminar feature described being be the avoidance of L4 by FB projections in visual cortex 59. Cells giving rise to FF and FB projections can form distinct populations, but do not always occupy different layers 111 (Figure 5b); furthermore, these subclasses can receive different patterns of long-range synaptic input 112. The existence of multiple genetically distinct subclasses of IT neuron with characteristic long-range projections might provide a cellular explanation for inter-areal connectivity patterns in rodents, including but not limited to its hierarchical organization.
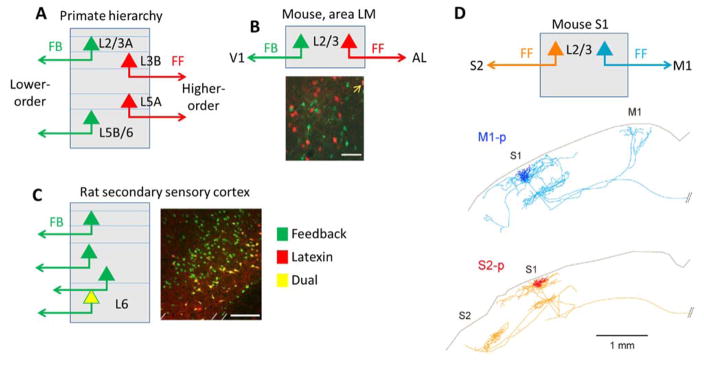
Figure 5.
Hypothesized homologous hodology of inter-areal connectivity. A, In primate neocortex, FF and FB streams have characteristic layers of origin and termination. B, In mouse visual cortex, FF and FB projections from an intermediate area (LM) arise from distinct IT subclasses that are intermingled within L2/3, as demonstrated by retrograde tracer injections into the upstream and downstream areas (adapted with permission from Ref. 111). Scale bar 50 μm. C, In rat secondary sensory cortices, a subclass of L6 neurons expressing latexin project back to the corresponding primary sensory region but not to thalamus, higher-order cortex, or contralateral cortex (adapted with permission from Ref. 82). Scale bar 200 μm. D, In mouse S1, two distinct subclasses of L2/3 neurons project to M1 and S2, both of which would be considered FF projections. Reconstructed neurons are adapted with permission from Ref. 66. Note the callosally projecting axons of both neurons (branches to the right), a defining feature of IT neurons.
One example of subclass-specific inter-areal IT projections comes from a study of deep-layer neurons in the rat 82 (Figure 5c). In secondary visual, auditory, and somatosensory cortices, a molecularly distinct subset of deep-layer neurons (expressing latexin and Nr4a2) send FB-type projections to the corresponding primary areas, but rarely to higher-order cortex, thalamus, or the contralateral hemisphere. It thus appears likely that these cells express a gene module including not only latexin and Nr4a2, but also other molecules that during development direct their axonal projections toward primary cortex.
Extrapolating from this relatively clear example, one might hypothesize that the apparently complex global pattern of corticocortical connections could be explained by the existence of a relatively small number of IT subclasses that are homologous across regions. Analogously to the way common gene modules guide the differentiation of top-level EC classes (PT vs. IT vs. CT), these IT subclasses would express common gene modules enforcing common characteristics including long-range axon targets, input connectivity, intrinsic physiology, somatodendritic morphology, and in some cases sublamination of both somas and axon terminals. Similarly, just as area-specific genes modulate the top-level EC classes by directing the precise subcortical structures they target (see below), area-specific genes might regionally diversify the common IT subtypes, for example by guiding top-down projections from V2 to V1, rather than to A1. Importantly, the IT subclasses giving rise to FF and FB projections need not occupy different layers in all species. Thus, even though the organization of inter-areal projections might appear different between primate and rodent at the laminar level, it may be homologous at the level of cellular subclasses.
If such homologous IT subclasses exist, they are unlikely to be restricted to subclasses producing FF and FB projections. For example, within superficial barrel cortex, distinct subclasses of IT neurons project to motor cortex and secondary somatosensory cortex, although both would be considered FF pathways. These subclasses have differences in intrinsic physiology, and encode information differently in vivo 64–66. While present data do not rule out these subclasses being specific to barrel cortex, it has been hypothesized that these two output streams are homologous to the dorsal and ventral streams of the visual and auditory pathways, specialized for processing information on stimulus location and identity respectively 66. Indeed, the dorsal and ventral projection streams of primate visual cortex also originate from neurons with different firing patterns, presumably corresponding to different IT subclasses 28, 113. It is therefore conceivable that a common set of genes control the long-range projections, physiology, and possibly information-coding of dorsal-type and ventral-type IT neurons across multiple areas and species. Combinatorial expression of a relatively small set of genetic modules like those just hypothesized could be sufficient to define the complex set of subtype- and area-specific corticocortical projections found in the mammalian brain.
Homologous inhibitory circuits mediate diverse effects
Recent research has greatly elucidated the development, connectivity, and function of different types of cortical interneurons. Their classification remains an actively pursued issue (e.g. Ref. 114), but in an increasingly adopted scheme they can be grouped into three genetically defined top-level classes, expressing parvalbumin (Pvalb), somatostatin (Sst) or serotonin receptor type 3a (Htr3a) (Table 2) 115. These classes contain a very large number of subtypes, which are outside the scope of this review and have been extensively reviewed elsewhere (e.g. Refs. 13, 116–118). We focus here on one particular aspect: how serially homologous inhibitory circuits may mediate diverse control of cortical processing during behavior.
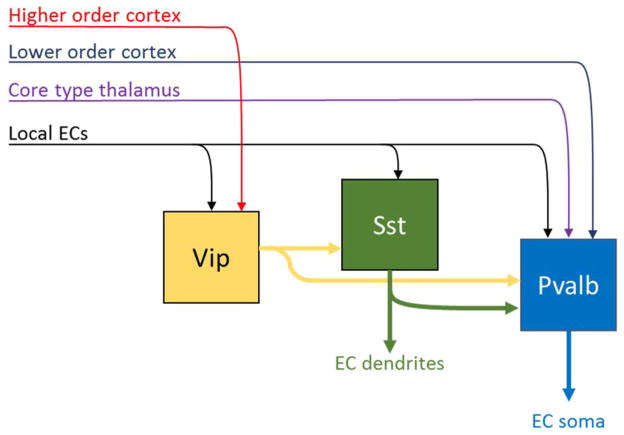
Classes of interneurons, like classes of ECs, form systematically asymmetric connections that define a sequentially organized network, and recent evidence indicates that this organization is conserved between multiple areas (Figure 6). The overall most upstream interneurons in this inhibitory network are those expressing vasoactive intestinal peptide (Vip), a major subclass of Htr3a neurons 119–122. These cells are most abundant in L2/3, and are characterized by a narrowly focused descending axon, a trait shared with a class of interneurons of lower L1 that have been termed “single bouquet cells” 122. Both Vip and lower L1 interneurons receive strong excitatory input from corticocortical axons in L1 as well as local ECs 120, 123–125, and are also excited by ionotropic receptors for acetylcholine and serotonin 116, 120, 124.
Figure 6.
Sequential hodology of three major inhibitory cell classes. All classes receive inputs from local ECs. Vip cells (a subclass of Htr3a interneurons) receive input from higher-order cortex, and inhibit primarily other interneuron classes. Sst cells inhibit Pvalb cells and the dendrites of ECs. Pvalb cells receive strong feedforward inputs from thalamus and lower-order cortex, and inhibit ECs perisomatically. Note that multiple additional interneuron classes exist, whose hodology is not yet fully established.
The primary targets of Vip and single-bouquet cells are other interneurons, especially Sst interneurons as well as Pvalb-positive basket cells 119–122. Sst and Pvalb cells receive excitatory input from local neurons, with Pvalb cells also receiving strong inputs from core-type thalamic and FF corticocortical axons 51, 126. Sst and Pvalb interneurons inhibit ECs on their dendrites and somata respectively, and Sst cells also inhibit Pvalb cells in a largely unidirectional manner 121, 127.
The activity of different interneuron classes is modulated by behavior, in a manner that appears to differ between cortical regions. In barrel cortex, whisking leads to increased Vip firing, primarily through projections from motor cortex 120. Consistent with the strong inhibition of Sst cells by Vip cells, whisking hyperpolarizes Sst cells 128, which in turn causes ECs’ apical dendrites to be released from inhibition, potentially explaining the enhanced dendritic calcium activity seen during active whisking 128, 129.
In V1, locomotion excites Vip cells 124, and causes diverse speed-dependent changes in the activity of ECs 130, including increased visually-driven activity in superficial layers 124, 131–133 that again appears to result from Vip cell-mediated disinhibition 124. In A1 however, locomotion decreases sensory responses in L2/3 IT and Pvalb neurons 46, 134, even though locomotion again increases the activity of A1 Vip cells 124, which again inhibit Sst and Pvalb interneurons 119. Thus, the effects of locomotion on the different sensory cortices can be quite different, despite their apparently conserved hodology. Intriguingly, these opposing effects of locomotion on sensory responses in superficial visual and auditory cortex can both be mimicked by optogenetic stimulation of inputs from higher order cortices 125, 134.
How could two cortical circuits with apparently identical hodology show opposite modulation by behavior and top-down cortical input? Such a phenomenon is not unprecedented: in electronics, for example, a circuit of a single topology can act as an amplifier or an attenuator, depending on the precise relative impedances of its constituent components. Analogously, quantitative differences in the parameters of serially homologous cortical circuits might explain how activation of one input pathway causes opposite-signed effects on the activity of a single cell class in different areas. For example, the differing effects of locomotion on superficial ECs of V1 and A1 might be explained by differences in the strength of inhibition that ECs, Sst, and Pvalb cells receive from Vip cells; by differences in the susceptibility of different interneuron classes to neuromodulation; or by differences in the strengths of local and top-down inputs received by different interneuron classes. More generally, quantitative differences between areas may control how animals integrate top-down and bottom-up information in a manner appropriate to each modality and behavior. For example, enhanced integration of top-down and bottom-up information during active whisking has been hypothesized to allow for computation of object locations by the interaction of whisker position and touch 129, 135. Integration of optic flow and running speed has been proposed to allow V1 neurons to estimate an animal’s velocity through the world 130. Finally, suppression of auditory cortical activity during running might enable the animal to focus more on the visual and somatosensory modalities critical to rodent navigation 46, 134. Thus, quantitative variations in serially homologous circuits might adapt different cortical regions to the ethological role of each sense.
Developmental basis of serially homologous circuits
The serially homologous organization of neocortex – like serial homology throughout the body – occurs because the developmental precursor populations of different regions follow homologous genetic programs, leading to similar cell types arranged in a similar organization. Again, space and citation limits preclude appropriate acknowledgement of a vast developmental literature, for which we refer the reader to other recent reviews 11, 13, 116, 136. The top-level EC classes are developmentally specified by mutually suppressive interactions between transcription factors including Fezf2 and Ctip2 for PT, Satb2 for IT, and Tbr1 for CT neurons. The relationship between gene expression and top-level cell classes appears to be conserved between cortical regions 11. Genetic modules downstream of these top-level transcription factors control receptors and molecular pathways involved in axon guidance and synapse formation, giving each class its characteristic connectivity profile 11, 137, 138. Experimental manipulation of these transcription factors in postmigratory neurons changes their connectivity and physiological properties, confirming that it is the genetically specified cell class, rather than laminar location per se that is the fundamental determinant of cortical connectivity 139–141.
What developmental mechanisms account for differences between cortical regions? The developing neocortex shows graded expression of multiple transcription factors across its surface, through which neocortical arealization is developmentally orchestrated (e.g. 56, 142). These gradations modulate the common cortical developmental plan, resulting for example in differences in long-range axon targeting, and attracting different types of thalamic input 11, 56, 136. Thalamocortical innervation in turn sculpts cortical organization, and appears to underlie inter-areal differences in L4 architecture. Indeed, in primary sensory cortex, TC innervation and activity is developmentally required for expression of L4-specific genes such as the M2 muscarinic receptor 143, for the formation of whisker barrels 144, and for the retraction of apical dendrites to form spiny stellate cells 145. The characteristic architectural features of L4 appear to be controlled by the patterns of sensory innervation the neocortex receives, as evidenced for example by the adaptation of barrel cortex to ectopic numbers of whiskers 144. Such input-driven malleability of L4 might help accelerate the evolution of new sensory strategies.
Outside of L4, there is as yet little evidence for major inter-areal differences in local circuit hodology. However, as noted above, hodologically similar circuits can evidently operate differently in different cortical areas. We have suggested that such differences can result from relatively small changes in quantitative parameters, such as the number of cells of particular classes, or their precise intrinsic and synaptic parameters. Although the developmental basis for such differences are not yet known, the existence of physiological differences between areas is supported not only by differences in cell counts, by also the fact that neurons of a single cell class can differ in ion channel expression between regions 93. Such quantitative differences may in turn be imposed by areal differences in gene expression, as well as the differing electrical activity patterns of multiple regions.
These observations suggest a hypothesis for how a common cortical circuit plan is modulated between areas. First, intrinsic differences in gene expression give each area its characteristic long-range patterns of input and output, as well as establishing quantitative differences in circuit parameters that fine-tune local circuit dynamics for the type of information processing that will occur there. Second, differences in extrinsic innervation and afferent activity patterns sculpt the circuits of thalamorecipient neurons (particularly in L4 sensory cortex), to adapt their architecture to particular classes of inputs.
Is cortical organization homologous across species?
How similar is cortical architecture between species? A comparison of the rodent literature reviewed here with cat, tree shrew, and primate studies might give the impression that the hodology of these species is very different. In fact, there are as yet few data that indicate major differences in hodology between species. Evidence for the sequential hodology illustrated in Figures 2, 4, and 6 for example, comes largely from experimental paradigms as yet applied only in rodent, but there are insufficient data to suggest this organization should not hold in other mammals. Similarly, while modern genetic methods allow detailed study of rodent inhibitory circuits, most such techniques are not yet available in other species.
As with differences between areas of mouse neocortex, the greatest differences between species appear to be in L4. L4 has a deep homology across species: it is found in marsupials 146, and even non-mammalian vertebrates such as birds and turtles (which lack neocortex) contain cells proposed as homologous to L4 neurons based on gene expression patterns 147. The cytoarchitectural idiosyncrasies of L4 appear strongest in areas devoted to ethologically important modalities. Rodents rely heavily on their vibrissae, and their S1 barrels have striking specializations compared to other sensory areas. In highly visual primates and humans, L4 in V1 is stratified into distinct sublayers receiving different input streams 28. By contrast, the star-nosed mole’s somatosensory L4 contains a map of its nose 148.
The question of how primate and especially human neocortex differs from that of other mammals has long intrigued neuroscientists. There is evidence that primate neocortex has a different developmental profile to rodent 136. In primates, L2/3 appears relatively expanded compared to carnivorans and rodents, which has been suggested to arise from increased proliferation that may in turn be allowed by longer gestation 149. Expansion of L2/3 has thus been suggested as one of the key evolutionary features of primate neocortex, but the intriguing implication – that L2/3 IT neurons’ circuits have somehow been modified with this adaptation – remains largely unexplored.
Outlook
Recent years have seen tremendous advances in our understanding of neocortical function, but an increasing fraction of this work is focused on a handful of cortical areas in a very specific model organism: the C57BL/6 strain of M. musculus. This intense focus is likely to facilitate progress, akin to the role of E. coli in the early days of molecular biology. The serially homologous nature of cortical circuits suggests that what we learn in the mouse will guide us towards general organizing principles, which can then be tested by more targeted investigations in other mammalian species. However, while recent work has suggested the broad outlines of how homologous connectivity may lead to common processing strategies in multiple areas, a great deal more work needs to be done before such principles are firmly established.
First, we must continue to work towards a consensus about the cell types that make up cortical circuits. For the top-level classification of ECs and interneurons, such a consensus is now emerging. It is now apparent that most if not all cortical regions contain homologous cell classes generated through similar cell-fate specification mechanisms: for excitatory neurons, the IT, PT, and CT classes; and for inhibitory neurons, the Pvalb, Sst, and Htr3a classes. This classification provides a unified framework that is consistent at multiple levels including development, molecular biology, local and long-range connectivity, intrinsic physiology, and in vivo activity, all of which appear to be broadly conserved across areas. The next frontier is to understand the extent to which the subclasses of these top-level classes are also homologously specified. Progress on this question will be accelerated by continued development of molecular markers, transgenic mice, and other tools that systematically and reliably identify and manipulate neuronal subclasses across areas (e.g. Refs. 12, 150).
Second, we must continue to clarify the input and output connectivity of these cell types at all length scales. While the local connectivity and long-range outputs of top-level EC and interneuron classes appears homologous across many areas, it is not yet clear whether the same will be true for subclasses. Furthermore, it is not yet known whether the long-range inputs of even the top-level classes are homologous across areas. For example, PT neurons of barrel cortex receive very little input from POm thalamus 16; do PT neurons elsewhere also not receive matrix-type thalamic input? The recent development of optogenetic circuit tracing 62 has enabled studies of long-range inputs to different cortical cell classes; when this technique is systematically applied to numerous areas, this question and many others can be answered, providing general principles of cortical connectivity across multiple regions.
Third, the relationship of IT subclasses to inter-areal connectivity must be established. Cortical areas are interconnected through seemingly myriad long-range projections. Conceivably, this complexity could arise from a small number of basic genetically specified IT subclasses, modulated by area-specific molecular gradients or other factors. Understanding the projection patterns of IT subclasses thus holds the potential to illuminate the cellular basis of inter-areal connectivity, including but not limited to the hierarchical organization seen at a macroscopic scale. CT neurons, lacking inter-areal branches, seem to be receivers but not senders in these corticocortical networks. How PT axons’ intracortical projections contribute to this organization needs to be further evaluated, particularly the possibility that they systematically provide feedback signals. A cellular understanding of IT subclasses would give information on not just the anatomical connections of the different subclasses but also their physiological properties, and provide a framework to understand the types of information transmitted by different projection classes in vivo.
Fourth, how does the specific connectivity and physiology of different cell classes contribute to their in vivo firing patterns? The in vivo coding strategies of top-level EC and interneuron classes seem broadly homologous across regions, as do their profiles of connectivity and intrinsic physiology. Understanding how each cell class’s connectivity and physiology shapes its coding strategy – and understanding whether and how this differs between further subclasses – will be critical to understanding cortical information processing.
Fifth, in what ways are serially homologous circuit patterns modified among cortical areas? What are the developmental mechanisms that specify these differences, and what are their functional consequences? We have suggested that the primary differences between areas are quantitative rather than qualitative. Assessing this will entail quantitative comparisons between areas at multiple levels, ranging from functional synaptic connectivity and intrinsic physiology to cell densities and morphology. Relatively few genes show different expression patterns between cortical regions; understanding how these modify the development of cortical connectivity and physiology will be important to understand its adult function. Understanding how quantitative differences in circuit patterns underlie different in vivo functions will require comparisons of recordings between multiple areas, most likely during several types of behaviors.
Finally, how similar is the cortical organization of different species? In this short review, we have not attempted a systematic phylogenetic comparison, but simply drawn on a few examples of homologous organization. A large number of observations made in individual species remain to be evaluated in others. For example, two observations for which there is good evidence for serial homology in the rodent – the sequential hodology of local excitatory and inhibitory circuits, and the cell-type dependence of firing sparseness – remain to be thoroughly tested in other species. Comparative studies will remain essential for understanding exactly what is similar and specialized about neocortical circuits across different areas and mammalian species, and exactly how circuit organization relates to behavior.
Acknowledgments
We thank K. Svoboda, N. Steinmetz, and N. Yamawaki, and M. Carandini for comments. KDH is supported by grants from the Wellcome Trust (095668), EPSRC (I005102, K015141), and Simons Foundation. GMGS is supported by grants from NIH (NS061963, NS087479, DC013272) and the Whitehall Foundation.
Footnotes
The unqualified word homology refers instead to a correspondence of structures between species, deriving from a common ancestral form.
Contributor Information
Kenneth D. Harris, Email: Kenneth.harris@ucl.ac.uk.
Gordon M. G. Shepherd, Email: G-shepherd@northwestern.edu.
References
- 1.Douglas RJ, Martin KA, Whitteridge D. A Canonical Microcircuit for Neocortex. Neural Computation. 1989;1:480–488. [Google Scholar]
- 2.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013;503:51–58. doi: 10.1038/nature12654. [DOI] [PubMed] [Google Scholar]
- 5.Braitenberg VB, Schuz A. Cortex: statistics and geometry of neuronal connectivity. Springer; Berlin: 1998. [Google Scholar]
- 6.Oberlaender M, et al. Cell type-specific three-dimensional structure of thalamocortical circuits in a column of rat vibrissal cortex. Cerebral cortex. 2012;22:2375–2391. doi: 10.1093/cercor/bhr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanyants A, Martinez LM, Ferecsko AS, Kisvarday ZF. The fractions of short- and long-range connections in the visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3555–3560. doi: 10.1073/pnas.0810390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmeyer D. Excitatory neuronal connectivity in the barrel cortex. Front Neuroanat. 2012;6:24. doi: 10.3389/fnana.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko H, et al. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepherd GMG. Corticostriatal connectivity and its role in disease. Nature reviews. Neuroscience. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nature reviews. Neuroscience. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang ZJ. Toward a Genetic Dissection of Cortical Circuits in the Mouse. Neuron. 2014;83:1284–1302. doi: 10.1016/j.neuron.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Economo C. The Cytoarchitectonics of the Human Cerebral Cortex. Oxford University Press; London: 1929. [Google Scholar]
- 15.Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Lorente de No R. Cerebral Cortex: Architecture, Intracortical Connections, Motor Projections. In: Fulton JF, editor. Physiology of the Nervous System. 3. Oxford University Press; London: 1949. pp. 288–330. [Google Scholar]
- 21.Evarts EV, Shinoda Y, Wise SP. Neurophysiological approaches to higher brain functions. John Wiley & Sons; New York: 1984. [Google Scholar]
- 22.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends in neurosciences. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 23.Clasca F, Rubio-Garrido P, Jabaudon D. Unveiling the diversity of thalamocortical neuron subtypes. The European journal of neuroscience. 2012;35:1524–1532. doi: 10.1111/j.1460-9568.2012.08033.x. [DOI] [PubMed] [Google Scholar]
- 24.Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. The Journal of comparative neurology. 2005;481:127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- 25.Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cerebral cortex. 2010;20:2265–2276. doi: 10.1093/cercor/bhq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith PH, Uhlrich DJ, Manning KA, Banks MI. Thalamocortical projections to rat auditory cortex from the ventral and dorsal divisions of the medial geniculate nucleus. The Journal of comparative neurology. 2012;520:34–51. doi: 10.1002/cne.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura A, Donishi T, Sakoda T, Hazama M, Tamai Y. Auditory thalamic nuclei projections to the temporal cortex in the rat. Neuroscience. 2003;117:1003–1016. doi: 10.1016/s0306-4522(02)00949-1. [DOI] [PubMed] [Google Scholar]
- 28.Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nature reviews. Neuroscience. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Martin A, et al. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature. 2014 doi: 10.1038/nature12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko T. Local connections of excitatory neurons in motor-associated cortical areas of the rat. Frontiers in neural circuits. 2013;7:75. doi: 10.3389/fncir.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooks BM, et al. Laminar organization of long-range excitatory input to mouse motor cortex. Journal of Neuroscience. 2013;33:748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nature neuroscience. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouchelon G, et al. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014 doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick D. The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cerebral cortex. 1996;6:329–341. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- 36.Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:13. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiler N, Wood L, Yu J, Solla SA, Shepherd GMG. Top-down laminar organization of the excitatory network in motor cortex. Nature neuroscience. 2008;11:360–366. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooks BM, et al. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 2011;9:e1000572. doi: 10.1371/journal.pbio.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schubert D, Kötter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2961–2970. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Matney CJ, Blankenship A, Hestrin S, Brown SP. Layer 6 corticothalamic neurons activate a cortical output layer, layer 5a. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:9656–9664. doi: 10.1523/JNEUROSCI.1325-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CC, Sherman SM. Modulator property of the intrinsic cortical projection from layer 6 to layer 4. Front Syst Neurosci. 2009;3:3. doi: 10.3389/neuro.06.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staiger JF, et al. Functional Diversity of Layer IV Spiny Neurons in Rat Somatosensory Cortex: Quantitative Morphology of Electrophysiologically Characterized and Biocytin Labeled Cells. Cerebral cortex. 2004 doi: 10.1093/cercor/bhh029. [DOI] [PubMed] [Google Scholar]
- 44.Peters A, Kara DA. The neuronal composition of area 17 of rat visual cortex. I. The pyramidal cells. The Journal of comparative neurology. 1985;234:218–241. doi: 10.1002/cne.902340208. [DOI] [PubMed] [Google Scholar]
- 45.Smith PH, Populin LC. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. The Journal of comparative neurology. 2001;436:508–519. doi: 10.1002/cne.1084. [DOI] [PubMed] [Google Scholar]
- 46.Zhou M, et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nature neuroscience. 2014;17:841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen BJ, Chelaru MI, Dragoi V. Correlated variability in laminar cortical circuits. Neuron. 2012;76:590–602. doi: 10.1016/j.neuron.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MA, Jia X, Zandvakili A, Kohn A. Laminar dependence of neuronal correlations in visual cortex. J Neurophysiol. 2013;109:940–947. doi: 10.1152/jn.00846.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- 50.Van Hooser SD, Roy A, Rhodes HJ, Culp JH, Fitzpatrick D. Transformation of receptive field properties from lateral geniculate nucleus to superficial V1 in the tree shrew. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11494–11505. doi: 10.1523/JNEUROSCI.1464-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nature neuroscience. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 52.Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2002;357:1717–1727. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 54.Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nature neuroscience. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- 55.Shepherd GM. Intracortical cartography in an agranular area. Front Neurosci. 2009;3:337–343. doi: 10.3389/neuro.01.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alfano C, Studer M. Neocortical arealization: evolution, mechanisms, and open questions. Developmental neurobiology. 2013;73:411–447. doi: 10.1002/dneu.22067. [DOI] [PubMed] [Google Scholar]
- 57.Rowell JJ, Mallik AK, Dugas-Ford J, Ragsdale CW. Molecular analysis of neocortical layer structure in the ferret. The Journal of comparative neurology. 2010;518:3272–3289. doi: 10.1002/cne.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Cabezas MA, Barbas H. Area 4 has layer IV in adult primates. The European journal of neuroscience. 2014;39:1824–1834. doi: 10.1111/ejn.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coogan TA, Burkhalter A. Hierarchical organization of areas in rat visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:3749–3772. doi: 10.1523/JNEUROSCI.13-09-03749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain research. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- 61.Markov NT, Kennedy H. The importance of being hierarchical. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature neuroscience. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 63.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato TR, Svoboda K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4256–4260. doi: 10.1523/JNEUROSCI.3774-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature. 2013;499:336–340. doi: 10.1038/nature12236. [DOI] [PubMed] [Google Scholar]
- 66.Yamashita T, et al. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–1490. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 67.Bureau I, Shepherd GM, Svoboda K. Precise development of functional and anatomical columns in the neocortex. Neuron. 2004;42:789–801. doi: 10.1016/j.neuron.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Staiger JF, Bojak I, Miceli S, Schubert D. A gradual depth-dependent change in connectivity features of supragranular pyramidal cells in rat barrel cortex. Brain structure & function. 2014 doi: 10.1007/s00429-014-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shepherd GMG, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci. 2005;25:5670. doi: 10.1523/JNEUROSCI.1173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson CT, Sheets PL, Kiritani T, Shepherd GMG. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nature neuroscience. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 76.Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8273–8284. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- 78.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 79.Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nature neuroscience. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- 80.Velez-Fort M, et al. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron. 2014;83:1431–1443. doi: 10.1016/j.neuron.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watakabe A, et al. Area-specific substratification of deep layer neurons in the rat cortex. The Journal of comparative neurology. 2012;520:3553–3573. doi: 10.1002/cne.23160. [DOI] [PubMed] [Google Scholar]
- 82.Bai WZ, Ishida M, Arimatsu Y. Chemically defined feedback connections from infragranular layers of sensory association cortices in the rat. Neuroscience. 2004;123:257–267. doi: 10.1016/j.neuroscience.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 83.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 84.Tamamaki N, Tomioka R. Long-Range GABAergic Connections Distributed throughout the Neocortex and their Possible Function. Front Neurosci. 2010;4:202. doi: 10.3389/fnins.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5990–5999. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueta Y, Hirai Y, Otsuka T, Kawaguchi Y. Direction- and distance-dependent interareal connectivity of pyramidal cell subpopulations in the rat frontal cortex. Frontiers in neural circuits. 2013;7:164. doi: 10.3389/fncir.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson A, et al. A circuit for motor cortical modulation of auditory cortical activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14342–14353. doi: 10.1523/JNEUROSCI.2275-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veinante P, Deschenes M. Single-cell study of motor cortex projections to the barrel field in rats. The Journal of comparative neurology. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- 90.Sheets PL, et al. Corticospinal-specific HCN expression in mouse motor cortex: Ih-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. Journal of Neurophysiology. 2011;106:2216–2231. doi: 10.1152/jn.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suter BA, Migliore M, Shepherd GM. Intrinsic electrophysiology of mouse corticospinal neurons: a class-specific triad of spike-related properties. Cerebral cortex. 2013;23:1965–1977. doi: 10.1093/cercor/bhs184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dembrow N, Johnston D. Subcircuit-specific neuromodulation in the prefrontal cortex. Frontiers in neural circuits. 2014;8:54. doi: 10.3389/fncir.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller MN, Okaty BW, Nelson SB. Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13716–13726. doi: 10.1523/JNEUROSCI.2940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tseng GF, Prince DA. Heterogeneity of rat corticospinal neurons. The Journal of comparative neurology. 1993;335:92–108. doi: 10.1002/cne.903350107. [DOI] [PubMed] [Google Scholar]
- 95.Phillips CG, Porter R. Corticospinal neurones: their role in movement. Academic Press; London: 1977. [PubMed] [Google Scholar]
- 96.Christophe E, et al. Two populations of layer v pyramidal cells of the mouse neocortex: development and sensitivity to anesthetics. J Neurophysiol. 2005;94:3357–3367. doi: 10.1152/jn.00076.2005. [DOI] [PubMed] [Google Scholar]
- 97.de Kock CP, Sakmann B. High frequency action potential bursts (>or= 100 Hz) in L2/3 and L5B thick tufted neurons in anaesthetized and awake rat primary somatosensory cortex. J Physiol. 2008;586:3353–3364. doi: 10.1113/jphysiol.2008.155580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beloozerova IN, et al. Activity of different classes of neurons of the motor cortex during postural corrections. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7844–7853. doi: 10.1523/JNEUROSCI.23-21-07844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swadlow HA. Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: receptive fields and axonal properties. J Neurophysiol. 1989;62:288–308. doi: 10.1152/jn.1989.62.1.288. [DOI] [PubMed] [Google Scholar]
- 100.da Costa NM, Martin KA. Selective targeting of the dendrites of corticothalamic cells by thalamic afferents in area 17 of the cat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13919–13928. doi: 10.1523/JNEUROSCI.2785-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang ZW, Deschenes M. Projections to layer VI of the posteromedial barrel field in the rat: a reappraisal of the role of corticothalamic pathways. Cerebral cortex. 1998;8:428–436. doi: 10.1093/cercor/8.5.428. [DOI] [PubMed] [Google Scholar]
- 102.Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 103.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 104.Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bortone DS, Olsen SR, Scanziani M. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron. 2014;82:474–485. doi: 10.1016/j.neuron.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sirota MG, Swadlow HA, Beloozerova IN. Three channels of corticothalamic communication during locomotion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5915–5925. doi: 10.1523/JNEUROSCI.0489-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Markov NT, et al. Cortical high-density counterstream architectures. Science. 2013;342:1238406. doi: 10.1126/science.1238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zingg B, et al. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berezovskii VK, Nassi JJ, Born RT. Segregation of feedforward and feedback projections in mouse visual cortex. The Journal of comparative neurology. 2011;519:3672–3683. doi: 10.1002/cne.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mao T, et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeFelipe J, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature reviews. Neuroscience. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou M, et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nature neuroscience. 2014 doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taniguchi H. Genetic dissection of GABAergic neural circuits in mouse neocortex. Frontiers in cellular neuroscience. 2014;8:8. doi: 10.3389/fncel.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nature neuroscience. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nature neuroscience. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nature neuroscience. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cruikshank SJ, et al. Thalamic control of layer 1 circuits in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu Y, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang S, et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang W, Carrasquillo Y, Hooks BM, Nerbonne JM, Burkhalter A. Distinct balance of excitation and inhibition in an interareal feedforward and feedback circuit of mouse visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17373–17384. doi: 10.1523/JNEUROSCI.2515-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gentet LJ, et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nature neuroscience. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 129.Xu NL, et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- 130.Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M. Integration of visual motion and locomotion in mouse visual cortex. Nature neuroscience. 2013;16:1864–1869. doi: 10.1038/nn.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nature neuroscience. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nature neuroscience. 2009;12:492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deck M, et al. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harwell CC, et al. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73:1116–1126. doi: 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.De la Rossa A, et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nature neuroscience. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- 140.Jensen KF, Killackey HP. Subcortical projections from ectopic neocortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:964–968. doi: 10.1073/pnas.81.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Imai H, Yamamoto T, Katsuyama Y, Kikkawa S, Terashima T. Subcortically and callosally projecting neurons are distinct neuronal pools in the motor cortex of the reeler mouse. The Kobe journal of medical sciences. 2012;58:E86–95. [PubMed] [Google Scholar]
- 142.O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 143.Chou SJ, et al. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. The European journal of neuroscience. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Callaway EM, Borrell V. Developmental sculpting of dendritic morphology of layer 4 neurons in visual cortex: influence of retinal input. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7456–7470. doi: 10.1523/JNEUROSCI.5222-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica) Brain, behavior and evolution. 2009;73:206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Catania KC. Evolution of brains and behavior for optimal foraging: a tale of two predators. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 1):10701–10708. doi: 10.1073/pnas.1201885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hutsler JJ, Lee DG, Porter KK. Comparative analysis of cortical layering and supragranular layer enlargement in rodent carnivore and primate species. Brain research. 2005;1052:71–81. doi: 10.1016/j.brainres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 150.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]