Quiescence, self-renewal, and differentiation are essential properties of all adult stem cells that ultimately allow tissue homeostasis. Within the bone marrow (BM), haematopoietic stem cells (HSC), which are responsible for producing all blood elements, find an adequate microenvironment where these properties are finely tuned to support the long-term maintenance and function of HSC.1 Technical advances over the last decade have allowed identifying the critical components of this haematopoietic environment, and have validated and refined the classic concept of the niche: a local tissue environment that directly maintains and regulates a particular kind of stem cell or progenitor.1 Perivascular stromal cells and endothelial cells have been shown to be critical for maintaining HSC through secretion of factors that globally support a proliferative status of HSC. For example, non-myelinating Schwann cells induce quiescence through the anti-proliferative cytokine transforming growth factor β-1 (TGF-β), and megakaryocytes, a mature haematopoietic cell type, maintain HSC quiescence by producing TGF-β, thrombopoietin or PF4. Additionally, the endothelium is able to promote HSC proliferation through interactions mediated by E-selectin, a receptor classically involved in leukocyte trafficking (reviewed in ref. 1).
Traditionally, the model of HSC niche has been unidirectional, such that only parenchymal elements in the BM exerted a control over HSC. In this unidirectional model, perturbations in stromal cells of the niche ultimately influenced HSC behavior. It remained unclear, however, whether the stromal niche (or the niche as a whole) was reciprocally modulated by the HSC. Previous studies noted, however, that alterations in the BM microenvironment appeared with hematological malignancies. Remodeling of the BM niche by leukemic stem cells reinforced a leukemia-supporting niche,2 a property that has emerged as a critical event in the development of blood neoplasms. One of the key factors in this remodeling is the aberrant secretion of cytokines, such as thrombopoietin or CCL3, by the leukemic blasts. Using a completely different system, Zon and colleagues used live imaging in the zebrafish to demonstrate that the haematopoietic niche “reacts” to the arrival of HSC3 by triggering the remodeling of endothelial cells around the incoming HSC, leading to the formation of a cellular pocket. These series of studies have given strength to the possibility that the primitive haematopoietic compartment, leukemic or healthy, can influence their own environment.
Cytokines produced by many cell types have typically a broad range of actions and can act in an autocrine or a paracrine manner. Ironically, while this has been extensively studied in the stromal compartment of the niche, the fact that HSC and haematopoietic progenitors also produce a large variety of cytokines has been largely disregarded. A remarkable example of a cytokine with autocrine functions is TGF-β,4 which triggers potent anti-proliferative effects on HSC but can also suppresses expression of critical niche factors by stromal cells. A key issue to understanding stem cell regulation is therefore how the production of cytokines like TGF-β is regulated in the complex multicellular environment of the BM, and what are the type of cells that produce them. Our own work has revealed that the BM microenvironment can be regulated by HSC and its more mature progeny, and that this control occurs by modulating the secretion of TGF-β.5 Dysregulated production of TGF-β by primitive progenitors affected not only stromal components of the niche but also the whole population of HSC, indicating that cytokine-producing precursors are bona fide components of the BM microenvironment. The question then is how do HSC limit TGF-β release at the core of the haematopoietic niche?
While searching for the E-selectin ligand on HSC that transduced its proliferative effects in HSC we discovered that deficiency in one such ligand, E-selectin ligand-1 (ESL-1), reproduced the proliferative defects seen in mice deficient in E-selectin. Unexpectedly, transplantation of HSC deficient in this ligand demonstrated that the proliferative arrest was transmitted to wild-type progenitors that shared the same environment. Previous studies had demonstrated that ESL-1 sequestered TGF-β at the Golgi and prevented its secretion,5 which led us to discover that this glycoprotein regulated HSC proliferation not by binding E-selectin, but rather by limiting the levels of this cytokine in the BM milieu. Several niche cells that produce CXCL12, including reticular cells (CAR cells) and endothelial cells, were significantly less frequent when ESL1-deficient HSC were present in the BM, whereas osteoblasts, a different type of niche cell, were resistant to the absence of ESL-1, suggesting that the effects of TGF-β in the niche had predilection for certain microenvironments. The dominant effect of ESL-1 mutant precursors over neighboring wild-type progenitors was also reproducible in vitro, when no other cell types were present, demonstrating that this phenomenon accounted for direct autocrine and paracrine repression of HSC proliferation (Fig. 1).5
Figure 1.
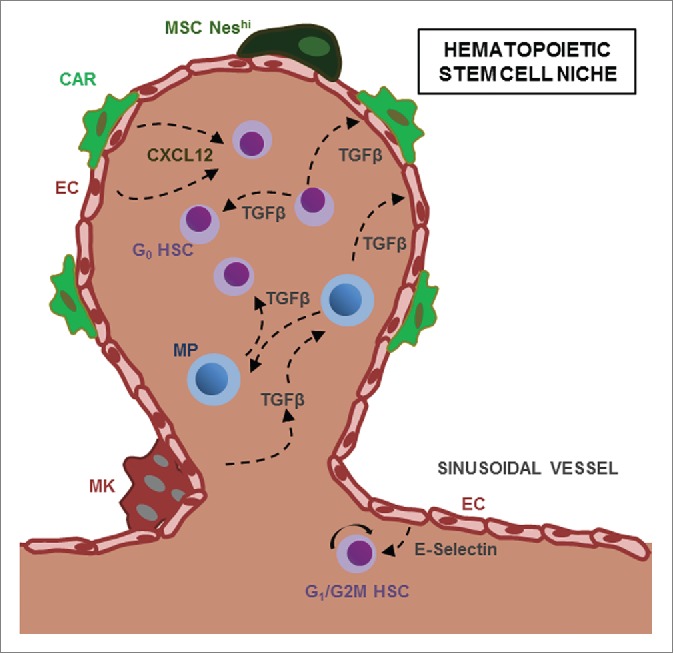
Bidirectional crosstalk between HSC and their microenvironment. HSC and Myeloid Progenitors (MP) are found in perivascular structures and are regulated by cytokines such as CXCL12 or TGF-β secreted by niche cells, including NestinHI mesenchymal stem cells (NesHI MSC), CXCL12-abundant reticular (CAR) cells and megakaryocytes (MK). HSC and MP can reciprocally regulate neighboring progenitors and stromal niche cells through TGF-β.
The relevance of this proximity-based regulation, however, would be negligible in the “vast” space of the BM unless HSC clustered in certain areas. Such type of areas, named hemospheres, has been recently described and shown to contain microvessels, stromal cells, multiple HSC and a small fraction of haematopoietic progenitors.6 It is therefore conceivable that HSC within these clusters control their neighbor HSC through the secretion of cytokines or other regulatory proteins. Interestingly, these findings further complement the recent identification of a different haematopoietic -derived factor, Angiopoietin-1, that maintains HSC quiescence and promotes regeneration of the vascular niche after irradiation.7
While these observations are very recent and the number of identified factors that mediate this HSC-mediated niche regulation is still small, we propose that identification of the molecules involved in this reciprocal crosstalk should dramatically improve our understanding of how the HSC niche is functionally organized. The concept of HSC as modulators of their own niche can further pave the way to design new therapies using genetically modified HSC to treat hematological malignancies, or even new pharmacological approaches to improve engraftment in BM transplantation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Morrison SJ, Scadden DT. Nature 2014; 505(7483):327-34; PMID:24429631; http://dx.doi.org/ 10.1038/nature12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schepers K, et al. Cell Stem Cell 2013; 13(3):285-99; PMID:23850243; http://dx.doi.org/ 10.1016/j.stem.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamplin OJ, et al. Cell 2015; 160(1–2):241-52; PMID:25594182; http://dx.doi.org/ 10.1016/j.cell.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hatzfeld J, et al. J Exp Med 1991; 174(4):925-9; PMID:1717634; http://dx.doi.org/ 10.1084/jem.174.4.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leiva M, et al. Nat Commun 2016; 7:10222; PMID:26742601; http://dx.doi.org/ 10.1038/ncomms10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang L, et al. EMBO J 2013; 32(2):219-30; PMID:23188081; http://dx.doi.org/ 10.1038/emboj.2012.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou BO, et al. Elife 2015; 4:e05521; PMID:25821987 [DOI] [PMC free article] [PubMed] [Google Scholar]


