Abstract
Spermatozoa from a number of species can be cryopreserved and then subsequently used to fertilize eggs1. However, this technique has several limitations. First, the freezing protocol varies for each species and must be determined empirically, and for some species appropriate methods have not yet been identified1,2. Second, because these cells are fully differentiated, they will not undergo replication when thawed, and recombination of genetic information cannot occur. We now demonstrate, by using the recently developed spermatogonial transplantation technique3,4, that male germline stem cells can be successfully cryopreserved. Donor testis cells isolated from prepubertal or adult mice and frozen from 4 to 156 days at −196 °C were able to generate spermatogenesis in recipient seminiferous tubules. Relatively standard preservation techniques were used, suggesting that male germ cells from other species can also be stored for long periods. Because transplanted testis stem cells will ultimately undergo replication and meiotic recombination during spermatogenesis, one might consider these preserved male germ lines as biologically immortal.
After birth in mammals, female germline cells do not undergo replication, and their number decreases with age5. However, in the male, germline cell division and spermatogenesis continue throughout most or all of adult life. Spermatogenesis is complex, highly ordered and very productive6,7. At the foundation of this process is the spermatogonial stem cell, which both renews itself and provides a population of cells that increases in number and differentiates to form mature spermatozoa6–8. In most mammals the differentiation process is completed over the course of 30 to 60 days, and the population of differentiating cells increases dramatically. A single rat stem cell is capable of producing 4096 mature spermatozoa6. However, the efficiency is never 100%, and some cellular degeneration typically occurs at each differentiation step6,7.
Surprisingly, there are few reports of efforts to cryopreserve male germline stem cells. Early attempts to freeze testicular tissue met with only limited success (reviewed in ref. 9, 10). However, in one study, cytological evidence of spermatogenesis was observed following transplantation of frozen and thawed immature rat testis pieces to the scrotum of a castrated adult10.
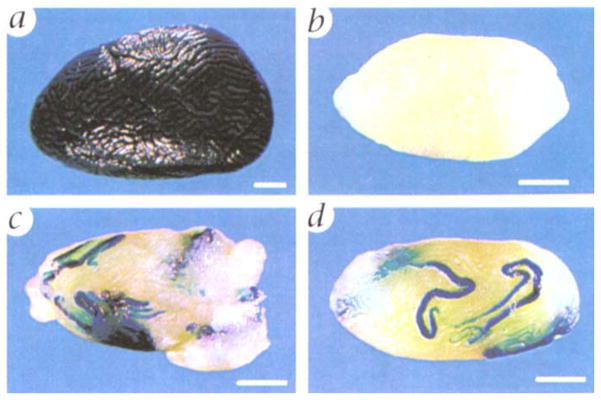
To determine whether male germ cells could be cryopreserved in suspension, testis cells were collected from prepubertal or adult mice carrying a lacZ transgene that allows round spermatids and cells in later steps of spermiogenesis to be stained blue when incubated with X-gal (Fig. 1a)3,4,11. This provides a useful marker of testis cells, because somatic cells do not stain and a single spermatogonial stem cell expands into a large clone of round spermatids6 making identification of stem cell progeny easy and unequivocal3,4. Donor testis cells were collected and frozen slowly at −70 °C, then subsequently stored for varying periods of time at −196 °C, using techniques similar to those generally employed for somatic cells12. The cells were then thawed and transplanted into the seminiferous tubules of recipient mice in which endogenous spermatogenesis had been destroyed by busulfan treatment (Fig. 1b)3,4,13. Following transfer to recipient tubules, these donor cells established spermatogenesis. Areas of the recipient testes populated by cryopreserved donor cells could be readily identified by blue staining (Fig. 1, c and d). Donor cells from both adult (Fig. 1c) and prepubertal (Fig. 1d) animals were effective in colonizing recipient testes. Areas not repopulated by donor cells will not stain blue3,4.
Fig. 1.
Morphology of testes with transplanted stem cells previously stored at −196 °C. a, Testis of donor mouse (designated ZFlacZ), which carries an E. coli β-galactosidase transgene (lacZ) that allows round spermatids and later stages of spermatogenesis to be stained blue following incubation with 5-bromo-4-chloro-3-in-dole-β-D-galactosidase (X-gal)3,4,11. b, Testis of recipient male (C57BL/6 × SJL) F1 treated with busulfan (36 mg/kg) to destroy endogenous spermatogenesis3,4,13. c, Left testis of recipient male 891 (Table 1) that received donor testis cells isolated from adult mice (8–16 weeks of age) and frozen 7 days. The testis has been bisected to allow penetration of fixative and stain, d, Left testis of recipient male 771 (Table 1) that received donor testis cells isolated from prepubertal mice (6–14 days of age) and frozen 111 days. Following incubation with X-gal, blue tubules in c and d identify areas of spermatogenesis from frozen donor cells. Scale bar, 1 mm.
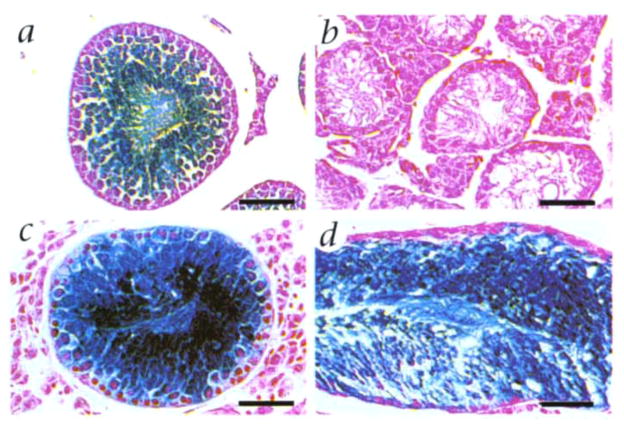
To assess the morphological fidelity of donor cell spermatogenesis, microscopic sections of recipient seminiferous tubules were examined. In donor testes, all spermatogenic stages from round spermatids to mature elongating spermatids were stained blue (Fig. 2a). In busulfan-treated recipient testes, which did not receive transplanted donor cells, the seminiferous tubules lacked germ cell stages and contained only Sertoli cells lining the tubule (Fig. 2b). However, after transplantation of donor cells, blue-stained tubules contained germ cell elements, from spermatogonia to mature spermatozoa (Fig. 2, c and d). The structure and pattern of spermatogenesis resulting from donor cells resembled that seen in control testes (Fig. 2a), and was similar for donor cells from adult (Fig. 2c) and prepubertal (Fig. 2d) mice. Previous studies have demonstrated that spermatozoa produced from transplanted testis cells can fertilize eggs, which subsequently develop into normal offspring4.
Fig. 2.
Microscopic appearance of spermatogenesis in recipient seminiferous tubules following microinjection of donor testis cells preserved at −196 °C. a, Seminiferous tubule of donor testis from transgenic ZFlacZ mouse. Round spermatids and more mature stages stain blue following incubation with X-gal (Fig. 1a)3,4,11. When staining is intense, immature stages also appear blue. b, Seminiferous tubule from recipient mouse treated with busulfan. No germ cell stages are present. Only Sertoli cells remain. c and d, Seminiferous tubules from busul-fan-treated recipient mice 891 and 771, respectively (Table 1). Blue staining of germ cells indicates their origin from transplanted donor cells that had been frozen 7 and 111 days, respectively. Background stain in all sections is neutral fast red. Scale bar, 50 μm.
Four separate experiments, in which testis cells were frozen between 4 and 156 days, are summarized in Table 1. In each experiment, cells stored at −196 °C were able to repopulate recipient testes and to generate normal spermatogenesis in recipients. In total, 22 of 30 (73%) recipient testes showed spermatogenesis from transplanted cells. In successfully reconstituted testes, from 1 to more than 12 seminiferous tubules were colonized. Because of intertwining among the convoluted tubules6,7,14, it was not possible to accurately count more than 12 stained tubules in a single testis. The overall rate of successful colonization was not decreased by the time at −196 °C, because in the least successful experiment the cells were frozen only 4 days. Variation in colonization of recipient testes by donor cells is generally correlated with the number of cells microinjected and the surface area of the testis covered (ref. 4 and unpublished observations). The later parameter provides an estimate of the degree of filling of the seminiferous tubules in the testis.
Table 1.
Spermatogenesis from frozen testis stem cells transplanted to recipient mouse testes
| Experiment | Recipient mouse | Time donor cells frozen (days) | Frozen donor cell concentration (no. × 10−6) | Percent testis surface area covereda (right, left) | Time to analysisb (days) | Number of testis tubules with donor cellsc (right, left) |
|---|---|---|---|---|---|---|
| 1 | 887 | 4 | 28 | 90, 90 | 133 | ≥12, 8 |
| 888 | 4 | 28 | 85, 90 | 133 | 0, 0 | |
| 889 | 4 | 28 | 80, 90 | 133 | 0, 0 | |
| 890 | 4 | 28 | 30, 25 | 133 | 0, 0 | |
| 2 | 891 | 7 | 32 | 80, 80 | 124 | 6, ≥12 |
| 892 | 7 | 32 | 85, 75 | 124 | 5, 3 | |
| 893 | 7 | 32 | 85, 45 | 124 | 3, 1 | |
| 894 | 7 | 32 | 95, 70 | 124 | ≥12, 9 | |
| 3 | 895 | 9, 13 | 46 | 85, 95 | 117 | 8, ≥12 |
| 896 | 9, 13 | 46 | 95, 95 | 117 | ≥12, ≥12 | |
| 897 | 9, 13 | 46 | 55, 80 | 117 | ≥12, ≥12 | |
| 4 | 771 | 111 | 18 | 50, 70 | 108 | 3, 8 |
| 789 | 133 | 12 | 90, 80 | 162 | 0, 5 | |
| 791 | 135 | 15 | 70, 70 | 160 | 4, 0 | |
| 798 | 156 | 11 | 80, 50 | 139 | 7, 5 |
Recipient mice and donor cells described in Fig 1. Busulfan treatment in experiment 1 (33 mg/kg); 2 (35–37 mg/kg), 3 (33–40 mg/kg), 4 (32 mg/kg). Age of males supplying donor cells in experiment 4, 6–14 days; in other experiments, 6–16 weeks. In experiment 3, cells frozen 9 and 13 days were mixed. Presence of spermatogenesis from microinjected frozen donor cells is readily detected by staining (Fig. 1).
Percent of surface seminiferous tubules in recipient testes filled by donor cell suspension4.
Number of days from microinjection of cells to analysis; also time available for spermatogenesis. Mouse spermatogenesis takes 35 days from stem cell to mature spermatozoa6,7.
Number of seminiferous tubules in recipient that stain blue, indicating the presence of spermatogenesis from donor cells. When there are more than 12 tubules stained, total number cannot be accurately determined because tubules are interwoven. Therefore, 12 indicates 12 or more.
Successful cryopreservation of spermatogonial stem cells by routine procedures is surprising considering the difficulty that has been associated with freezing mature spermatozoa1,2. Indeed, it has been difficult to establish a simple, reliable cryopreservation system for mouse spermatozoa2, although the dramatic increase in the number of transgenic mouse lines has stimulated considerable effort in this area. Successful spermatogonial stem cell cryopreservation probably results from the disparate morphologic characteristics of the reproductive cell populations6. During the differentiation process from spermatogonia to spermatozoa, there are major structural modifications, including the loss of cytoplasm and restructuring of nuclear DNA (ref. 15, 16), which may render spermatozoa more sensitive to freezing and thawing. The stem cell appears able to respond to freezing much as a typical somatic cell does and to recover with full functional capability. Given the apparent morphological similarity among spermatogonia of various species6,14,17, we believe that the stem cells of most or all mammalian species can be stored indefinitely at −196 °C, then subsequently used to generate functional spermatogenesis and mature spermatozoa in the appropriate seminiferous tubule microenvironment. A testis of the species of donor cell origin will likely provide the most compatible site for regeneration of spermatogenesis, yet there is evidence that testes of other species may also be suitable as a surrogate host18.
In these experiments, recipient males were maintained only long enough to generate spermatogenesis and mature spermatozoa from transplanted cells. Previous work has shown that donor cell-derived spermatozoa can fertilize eggs effectively. In those studies, 80% of the progeny from one recipient male were shown to carry the donor haplotype4. Although this high level of colonization can be achieved, it is not essential to perpetuate the germ line, because intracytoplasmic injection of a single spermatozoa into the oocyte will result in fertilization and birth of live young in mice and humans19,20. Furthermore, the recent demonstration that intracytoplasmic injection of a round spermatid can produce young means that spermatogenesis in the recipient must only proceed two-thirds of the way to completion21,22. The complicated process of spermatozoa shaping, from round spermatid to mature spermatozoa, that occupies the last one-third of the differentiation pathway is not required if the haploid male germ cell nucleus is injected into the oocyte19–22.
Cryopreservation of spermatogonial stem cells is likely to have far-reaching consequences. Testis cells from unique experimental or livestock animals of any age, from prepuberty to maturity, can now be frozen with the expectation that they will generate spermatogenesis at a later date. The germ line of the animal could then be reclaimed when needed. In addition, identifying the conditions necessary to culture and to modify spermatogonial stem cells before or after freezing will dramatically increase the value of these cells. Thus, this technique is far different than cryopreservation of mature spermatozoa, for which the protocol must be determined for each species and which then preserves only a static cell population no longer able to undergo genetic recombination or proliferation.
One must now begin to address the medical issue of whether males likely to lose germ cells (for example, following chemotherapy) should have testicular biopsy material cryopreserved for possible reintroduction following cessation of treatment. In addition, males with azoospermia may be benefited by this technique. Although mature spermatozoa are often absent in these individuals, or few in number, the stem cells present in the testes may be capable of generating spermatogenesis in a surrogate. Ideally testis cells could be cryopreserved and periodically expanded in number, when appropriate techniques are developed, for introduction into a testis. The recent demonstration that rat spermatogenesis will proceed normally in the seminiferous tubules of an immunodeficient mouse suggests that xenogeneic spermatogonial transplantation may be possible for other species, and thus will facilitate the identification of surrogates18. Probably the widest use of cryopreservation will be for the germ lines of valuable experimental males in research, agriculture animals that die before puberty or are too old to breed yet have valuable germ lines, and males from exotic or endangered species. For example, the genetic diversity of a species with a small number of individuals might be partially protected by cyropreservation of testicular tissue from males of all ages. The possibility of preserving a male germ line indefinitely will be enormously valuable in veterinary medicine as well as human medicine. An important aspect of the technique is that in biological terms cyropreservation of the germ line effectively establishes the potential of generating at any time clones of the original male following spermatogonial transplantation to multiple recipients. When the ability to culture these cells is achieved, the procedure of cryopreservation will have even greater use. The essence of biology is the proliferation and meiotic recombination that occurs in the germ cells, and transplantation, cryopreservation and eventual culture of the spermatogonial stem cell provide a new and unique entry into this fundamental process.
Note added in proof: Using the techniques described above, we have now cryopreserved rat testis cells for up to 56 days, and they subsequently generated rat spermatogenesis when transplanted to the testis of an immunodeficient mouse (xenogeneic spermatogonial transplantation procedure from ref. 18).
Methods
Donor cells were collected by enzymatic digestion of transgenic testes (ref. 23, 24) and were suspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 6 mM lactate, 0.5 mM pyruvate, 30 mg/l penicillin, and 50 mg/l streptomycin (designated DMEM-C); at a concentration of 16–40 × 106 cells/ml. Freezing medium (FBS, DMEM-C, DMSO in a ratio of 1:3:1) was added slowly by drops to equal the original cell volume and mixed25. Cells were dispensed 1.0 ml per freezing vial and placed in an insulated container at −70 °C for at least 12 h and then stored in liquid nitrogen (−196 °C). The cells were thawed by swirling in a 37 °C water bath, and DMEM-C was added slowly by drops to 3 times the volume in the vial. Recovery of cells following freezing ranged from 47% to 61% (mean = 55), and viability of the cells ranged from 50% to 66% (mean = 60). Thus, about one-third of the original cell population survived the procedure. Aggregation of cells during freezing and thawing was the primary cause of cell loss. After thawing, the cells for an experiment were pooled and centrifuged at 600g for 5 min at 16 °C, the supernatant was removed, and the pellet was resuspended in injection medium4. Microinjection of seminiferous tubules with donor cells and analysis of recipient mice was as previously described4.
Acknowledgments
We thank our colleagues for suggestions regarding these studies. In particular we are grateful to L. Russell for advice on the morphology of the spermatogenic process, and to R. Behringer and E. Sandgren for comments on the manuscript. We thank J. Zalles for histologic slide preparation, J. Hayden for assistance with photography and C. Pope for manuscript preparation. Financial support for the work was from the National Institute of Health, US Department of Agriculture, and Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
References
- 1.Hafez ESE. Preservation and cryopreservation of gametes and embryos. In: Hafez ESE, editor. Reproduction in Farm Animals. 6. Lea & Febiger; Philadelphia: 1993. pp. 503–525. [Google Scholar]
- 2.Tada N, et al. Cryopreservation of mouse spermatozoa in the presence of raffinose and glycerol. Reprod Fert. 1990;89:511–516. doi: 10.1530/jrf.0.0890511. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL, Zimmermann JW. Spermatogenesis following male germ cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker TG. Oogenesis and ovarian development. In: Balin H, Glasser S, editors. Reproductive Biology. Excerpta Medica; Amsterdam: 1972. pp. 398–437. [Google Scholar]
- 6.Russell LD, Ettlin RA, Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Cache River Press; Clearwater, Florida: 1990. Mammalian spermatogenesis; pp. 1–40. [Google Scholar]
- 7.Ewing LL, Davis JC, Zirkin BR. Regulation of testicular function: A spatial and temporal view. In: Greep RO, editor. International Review of Physiology. University Park Press; Baltimore: 1980. pp. 41–115. [PubMed] [Google Scholar]
- 8.De Rooij DG, Van Dissel-Emiliani FMF, Van Pelt AMM. Regulation of spermatogonial proliferation. Annu NY Acad Sci. 1992;364:140–153. doi: 10.1111/j.1749-6632.1989.tb25894.x. [DOI] [PubMed] [Google Scholar]
- 9.Gosden RG. Transplantation of ovaries and testes. In: Edwards RG, editor. Fetal Tissue Transplants in Medicine. Cambridge Univ. Press; New York: 1992. pp. 253–280. [Google Scholar]
- 10.Deanesly RJ. Spermatogenesis and endocrine activity in grafts of frozen and thawed rat testis. Endocrinology. 1954;11:201–206. doi: 10.1677/joe.0.0110201. [DOI] [PubMed] [Google Scholar]
- 11.Zambrowicz BP, et al. Expression of a mouse Zfy-1/lacZ transgene in the somatic cells of the embryonic gonad and germ cells of the adult testis. Development. 1994;120:1549–1559. doi: 10.1242/dev.120.6.1549. [DOI] [PubMed] [Google Scholar]
- 12.Freshney RI. Instability, variation, and preservation. In: Freshney RI, editor. Culture of Animal Cells. 3. Wiley-Liss; New York: 1994. pp. 253–266. [Google Scholar]
- 13.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: Cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987;176:259–268. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 14.Dym M. The male reproductive system. In: Weiss L, editor. Histology Cell and Tissue Biology. 5. Elsevier Science Pub. Co; New York: 1993. pp. 1000–1053. [Google Scholar]
- 15.Meistrich ML. Nuclear morphogenesis during spermiogenesis. In: de Kretser D, editor. Molecular Biology of the Male Reproductive System. Academic Press; New York: 1993. pp. 67–97. [Google Scholar]
- 16.Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermiogenesis. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. Oxford Univ. Press; New York: 1993. pp. 332–376. [Google Scholar]
- 17.Setchell BP. Spermatogenesis and spermatozoa. In: Austin CR, Short RV, editors. Reproduction in Mammals: Germ Cells and Fertilization. 2. Cambridge Univ. Press; New York: 1982. pp. 63–101. [Google Scholar]
- 18.Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis. Nature. :381. doi: 10.1038/381418a0. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121:2397–2405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- 20.Aitken RJ, Irvine DS. Fertilization without sperm. Nature. 1996;379:493–495. doi: 10.1038/379493a0. [DOI] [PubMed] [Google Scholar]
- 21.Fishel S, et al. Pregnancy after intracytoplasmic injection of spermatid. Lancet. 1995;345:1641–1642. doi: 10.1016/s0140-6736(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 22.Tesarik J, Mendoza C, Testart J. Viable embryos from injection of round spermatids into oocytes. N Engl J Med. 1995;333:525. doi: 10.1056/NEJM199508243330819. [DOI] [PubMed] [Google Scholar]
- 23.Bellvé AR, et al. Spermatogenic cells of the prepubertal mouse. Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellvé AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- 25.Robertson EJ. Embryo-derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. IRL Press; Oxford: 1987. pp. 71–112. [Google Scholar]