The Ras/Raf/MEK/ERK pathway plays a crucial role in physiological processes and its aberrant activity drives various tumor entities.1 The three RAF isoforms (A-Raf, B-Raf and Raf-1) are major ERK pathway regulators. Commensurate with its role as a signaling hub, B-Raf, the most potent MEK activator, represents the most frequently mutated protein kinase in cancer. Knock-out/knock-in approaches delivered critical insights into the unique and overlapping functions of Raf isoforms. If Braf deficiency is introduced via the germline, Braf−/− mice display a lethal placental phenotype and, if deficiency is limited to the nervous system, a myelination defect restricts viability to the first 5 postnatal weeks (see Refs. 2, 3 for discussion and references).
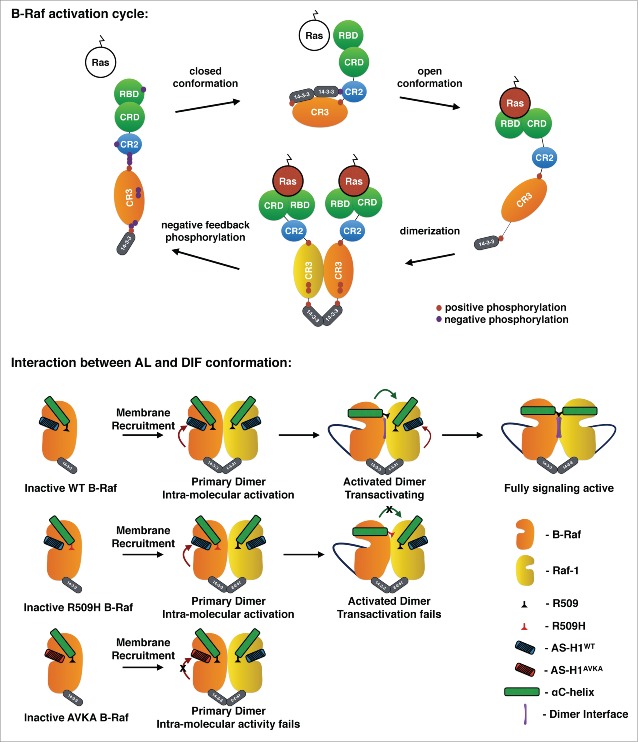
Tight regulation of B-Raf activity is achieved by the incompletely understood protein-protein interaction and (de)phosphorylation events guiding B-Raf through its activation cycle.1,4 In its inactive state, B-Raf resides in an auto-inhibited conformation in the cytoplasm that is stabilized by 14-3-3 proteins binding to phospho-S365 in the N-terminal regulatory moiety and the C-terminus of the kinase (Fig. 1). Upon interaction with Ras, 14-3-3 is displaced from phospho-S365, thereby exposing the kinase domain of B-Raf, which now forms either homo- or heterodimers.1 In addition, Ras promotes the in cis auto-phosphorylation of T599 and S602 in the evolutionary conserved TVKS-motif of the B-Raf activation loop (AL).1,5 These phosphorylations induce conformational changes in the kinase domain, leading to the alignment of the so-called C- and R-spine residues, ATP uptake and ultimately MEK phosphorylation.1 These conformational changes expose, the dimer interface (DIF), a set of amino acid residues forming a contact zone essential for the allosteric activation of the receiver by the activator protomer.1,4 The relevance of TVKS-motif phosphorylation was demonstrated by experiments showing that substitution of T599/S602 by alanine (AVKA) and phospho-mimetic (EVKD) residues impairs Ras-induced activity and confers transforming properties to B-Raf, respectively.3,5 The dominance of V600 substitutions in tumor-associated BRAF mutations further underscores that AL phosphorylation mimicking mutations induce conformational changes that cut the B-Raf activation cycle short. Indeed, B-RafV600E signals independently of RAS, 14-3-3 binding, critical phosphorylation sites and, although it forms particularly stable dimers in its normal state, an intact DIF.3,4 Thus, various lines of evidence suggest that AL phosphorylation induces conformational changes in the B-Raf kinase domain, promoting both dimerization and kinase activity (Fig. 1) and that, once this conformation is stabilized by V600E mutation-specific effects,1,4 AL phosphorylation becomes redundant.
Figure 1.
Upper sketch: Model of the B-Raf activation cycle. Negative feedback phosphorylations disrupt the B-Raf/Raf-1 hetero-dimer, leading to re-formation of the closed B-Raf monomer.1 The regulatory moiety consists of the Ras-binding domain, the cysteine-rich domain and the conserved region 2 encompassing S365. Lower sketch: Interplay between AL phosphorylation, dimerization and transactivation. Top: Dimerization of B-Raf with Raf-1 induces AL phosphorylation (red arrow) followed by conformational changes promoting DIF formation and Raf-1 transactivation. Middle: The R509H mutation prevents DIF formation and Raf-1 transactivation. Bottom: The AVKA mutation in B-Raf precludes the conformational change leading to DIF formation and Raf-1 transactivation.
In addition to point mutations like BRAFV600E, chromosomal rearrangements represent alternative tumor-associated BRAF alterations. The resulting fusion oncoproteins lack the N-terminal regulatory moiety, but expose an intact kinase domain with increased homo-dimerization potential.1,6 Hence, they are regarded as constitutively active, although little is known about their regulatory requirements, e.g. whether they require AL phosphorylation.
To further investigate the relevance of the TVKS-motif for B-Raf signaling in development and physiology, we generated a conditional knock-in allele allowing the production a B-Raf mutant with alanine substitutions of T599 and S602.3 Although this BrafAVKA allele produces a kinase with significantly impaired activity, mice homozygous for this allele were surprisingly viable, fertile and had a normal life span. Nevertheless, BrafAVKA mice presented with mild abnormalities in the haematopoietic system, a distinct facial morphology, reduced MEK/ERK phosphorylation in the brain and slight gait abnormalities. Thus, genetic impairment of AL phosphorylation does not phenocopy the lethality of Braf−/− mice, further supporting a scaffolding role for B-Raf. This concept is supported by (pre)clinical observations showing that drug-bound or kinase-dead B-Raf provokes paradoxical ERK-pathway activation, a phenomenon that underlies therapy resistance, inhibitor promoted secondary neoplasms and restricts the application of clinically approved B-Raf inhibitors to BRAFV600E/K mutant tumors.1,7 Interestingly, however, B-RafAVKA did not provoke paradoxical ERK activation like the kinase-dead B-RafD594A mutant,3,4,7 suggesting that the inability of B-RafAVKA to undergo AL phosphorylation precludes a proper DIF conformation in which it can trans-activate another protomer, e.g., Raf-1 (Fig. 1). Consequently, Raf‐1, which still interacts with B‐RafAVKA, cannot be trans-activated, ultimately resulting in impaired MEK activation (Fig. 1). Interestingly, this phenotype mimics the DIF mutant B-RafR509H that also interacts with Raf-1, but fails to trans-activate its dimerization partner.4 These related phenotypes further illustrate the tight relation between AL phosphorylation and dimerization, as also proposed by structural studies (see Refs. 1, 3 for discussion and references).
Given the mild phenotype of BrafAVKA mice, a pharmacological strategy mimicking the effects of the AVKA mutation might allow quenching B-Raf activity without inducing debilitating side-effects, incl. paradoxical ERK activation. Therefore, we analyzed the requirement for the TVKS-motif in B-Raf oncoproteins. As expected from structural studies showing that B-RafV600E is locked in an active conformation1, alanine substitution of T599/S602 did not affect its signaling potential. In contrast, the AVKA mutation reduced the transformation potential of tumor-associated B-Raf oncoproteins such as the dimer promoting B-RafE586K mutant or the FAM131B-B-Raf fusion protein. These findings have two implications. Firstly, they highlight a strategy to target tumors with non-V600E mutants that might resist V600E/K selective drugs, as it has been observed for the vemurafenib-insensitive fusion proteins.3,6 Secondly, our findings illustrate that B-Raf fusions and oncogenic non-V600E point mutants are not as constitutively active as previously thought, but still retain a certain level of regulation that could be exploited pharmacologically.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Lavoie H, Therrien M. Nat Rev Mol Cell Biol 2015; 16:281-98; PMID:25907612; http://dx.doi.org/ 10.1038/nrm3979 [DOI] [PubMed] [Google Scholar]
- [2].Desideri E, et al.. Cell 2015; 161:967-70; PMID:26000477; http://dx.doi.org/ 10.1016/j.cell.2015.04.045 [DOI] [PubMed] [Google Scholar]
- [3].Köhler M, et al.. EMBO J 2016; 35:143-61; PMID:26657898; http://dx.doi.org/ 10.15252/embj.201592097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Röring M, et al.. EMBO J 2012; 31:2629-47; PMID:22510884; http://dx.doi.org/ 10.1038/emboj.2012.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang BH, Guan KL. EMBO J 2000; 19:5429-39; PMID:11032810; http://dx.doi.org/ 10.1093/emboj/19.20.5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sievert AJ, et al.. Proc Natl Acad Sci U S A 2013; 110:5957-62; PMID:23533272; http://dx.doi.org/ 10.1073/pnas.1219232110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heidorn SJ, et al.. Cell 2010; 140:209-21; PMID:20141835; http://dx.doi.org/ 10.1016/j.cell.2009.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]