ABSTRACT
Epidermal cells are an important regenerative source for skin wound healing. Aged epidermal cells have a low ability to renew themselves and repair skin injury. Ultraviolet (UV) radiation, particularly UVB, can cause photo-aging of the skin by suppressing the viability of human epidermal cells. A chorion-derived stem cell conditioned medium (CDSC-CNM) is thought to have regenerative properties. This study aimed to determine the regenerative effects of CDSC-CNM on UVB-induced photo-aged epidermal cells. Epidermal cells were passaged four times and irradiated with quantitative UVB, and non-irradiated cells served as a control group. Cells were then treated with different concentrations of CDSC-CNM. Compared to the non-irradiated group, the proliferation rates and migration rates of UVB-induced photo-aged epidermal cells significantly decreased (p < 0.05) with increasing intracellular radical oxygen species (ROS) generation and DNA damage. After treatment with CDSC-CNM, photo-aged epidermal cells significantly improved their viability, and their ROS generation and DNA damage decreased. The secretory factors in CDSC-CNM, including epidermal growth factor (EGF), transforming growth factor-β (TGF-β), interleukin (IL)-6, and IL-8 and the related signaling pathway protein levels, increased compared to the control medium (CM). The potential regenerative and reparative effects of CDSC-CNM indicate that it may be a candidate material for the treatment of prematurely aged skin. The functions of the secretory factors and the mechanisms of CDSC-CNM therapy deserve further attention.
KEYWORDS: cell cycle, DNA damage, epidermal cell, photo-aging, ROS
Introduction
Epidermal cells are an important regenerative source for skin wound healing. Aged epidermal cells have a low ability to renew themselves and repair skin injury. Photo-aging is the superimposition of chronic UV-induced damage on intrinsic skin aging and accounts for most age-related changes in skin appearance.1 UV radiation from the sun induces several harmful responses, including erythema, edema, sunburn, wrinkles, hyper-pigmentation, immunosuppression and even skin cancer.2 Generally speaking, UV contains UVA (320 – 400 nm), UVB (280 – 320 nm), UVC (200 – 280 nm) and VUV (vacuum UV, 100 – 200 nm). Although VUV and UVC are absorbed by oxygen and the ozone sphere, UVB and UVA reach our skin and contribute significantly to photo-aging. Short wavelength ultraviolet radiation (UVB) injures the epidermis, and longer wavelength UV radiation (UVA) penetrates to the dermis. Although UVA accounts for more than 90% of the total UV radiation and is constant throughout the year, UVB photons are one thousand times more capable of causing sunburn than UVA and cause skin photo-aging by suppressing the viability of human epidermal cells.3,4
Photo-aging is defined as the accelerated aging of the skin from exposure to sunlight. It causes fine lines, discoloration and stratum corneum thickening. These changes are mostly triggered by increased cellular ROS and ultimately induce mitochondrial DNA deletions with extracellular matrix degradation.5 Various methods have been developed to inhibit UV damage to human skin, including plant compounds, fillers of autologous graft and botox injections,6-8 but their therapeutic efficacy and safety are not always satisfactory. The secretory factors of adipose- and bone marrow-derived stem cells have also been used to treat wrinkles in prematurely aging skin.9-11 However, there is a lack of studies on the photo-aging reparative potential of chorion-derived stem cells (CDSCs) isolated from the human placenta. The placenta is the nutrition source for fetal development, and recent reports revealed the presence of abundant growth factors in the supernatant of cells from the placenta, including basic fibroblast growth factor (b-FGF), EGF and TGF-β.12,13 These cytokines are known to have regenerative properties in wound healing. Moreover, mitogen-activated protein kinases (MAPKs) are members of the serine/threonine kinase family and include p38 MAPK, c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinases 1 and 2 (Erk1/2). MAPKs are activated by external stress stimuli, such as heat shock, cytokines, and UV radiation, and are involved in cellular proliferation, survival, and apoptosis. UVB radiation triggers apoptosis in human keratinocytes and is mediated by several cellular pathways, including MAPK-regulated signaling pathways14 and, to a large extent, the Bcl-2/Mcl-1-inhibitable process.15-17 The Erk signaling pathway plays a crucial role in regulating normal cell proliferation, survival, and differentiation.18,19
To obtain evidence of the regenerative and reparative effects of CDSC-CNM and to further understand the mechanism underlying the protective effect of CDSC-CNM against UVB-induced skin photo-aging, we examined the cell vitality, ROS formation and DNA damage of photo-aged epidermal cells. Secretory CDSC factors and the protein levels of related signaling pathways in UVB-irradiated keratinocytes after CDSC-CNM treatment were assessed.
Results
CDSC characteristics
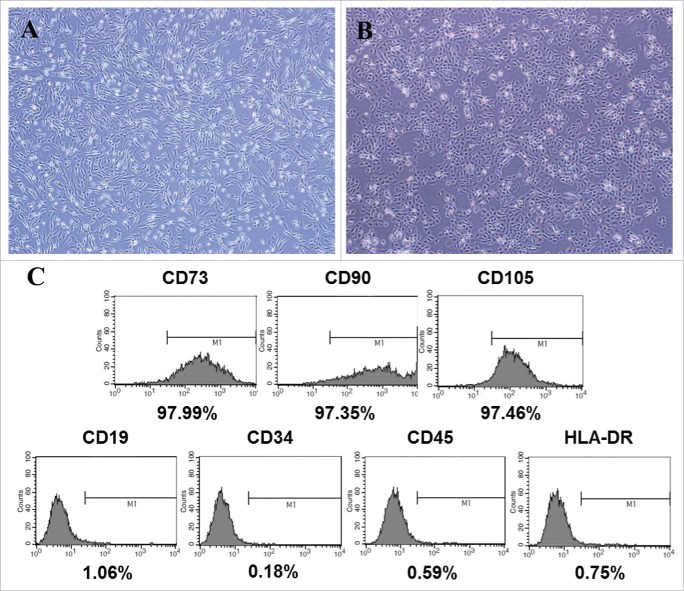
CDSCs have a fibroblast-like morphology (Fig. 1A). Flow cytometry revealed that CDSCs expressed a specific mesenchymal stromal cell (MSC) phenotype in which cells were positive for CD73, CD90 and CD105 and negative for CD19, CD34, CD45 and HLA-DR (Fig. 1C).
Figure 1.
Characteristics of MSCs derived from the placenta. (A) Morphology of MSCs derived from the placenta. The morphology of CDSCs exhibited the typical fibroblast-like morphology of MSCs. (B) Morphology of normal HaCaT cells cultured in a growth medium. (C) Flow cytometric analysis revealed that CDSCs were positive for specific MSC phenotype markers (CD73, CD90 and CD105) and negative for MSC phenotype markers (CD19, CD34, CD45 and HLA-DR).
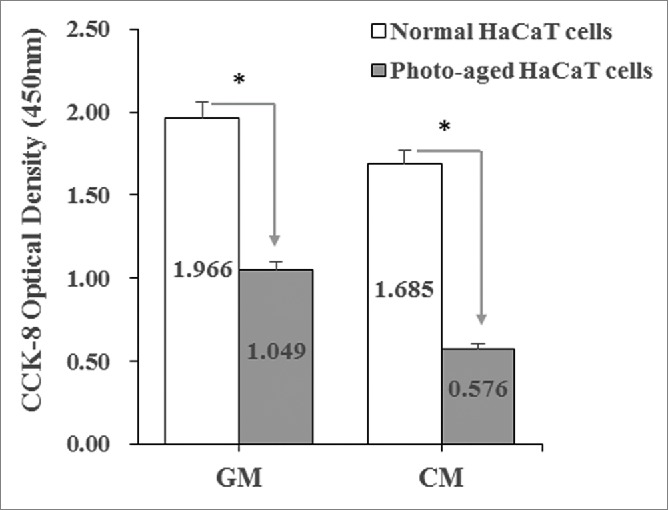
Photo-aged HaCaT cells showed decreased proliferation activities
Normal human immortalized keratinocytes (HaCaT cells) were cultured in a growth medium (GM) (Fig. 1B). The GM was consisted of DMEM with 10% FBS and 1% penicillin–streptomycin (0.1 mg/mL penicillin and 100 U/mL streptomycin). The formation of photo-aged HaCaT cells induced by UVB was confirmed by significantly decreasing cellular proliferation rates (Fig. 2) compared with normal keratinocytes cultured in either GM or CM. The CM was consisted of DMEM with 1% FBS and 1% penicillin–streptomycin.
Figure 2.
The formation of photo-aged HaCaT cells induced by UVB was confirmed. Proliferation rates of photo-aged HaCaT cells decreased compared with normal keratinocytes cultured in a growth medium (GM) or control medium (CM).
CDSC-CNM improved cellular proliferation rates of photo-aged HaCaT cells
The proliferation rates of photo-aged HaCaT cells cultured in CDSC-CNM significantly increased compared with those cultured in the CM. The proliferation rates were highest for photo-aged HaCaT cells cultured in 75% CDSC-CNM (Fig. 3), even though this proliferation rate was lower than that of normal HaCaT cells cultured in CM.
Figure 3.
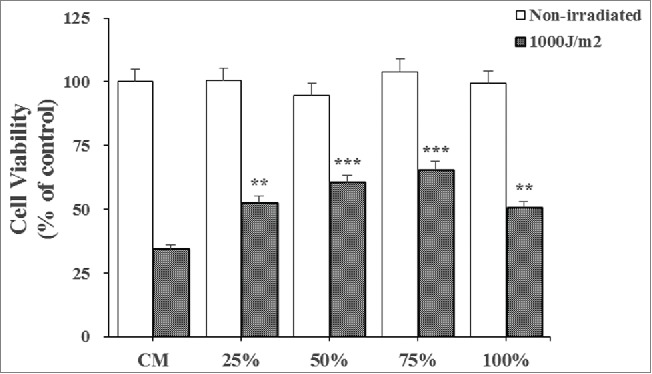
Effects of the chorion-derived stem cell conditioned medium (CDSC-CNM) on photo-aged HaCaT cell proliferation. To determine the proliferation rates, photo-aged HaCaT cells were cultured with different concentrations of CDSC-CNM for 72 h. Photo-aged HaCaT cells were cultured in CM as a control. The data are expressed as the mean of 6–8 replicates ± SD. * (P < 0.05), ** (P < 0.01) and *** (P < 0.001) indicate significant differences compared with non-irradiated cells in the same concentration of CDSC-CNM.
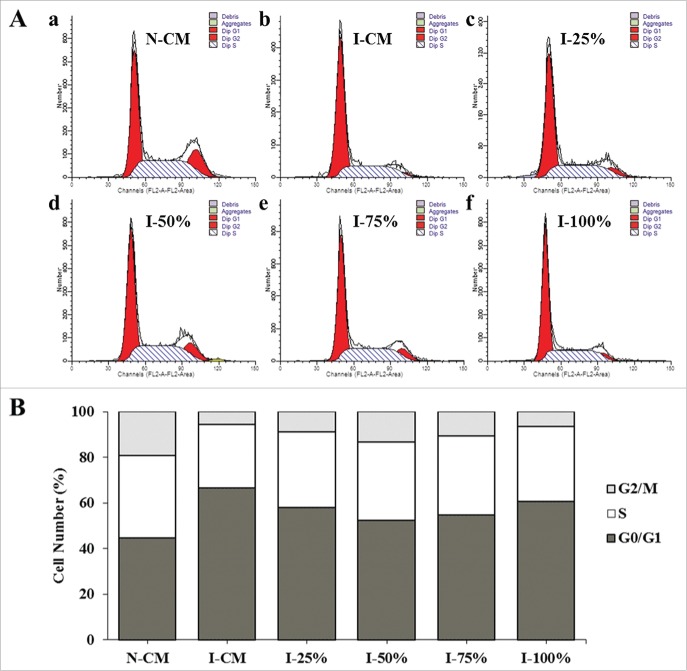
The effect of CDSC-CNM in modulating cell cycle progression of photo-aged HaCaT cells
To study the changes in the cell cycles of the induced cells, three cell subpopulations (G0/G1, S and G2/M) were evaluated by performing a flow cytometric measurement of the DNA distributions of the cells. A decrease in cell proportion was shown in the S and G2/M subpopulations of UVB-irradiated HaCaT cells compared with the normal group in CM, indicating low cell division and proliferative abilities of the photo-aged cells. However, after CDSC-CNM treatment in the photo-aged groups, the cell numbers in the S and G2/M phases increased by varying amounts (Fig. 4A). The data suggest that 50% and 75% CDSC-CNM prompted more cells to enter into the proliferative phase (Fig. 4B).
Figure 4.
Effects of the chorion-derived stem cell conditioned medium (CDSC-CNM) on the cell cycle progression of photo-aged HaCaT cells. After being treated with CDSC-CNM, HaCaT cells were stained with PI and analyzed using flow cytometry. (A) a: Non-irradiated HaCaT cells in a control medium (CM); b: UVB-irradiated HaCaT cells in CM; c-f: UVB-irradiated HaCaT cells treated with 25%, 50%, 75% and 100% CDSC-CNM, respectively. (B) The percentage of HaCaT cells in the G0/G1, S and G2/M phases following treatment with different concentrations of CDSC-CNM. Data are presented as the average ± SD from three independent experiments.
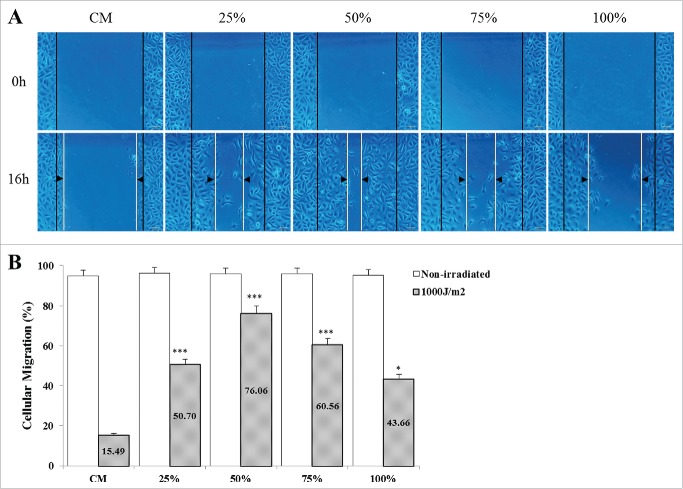
Photo-aged HaCaT cells cultured in CDSC-CNM showed improved cellular migration
The images indicated that CDSC-CNM has the ability to accelerate the cellular migration of photo-aged HaCaT cells. The 50% CDSC-CNM showed the fastest speed of cellular scratch healing (Fig. 5A), and non-irradiated HaCaT cells closed the scratch at 16 h. Photo-aged HaCaT cells cultured in 50% as well as in 75% CDSC-CNM showed significantly increased cellular migration rates compared with those grown in the CM (Fig. 5B).
Figure 5.
Effects of the chorion-derived stem cell conditioned medium (CDSC-CNM) on photo-aged HaCaT cell migration. Images were acquired at 0 and 16 h after incubation using fluorescence or a phase-contrast microscope in an in vitro scratch assay. (A) HaCaT cells were cultured with different concentrations of CDSC-CNM. The dotted lines define the areas of cells migration. The non-irradiated HaCaT cells closed the scratch at 16 h (photo not shown). (B) The rate of migration was measured by quantifying the total distance that the HaCaT cells had moved relative to the scratch (marked by imaginary dotted lines). The data are expressed as the mean of 6-8 replicates ± SD.* (P < 0.05) and *** (P < 0.001) indicate significant differences compared with non-irradiated cells in the same concentrations of CDSC-CNM.
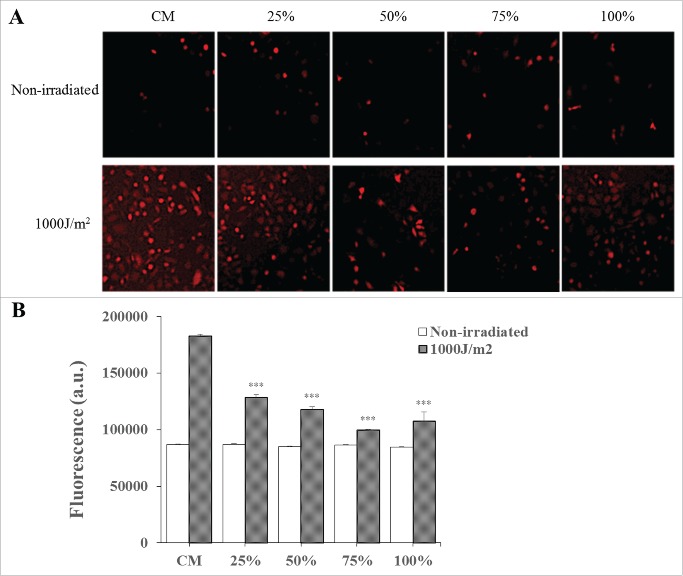
Antioxidant activity in UVB-irradiated HaCaT cells from CDSC-CNM
The images showed the ROS generation in HaCaT cells after UVB radiation in the CM and in the presence of various concentrations of CDSC-CNM (Fig. 6A). As observed, a certain fluorescence signal was observed due to the basal metabolism of HaCaT cells in the absence of irradiation. When cells were irradiated with 1000 J/m2 in CM, a fluorescence signal showed a significant increase (97%) compared with basal condition (0%) due to UVB-induced ROS generation. In the presence of CDSC-CNM, the fluorescence intensity decreased significantly (p<0 .001) compared to irradiated cells in CM. The ability of CDSC-CNM to inhibit ROS generation showed a 56% ROS decrease at 50% CDSC-CNM and a 78% ROS decrease at 75% CDSC-CNM compared to irradiated cells in CM (Fig. 6B).
Figure 6.
Measurement of intracellular ROS generation by the H2DCFDA fluorescent probe. (A) CDSC-CNM decreases UVB-induced ROS in photo-aged HaCaT cells. (B) Total fluorescence is expressed as arbitrary units. The data are expressed as the mean ± SD. *** (P < 0.001) indicates significant differences compared with non-irradiated cells in the same concentrations of CDSC-CNM.
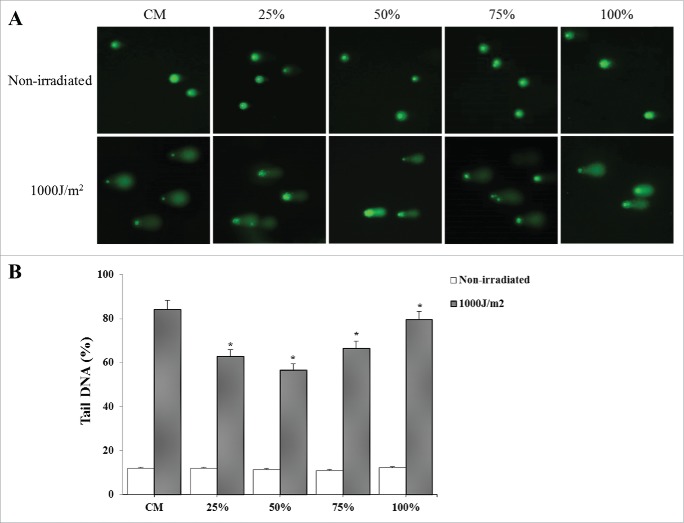
Effects of CDSC-CNM on UVB-induced DNA damage
The degree of nuclear DNA damage in individual cells exposed to UVB was shown by comet assay. The fluorescent images showed representative pictures of the effect of combined extract on comet electrophoresis gels for HaCaT cells that were either irradiated at 1000 J/m2 or not irradiated and the corresponding gels' quantification (Fig. 7A). The comet score of photo-aged HaCaT cells increased considerably, indicating increased DNA damage. By contrast, the presence of 50% CDSC-CNM significantly reduced the DNA damage (p < 0.05) by 26% compared with irradiated cells in CM (Fig. 7B).
Figure 7.
The comet assay of photo-aged HaCaT cells. (A) CDSC-CNM decreases UVB-induced DNA strand break formation in HaCaT cells. To evaluate DNA damage, 50 cells (nuclei) per slide were analyzed. (B) Total damage was expressed in arbitrary units and determined as described in the materials and methods section. The data are expressed as the mean ± SD. * (P < 0.05) indicates significant differences compared with non-irradiated cells in the same concentrations of CDSC-CNM.
Analysis of CDSC-CNM
To investigate growth factors secreted from CDSCs, we performed a cytokine array that is related to epidermic cells. CDSC-CNM contains multiple growth factors, such as EGF, TGF-β, interleukin (IL)-6 and IL-8. The spots were proportional to standard positive spots; CDSC-CNM contained 5-fold higher IL-6, 4-fold higher IL-8 and 2-fold higher monocyte chemotactic protein 1 (MCP-1) than did the CM (Fig. 8).
Figure 8.
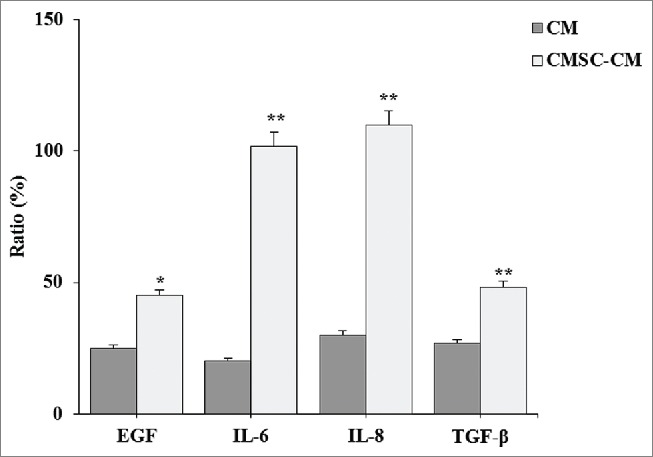
Analysis of cytokines in CDSC-CNM. CDSCs were incubated in DMEM containing 10% FBS and 1% antibiotic-antimycotic. After 72 h, the supernatant was collected and examined with the cytokine ELISA kit. Secretory factor cytokine levels in the chorion-derived stem cell-conditioned medium (CDSC-CNM) were compared with the control medium (CM). The data are expressed as the mean of 6–8 replicates ± SD. * (P < 0.05) and ** (P < 0.01) indicate significant differences compared with CM.
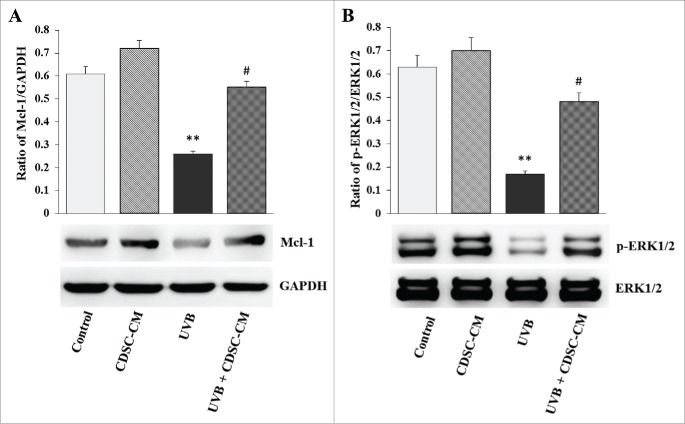
Mcl-1 and phosphorylated Erk1/2 (p-Erk1/2) protein levels in HaCaT cells after treatment with CDSC-CNM
The exposure of HaCaT cells to UVB radiation considerably reduced the expression of the Mcl-1 and p-Erk1/2 proteins. However, this was reversed by CDSC-CNM. The results presented here show that the Mcl-1 protein levels of photo-aged HaCaT cells increased after CDSC-CNM treatment (Fig. 9A). CDSC-CNM modulated Erk1/2 signaling in UVB-irradiated cells to favor cell survival. Specifically, CDSC-CNM prevented the UVB-mediated dephosphorylation of Erk1/2 (Fig. 9B).
Figure 9.
CDSC-CNM regulates the related signaling pathway protein levels and prevents UVB-induced apoptosis. Non-irradiated HaCaT cells were cultured in the control medium (CM) and CDSC-CNM. UVB-irradiated HaCaT cells were also treated with CM and CDSC-CNM. (A) HaCaT cell lysates were electrophoresed in SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted with antibodies specific for Mcl-1 and GADPH. (B) HaCaT cell lysates were electrophoresed in SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted with antibodies specific for Erk1/2 and p-Erk1/2. The data are expressed as the mean ± SD. ** (P < 0.01) indicates significant differences compared with control cells. # (P < 0.05) indicates significant differences compared with UVB-irradiated cells treated with CM.
Discussion
Photo-aging can cause wrinkles, hyper-pigmentation, epidermal hyperplasia and even skin cancer. UVB effects the epidermal basal cell layer of the skin, causing sunburn, tanning and pigmented spots and accelerating skin aging.20 With improvements in health care, more and more people are paying attention to photo-aging and are careful to protect their skin from the sun. Sunscreen is a topical measure to prevent skin from inadvertent UV exposure.21 Dietary plant compounds may significantly contribute to lifelong protection of the skin. Carnosol, naringenins, rosmarinic acid,22 polyphenols of green tea and prunella vulgaris extract have been postulated to be effective agents for attenuating the cell-damaging effects of both UV radiation and ionizing radiation.6,23-25 Moreover, fillers of autologous young fibroblasts isolated from gingiva and autologous adipose cells from the abdomen have been used to treat wrinkles. Many methods have been used for skin repair, such as stem cell injection and somatic cells reprogramming for tissue regeneration.26 However, efficacious and safe treatments to repair photo-injured skin need further research. In this study, CDSC-CNM has shown efficient regenerative and reparative effects on UVB-irradiated keratinocytes.
CDSCs were isolated from the human placenta, where maternal and fetal nutrient and gas exchange occurs. The human placenta, previously considered disposable postpartum tissue, is an abundant source of human stem cells that are capable of proliferation and differentiation into the mesodermal lineage in vitro.27 The chorion is the outer membrane surrounding the fetus and comprises mesenchymal and trophoblastic regions. The stem cells derived from fetal membrane have immunoregulatory action because fetal tolerance inhibits T-cell proliferation and the activation of natural killer cells.28
As shown in our experiments, CDSCs present with a fibroblast-like morphology and express multiple markers that are characteristic of MSCs.29,30 These placenta-derived MSCs may be less vulnerable to immunological reactions and may have a rich secretion function. The results of cell proliferation, cycle and migration indicate that CDSC-CNM has the capacity to promote cell division and increase the survival of UVB-irradiated keratinocytes. Our results reveal that the supernatant of CDSCs may have a mechanism of photoprotective properties related to its capacity to decrease the generation of intracellular radical species, such as H2O2, OH and peroxynitrite, that have been directly linked to DNA oxidative damage.31,32 Intracellular ROS is one of the most damaging effects of UV radiation on skin. The excited oxygen electrons of ROS alter the redox state of the cells, inducing apoptosis and tissue injury and contributing to altered cell growth and differentiation or to the development of skin cancer.33 As our results showed a decreased number of UV-induced DNA breaks, CDSC-CNM may either attenuate the genotoxic effects of UV radiation or protect the DNA repair machinery.
The molecular mechanism of photo-aging is mostly triggered by ROS-mediated activation of cell surface receptors, which leads to the stimulation of stress-associated kinase pathways that are mediated by the activator protein 1 (AP-1) nuclear transcription factor.34 AP-1 prompts prostaglandin release, keratinocyte proliferation and a loss of collagen synthesis,35 resulting in the gene expression of matrix metalloproteinase (MMP) to inhibits membrane TGF-β1 receptor gene expression.36-38 Together, these factors contribute to accelerate extracellular matrix degradation with subsequent damage to lipids, proteins and mitochondrial and nuclear DNA. Therefore, we speculate that the inhibition of UV-induced intracellular ROS is concomitant with a certain level of DNA protection. The potent ROS-scavenging capability of CDSC-CNM is important in the cellular reparative process.
In our experiments, 50% or 75% CDSC-CNM showed the best regenerative and reparative effects on photo-aged epidermal cells. In the cultivation process of CDSCs, active factors were secreted, but nutrients in the supernatant that maintain cell growth were self-consumed. In addition, the improved TGF-β signaling of photo-aged HaCaT cells in 50% or 75% CDSC-CNM might be caused by secretory factors that contain short-mRNAs, which interfere with the genetic programming and induce TGF-β receptor gene repair of photo-aged HaCaT cells. By contrast, the impairment of the TGF-β signaling pathway might result in decreasing activities of photo-aged HaCaT cells.
Many researchers are now focusing on stem cell secretory factors for regenerative medicine because stem cells still express a major histocompatibility complex (MHC) on their membranes, leading to a risk of donor rejection if used as transplanted foreign material.39 Various experiments have shown that factors secreted by stem cells into the conditioned medium have regenerative properties. For example, an adipose-derived stem cell-conditioned medium can induce proliferation and type I collagen synthesis and inhibit MMP-1 expression in UVB-irradiated HDFs.10,40 These effects may be attributed to the antioxidant action of the conditioned medium of adipose-derived stem cells, as previously reported by Kim et al.41 Similar to adipose-derived stem cells, CDSCs also release various active factors into their conditioned medium. Recent reports have demonstrated that high levels of cytokines can be extracted from chorion-derived materials and that placenta-derived stem cells secrete various growth factors, including b-FGF, EGF, TGF-β, IL-6, IL-8, IL-10 and tissue inhibitors of metalloproteinases.42 These factors might play a vital role in the regenerative and reparative effects on UVB-irradiated keratinocytes. EGF is specialized to accelerate the proliferation of epidermal cells. The TGF-β level of a supernatant is related to the vitality of cells, and the TGF-β–smad signaling pathway is well known to be important in the migration of keratinocytes.36,43 Moreover, IL-6 and IL-8 induce epithelialization by triggering the migration of fibroblasts and keratinocytes.44,45 Furthermore, various molecules and mechanisms may be involved in the reparative process, including the inhibition of the UVB-induced p53 reporter, the activation of the Wnt/β-catenin signaling pathway and high NF-E2-related factor 2 (Nrf2) activity that protects basal keratinocytes from ROS damage.46-48 CDSC-CNM prevents UVB-induced apoptotic cell death in human HaCaT cells by modulating Bcl-2, Mcl-1, and Bax expression as well as by regulating MAPK and mitochondrial signaling pathways. IL-6 in the conditioned medium induced an increase in Mcl-1 protein levels in HaCaT cells by regulating the JAK/STAT signaling pathway. The level of p-Erk1/2 protein, which is correlated with the activation of MAPK signaling, also increased after treatment with CDSC-CNM. Therefore, secretory factors modulate the expression of anti- and pro-apoptotic effectors to safeguard HaCaT cells against UVB radiation. However, the definite mechanisms of the secretory factors of CDSCs need further study.
In conclusion, this study demonstrated the regenerative and reparative effects of CDSC-CNM on photo-aged epidermal cells in vitro. Considering all of the above-mentioned evidence after CDSC-CNM treatment, the increased cell survival and the decreased intracellular ROS generation and DNA damage, we suggest that CDSC-CNM may be able to attenuate further major events leading to photo-aging. Although molecular mechanism research and future in vivo studies are required, we believe that CDSC-CNM is potentially applicable in aging resistance and skin photo-aging treatment.
Materials and methods
Ethics statement: Tissues were obtained from human subjects after they provided informed consent. The protocol was approved by the national ethics committee in China.
Materials
The HaCaT cells were obtained from ATCC Cell Lines Service (Manassas, VA, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS) and penicillin–streptomycin were obtained from Gibco (Carlsbad, CA, USA). A human MSC analysis kit was obtained from BD Biosciences (San Jose, CA, USA). UVB light was purchased from Philips (Germany). Cell count kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). Propidium iodide was purchased from Sigma (St Louis, MO, USA). 2', 7'-Dichlorodihydrofluorescein diacetate (H2DCFDA) was purchased from KeyGEN Biotechnologies Co. (China). The reagent kit for a single-cell gel electrophoresis assay was obtained from Trevigen Inc. (Gaithersburg, MD, USA). SYBR Green I was purchased from Gibco (Carlsbad, CA, USA). Human EGF enzyme-linked immuno sorbent assay (ELISA), human TGF-β ELISA, human IL-6 ELISA and human IL-8 ELISA kits were purchased from Rapidbio (West Hills, CA, USA). The primary anti-Mcl-1, -phospho-Erk1/2 (Thr202/Tyr204) and -Erk1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA). All other chemicals were analytical grade.
Cell isolation and culture
CDSC isolation and culture
Informed consent was obtained from all subjects in accordance with the regulations of the Department of Obstetrics and Gynecology, Chinese PLA General Hospital, Beijing, China.
Human placenta tissue was obtained from tissue that was disposed after cesarean section. The obtained samples were washed with PBS and digested with 0.1% collagenase type II (Sigma-Aldrich, St Louis, MO, USA) in a thermostatic shaker THZ-881K (China) at 250 r/min for 90 min at 37°C. The digested samples were washed twice with PBS and centrifuged at 2000 r/min for 10 min. After centrifugation, the supernatant was discarded. The pellet was resuspended in GM, seeded in culture dishes, and incubated overnight at 37°C in a 5% CO2 atmosphere. To remove debris, cells were washed with PBS, and fresh culture medium was added after 48 h. The medium was then exchanged every 72 hours until the cells reached 90% confluence and were passaged after trypsinization. The cells used for analysis were from passages 3 to 5. The CDSC morphology was photographed with the use of an inverted microscope (Olympus IX71, Tokyo, Japan).
HaCaT cell culture
HaCaT cells were cultured in GM in a humidified atmosphere with 5% CO2 at 37°C. The HaCaT cells were trypsinized when the cells reached 90% confluence and were seeded in 96- or 24-well plates, depending on the assay.
Detection of mesenchymal stem cell-specific antigens
For flow cytometry analysis, cultured CDSCs were trypsinized and resuspended in FBS. The cells were incubated with monoclonal fluorescein antibodies for CD73, CD90, CD105, HLA-DR, CD19, CD34 and CD45 for 30 min. As a negative control, cells were incubated with isotype control immunoglobulin G1. Incubated cells were fixed with 0.5% paraformaldehyde and analyzed on a flow cytometer-fluorescence-activated cell sorting (FACS) Verse (Becton Dickinson, Franklin Lakes, NJ, USA) with the use of FACSuite software.
CDSC-CNM collection
CDSC-CNM was collected from the supernatant of a high-density CDSC culture. Then, 7 × 105/mL passage-4 CDSCs were seeded in the GM in 100-mm culture dishes. When cells grew at a density of 3.5 × 105/cm2, the GM was exchanged with fresh DMEM containing 1% FBS and 1% penicillin–streptomycin which is the same as CM for 72 hours as previously performed by Potapova et al.49 The supernatant was then collected, centrifuged at 200 g for 10 min, and maintained at −20°C until use.
Experiments
UVB radiation-inducing photo-aged HaCaT cells
UVB-induced HaCaT cells were prepared based on a procedure published by Abu-Yousif et al.5 Briefly, three replicates of HaCaT cells were cultured in GM in 5% CO2 at 37°C for 24 hours. The HaCaT cells were then washed with PBS and exposed to UVB with 3 to 4 drops of PBS. UVB irradiation was provided by UVB lamps (Philips, Germany) at 1000 J/m2. The PBS was then replaced with different concentrations of CDSC-CNM. In addition, non-UVB irradiated HaCaT cells were cultured in different plates with similar washing and medium replacement procedures. The cells were subsequently incubated for assays.
Treatment with CDSC-CNM
Immediately after UVB irradiation (1000J/m2), HaCaT cells were treated with CM and 25%, 50%, 75% or 100% CDSC-CNM diluted in DMEM supplemented with 1% FBS. The CM also consisted of DMEM plus 1% FBS. Non-irradiated HaCaT cells were cultured in GM and CM. All cells were cultured at 37°C in 5% CO2, and the medium was replaced every 72 hours. Each treatment was replicated 3 times. The CDSC-CNM concentration that resulted in the best proliferation rate was used in subsequent experiments to assess the migration ability, ROS generation, and DNA damage of HaCaT cells.
Measurements
Proliferation analysis
Cellular viability was determined using a CCK-8 assay. HaCaT cells (2 × 103 cells/well) were seeded in 96-well plates. After UVB irradiation, HaCaT cells were treated with GM, CM and different concentrations of CDSC-CNM for 72 hours. The medium was removed, the cells were washed with PBS, and 100 µL of solution consisting of 10% CCK-8 and 90% fresh serum-free DMEM (containing 1% penicillin–streptomycin) were added to each well. The plates were wrapped with aluminum foil and incubated at 37°C in 5% CO2 for 2 hours. The supernatant was measured with a Synergy2 multi-detection microplate reader (Biotek, VT, USA) at 450 nm. The optical density of each well was calculated against the relative HaCaT cell standard curve. HaCaT cell proliferation was photographed with the use of an inverted microscope. The proliferation analysis was conducted in triplicate.
Cell cycle
HaCaT cells (1 × 104 cells/well) were seeded in 6-well plates. After 1000J UVB irradiation, HaCaT cells were treated with CM and different concentrations of CDSC-CNM for 72 hours. Normal HaCaT cells were treated with CM as a control. After fixation with 75% ethanol, single-cell suspensions were digested with DNase-free RNase in PBS containing 5 µg/mL propidium iodide for DNA staining (30 min at 37°C). The propidium iodide fluorescence and forward light scattering were detected with a flow cytometer equipped with cellquest (Largo, FL, USA) software. The percentage of cells in every phase of the cell cycle was calculated.
Cellular migration assay
A cellular migration assay was performed based on a method by Liang et al.50 with slight modification. Briefly, HaCaT cells were cultured in a 24-well black plate until 90-100% confluence was reached. After UVB irradiation, all wells were linearly scratched with a p200 pipet tip through the center of the well bottom, followed by incubation with CM and 25%, 50%, 75% or 100% CDSC-CNM diluted in DMEM supplemented with 1% FBS. The images were taken using an inverted microscope. For each image, distances between one side of the scratch and the other were measured at certain intervals (mm) using Image Pro-Plus software (Media Cybernetics). The capacity for migration was determined by comparing the images from time 0 to the last time point to obtain the distance of each scratch closure on the basis of the distances measured by software. At least three readings of distance for each sample were obtained, and each experiment was repeated at least three times.
ROS generation evaluation
To study the potential protective effect of CDSC-CNM on the attenuation of intracellular ROS generation induced by UVB radiation, H2DCFDA was used to monitor the intracellular ROS generation induced by UVB radiation.51 H2DCFDA is a probe that becomes fluorescent when oxidized by free radicals and is especially sensitive to H2O2, OH and peroxynitrite at the intracellular level. Cells were cultured in a 96-well black plate until 90-100% confluence was reached. The cells were then were exposed to UVB light, treated with CM and different concentrations of CDSC-CNM, and labeled with H2DCFDA. The supernatant was measured with the use of a Synergy2 multi-detection microplate reader (Biotek, VT, USA).
Alkaline comet assay
To study the level of nuclear DNA damage in individual cells exposed to UVB, a comet assay was performed as previously reported.23 A total of 50 nuclei by duplicate were utilized to determine the comet score. The gels were analyzed by fluorescence microscopy (Olympus U-RFL-T, Tokyo, Japan) after being dyed with SYBR Green I. DNA damage was quantified by calculating the tail and nuclear proportion using comet assay software.
Cytokine assay
To analyze the reparative and regenerative factors of epidermal cells in CDSC-CNM, the supernatant collected from CDSCs was centrifuged at 2000-3000 r/min for 20 min. The supernatant then was detected by a series of human cytokine ELISA kits in accordance with the manufacturer's instructions. CDSC-CNM was detected for EGF, TGF-β, IL-6 and IL-8. The CM was also detected as a control.
Western blot
HaCaT cells were exposed to UVB (1000J/m2) and harvested after being treated with different concentrations of CDSC-CNM for 24 h. Cells were lysed in a 5× SDS sample buffer. After the samples were boiled, equal amounts of total lysates were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were soaked in a blocking solution (5% skim milk and 0.2% Tween 20-PBS) for 1 h and then incubated with anti-Mcl-1, anti-Erk1/2, and anti-p-Erk1/2 antibodies for 1 h. GADPH (R&D Systems, Wiesbaden, Germany) was used for internal loading control. After being washed with Tween 20-PBS, the membranes were incubated with appropriate HRP-conjugated secondary antibodies for 1 h. Specific bands were visualized using an ECL method (ECL+; Amersham Biosciences).
Statistical analysis
The data were presented as an average ± standard error of 4–8 determinations, depending on the assay. One-way analysis of variance (ANOVA) and statistical comparisons of the different treatments were performed using Tukey's test with SPSS software, Version 12.0 (SPSS Inc., Chicago, IL). A tied-P value of < 0.05 was considered statistically significant.
Abbreviations
- AP-1
activator protein 1
- b-FGF
basic fibroblast growth factor
- CDSC-CNM
chorion-derived stem cell conditioned medium
- CDSCs
chorion-derived stem cells
- CM
control medium
- DMEM
Dulbecco's modified Eagle medium
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- GM
growth medium
- IL
interleukin
- MAPKs,
mitogen-activated protein kinases
- MCP-1
monocyte chemotactic protein 1
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- MSC
mesenchymal stromal cell
- p-Erk1/2
phosphorylated Erk1/2
- PBS
phosphate-buffered saline
- ROS
radical oxygen species
- TGF-β
transforming growth factor-β
- UV
ultraviolet
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported in part by the National Nature Science Foundation of China (81571905, 81171798, 81421064, 81230041), Beijing Municipal Natural Science Foundation (Grant No. 7142124) and the National Basic Science and Development Program (973 Program, 2012CB518105).
References
- [1].Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol 2007; 157:874-87; PMID:17711532; http://dx.doi.org/ 10.1111/j.1365-2133.2007.08108.x [DOI] [PubMed] [Google Scholar]
- [2].Marionnet C, Tricaud C, Bernerd F. Exposure to non-extreme solar UV daylight: spectral characterization, effects on skin and photoprotection. Int J Mol Sci 2015; 16:68-90; http://dx.doi.org/ 10.3390/ijms16010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sola Y, Lorente J. Contribution of UVA irradiance to the erythema and photoaging effects in solar and sunbed exposures. J Photochem Photobiol B 2015; 143:5-11; PMID:25579807; http://dx.doi.org/ 10.1016/j.jphotobiol.2014.10.024 [DOI] [PubMed] [Google Scholar]
- [4].Svobodova A, Psotova J, Walterova D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2003; 147:137-45; PMID:15037894; http://dx.doi.org/ 10.5507/bp.2003.019 [DOI] [PubMed] [Google Scholar]
- [5].Abu-Yousif AO, Smith KA, Getsios S, Green KJ, Van Dross RT, Pelling JC. Enhancement of UVB-induced apoptosis by apigenin in human keratinocytes and organotypic keratinocyte cultures. Cancer Res 2008; 68:3057-65; PMID:18413777; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2763 [DOI] [PubMed] [Google Scholar]
- [6].Vostalova J, Zdarilova A, Svobodova A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes. Arch Dermatol Res 2010; 302:171-81; PMID:19862537; http://dx.doi.org/ 10.1007/s00403-009-0999-6 [DOI] [PubMed] [Google Scholar]
- [7].Kim MJ, Woo SW, Kim MS, Park JE, Hwang JK. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J Asian Nat Prod Res 2014; 16:1139-47; PMID:25465718; http://dx.doi.org/ 10.1080/10286020.2014.983092 [DOI] [PubMed] [Google Scholar]
- [8].El-Mahdy MA, Zhu Q, Wang QE, Wani G, Patnaik S, Zhao Q, Arafa E, Barakat B, Mir SN, Wani AA. Naringenin protects HaCaT human keratinocytes against UVB-induced Apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochem Photobiol 2008; 84:307-16; PMID:18086244; http://dx.doi.org/ 10.1111/j.1751-1097.2007.00255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang JA, Chung HM, Won CH, Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther 2010; 10:495-503; PMID:20218919; http://dx.doi.org/ 10.1517/14712591003610598 [DOI] [PubMed] [Google Scholar]
- [10].Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 2009; 53:96-102; PMID:18829265; http://dx.doi.org/ 10.1016/j.jdermsci.2008.08.007 [DOI] [PubMed] [Google Scholar]
- [11].Xu Y, Zhang JA, Xu Y, Guo SL, Wang S, Wu D, Wang Y, Luo D, Zhou BR. Antiphotoaging effect of conditioned medium of dedifferentiated adipocytes on skin in vivo and in vitro: a mechanistic study. Stem Cells Dev 2015; 24:1096-111; PMID:25517994; http://dx.doi.org/ 10.1089/scd.2014.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013; 10:493-500; PMID:23902526; http://dx.doi.org/ 10.1111/iwj.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun 2013; 438:410-9; PMID:23899518; http://dx.doi.org/ 10.1016/j.bbrc.2013.07.088 [DOI] [PubMed] [Google Scholar]
- [14].Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16:103-19; PMID:22239440; http://dx.doi.org/ 10.1517/14728222.2011.645805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Assefa Z, Van Laethem A, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta 2005; 1755:90-106; PMID:15964692 [DOI] [PubMed] [Google Scholar]
- [16].Sitailo LA, Jerome-Morais A, Denning MF. Mcl-1 functions as major epidermal survival protein required for proper keratinocyte differentiation. J Invest Dermatol 2009; 129:1351-60; PMID:19037233; http://dx.doi.org/ 10.1038/jid.2008.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahashi H, Honma M, Ishida-Yamamoto A, Namikawa K, Miwa A, Okado H, Kiyama H, Iizuka H. In vitro and in vivo transfer of bcl-2 gene into keratinocytes suppresses UVB-induced apoptosis. Photochem Photobiol 2001; 74:579-86; PMID:11683038; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [18].Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 2005; 115:2618-24; PMID:16200194; http://dx.doi.org/ 10.1172/JCI26273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J 2010; 277:2-21; PMID:19843174; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07366.x [DOI] [PubMed] [Google Scholar]
- [20].Reich A, Medrek K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int J Mol Sci 2013; 14:8456-66; PMID:23594996; http://dx.doi.org/ 10.3390/ijms14048456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Latha MS, Martis J, Shobha V, Sham SR, Bangera S, Krishnankutty B, Bellary S, Varughese S, Rao P, Naveen KB. Sunscreening agents: a review. J Clin Aesthet Dermatol 2013; 6:16-26; PMID:23320122 [PMC free article] [PubMed] [Google Scholar]
- [22].Park M, Han J, Lee CS, Soo BH, Lim KM, Ha H. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp Dermatol 2013; 22:336-41; PMID:23614740; http://dx.doi.org/ 10.1111/exd.12138 [DOI] [PubMed] [Google Scholar]
- [23].Psotova J, Svobodova A, Kolarova H, Walterova D. Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J Photochem Photobiol B 2006; 84:167-74; PMID:16631374; http://dx.doi.org/ 10.1016/j.jphotobiol.2006.02.012 [DOI] [PubMed] [Google Scholar]
- [24].Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells 2012; 4:53-61; PMID:22993662; http://dx.doi.org/ 10.4252/wjsc.v4.i6.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jones GN, Moschidou D, Puga-Iglesias TI, Kuleszewicz K, Vanleene M, Shefelbine SJ, Bou-Gharios G, Fisk NM, David AL, De Coppi P, et al.. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. Plos One 2012; 7:e43395; PMID:22962584http://dx.doi.org/ 10.1371/journal.pone.0043395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao Z, Xu M, Wu M, Ma K, Sun M, Tian X, Zhang C, Fu X. Direct reprogramming of human fibroblasts into sweat gland-like cells. Cell Cycle 2015; 14:3498-505; PMID:26566868; http://dx.doi.org/ 10.1080/15384101.2015.1093707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kmiecik G, Niklinska W, Kuc P, Pancewicz-Wojtkiewicz J, Fil D, Karwowska A, Karczewski J, Mackiewicz Z. Fetal membranes as a source of stem cells. Adv Med Sci 2013; 58:185-95; PMID:24327530; http://dx.doi.org/ 10.2478/ams-2013-0007 [DOI] [PubMed] [Google Scholar]
- [28].Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta 2009; 30:2-10; PMID:18995896; http://dx.doi.org/ 10.1016/j.placenta.2008.09.009 [DOI] [PubMed] [Google Scholar]
- [29].Fariha MM, Chua KH, Tan GC, Tan AE, Hayati AR. Human chorion-derived stem cells: changes in stem cell properties during serial passage. Cytotherapy 2011; 13:582-93; PMID:21231803; http://dx.doi.org/ 10.3109/14653249.2010.549121 [DOI] [PubMed] [Google Scholar]
- [30].Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 2006; 194:664-73; PMID:16522395; http://dx.doi.org/ 10.1016/j.ajog.2006.01.101 [DOI] [PubMed] [Google Scholar]
- [31].Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 2011; 20:108-20; PMID:19995679; http://dx.doi.org/ 10.1016/j.jtv.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang L, Liu Y, Wu S. The roles of nitric oxide synthase and eIF2alpha kinases in regulation of cell cycle upon UVB-irradiation. Cell Cycle 2010; 9:38-42; PMID:20016280; http://dx.doi.org/ 10.4161/cc.9.1.10268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zanoni TB, Hudari F, Munnia A, Peluso M, Godschalk RW, Zanoni MV, den Hartog GJ, Bast A, Barros SB, Maria-Engler SS, et al.. The oxidation of p-phenylenediamine, an ingredient used for permanent hair dyeing purposes, leads to the formation of hydroxyl radicals: Oxidative stress and DNA damage in human immortalized keratinocytes. Toxicol Lett 2015; 239:194-204; PMID:26456176; http://dx.doi.org/ 10.1016/j.toxlet.2015.09.026 [DOI] [PubMed] [Google Scholar]
- [34].Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J BIOL CHEM 2006; 281:27389-97; PMID:16849327; http://dx.doi.org/ 10.1074/jbc.M602355200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996; 379:335-9; PMID:8552187; http://dx.doi.org/ 10.1038/379335a0 [DOI] [PubMed] [Google Scholar]
- [36].Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am J Pathol 2004; 165:741-51; PMID:15331399; http://dx.doi.org/ 10.1016/S0002-9440(10)63337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hwang E, Lee DG, Park SH, Oh MS, Kim SY. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type I and MMP-1 expression. J Med Food 2014; 17:985-95; PMID:25019675; http://dx.doi.org/ 10.1089/jmf.2013.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Quan T, Wang F, Shao Y, Rittie L, Xia W, Orringer JS, Voorhees JJ, Fisher GJ. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol 2013; 133:658-67; PMID:23096713; http://dx.doi.org/ 10.1038/jid.2012.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rossignol J, Boyer C, Thinard R, Remy S, Dugast AS, Dubayle D, Dey ND, Boeffard F, Delecrin J, Heymann D, et al.. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med 2009; 13:2547-58; PMID:20141619; http://dx.doi.org/ 10.1111/j.1582-4934.2008.00657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Song SY, Jung JE, Jeon YR, Tark KC, Lew DH. Determination of adipose-derived stem cell application on photo-aged fibroblasts, based on paracrine function. Cytotherapy 2011; 13:378-84; PMID:21062113; http://dx.doi.org/ 10.3109/14653249.2010.530650 [DOI] [PubMed] [Google Scholar]
- [41].Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 2008; 49:133-42; PMID:17870415; http://dx.doi.org/ 10.1016/j.jdermsci.2007.08.004 [DOI] [PubMed] [Google Scholar]
- [42].Passeron T, Namiki T, Passeron HJ, Le Pape E, Hearing VJ. Forskolin protects keratinocytes from UVB-induced apoptosis and increases DNA repair independent of its effects on melanogenesis. J Invest Dermatol 2009; 129:162-6; PMID:18580960; http://dx.doi.org/ 10.1038/jid.2008.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev 2005; 16:15-34; PMID:15733830; http://dx.doi.org/ 10.1016/j.cytogfr.2004.11.002 [DOI] [PubMed] [Google Scholar]
- [44].Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol 2007; 156:1163-71; PMID:17441960; http://dx.doi.org/ 10.1111/j.1365-2133.2007.07867.x [DOI] [PubMed] [Google Scholar]
- [45].Michel G, Kemeny L, Peter RU, Beetz A, Ried C, Arenberger P, Ruzicka T. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. Febs Lett 1992; 305:241-3; PMID:1299623; http://dx.doi.org/ 10.1016/0014-5793(92)80677-9 [DOI] [PubMed] [Google Scholar]
- [46].Lee J, Shin YK, Song JY, Lee KW. Protective mechanism of morin against ultraviolet B-induced cellular senescence in human keratinocyte stem cells. Int J Radiat Biol 2014; 90:20-8; PMID:23952478; http://dx.doi.org/ 10.3109/09553002.2013.835502 [DOI] [PubMed] [Google Scholar]
- [47].Zhang C, Chen P, Fei Y, Liu B, Ma K, Fu X, Zhao Z, Sun T, Sheng Z. Wnt/β-catenin signaling is critical for dedifferentiation of aged epidermal cells in vivo and in vitro. Aging Cell 2012; 11:14-23; PMID:21967252; http://dx.doi.org/ 10.1111/j.1474-9726.2011.00753.x [DOI] [PubMed] [Google Scholar]
- [48].Schafer M, Dutsch S, Auf DKU, Werner S. Nrf2: a central regulator of UV protection in the epidermis. Cell Cycle 2010; 9:2917-8; PMID:20699662; http://dx.doi.org/ 10.4161/cc.9.15.12701 [DOI] [PubMed] [Google Scholar]
- [49].Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007; 25:1761-8; PMID:17395769; http://dx.doi.org/ 10.1634/stemcells.2007-0022 [DOI] [PubMed] [Google Scholar]
- [50].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2:329-33; PMID:17406593; http://dx.doi.org/ 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- [51].Herranz-Lopez M, Fernandez-Arroyo S, Perez-Sanchez A, Barrajon-Catalan E, Beltran-Debon R, Menendez JA, Alonso-Villaverde C, Segura-Carretero A, Joven J, Micol V. Synergism of plant-derived polyphenols in adipogenesis: perspectives and implications. Phytomedicine 2012; 19:253-61; PMID:22280831; http://dx.doi.org/ 10.1016/j.phymed.2011.12.001 [DOI] [PubMed] [Google Scholar]