ABSTRACT
We demonstrate in the present study that young host mice rejuvenate aged hair follicles after transplantation. Young mice promote the hair shaft growth of transplanted old hair follicles, as well as young follicles, in contrast to old host mice, which did not support hair-shaft growth from transplanted old or young follicles. Nestin-expressing hair follicle-associated pluripotent (HAP) stem cells of transplanted old and young hair follicles remained active in young host nude mice. In contrast, the nestin-expressing HAP stem cells in young and old hair follicles transplanted to old nude mice were not as active as in young nude host mice. The present study shows that transplanted old hair follicles were rejuvenated by young host mice, suggesting that aging may be reversible.
KEYWORDS: aged, GFP, growth, hair follicle, hair shaft, mouse, nestin, rejuvenation, stem cell, transplant, young
Introduction
Recently, parabiosis experiments pairing old and young mice have suggested that some features of aging organs in old mice, in particular stem cells, can be reversed by factors in blood of young mice,1 including brain,2,3 spinal cord,4 and heart.5
The hair follicle, which cycles through telogen (resting), anagen (growing), and catagen (regression) throughout the life of mammals, undergoes obvious age-related changes including hair loss.6 The hair follicle contains hair-follicle-associated pluripotent (HAP) stem cells,7-21 which may also deteriorate during aging.
Instead of using complex parabiosis surgery,3 we used hair-follicle subcutaneous transplantation in order to determine if the hair follicle, including its ability to produce hair shafts, and its HAP stem cells, can be rejuvinated.
Results and discussion
Young host nude mice rejuvenate aging hair follicles to regrow long hair shafts
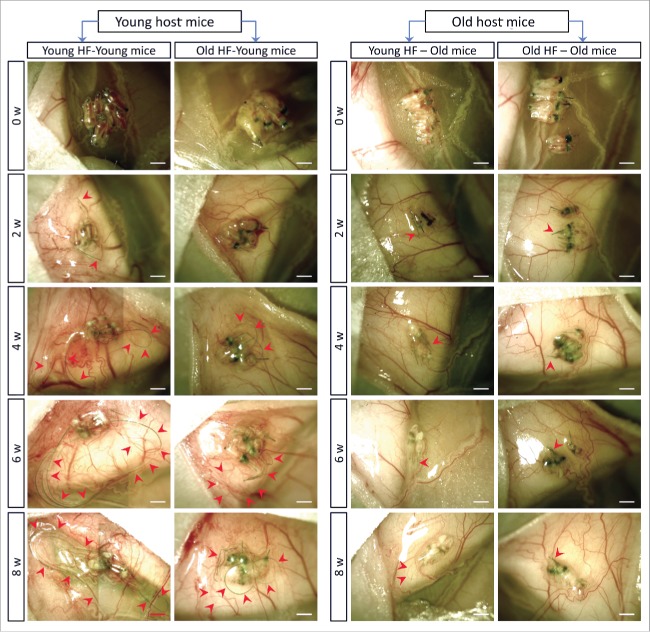
We transplanted young whisker hair follicles subcutaneously into both young and old nude mice. We also transplanted old whisker hair follicles into young and old nude mice (Fig. 1). In young nude mice, the transplanted young hair follicles started to establish blood vessel connections and hair shafts began to grow by week 2, at which time the average length of the 10 longest hair shafts was 1.03 mm ± 0.16 mm. By week 4, the growth rate of the hair shafts began to increase and the average of the 10 longest hair-shaft length was 4.37 mm ± 0.88 mm. By week 6, the hair-shaft length was 5.99 mm ± 1.69 mm, and at week 8, the hair-shaft length was 6.52 mm ± 1.49 mm. The old hair follicles transplanted to young nude mice also established blood vessel connections by week 2, at which time the average length of the 10 longest hair shafts was 0.73 mm ± 0.12 mm. The hair-shaft growth rate increased by week 4, having a hair-shaft length of 1.98 mm ± 0.55 mm and continued to grow at week 6 with a hair-shaft length of 2.70 mm ± 0.81 mm, and at week 8 with a hair-shaft length of 3.62 mm ± 0.59 mm. The growth rate of old hair follicles in young nude mice was somewhat slower than young hair follicles (Figs. 2 and 3).
Figure 1.
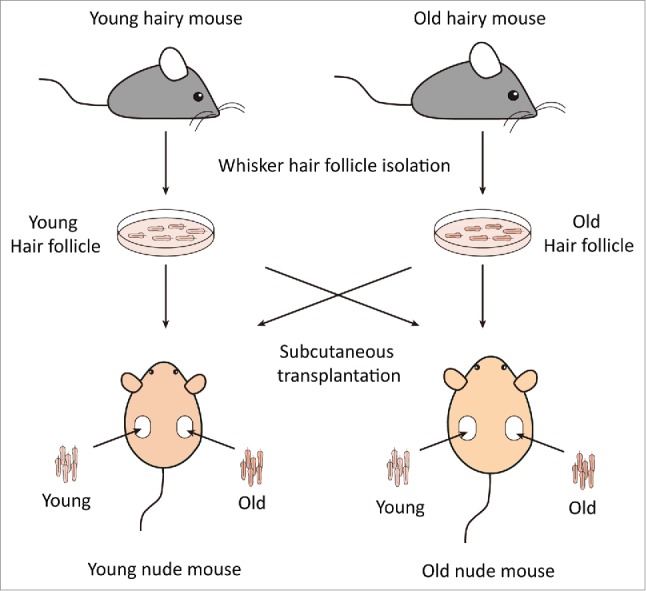
Experimental scheme for subcutaneous transplantation of whisker hair follicles. Whisker hair follicles were first isolated from both young and old nestin-driven green fluorescent protein (ND-GFP) transgenic hairy mice and placed into culture medium. Both young and old hair follicles were subsequently transplanted into the subcutis on both flanks of young and old non-transgenic nude mice. Please see the Materials and Methods for details.
Figure 2.
Comparsion of hair-shaft growth from young and old hair follicles transplanted in young or old nude mice. Whisker follicles were isolated from young and old ND-GFP transgenic hairy mice and transplanted to young and old non-transgenic nude mice. Hair-shaft length was measured with the Dino-Lite microscope imager. Please see Materials and Methods for details.
Figure 3.
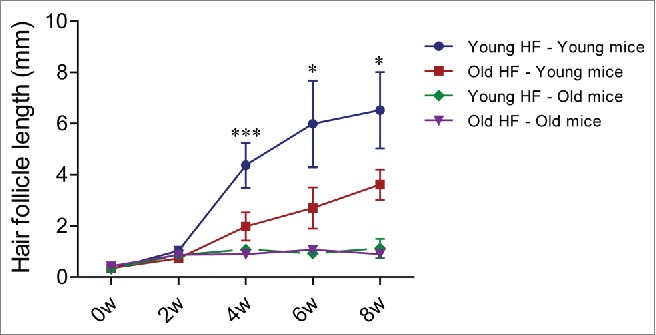
Quantitative time-course growth data of the average of the 10 longest hair shafts in the different groups after hair-follicle subcutaneous transplantation. Please see legend to Figure 2 for details.
In contrast, in old nude mice, both transplanted young and old hair follicles failed to regrow extensive hair shafts (Figs. 2 and 3). At week 2 and week 4, both the young and old transplanted hair follicles had less blood vessel connections with old host mice, in contrast to blood vessel connections in young mice.
Therefore, our results showed that both young and old hair follicles can regrow extensive hair shafts when transplanted to young nude mice, while neither young nor old hair follicles can regrow extensive hair shafts when transplanted to old nude mice. These results suggest that young nude mice can provide a more suitable environment to subcutaneously-transplanted hair follicles, both young and old, than old nude host mice. These results also suggest a large influence of the host nude on the donated hair follicles, due to the fact that both young and old hair follicles fail to regrow long hair shafts in old host mice. Old hair follicles had the capability to regrow long hair shafts when transplanted to young host mice, suggesting that old hair follicles can be rejuvenated by young host mice.
Hair follicle-associated pluripotent (HAP) stem cell behavior in transplanted follicles in young and old mice
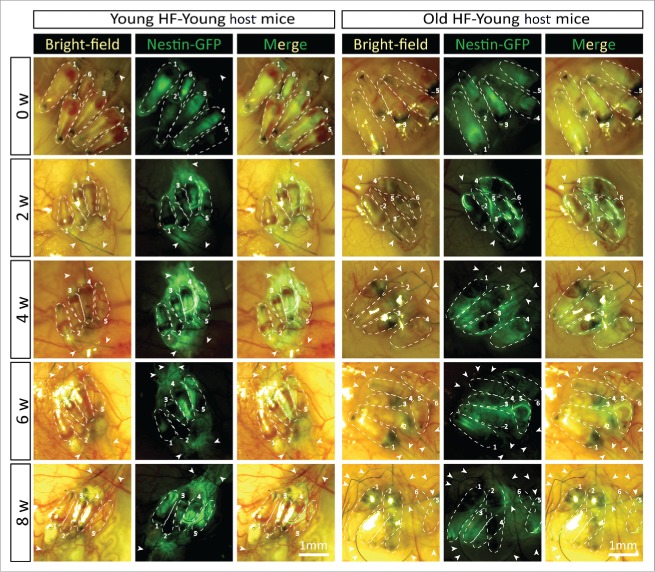
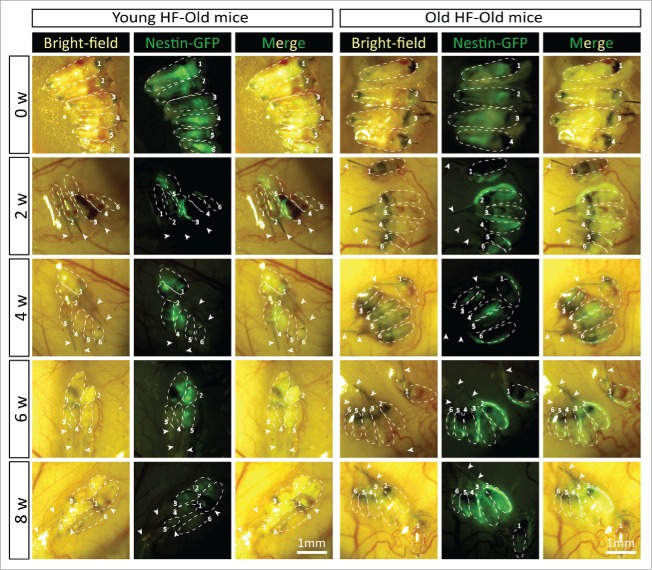
In young nude host mice, HAP stem cells in the transplanted follicles were active throughout the 8-week experimental period as can be seen by their expression of nestin-driven green fluorescent protein (ND-GFP). ND-GFP expressing cells were widely distributed in both young and old hair follicles transplanted to young host nude mice. HAP stem cells were located in various areas of the follicle, including the follicle sensory nerve, hair matrix bulb and outer-root sheath. HAP stem cells surrounded the hair bulb at week 8 suggesting their role in hair-shaft regrowth (Fig. 4). In old hair follicles of old nude mice, most of the ND-GFP expressing cells were located in the attached sensory nerves but not in the center of the hair follicle as they were in young follicles transplanted to young mice (Fig. 5). Thus, the subcutaneous environment has a strong influence on the HAP stem cells of young and old hair follicles.
Figure 4.
Time-course comparison of ND-GFP fluorescence of HAP stem cells and their location in young and old hair follicles transplanted to young nude host mice. ND-GFP expressing HAP stem cells were located in various areas: sensory nerve, hair matrix bulb area, and outer-root sheath area. HAP stem cell ND-GFP fluorescence was imaged with the Dino-Lite. In the fluorescence images, each follicle is outlined with a dashed line and numbered for comparison with the brightfield images where the follicles are numbered. Please see the Materials and Methods for details.
Figure 5.
Time-course comparison of ND-GFP fluorescence of HAP stem cells in young and old hair follicles transplanted to old nude host mice. After subcutaneous transplantation. ND-GFP-expressing HAP stem cells in young hair follicles of old nude mice were located mainly in the center of hair follicles (arrows), while in old hair follicles, the ND-GFP expressing HAP stem cells were located mainly in the attached sensory nerves (arrow). HAP stem cell ND-GFP fluorescence was imaged with the Dino-Lite. In the fluorescence images, each follicle is outlined with a dashed line and are also numbered for comparison with the brightfield images where the follicles are numbered. Please see the Materials and Methods for details.
Recent results have shown that young blood rejuvenates aging organs, possibly via stem cells.1 Similarly, the young subcutaneous environment stimulates or rejuvenates hair-shaft growth in old follicles, also possibly via stem cells. We observed that both young and old hair follicles established blood vessel connections within 2 weeks after transplantation to young mice (Fig. 2). In contrast, in old nude mice, both young and old hair follicles failed to establish sufficient blood vessel connections. This result indicated that hair-shaft regrowth failure of transplanted hair follicles in old host mice may be due to a deficiency in angiogenesis in old nude mice.
The rejuvenated hair shaft regrowth capability of aging hair follicles effected by subcutaneous transplantation into young nude mice suggests that the aging hair loss (alopecia) may be reversible. Furthermore, this hair follicle aging model is a relatively short-term experiment compared to other life-long aging experiments and an efficient tool to test hair-growth promoting drugs.
Circadian-clock genes have been shown to regulate the hair growth cycle, possibly by modulating the cell cycle in the secondary hair germ.22 The circadian genes may play a role in aging of the hair follicle which is manifested in hair loss and color.23 TP53 can convert CDKN1A (p21CIP1)-induced irreversible senescence into reversible quiescence possibly by inhibiting the mechanistic target of rapamycin (MTOR).24,25 High doses of doxorubicin and nutlin-3a, an inhibitor of the interaction between TP53 and its major negative regulator HDM2, promoted reversible quiescence, instead of irreversible senescence.25,26 MTOR inhibitors, such as rapamycin, may be able to rejuvenate stem cells within the aging hair follicle, as well as restore the responsiveness of the aging stem cells to stimuli.27 Future experiments will also focus on the role of stem cells in the aging hair follicle and pharmaceutical rejuvenation of this process, such as with rapamycin.
Materials and methods
Mice
Nestin-driven green fluorescent protein (ND-GFP) transgenic immunocompetent mice and non-transgenic nude mice (AntiCancer, Inc., San Diego, CA), at different ages, were used. ND-GFP is expressed in the hair-follicle associated pluripotent (HAP) stem cells.7,28 Mice were kept in a barrier facility under HEPA filtration. Mice were fed with an autoclaved laboratory rodent diet. All mouse surgical procedures and imaging were performed with the animals anesthetized by subcutaneous injection of a ketamine mixture (0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1, as described above.
Isolation and subcutaneous transplantation of whisker hair follicles
Mice were anesthetized with the ketamine mixture described above. The whisker pad of the nestin-GFP transgenic mice was removed, and its inner surface was exposed and dissected under a binocular microscope with a small scissors to separate each follicle. The isolated follicles were quickly washed in PBS for 2 seconds and maintained in DMEM (GIBCO Life Technologies, Grand Island, NY) for transplantation.
Both young and old hair follicles were then transplanted into the subcutis on both flanks of young and old nude mice (Fig. 1). An “L” shape incision in the skin on the back of nude mice was made using fine scissors and the hair follicle was carefully inserted under the skin. On day-0, after obtaining images with the portable Dino-Lite microscope imager (AM4113TGFBW Dino-Lite Premier; AnMo Electronics Corporation, Taiwan),29 the skin was closed with a 6–0 suture. The host young nude mice were approximately 4 weeks old, and aging host nude mice were approximately 7.5 months old. Donor young hairy ND-GFP mice were approximately 6 weeks old, while the donor aging hairy ND-GFP mice were 14 months old. Four groups were set up as follows: young transplanted hair follicle – young host nude mice group (Young HF-Young mice); old transplanted hair follicle – young host nude mice (Old HF-Young mice); young transplanted hair follicle – old host nude mice (Young HF-Old mice); and old transplanted hair follicle – old host nude mice (Old HF-Old mice). Each group consisted of 17-20 hair follicles. The skin flap, which contained the transplanted hair follicles was exposed and observed every 2 weeks (0, 2, 4, 6, and 8 weeks). The transplanted hair follicles were exposed by making a larger “L” shape incision around the edge of the scar in the skin which resulted from transplantation of the hair follicles as described above, on the back of nude mice, and avoiding the hair follicles and connected blood vessels associated with them. The skin was lifted and the skin-flap opened in order to obtain images with the portable Dino-Lite microscope imager. After obtaining the images, the incision was closed with a 6–0 suture.
Measurement of hair shaft length
In vivo hair-follicle images, obtained with the Dino-Lite, were used to determine the length of each hair shaft using Image Pro Plus 6.0 software. The average length of the 10 longest hair shafts in each group is presented as the mean ± SEM. Group differences were obtained using the ANOVA test. The significance level for all tests was P < 0.05.
Dedication
This paper is dedicated to the memory of A.R. Moossa, M.D.
Funding
The present work was supported in part by the National Institute of Neurological Disorders and Stroke grant NS086217 and the National Natural Science Foundation of China (#81571211) and Shanghai Natural Science Foundation (#14ZR1449300).
References
- [1].Scudellari M. Aging research: Blood to blood. Nature 2015; 517:426-9; PMID:25612035; http://dx.doi.org/ 10.1038/517426a [DOI] [PubMed] [Google Scholar]
- [2].Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014; 344:630-4; PMID:24797482; http://dx.doi.org/ 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al.. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 2014; 20:659-63; PMID:24793238; http://dx.doi.org/ 10.1038/nm.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012; 10:96-103; PMID:22226359; http://dx.doi.org/ 10.1016/j.stem.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, et al.. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013; 153:828-39; PMID:23663781; http://dx.doi.org/ 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Paus R, Cotsarelis G. The biology of hair follicles. N Eng J Med 1999; 341:491-7; http://dx.doi.org/ 10.1056/NEJM199908123410706 [DOI] [PubMed] [Google Scholar]
- [7].Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 2003; 100:9958-61; PMID:12904579;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair follicle cells. Proc Natl Acad Sci USA 2004; 101:13291-5; PMID:15331785; http://dx.doi.org/ 10.1073/pnas.0405250101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005; 102:5530-4; PMID:15802470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA 2005; 102:17734-8; PMID:16314569; http://dx.doi.org/ 10.1073/pnas.0508440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008; 7:1865-9; PMID:18583926; http://dx.doi.org/ 10.4161/cc.7.12.6056 [DOI] [PubMed] [Google Scholar]
- [12].Amoh Y, Mii S, Aki R, Hamada Y, Kawahara K, Hoffman RM, Katsuoka K. Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle and lower part of the vibrissa hair follicle. Cell Cycle 2012; 11:3513-7; PMID:22918245; http://dx.doi.org/ 10.4161/cc.21803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem 2009; 107:1016-20; PMID:19507228; http://dx.doi.org/ 10.1002/jcb.22204 [DOI] [PubMed] [Google Scholar]
- [14].Amoh Y, Hamada Y, Aki R, Kawahara K, Hoffman RM, Katsuoka K. Direct transplantation of uncultured hair-follicle pluripotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. J Cell Biochem 2010; 110:272-7; PMID:20411592 [DOI] [PubMed] [Google Scholar]
- [15].Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, et al.. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 2011; 10:830-9; PMID:21330787; http://dx.doi.org/ 10.4161/cc.10.5.14969 [DOI] [PubMed] [Google Scholar]
- [16].Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 2011; 112:2046-50; PMID:21465525; http://dx.doi.org/ 10.1002/jcb.23122 [DOI] [PubMed] [Google Scholar]
- [17].Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 2013; 114:1674-84; PMID:23444061; http://dx.doi.org/ 10.1002/jcb.24509 [DOI] [PubMed] [Google Scholar]
- [18].Mii S, Uehara F, Yano S, Tran B, Miwa S, Hiroshima Y, Amoh Y, Katsuoka K, Hoffman RM. Nestin-expressing stem cells promote nerve growth in long-term 3-dimensional Gelfoam®-supported histoculture. PLoS One 2013; 8:e67153; PMID:23840607; http://dx.doi.org/ 10.1371/journal.pone.0067153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duong J, Mii S, Uchugonova A, Liu F, Moossa AR, Hoffman RM. Real-time confocal imaging of trafficking of nestin-expressing multipotent stem cells in mouse whiskers in long-term 3-D histoculture. In Vitro Cell Dev Biol Anim 2012; 48:301-5; PMID:22580909; http://dx.doi.org/ 10.1007/s11626-012-9514-z [DOI] [PubMed] [Google Scholar]
- [20].Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 2015; 14, 2362-6; PMID:25970547; http://dx.doi.org/ 10.1080/15384101.2015.1042633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Li L, Katsuoka K, Hoffman RM, Amoh Y. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle-associated pluripotent stem cells. Tissue Eng Part C 2015; 21:825-31; PMID:25743086; http://dx.doi.org/ 10.1089/ten.tec.2014.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, Paus R, Takahashi JS, Andersen B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet 2009; 5:e1000573; PMID:19629164; http://dx.doi.org/ 10.1371/journal.pgen.1000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geyfman M, Andersen B. Clock genes, hair growth and aging. Aging (Albany NY) 2010; 2:122-8; PMID:20375466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA 2010; 107:9660-4; PMID:20457898; http://dx.doi.org/ 10.1073/pnas.1002298107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Galluzzi L, Kepp O, Kroemer G. TP53 and MTOR crosstalk to regulate cellular senescence. Aging (Albany NY) 2010; 2:535-7; PMID:20876940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leontieva OV, Gudkov AV, Blagosklonny MV. Weak p53 permits senescence during cell cycle arrest. Cell Cycle 2010; 9:4323-7; PMID:21051933; http://dx.doi.org/ 10.4161/cc.9.21.13584 [DOI] [PubMed] [Google Scholar]
- [27].Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res 2008; 11:801-8; PMID:18729812; http://dx.doi.org/ 10.1089/rej.2008.0722 [DOI] [PubMed] [Google Scholar]
- [28].Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, Keyes WM, Mills AA, Gleiberman A, Zhang MQ, et al.. Neural potential of a stem cell population in the hair follicle. Cell Cycle 2007; 6:2161-70; PMID:17873521; http://dx.doi.org/ 10.4161/cc.6.17.4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hiroshima Y, Maawy A, Sato S, Murakami T, Uehara F, Miwa S, Yano S, Momiyama M, Chishima T, Tanaka K, et al.. Hand-held high-resolution fluorescence imaging system for fluorescence-guided surgery of patient and cell-line pancreatic tumors growing orthotopically in nude mice. J Surg Res 2014; 187:510-7; PMID:24373959; http://dx.doi.org/ 10.1016/j.jss.2013.11.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]