Teratomas, from the Greek work tera meaning monster, are germ cell tumors which contain well-differentiated cells from all 3 germ layers, such as hair, bone, teeth and neurons.1 How germ cells fail to follow their normal developmental program, and instead begin to differentiate into somatic tissues is a fascinating problem that has yet to be solved.
Gametogenesis begins early in embryogenesis when the primordial germ cells are specified as distinct from somatic cells.2 After specification, the primordial germ cells divide mitotically and migrate to the gonad. Once in the gonad, they initiate sex-specific differentiation, eventually leading to the production of either sperm or eggs. In the mouse, sex-specific differences are already detected in the embryonic gonad. In the ovary, germ cells initiate meiosis, then enter meiotic arrest until ovulation. In the testis, germ cells do not initiate meiosis and instead enter G1/G0 mitotic arrest until after birth. As they arrest, germ cells of both sexes also downregulate a set of pluripotency genes, which were necessary for the initial specification and maintenance of primordial germ cell fate (Fig. 1).
Figure 1.
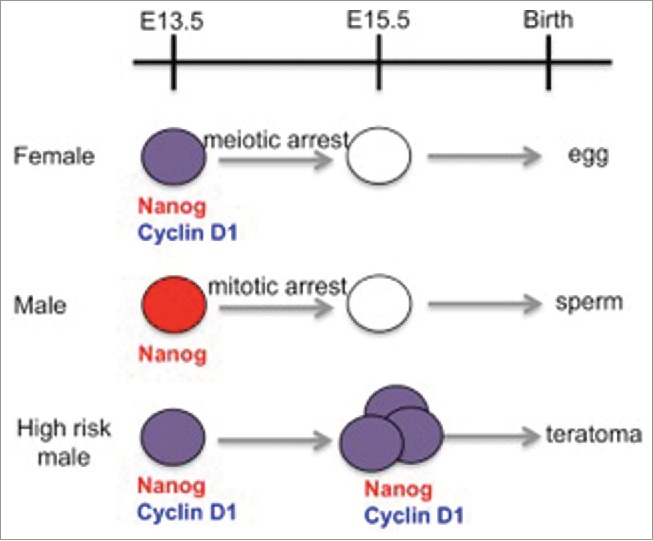
Timeline of germ cell development in the mouse embryo: A comparison of female, male and male germ cells from a high teratoma risk strain. Germ cells from the high risk strain fail to enter cell cycle arrest on schedule, and inappropriately express Nanog (red) and Cyclin D1 (blue). Age is given in days post conception.
It has long been thought that if male embryonic germ cells fail to arrest on schedule they will give rise to testicular teratomas in the adult.1 Studies carried out several years ago provided considerable support for this idea by correlating prolonged germ cell proliferation in the embryo with teratoma incidence in the adult.3,4 Moreover, these proliferating germ cells were found to ectopically express a number of genes, including the Cyclin D1 encoding gene Ccnd1.3,4 Although Cyclin D1 is a known human oncogene,5 the possibility that Cyclin D1 plays a similar role in teratoma initiation in mice has remained untested–until now.
In this volume of Cell Cycle, Jason Heaney and colleagues, including Denise Lanza and Emily Dawson as joint first authors, report that they have unequivocal evidence that aberrant expression of Cyclin D1 is required for teratoma formation.6 To build their case, Lanza et al., first establish that the Cyclin D1 protein is the only aberrantly expressed D-type Cyclin in germ cells that fail to enter cell cycle arrest on schedule. This was done by comparing expression in fetal germ cells from inbred strains of mice with different frequencies of teratoma incidence: a high risk strain (80% of males affected), a low risk strain (8% of male affected) and a completely resistant (i.e. wild type) strain. Next, the authors use a classic genetic suppression assay to establish that ectopic Cyclin D1 expression is what drives the tumor phenotype. They show that removal of the Ccnd1 gene in the high risk strain results in a dramatic reduction in teratoma incidence in the adult. Moreover, in the embryo these germ cells resemble normal male germ cells in that they exit mitosis on schedule, and no longer express the pluripotency marker, Nanog. With these results, Lanza et al., establish that aberrant Cyclin D1 expression plays a large and important role in misdirecting germ cell development.
Intriguing questions remain. How does ectopic Cyclin D1 protein derail germ cell development? Might forcing Cyclin D1 expression in a resistant strain of mice be all that is necessary to misdirect a germ cell into a terminally differentiated somatic cell type? Can the steps in the germ cell to somatic cell conversion pathway be identified? What is the underlying cause of ectopic Cyclin D1 expression in the high risk strain of mice? Cyclin D is normally not expressed in embryonic male germ cells, but it is detected in the adult germline where it is associated with spermatogonial differentiation7–Are the germ cells in this strain attempting to differentiate prematurely? On the other hand, in female embryos Cyclin D1 is expressed in germ cells, where it is limited to the cells about to enter meiosis4–Perhaps the underlying defect in the high risk strain is a failure to maintain male identity? The answers to these and other questions will provide insights into the biology of these curious tumors, an essential first step toward developing new therapies.
References
- [1].Bustamante-Marin X, Garness JA, Capel B. Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology. Int J Dev Biol 2013; 57:201-10; PMID:23784831; http://dx.doi.org/ 10.1387/ijdb.130136bc [DOI] [PubMed] [Google Scholar]
- [2].Lesch BJ, Page DC. Genetics of germ cell development. Nat Rev Genet 2012; 13:781-94; PMID:23044825; http://dx.doi.org/ 10.1038/nrg3294 [DOI] [PubMed] [Google Scholar]
- [3].Cook MS, Munger SC, Nadeau JH, Capel B. Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development 2011; 138:23-32; PMID:21115610; http://dx.doi.org/ 10.1242/dev.057000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heaney JD, Anderson EL, Michelson MV, Zechel JL, Conrad PA, Page DC, Nadeau JH. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development 2012; 139:1577-1586; PMID:22438569; http://dx.doi.org/ 10.1242/dev.076851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011; 11:558-72; PMID:21734724; http://dx.doi.org/ 10.1038/nrc3090 [DOI] [PubMed] [Google Scholar]
- [6].Lanza D, Dawson E, Rao P, Heaney JD. Misexpression of cyclin D1 in embryonic germ cells promotes testicular teratoma initiation. Cell Cycle; 2016; In press; PMID:16931914; http://dx.doi.org/ 10.1080/15384101.2016.1149272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol of Reprod 2000; 63:1893-8; PMID:11090462; http://dx.doi.org/ 10.1095/biolreprod63.6.1893 [DOI] [PubMed] [Google Scholar]


